Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications
Abstract
1. Introduction
2. Results
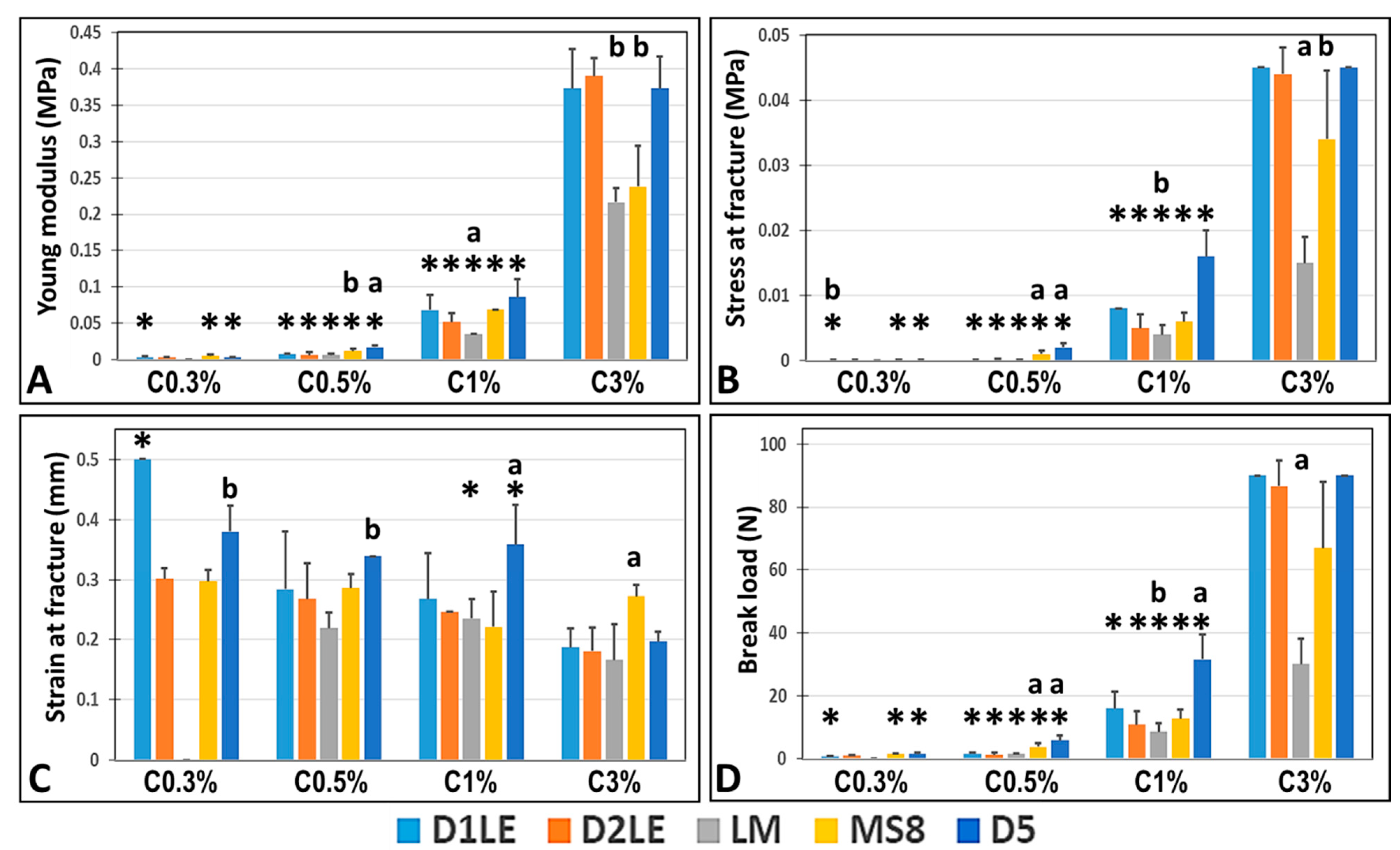
2.1. Biomechanical Properties of Agarose Biomaterials
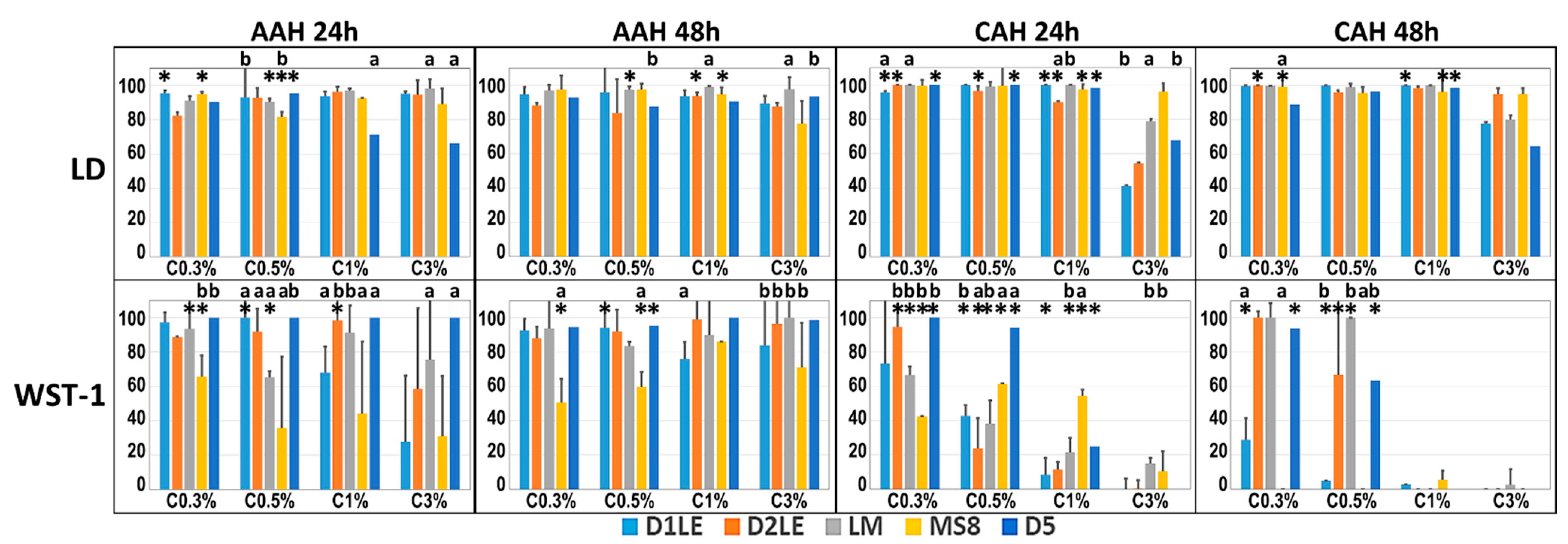
2.2. Indirect Effect on Cell Viability and Function
2.3. Direct Effect on Cell Viability and Function
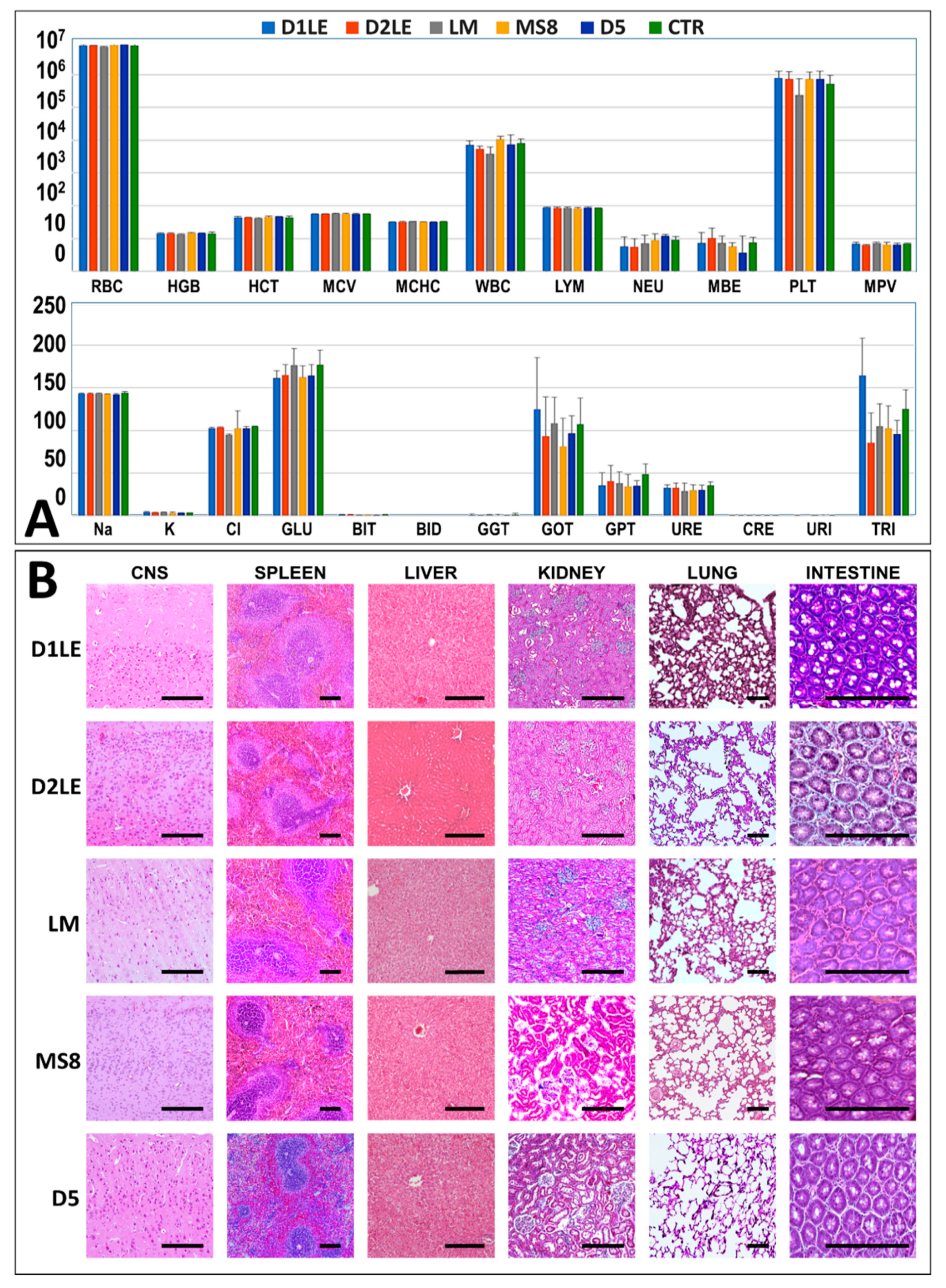
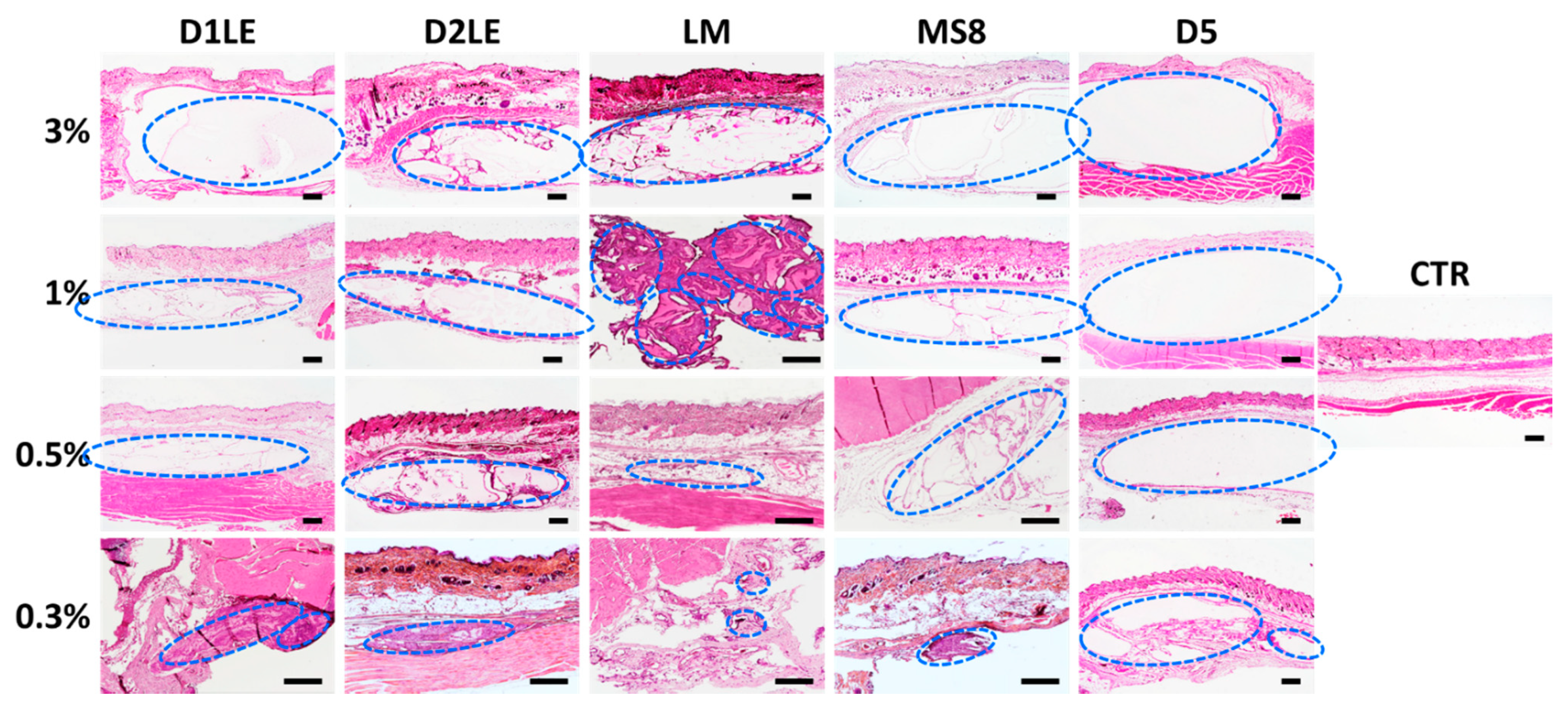
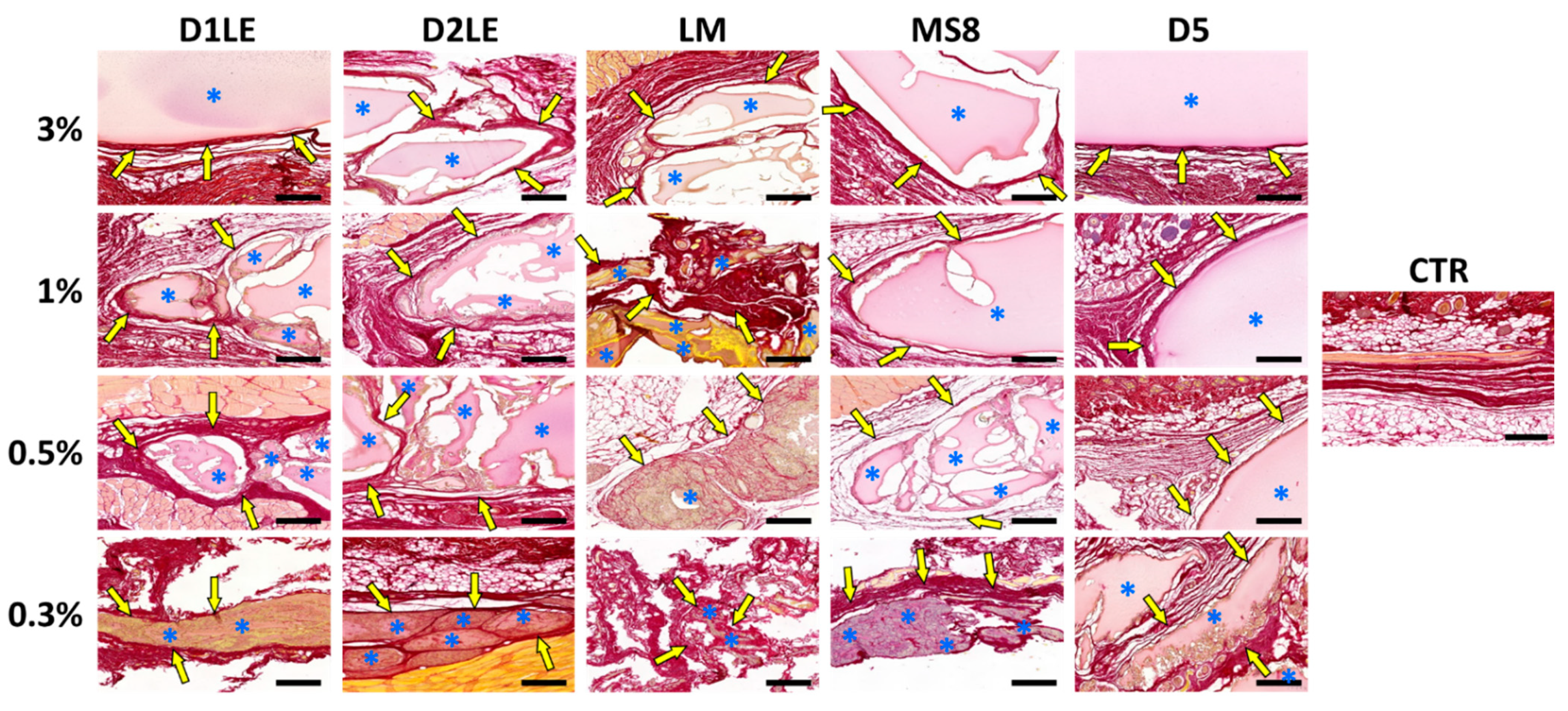
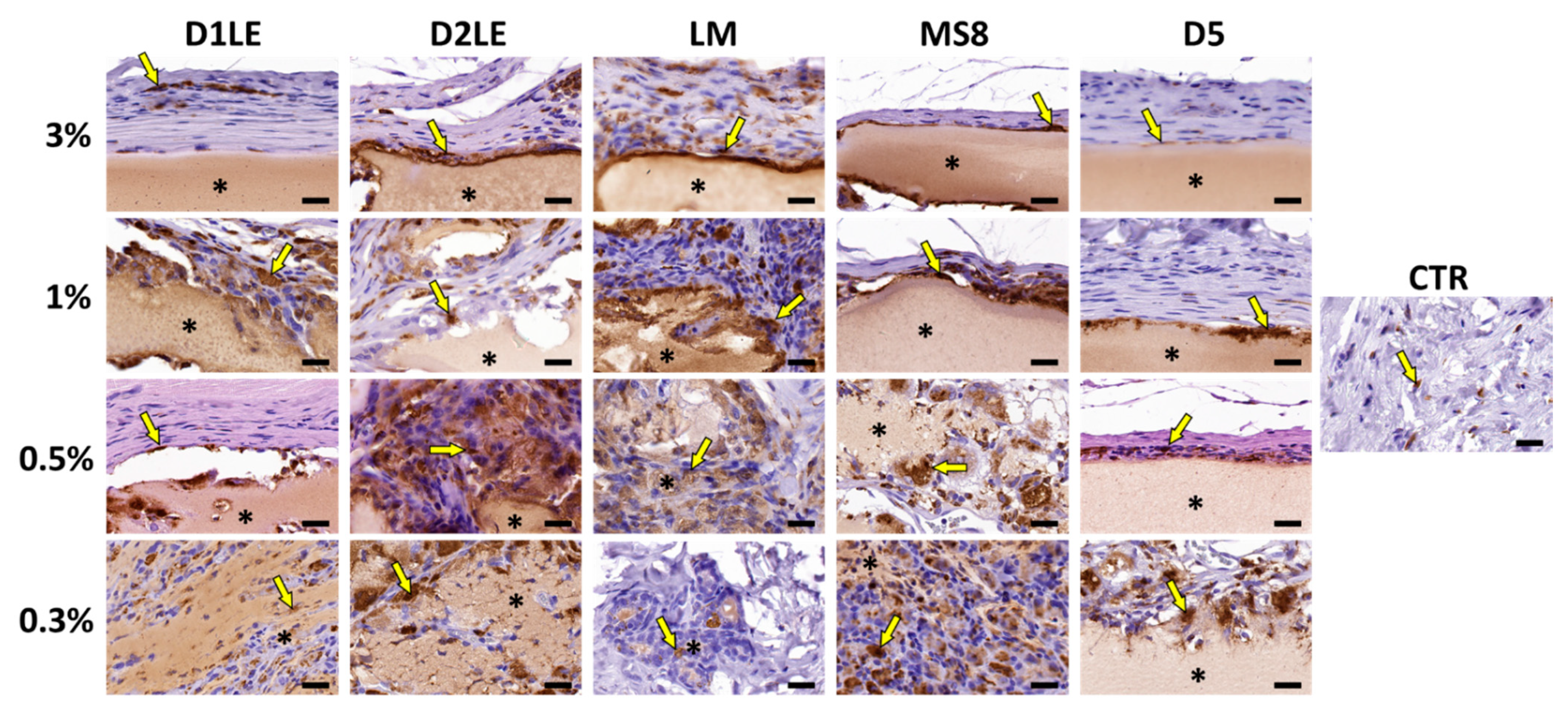
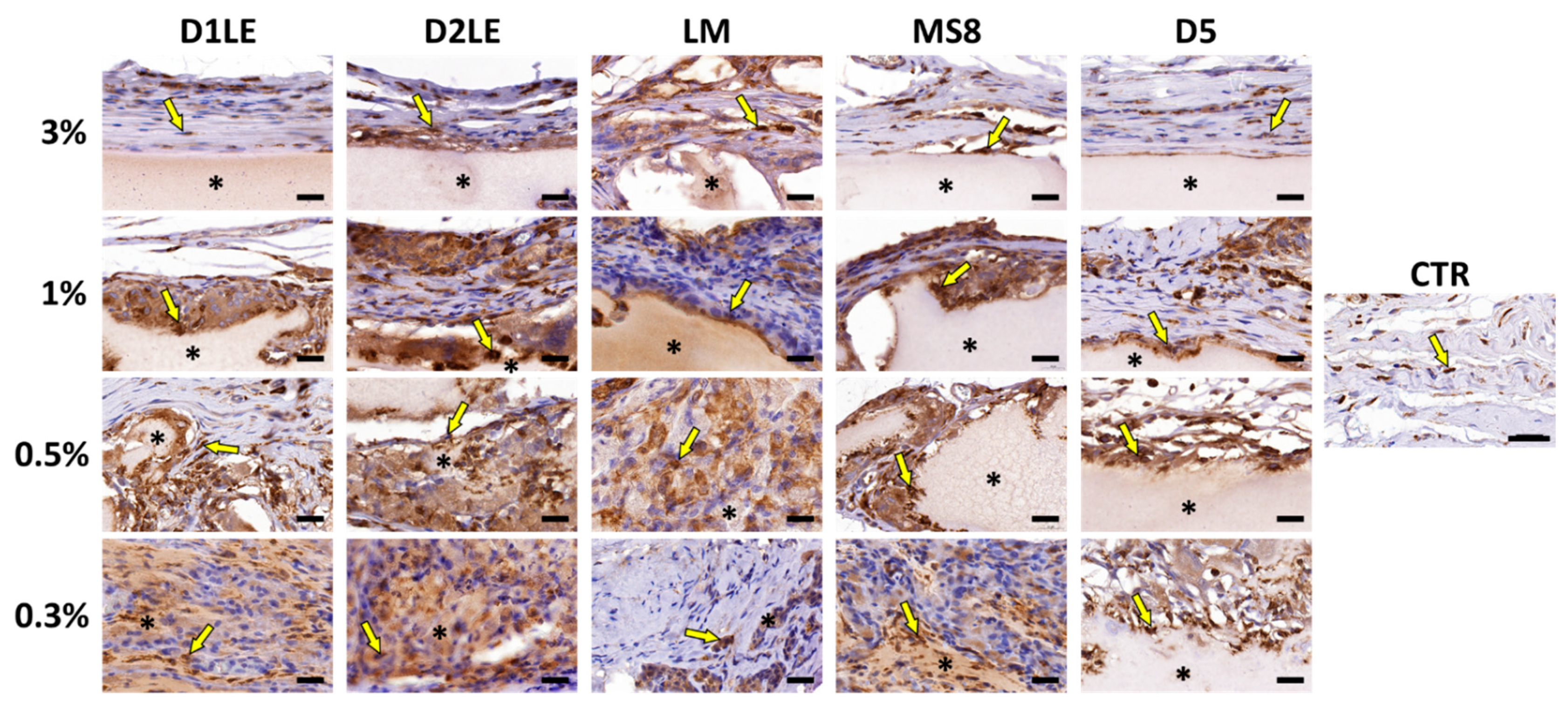
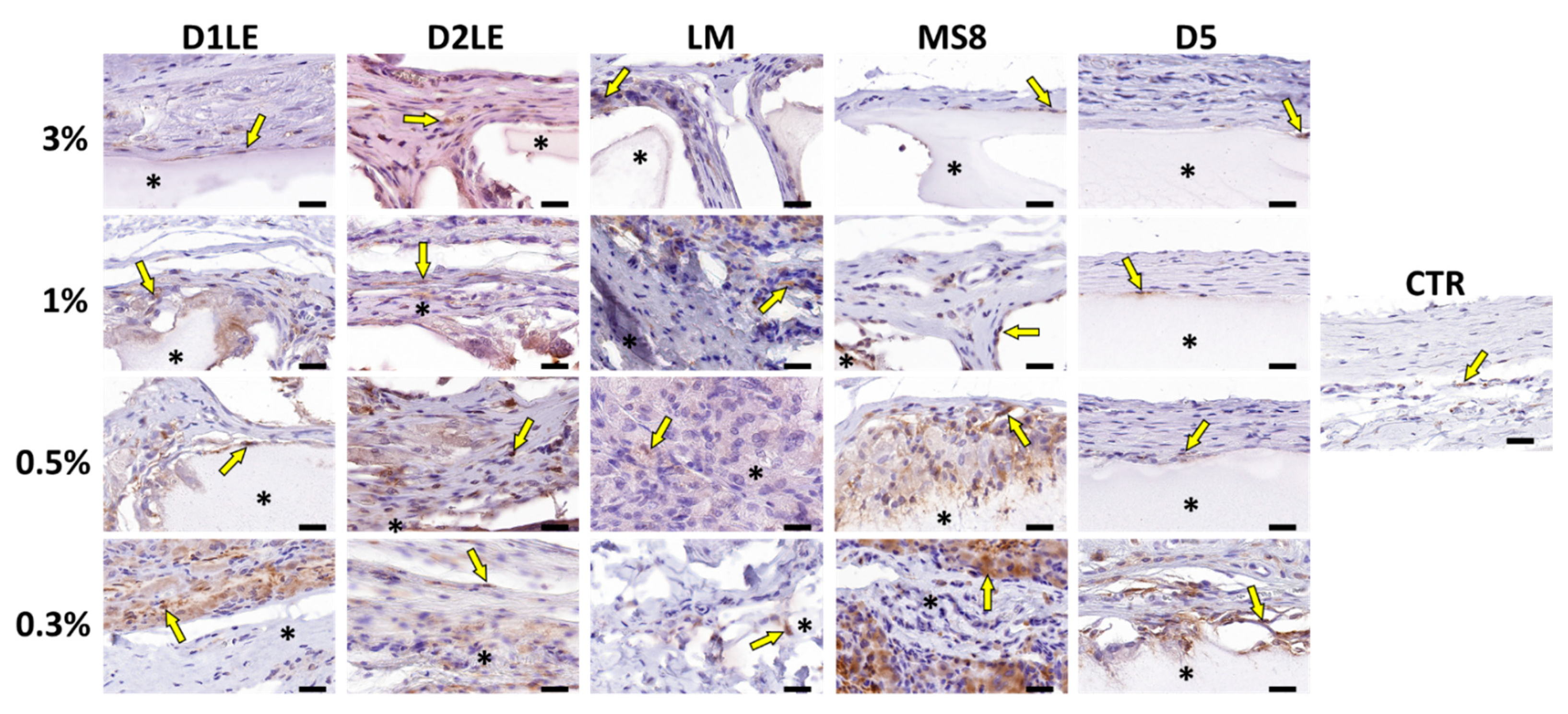
2.4. In Vivo Biocompatibility
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Human Cell Cultures
4.3. Generation of Agarose Hydrogels
4.4. Analysis of Biomechanical Properties
4.5. Ex Vivo Analysis of Biocompatibility, Cell Viability and Function
4.6. In Vivo Analysis
4.7. Histological Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Chandra, P.; Atala, A. Engineering Blood Vessels and Vascularized Tissues: Technology Trends and Potential Clinical Applications. Clin. Sci. (Lond.) 2019, 133, 1115–1135. [Google Scholar] [CrossRef]
- Ionescu, A.-M.; Alaminos, M.; de la Cruz Cardona, J.; de Dios García-López Durán, J.; González-Andrades, M.; Ghinea, R.; Campos, A.; Hita, E.; del Mar Pérez, M. Investigating a Novel Nanostructured Fibrin-Agarose Biomaterial for Human Cornea Tissue Engineering: Rheological Properties. J. Mech. Behav. Biomed. Mater. 2011, 4, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Torre, I.; Alonso, M.; Rodriguez-Cabello, J.-C. Elastin-Based Materials: Promising Candidates for Cardiac Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, P.; O’Leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, G.; Xu, F. Engineering Biomaterials and Approaches for Mechanical Stretching of Cells in Three Dimensions. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of Hydrogel Biomaterials with Controlled Mechanical Properties for Tissue Engineering Applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Jin, M.; Shi, J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part. B Rev. 2020. [Google Scholar] [CrossRef]
- Akshay Kumar, K.P.; Zare, E.N.; Torres-Mendieta, R.; Wacławek, S.; Makvandi, P.; Černík, M.; Padil, V.V.T.; Varma, R.S. Electrospun Fibers Based on Botanical, Seaweed, Microbial, and Animal Sourced Biomacromolecules and Their Multidimensional Applications. Int. J. Biol. Macromol. 2021, 171, 130–149. [Google Scholar] [CrossRef]
- Santos, J.M.; Palma, P.J.; Ramos, J.C.; Cabrita, A.S.; Friedman, S. Periapical Inflammation Subsequent to Coronal Inoculation of Dog Teeth Root Filled with Resilon/Epiphany in 1 or 2 Treatment Sessions with Chlorhexidine Medication. J. Endod. 2014, 40, 837–841. [Google Scholar] [CrossRef]
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl. Sci. 2019, 9, 3942. [Google Scholar] [CrossRef]
- Palma, P.J.; Marques, J.A.; Falacho, R.I.; Correia, E.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures. J. Funct. Biomater. 2019, 10, 14. [Google Scholar] [CrossRef]
- Pollot, B.E.; Rathbone, C.R.; Wenke, J.C.; Guda, T. Natural Polymeric Hydrogel Evaluation for Skeletal Muscle Tissue Engineering. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2018, 106, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.L.; Padalhin, A.R.; Kim, B.; Lee, B.-T. Preparation and Evaluation of BCP-CSD-Agarose Composite Microsphere for Bone Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2263–2272. [Google Scholar] [CrossRef]
