The Impact of Hanseniaspora vineae Fermentation and Ageing on Lees on the Terpenic Aromatic Profile of White Wines of the Albillo Variety
Abstract
:1. Introduction
2. Results
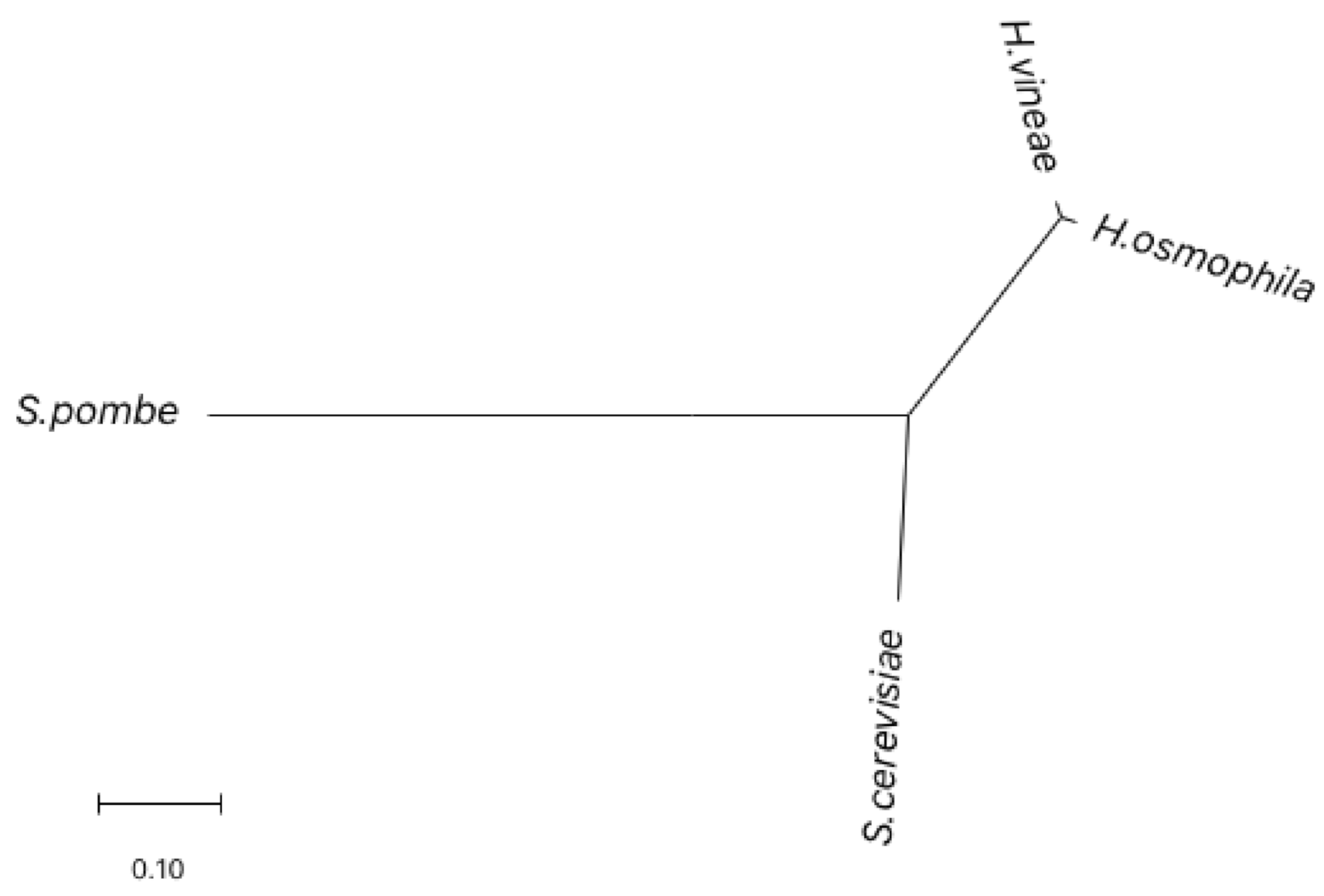
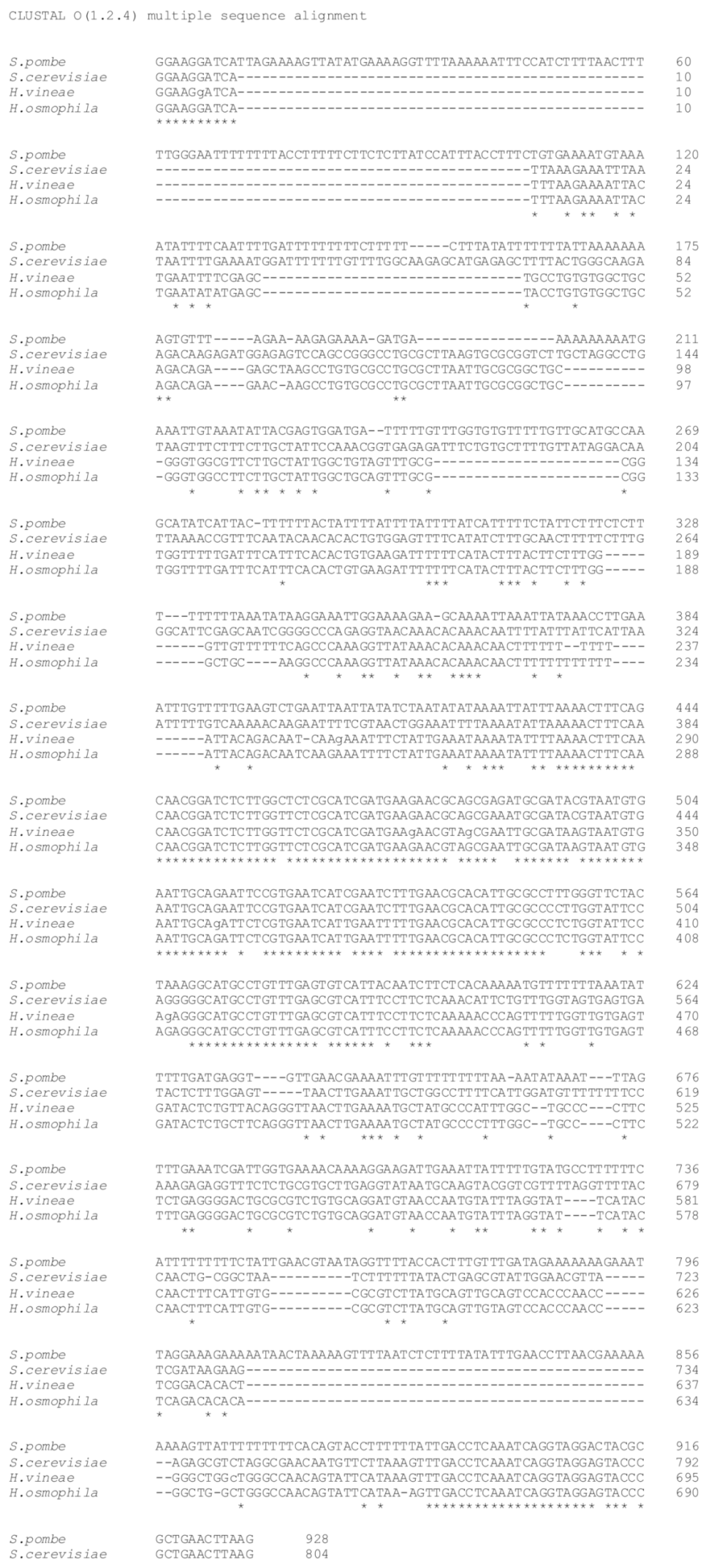
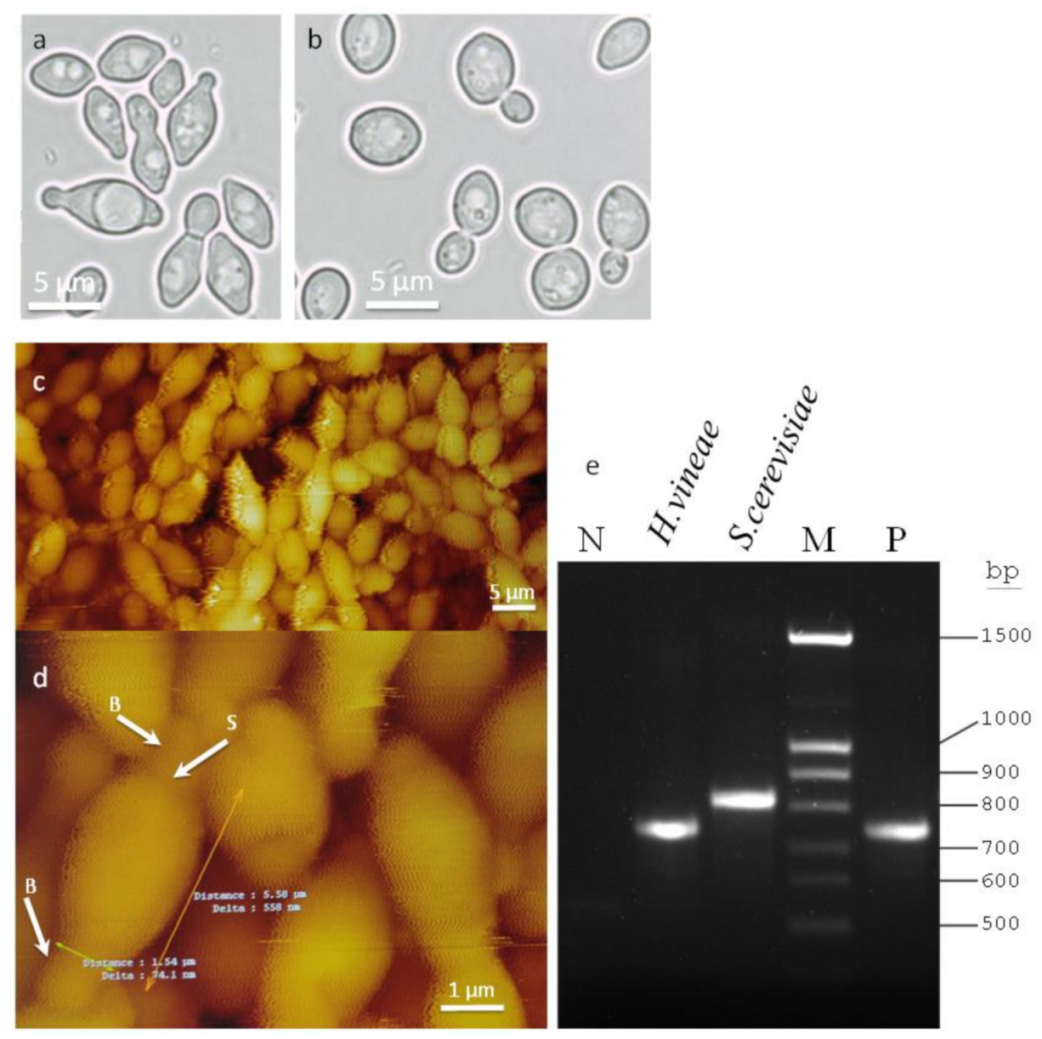
2.1. Morphology and Molecular Identification of H. vineae
2.2. General Oenological Parameters
2.3. Aroma Compounds
2.3.1. Terpenes and Polyoxygenated Terpenes
2.3.2. Aldehydes, C6 Compounds, Alcohols, and Volatile Phenols
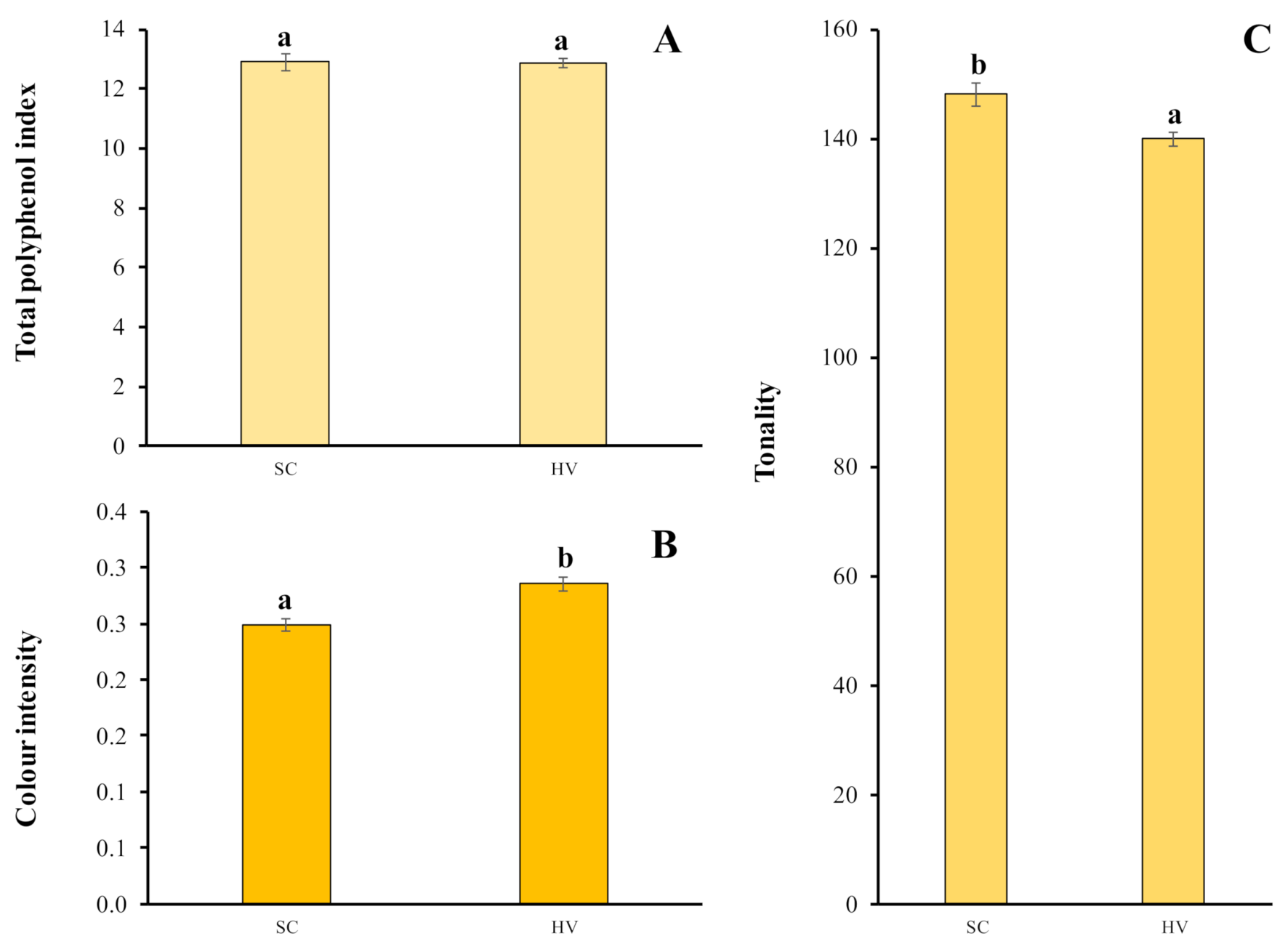
2.4. Total Polyphenol Index and Chromatic Characteristics
2.5. Polysaccharides Content
3. Discussion
3.1. General Oenological Parameters
3.2. Terpenes and Polyoxygenated Terpenes
3.3. Aldehydes, C6 Compounds, Alcohols and Volatile Phenols
3.4. Total Polyphenol Index and Chromatic Characteristics
3.5. Polysaccharides Content
4. Materials and Methods
4.1. Yeasts and Inoculations
4.2. Atomic Force Microscopy
4.3. PCR Analysis of the rDNA–ITS Region
4.4. Musts and Fermentations
4.5. Oenological Parameters Analysis
4.6. Terpenes and Aroma Composition by GC-MS
4.7. Total Polyphenol Index and Chromatic Characteristics
4.8. Polysaccharides by HPLC-RI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. A Review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Del Fresno, J.M.; Escott, C.; Loira, I.; Herbert-Pucheta, J.E.; Schneider, R.; Carrau, F.; Cuerda, R.; Morata, A. Impact of Hanseniaspora vineae in Alcoholic Fermentation and Ageing on Lees of High-Quality White Wine. Fermentation 2020, 6, 66. [Google Scholar] [CrossRef]
- Giorello, F.; Valera, M.J.; Martin, V.; Parada, A.; Salzman, V.; Camesasca, L.; Fariña, L.; Boido, E.; Medina, K.; Dellacassa, E.; et al. Genomic and Transcriptomic Basis of Hanseniaspora vineae’s Impact on Flavor Diversity and Wine Quality. Appl. Environ. Microbiol. 2018, 85, e01959-18. [Google Scholar] [CrossRef] [Green Version]
