Abstract
Thioredoxin interacting protein (TXNIP) is a metabolism- oxidative- and inflammation-related marker induced in cardiovascular diseases and is believed to represent a possible link between metabolism and cellular redox status. TXNIP is a potential biomarker in cardiovascular and ischemic diseases but also a novel identified target for preventive and curative medicine. The goal of this review is to focus on the novelties concerning TXNIP. After an overview in TXNIP involvement in oxidative stress, inflammation and metabolism, the remainder of this review presents the clues used to define TXNIP as a new marker at the genetic, blood, or ischemic site level in the context of cardiovascular and ischemic diseases.
1. Introduction
Cardiovascular diseases remain a major cause of death worldwide and are increasing due to the ageing population and poor eating habits. The pathological changes are originally characterized by metabolic disorders and endothelial dysfunction. Oxidative stress plays an important role and induces vascular-related gene expression, promoting local inflammatory response and cell life and death dysregulation. When oxidative stress occurs, vascular walls produce excessive reactive oxygen species (ROS), which causes damage to the structure and function of endothelial cells. That enhances the inflammatory response of the vascular wall and impairs vascular function or revascularization. ROS are produced continuously during cell metabolism and are used as mediators in many biological processes. Specifically, ROS reversibly activate signaling pathways that trigger adaptation systems in the cell. Previous works have associated excessive ROS with age-related pathologies [1,2,3,4,5,6,7]. However, recent reviews still report that excessive ROS can lead to diseases and pathological conditions [8,9,10,11]. Thioredoxin interacting protein (TXNIP) is a metabolism- oxidative- and inflammation-related marker induced in cardiovascular pathologies and could represent an emergent link between physiopathology and cardiovascular events. More precisely, TXNIP has been widely described as a pro-oxidant compound [12,13], but it is also a regulator of metabolism [14,15,16,17], a modulator of the inflammatory [18,19] or angiogenic response [20,21], and an antiproliferative and pro-apoptotic agent [22,23]. Clinically, genetic association studies have shown that polymorphisms affecting TXNIP expression are linked to hypertension and arterial stiffness and increase the risk of coronary heart disease [24,25,26]. Epigenetic modifications of TXNIP are also associated with risks of cardiovascular diseases [27,28]. Additionally, blood or mononuclear blood cells’ mRNA TXNIP levels have been related to coronary and heart diseases [29,30,31].
Finally, in this review, we propose an overview of half a decade of work analyzing TXNIP as a marker of cardiovascular risk and diseases. The mechanisms involved are specified and TXNIP is identified as a potential target for preventive and curative medicine in cardiovascular and ischemic diseases.
2. TXNIP is a Multifunctional Protein
TXNIP is a 46-kDa ubiquitously expressed protein that contains 391 amino acid residues and is encoded on chromosome 1q21.1. TXNIP is an α-arrestin protein that regulates pleiotropic biological responses [32,33,34]. TXNIP appears to perform certain functions through multiple binding partners [35], which are summarized in Table 1.

Table 1.
The multiple signaling partners of TXNIP and pleiotropic effects.
2.1. TXNIP is a Protein Shuttle
The structure of TXNIP and the presence of “arrestin-like” domains suggest the ability of this protein to interact with other proteins but also to participate in cell trafficking and particularly in the transport of proteins with which it interacts. TXNIP is almost exclusively localized in the nucleus in healthy cells [36,133]. Nevertheless, TXNIP is able to translocate into the mitochondria under fructose stimulation, into the cytosol, and to the membrane in response to physiological levels of ROS [134]. During its translocation, it also carries several proteins. Thioredoxin (TRX), a major antioxidant enzyme, is carried by TXNIP to the membrane [36]. This interaction is central to the regulation of the TRX-TXNIP system. In addition to its role with TRX, TXNIP can also interact with HIF-1α. TXNIP leads HIF1- α outside the nucleus to the cytosol where the factor will be degraded [38]. TXNIP is also responsible for the nuclear translocation of NF-κB [39,40]. In parallel, TXNIP is degraded by the ubiquitin-proteasome using the protein ITCH. Interestingly, the regulation of TXNIP lifetime appears to be crucial for the control of TRX oxidoreductase activity [41]. Finally, the overexpression of ITCH in cardiomyocytes is reported to ameliorate reactive oxygen species-induced cardiotoxicity through the thioredoxin system [42]. In this case, TXNIP degradation is driven by ITCH binding to the “arrestin-like” domains [43].