- Oliveira, I.; Carvalho, A.L.; Radhouani, H.; Gonçalves, C.; Oliveira, J.M.; Reis, R.L. Promising Biomolecules. Adv. Exp. Med. Biol. 2018, 1059, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. Hydrogels of Agarose, and Methacrylated Gelatin and Hyaluronic Acid Are More Supportive for in Vitro Meniscus Regeneration than Three Dimensional Printed Polycaprolactone Scaffolds. Int. J. Biol. Macromol. 2019, 122, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Rico-Sánchez, L.; Garzón, I.; González-Andrades, M.; Ruíz-García, A.; Punzano, M.; Lizana-Moreno, A.; Muñoz-Ávila, J.I.; Sánchez-Quevedo, M.D.C.; Martínez-Atienza, J.; Lopez-Navas, L.; et al. Successful Development and Clinical Translation of a Novel Anterior Lamellar Artificial Cornea. J. Tissue Eng. Regen. Med. 2019, 13, 2142–2154. [Google Scholar] [CrossRef]
- Carriel, V.; Garzón, I.; Jiménez, J.-M.; Oliveira, A.-C.-X.; Arias-Santiago, S.; Campos, A.; Sánchez-Quevedo, M.-C.; Alaminos, M. Epithelial and Stromal Developmental Patterns in a Novel Substitute of the Human Skin Generated with Fibrin-Agarose Biomaterials. Cells Tissues Organs (Print) 2012, 196, 1–12. [Google Scholar] [CrossRef]
- Fernández-Valadés-Gámez, R.; Garzón, I.; Liceras-Liceras, E.; España-López, A.; Carriel, V.; Martin-Piedra, M.-Á.; Muñoz-Miguelsanz, M.-Á.; Sánchez-Quevedo, M.-C.; Alaminos, M.; Fernández-Valadés, R. Usefulness of a Bioengineered Oral Mucosa Model for Preventing Palate Bone Alterations in Rabbits with a Mucoperiostial Defect. Biomed. Mater. 2016, 11, 015015. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, X.; Jiang, Y.; Xing, L.; Xu, Y.; Lu, Y.; Ding, P.; Ma, J.; Xu, Y.; Gui, J. Platelet-Rich Plasma Combined with Agarose as a Bioactive Scaffold to Enhance Cartilage Repair: An in Vitro Study. J. Biomater. Appl. 2014, 28, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Ichanti, H.; Sladic, S.; Kalies, S.; Haverich, A.; Andrée, B.; Hilfiker, A. Characterization of Tissue Engineered Endothelial Cell Networks in Composite Collagen-Agarose Hydrogels. Gels 2020, 6, 27. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel Chitosan/Agarose/Hydroxyapatite Nanocomposite Scaffold for Bone Tissue Engineering Applications: Comprehensive Evaluation of Biocompatibility and Osteoinductivity with the Use of Osteoblasts and Mesenchymal Stem Cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Three-Dimensional Porous Scaffolds Based on Agarose/Chitosan/Graphene Oxide Composite for Tissue Engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Armisén, R. Agar and Agarose Biotechnological Applications. Hydrobiologia 1991, 221, 157–166. [Google Scholar] [CrossRef]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Scionti, G.; Moral, M.; Toledano, M.; Osorio, R.; Durán, J.D.G.; Alaminos, M.; Campos, A.; López-López, M.T. Effect of the Hydration on the Biomechanical Properties in a Fibrin-Agarose Tissue-like Model. J. Biomed. Mater. Res. A 2014, 102, 2573–2582. [Google Scholar] [CrossRef]
- Schuh, E.; Hofmann, S.; Stok, K.S.; Notbohm, H.; Müller, R.; Rotter, N. The Influence of Matrix Elasticity on Chondrocyte Behavior in 3D. J. Tissue Eng. Regen. Med. 2012, 6, e31–e42. [Google Scholar] [CrossRef] [PubMed]
- Cioroianu, A.R.; Storm, C. Normal Stresses in Elastic Networks. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2013, 88, 052601. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.G.; Mithieux, S.M.; Weiss, A.S. Engineered Tropoelastin and Elastin-Based Biomaterials. Adv. Protein Chem. Struct. Biol. 2009, 78, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Rubod, C.; Brieu, M.; Cosson, M.; Rivaux, G.; Clay, J.