- Martin, V.; Giorello, F.; Fariña, L.; Minteguiaga, M.; Salzman, V.; Boido, E.; Aguilar, P.S.; Gaggero, C.; Dellacassa, E.; Mas, A.; et al. De novo synthesis of benzenoid compounds by the yeast Hanseniaspora vineae increases the flavor diversity of wines. J. Agric. Food Chem. 2016, 64, 4574–4583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae–Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, D.; Duan, C.; Yan, G. Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process. Biochem. 2020, 90, 44–49. [Google Scholar] [CrossRef]
- Valera, M.J.; Olivera, V.; Pérez, G.; Boido, E.; Dellacassa, E.; Carrau, F. Effect of phenylalanine addition during vinification of Vitis vinifera cv Chardonnay with the yeast Hanseniaspora vineae. In Proceedings of the XVI Congreso Latinoamericano de Viticultura y Enología, Lima, Peru, 12 November 2019. [Google Scholar]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of lees in wine production. A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Giovani, G.; Rosi, I.; Bertuccioli, M. Quantification and characterization of cell wall polysaccharides released by non-Saccharomyces yeast strains during alcoholic fermentation. Int. J. Food Microbiol. 2012, 160, 113–118. [Google Scholar] [CrossRef]
- Aronsson, K.; Rönner, U.; Borch, E. Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae in relation to membrane permeabilization and subsequent leakage of intracellular compounds due to pulsed electric field processing. Int. J. Food Microbiol. 2005, 99, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of mannoproteins during Saccharomyces cerevisiae autolysis induced by pulsed electric field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef] [PubMed]