2.2. TXNIP in the TRX-TXNIP System
This system is highly conserved in almost all species, from bacteria to higher eukaryotes [135,136]. By virtue of its location, TRX is a protein of choice for fighting oxidative stress in the vessel. It exerts antioxidant activity by allowing the reduction of target proteins via the formation of disulfide bonds between two cysteine residues of its active site (Cys-Gly-Pro-Cys). This results in the oxidation of TRX and the reduction of residues on target proteins [32,44]. Then, TRX can be reduced by a flavoprotein oxidoreductase, called thioredoxin reductase (TrxR), which uses the NADPH as a co-factor, allowing it to be able to exert its antioxidant role [45]. TRX plays an important role in maintaining redox balance and cell signaling by participating in sulfhydryl reactions (e.g., reducing cysteine residues) and by interacting with various components of signaling pathways [46]. Several studies have shown that TRX activity could be modulated by a negative endogenous regulator: TXNIP [47,48]. TXNIP interacts with the catalytic center of reduced TRX and inhibits its reducing activity [44,48,49]. TXNIP deletion results in decreased ROS in vascular smooth muscle cells (VSMC), for example, and increases the antioxidant potential of TRX in vitro [135]. TXNIP is described as a pro-oxidant compound, but its role does not end with a simple modulation of the redox balance; it also acts as a transporter of TRX [37].
2.3. TXNIP and Its Role in Oxidative Stress
Oxidative stress is recognized as the first step in endothelial dysfunction, leading to vascular damage [2,50] and impaired revascularization [21]. The decreased expression of TRX, associated with increased expression of TXNIP, is implicated in endothelial dysfunction associated with aging and, in this context, increased expression of NADPH oxidase is also reported [51]. More interestingly, reduced endothelial expression of TXNIP is associated with an increased TRX and decreased NADPH oxidase expression protecting the endothelium from dysfunction induced by metabolic disorders [13,52]. Overexpression of TXNIP in vitro is associated with oxidative stress induced by mitochondrial ROS or NADPH oxidase [56]. Furthermore, given the role of TXNIP in inhibiting the antioxidant activity of TRX, its role in oxidative stress seems obvious [40,59,63,65,137,138]. Blocking its expression then induces a decrease in oxidative stress [139]. An in vitro study shows that TXNIP promotes nitrosative stress via its inhibitory action on TRX and repressing TXNIP, and thereby facilitates thioredoxin-mediated denitrosylation [140]. Interestingly, pharmacological inhibition of TXNIP in an in vivo model of ischemia-reperfusion has been associated with increased TRX activity [53]. A study in a mouse model of diabetes also shows the excess of ROS and the establishment of oxidative stress resulting from the decrease in the activity of TRX due to its inhibition by TXNIP [54]. Indeed, when glucose is high, TXNIP mediates the production of ROS via mitochondria and NADPH oxidase [56,57]. The resulting oxidative stress arises from overexpressed TXNIP, leading to endothelial dysfunction and impaired vasorelaxation [12,21].
2.4. TXNIP: Link between Oxidative Stress and Inflammation
TRX is a protein which, in addition to its role as an antioxidant, has anti-inflammatory properties. It is therefore a crucial protein in the protection of age-related vascular damage. Many articles suggest that TRX is an anti-inflammatory molecule at both intracellular and extracellular levels [141,142]. Conversely, TXNIP has a pro-inflammatory role leading to cardiac, vascular and endothelial dysfunctions [13,19,21,66,67,68,143,144]. Numerous studies conducted since the 2000s suggest that TXNIP may bind to the NLRP3 inflammasome, which enhances the inflammatory response, as reviewed almost a decade ago [65,69,70,71,72,73,74,75,76,77,78,79,80,81,82] and summarized in Figure 1. TXNIP and NLRP3 physically interact to activate the inflammasome [83]. The inflammasome is the multiprotein complex that controls the activation of caspase-1 in the innate immune system. Then, it causes the maturation of IL-1β. ROS are the major activators of the NLRP3 inflammasome. The physical interaction between TXNIP and NLRP3 could explain the activation of the inflammasome in a ROS-sensitive manner [18,84,85]. Zhou et al. suggested that under unstressed conditions, TXNIP is bound to TRX, and the NLRP-3 inflammasome is inactive due to a lack of interaction between TXNIP and NLRP3. However, under oxidative stress conditions, the generation of ROS facilitates TRX-TXNIP dissociation, thereby increasing NLRP3-TXNIP interaction [84] (Figure 1). These observations were then demonstrated in podocytes [86] or in response to LPS [87]. More recently, in adult mice with metabolic disorders, ROS accumulation results from endothelial dysfunction with decreased TRX and increased NADPH oxidase endothelial expression, leading to oxidative stress and NLRP3 inflammasome activation in the aortic wall [13,88]. In addition to the oxidative stress, TXNIP upregulation through the p38-FOXO1 axis seems critical for NLRP3 activation [89]. Endoplasmic reticulum stress is also associated with TXNIP-NLRP3 activation in the context of endothelial dysfunction [90]. Interestingly, the deletion of endothelial TXNIP in mice or in vivo anti-TXNIP treatment protects from oxidative stress and NLRP3 inflammasome activation [13,21]. Metformin or other compounds are also used to lower TXNIP aortic levels in vivo or endothelial levels in vitro in order to restrain NLRP3 activation and protect from endothelial dysfunction and cardiovascular risk factors [60,67,87,91,92,93,94,95,96,97,98,99,100,101]. The regulation of the NLRP3 inflammasome by the TRX-TXNIP complex is believed to be controlled by Nrf2 and AMPK [60,102,103,104,106,107]. The overexpression of TXNIP activates the TLR4-NF-κB-NLRP3 inflammasome signaling pathway with increased MyD88, NLPR3 inflammasome, and ASC expression, as well as the increased phosphorylation of lκBα and p65, thus promoting downstream NF-κB activation [109]. The blunted inflammatory response is associated with a decrease in NF-κB nuclear translocation. In fact, in hyperglycemic conditions, the overexpression of TXNIP leads to an increased expression of inflammation genes via chromatin modifications and by promoting nuclear translocation of NF-κB [39]. In addition, TXNIP also promotes inflammation in the endothelium in response to disturbed flow [111]. The expression of inflammatory markers and adhesion molecules such as ICAM-1, VCAM-1, and MCP-1 are diminished in VSMCs from TXNIP knockout mice [110]. This inflammation state can trigger cell senescence or cell death characterized as pyroptosis, which is hampered with the decrease of TXNIP [66,78,92,93,105]. The ROS-TXNIP-NLRP3 pathway can actually be enabled for a long time via an IL-1β-mediated positive feedback loop [145].