-C.; de Landsheere, L.; Gabriel, B. Biomechanical Properties of Human Pelvic Organs. Urology 2012, 79, 968.e17–968.e22. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.Y.; Hu, J.C.; Athanasiou, K. A Guide for Using Mechanical Stimulation to Enhance Tissue-Engineered Articular Cartilage Properties. Tissue Eng. Part. B Rev. 2018, 24, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Rodríguez, I.Á.; Garzón, I.; Martín-Piedra, M.Á.; Alfonso-Rodríguez, C.A.; García, J.M.; del Sánchez-Quevedo, M.C.; Alaminos, M. An Early and Late Cytotoxicity Evaluation of Lidocaine on Human Oral Mucosa Fibroblasts. Exp. Biol. Med. (Maywood) 2014, 239, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a Bioactive Material to Regulate Stem Cell Fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef]
- Xue, Y.Z.B.; Niu, Y.M.; Tang, B.; Wang, C.M. PCL/EUG Scaffolds with Tunable Stiffness Can Regulate Macrophage Secretion Behavior. Prog. Biophys. Mol. Biol. 2019, 148, 4–11. [Google Scholar] [CrossRef]
- Perrier-Groult, E.; Pérès, E.; Pasdeloup, M.; Gazzolo, L.; Duc Dodon, M.; Mallein-Gerin, F. Evaluation of the Biocompatibility and Stability of Allogeneic Tissue-Engineered Cartilage in Humanized Mice. PLoS ONE 2019, 14, e0217183. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.M.; Chato-Astrain, J.; de la Cardona Pérez, J.C.; Campos, F.; Pérez Gómez, M.; Alaminos, M.; Garzón Bello, I. Evaluation of the Optical and Biomechanical Properties of Bioengineered Human Skin Generated with Fibrin-Agarose Biomaterials. J. Biomed. Opt. 2020, 25, 1–16. [Google Scholar] [CrossRef]