- Palomero, F.; Morata, A.; Benito, S.; Calderón, F.; Suárez-Lepe, J.A. New genera of yeasts for over-lees aging of red wine. Food Chem. 2009, 112, 432–441. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Palomero, F.; González, C.; Suárez-Lepe, J.A. Schizosaccharomyces pombe: A Promising Biotechnology for Modulating Wine Composition. Fermentation 2018, 4, 70. [Google Scholar] [CrossRef] [Green Version]
- Palomo, E.S.; González-Viñas, M.A.; Díaz-Maroto, M.C.; Soriano-Pérez, A.; Pérez-Coello, M.S. Aroma potential of Albillo wines and effect of skin-contact treatment. Food Chem. 2007, 103, 631–640. [Google Scholar] [CrossRef]
- Culleré, L.; López, R.; Ferreira, V. Chapter 20—The Instrumental Analysis of Aroma-Active Compounds for Explaining the Flavor of Red Wines. In Red Wine Technology, 1st ed.; Antonio, M., Ed.; Academic Press an imprint of Elsevier: London, UK; San Diego, CA, USA, 2019; pp. 283–307. [Google Scholar] [CrossRef]
- ČUŠ, F.; Jenko, M. Influence of yeast on quality of Gewürztraminer wine. Food Technol. Biotechnol. 2013, 51, 547–553. [Google Scholar]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food. Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Song, M.; Xia, Y.; Tomasino, E. Investigation of a quantitative method for the analysis of chiral monoterpenes in white wine by HS-SPME-MDGC-MS of different wine matrices. Molecules 2015, 20, 7359–7378. [Google Scholar] [CrossRef] [Green Version]
- Marais, J. Terpenes in the aroma of grapes and wines: A Review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.; Yue, T.-X.; Zhang, Z.-W. Comparison between aroma compounds in wines from four Vitis vinifera grape varieties grown in different shoot positions. Food Sci. Technol. 2015, 35, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Guth, H. Identification of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3022–3026. [Google Scholar] [CrossRef]