- Campos, F.; Bonhome-Espinosa, A.B.; Chato-Astrain, J.; Sánchez-Porras, D.; García-García, Ó.D.; Carmona, R.; López-López, M.T.; Alaminos, M.; Carriel, V.; Rodriguez, I.A. Evaluation of Fibrin-Agarose Tissue-Like Hydrogels Biocompatibility for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 596. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Ramalingam, C.; Gandhi, M. Silver Nanoparticles Engineered by Thermal Co-Reduction Approach Induces Liver Damage in Wistar Rats: Acute and Sub-Chronic Toxicity Analysis. 3 Biotech. 2019, 9, 125. [Google Scholar] [CrossRef]
- Stokols, S.; Tuszynski, M.H. Freeze-Dried Agarose Scaffolds with Uniaxial Channels Stimulate and Guide Linear Axonal Growth Following Spinal Cord Injury. Biomaterials 2006, 27, 443–451. [Google Scholar] [CrossRef]
- Krouwels, A.; Melchels, F.P.W.; van Rijen, M.H.P.; Öner, F.C.; Dhert, W.J.A.; Tryfonidou, M.A.; Creemers, L.B. Comparing Hydrogels for Human Nucleus Pulposus Regeneration: Role of Osmolarity During Expansion. Tissue Eng. Part. C Methods 2018, 24, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Sen, S.; Huang, A.H.; Elliott, D.M.; Mauck, R.L. Engineered Disc-like Angle-Ply Structures for Intervertebral Disc Replacement. Spine (Phila Pa 1976) 2010, 35, 867–873. [Google Scholar] [CrossRef]
- Martin-Piedra, M.A.; Alfonso-Rodriguez, C.A.; Zapater, A.; Durand-Herrera, D.; Chato-Astrain, J.; Campos, F.; Sanchez-Quevedo, M.C.; Alaminos, M.; Garzon, I. Effective Use of Mesenchymal Stem Cells in Human Skin Substitutes Generated by Tissue Engineering. Eur. Cell Mater. 2019, 37, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef]