- Carrau, F.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts, FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Sánchez Arribas, A.; Martínez-Fernández, M.; Moreno, M.; Bermejo, E.; Zapardiel, A.; Chicharro, M. Analysis of total polyphenols in wines by FIA with highly stable amperometric detection using carbon nanotube-modified electrodes. Food Chem. 2013, 6, 1183–1192. [Google Scholar] [CrossRef]
- Sen, I.; Tokatli, F. Differentiation of wines with the use of combined data of UV-visible spectra and color characteristics. J. Food Compost. Anal. 2016, 45, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Pectins—Extraction, Purification, Characterization and Applications: Properties of Wine Polysaccharides; Masuelli, M., Ed.; Intech Open, Ltd.: London, UK, 2020; pp. 10–21. [Google Scholar]
- Romani, C.; Domizio, P.; Lencioni, L.; Gobbi, M.; Comitini, F.; Ciani, M.; Mannazzu, I. Polysaccharides and glycerol production by non-Saccharomyces wine yeasts in mixed fermentation. Quad. Vitic. Enol. Univ. Torino 2010, 31, 185–189. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. PCR protocols. A guide to methods and applications. In Amplification and Direct Sequencing of Fungal Ribosomal RNA Genesfor Phylo-genetics; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Bauer, R.; Nieuwoudt, H.; Bauer, F.F.; Kossmann, J.; Koch, K.R.; Esbensen, K.H. FTIR spectroscopy for grape and wine analysis. ACS Publ. 2008, 1371–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roda, R.; Martín, L.; Mislata, A.M.; Castaño, F.J.; Puxeu, M.; Ferrer-Gallego, R. Effects of fertigation by elicitors enriched in amino acids from vegetal and animal origins on Syrah plant gas exchange and grape quality. Food Res. Int. 2019, 125, 108630. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, J.; Peynaud, E.; Sudraud, P.; Ribéreau-Gayon, P. Análisis y control de los vinos, Tomo I. In Tratado de Enología; Ciencias y Técnicas del Vino: Buenos Aires, Argentina, 1980. [Google Scholar]
- Glories, Y. La Couleur Des Vins Rouges II. In Connaisance de la Vigne et du Vin; 1984; pp. 253–271. [Google Scholar]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-Da-Silva, J.M.; Laureano, O.; González, M.C.; Suárez-Lepe, J.A. Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
| Type | Compound | Odour Threshold | Odour | Saccharomyces cerevisiae | Hanseniaspora vineae Followed Sequentially by S. cerevisiae |
|---|---|---|---|---|---|
| Monoterpenes | Linalool | 25 a | Floral, lemon | 18.0 ± 3.8 a | 69.9 ± 16.1 b |
| terpinen-4-ol | 250 c | Pine | 15.8 ± 2.5 a | 20.7 ± 3.6 b | |
| Epoxylinalool | - | - | 18.4 ± 1.4 a | 18.3 ± 1.9 a | |
| β-citronellol | 18 b | Citric | 23.6 ± 2.0 a | 103.0 ± 7.9 b | |
| Geraniol | 20 a | Rose | 35.0 ± 2.3 a | 98.1 ± 10.1 b | |
| α-terpineol | 250 c | Pleasant, sweet | 3.2 ± 0.6 a | 6.1 ± 1.3 b | |
| Totals | 114.0 ± 12.5 a | 316.1 ± 41.0 b | |||
| Polyoxygenated terpenes | cis-furan-linalool oxide | 3000–4000 d | Earthy, leafy | 16.6 ± 1.4 b | 8.6 ± 1.4 a |
| trans-furan-linalool oxide | 3000–4000 d | Earthy, leafy | 20.7 ± 3.8 b | 10.5 ± 2.0 a | |
| cis-pyran linalool oxide | 3000–5000 e | - | 55.2 ± 7.0 b | 22.9 ± 5.4 a | |
| trans-pyran linalool oxide | 3000–5000 e | - | 24.1 ± 1.6 a | 57.6 ± 4.7 b | |
| 2,6-dimethyl-3,7-octadiene-2,6-diol | - | - | 4.7 ± 1.6 a | 9.4 ± 3.1 b | |
| 2,6-dimethyl-1,7-octadiene-3,6-diol | - | - | 5.8 ± 1.9 a | 16.0 ± 3.2 b | |
| 3,7-dimethyl-1,7-octanediol | - | - | 4.0 ± 1.6 a | 11.4 ± 1.6 b | |
| 8-hydroxylinalool | - | - | 25.5 ± 5.0 a | 51.2 ± 5.4 b | |
| Totals | 156.7 ± 23.9 a | 187.6 ± 26.8 b |
| Type | Compound | Odour Threshold | Odour | Saccharomyces cerevisiae | Hanseniaspora vineae Followed Sequentially by S. cerevisiae |
|---|---|---|---|---|---|
| Aldehydes | Benzaldehyde | 2000 a | Almonds | 4.2 ± 1.4 a | 17.8 ± 3.0 b |
| Trans-2-hexenal | 17 b | Green, apple | 90.0 ± 10.0 a | 799.1 ± 97.2 b | |
| Totals | 94.2 ± 11.4 a | 816.9 ± 100.2 b | |||
| C6 compounds | trans-3-hexen-1-ol | 400 c | Green | 178.6 ± 14.2 b | 102.8 ± 12.0 a |
| cis-2-hexen-1-ol | 400 d | - | 6.9 ± 3.2 a | 58.0 ± 12.8 b | |
| cis-3-hexen-1-ol | 400 c | Green | 5.7 ± 1.0 a | 6.2 ± 1.3 a | |
| 1-hexanol | 8000 e | Grass | 450.4 ± 27.4 b | 248.1 ± 25.6 a | |
| Totals | 641.5 ± 45.9 b | 415.2 ± 51.8 a | |||
| Alcohols | 1-octanol | 120 e | Waxy, green, citrus | 6.2 ± 2.6 a | 13.4 ± 2.1 b |
| 1-octen-3-ol | - | - | 22.0 ± 5.2 a | 92.1 ± 9.1 b | |
| benzyl alcohol | 200,000 b | Chemical, fruity | 28.0 ± 4.4 a | 43.0 ± 3.7 b | |
| Phenylethyl alcohol | 14,000 a | Rose petals | 4675.7 ± 976.3 a | 13,871.5 ± 1002.4 b | |
| Totals | 4731.9 ± 988.4 a | 14,020.1 ± 1017.3 b | |||
| Volatile phenols | Eugenol | 6 a | Clove, spicy | 0.4 ± 0.2 a | 4.3 ± 1.1 b |
| Isoeugenol | 6 a | Clove, spicy | 6.2 ± 1.1 a | 24.3 ± 3.1 b | |
| methyl salicylate | - | Sweet | 0.0 ± 0.0 a | 0.0 ± 0.0 a | |
| ethyl salicylate | - | Sweet | 0.0 ± 0.0 a | 0.0 ± 0.0 a | |
| Totals | 6.7 ± 1.3 a | 28.5 ± 4.2 bb |
| Polysaccharides Content (mg/L) | ||
|---|---|---|
| Wines fermented | by Saccharomyces cerevisiae | 194.15 ± 16.20 a |
| by Hanseniaspora vineae followed sequentially by S. cerevisiae | 203.97 ± 21.11 a | |