- Corradetti, B.; Taraballi, F.; Corbo, C.; Cabrera, F.; Pandolfi, L.; Minardi, S.; Wang, X.; Van Eps, J.; Bauza, G.; Weiner, B.; et al. Immune Tuning Scaffold for the Local Induction of a Pro-Regenerative Environment. Sci. Rep. 2017, 7, 17030. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R. Macrophage Reaction against Biomaterials in the Mouse Model—Phenotypes, Functions and Markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef]
- Sok, M.C.P.; Tria, M.C.; Olingy, C.E.; San Emeterio, C.L.; Botchwey, E.A. Aspirin-Triggered Resolvin D1-Modified Materials Promote the Accumulation of pro-Regenerative Immune Cell Subsets and Enhance Vascular Remodeling. Acta Biomater. 2017, 53, 109–122. [Google Scholar] [CrossRef]
- Santos, J.M.; Coelho, C.M.; Sequeira, D.B.; Marques, J.A.; Pereira, J.F.; Sousa, V.; Palma, P.J.; Santos, A.C. Subcutaneous Implantation Assessment of New Calcium-Silicate Based Sealer for Warm Obturation. Biomedicines 2021, 9, 24. [Google Scholar] [CrossRef]
- Campos, F.; Bonhome-Espinosa, A.B.; García-Martínez, L.; Durán, J.D.G.; López-López, M.T.; Alaminos, M.; Sánchez-Quevedo, M.C.; Carriel, V. Ex Vivo Characterization of a Novel Tissue-like Cross-Linked Fibrin-Agarose Hydrogel for Tissue Engineering Applications. Biomed. Mater. 2016, 11, 055004. [Google Scholar] [CrossRef]
- García-Martínez, L.; Campos, F.; Godoy-Guzmán, C.; Del Carmen Sánchez-Quevedo, M.; Garzón, I.; Alaminos, M.; Campos, A.; Carriel, V. Encapsulation of Human Elastic Cartilage-Derived Chondrocytes in Nanostructured Fibrin-Agarose Hydrogels. Histochem. Cell Biol. 2017, 147, 83–95. [Google Scholar] [CrossRef]