| Wines aged on lees (1 year) | Saccharomyces cerevisiae lees | 380.22 ± 16.20 b |
| Hanseniaspora vineae lees | 183.99 ± 21.11 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Fresno, J.M.; Escott, C.; Loira, I.; Carrau, F.; Cuerda, R.; Schneider, R.; Bañuelos, M.A.; González, C.; Suárez-Lepe, J.A.; Morata, A. The Impact of Hanseniaspora vineae Fermentation and Ageing on Lees on the Terpenic Aromatic Profile of White Wines of the Albillo Variety. Int. J. Mol. Sci. 2021, 22, 2195. https://doi.org/10.3390/ijms22042195
Del Fresno JM, Escott C, Loira I, Carrau F, Cuerda R, Schneider R, Bañuelos MA, González C, Suárez-Lepe JA, Morata A. The Impact of Hanseniaspora vineae Fermentation and Ageing on Lees on the Terpenic Aromatic Profile of White Wines of the Albillo Variety. International Journal of Molecular Sciences. 2021; 22(4):2195. https://doi.org/10.3390/ijms22042195
Chicago/Turabian StyleDel Fresno, Juan Manuel, Carlos Escott, Iris Loira, Francisco Carrau, Rafael Cuerda, Rémi Schneider, María Antonia Bañuelos, Carmen González, José Antonio Suárez-Lepe, and Antonio Morata. 2021. "The Impact of Hanseniaspora vineae Fermentation and Ageing on Lees on the Terpenic Aromatic Profile of White Wines of the Albillo Variety" International Journal of Molecular Sciences 22, no. 4: 2195. https://doi.org/10.3390/ijms22042195
APA StyleDel Fresno, J. M., Escott, C., Loira, I., Carrau, F., Cuerda, R., Schneider, R., Bañuelos, M. A., González, C., Suárez-Lepe, J. A., & Morata, A. (2021). The Impact of Hanseniaspora vineae Fermentation and Ageing on Lees on the Terpenic Aromatic Profile of White Wines of the Albillo Variety. International Journal of Molecular Sciences, 22(4), 2195. https://doi.org/10.3390/ijms22042195