- Campos, F.; Bonhome-Espinosa, A.B.; Vizcaino, G.; Rodriguez, I.A.; Duran-Herrera, D.; López-López, M.T.; Sánchez-Montesinos, I.; Alaminos, M.; Sánchez-Quevedo, M.C.; Carriel, V. Generation of Genipin Cross-Linked Fibrin-Agarose Hydrogel Tissue-like Models for Tissue Engineering Applications. Biomed. Mater. 2018, 13, 025021. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Rodríguez, C.-A.; Garzón, I.; Garrido-Gómez, J.; Oliveira, A.-C.-X.; Martín-Piedra, M.-Á.; Scionti, G.; Carriel, V.; Hernández-Cortés, P.; Campos, A.; Alaminos, M. Identification of Histological Patterns in Clinically Affected and Unaffected Palm Regions in Dupuytren’s Disease. PLoS ONE 2014, 9, e112457. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irastorza-Lorenzo, A.; Sánchez-Porras, D.; Ortiz-Arrabal, O.; de Frutos, M.J.; Esteban, E.; Fernández, J.; Janer, A.; Campos, A.; Campos, F.; Alaminos, M. Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 1923. https://doi.org/10.3390/ijms22041923
Irastorza-Lorenzo A, Sánchez-Porras D, Ortiz-Arrabal O, de Frutos MJ, Esteban E, Fernández J, Janer A, Campos A, Campos F, Alaminos M. Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications. International Journal of Molecular Sciences. 2021; 22(4):1923. https://doi.org/10.3390/ijms22041923
Chicago/Turabian StyleIrastorza-Lorenzo, Ainhoa, David Sánchez-Porras, Olimpia Ortiz-Arrabal, María José de Frutos, Emilio Esteban, Javier Fernández, Agustín Janer, Antonio Campos, Fernando Campos, and Miguel Alaminos. 2021. "Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications" International Journal of Molecular Sciences 22, no. 4: 1923. https://doi.org/10.3390/ijms22041923
APA StyleIrastorza-Lorenzo, A., Sánchez-Porras, D., Ortiz-Arrabal, O., de Frutos, M. J., Esteban, E., Fernández, J., Janer, A., Campos, A., Campos, F., & Alaminos, M. (2021). Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications. International Journal of Molecular Sciences, 22(4), 1923. https://doi.org/10.3390/ijms22041923