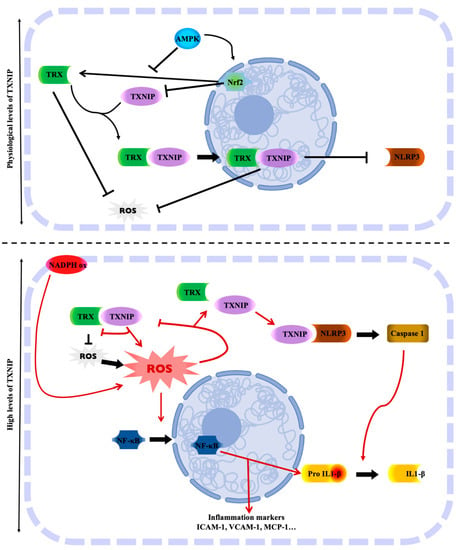
Figure 1.
TXNIP is the molecular link between the regulation of oxidative stress and inflammation. Physiological levels of TXNIP balance the regulation of TRX oxidative stress. TRX is free from TXNIP, whose level is controlled by several factors. However, high levels of TXNIP inhibit TRX activity, resulting in oxidative stress with the accumulation of ROS. The oxidative stress status allows TXNIP to activate the NLRP3 inflammasome and trigger cell inflammation. Created with BioRender.com.
2.5. TXNIP and Its Role in Metabolism
TXNIP has also attracted considerable attention due to its wide-ranging functions impacting several aspects of energy metabolism, as already reviewed elsewhere [14,15,16,146,147]. TXNIP is known to modulate cellular glucose utilization, the mitochondrial oxidation of metabolic fuels, and fasting-feeding transition [112,148]. TXNIP is implicated in adaptation to acidosis and, interestingly, it is associated with ATP generation [114]. Consequently, TXNIP appears to be an important regulator of glucose homeostasis via the regulation of gluconeogenesis in the liver [149]. In addition to metabolic disturbances, the total deletion of TXNIP induces the development of hemorrhages and hepatic steatosis which can lead to death [150]. Nevertheless, a recent genetic study in a family with homozygous nonsense mutations shows that suppression of TXNIP expression is non-lethal in humans [151]. A number of studies have therefore shown that TXNIP has a role to play in metabolic control, partially independent of its ability to bind to TRX [115,152]. Insulin and cellular glucose influx reciprocally regulate TXNIP expression in humans: glucose influx positively regulates TXNIP expression and its suppression by insulin [115,153]. These results are consistent with a recent study showing that IGF-1 could suppress TXNIP expression [116]. In addition, studies have shown that TXNIP induces the internalization of the glucose transporter GLUT1 and downregulates its transcription [113,117]. TXNIP overexpression in skeletal muscle cells reduces membrane GLUT1 expression, glucose uptake, and increases peripheric insulin resistance [118]. TXNIP deletion in murine embryonic fibroblast cells increases the levels of GLUT1 and the use of glucose by these same cells, and also increases lactate production [33,119]. Another study showed similar results with the GLUT4 transporter [120]. TXNIP is also overexpressed in the context of diabetes. Data from the literature identify TXNIP as a potential target in diabetes complications such as diabetic retinopathy, nephropathy, cardiomyopathy, and impaired post-ischemic revascularization [59,67,79,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176]. In a rat model of diabetic cardiomyopathy, TXNIP deletion in cardiomyocytes induces an improved inotropic response to β-adrenergic stimulation [144].
Finally, in mice, glucose intolerance induced by High Protein High Fat Low Carbohydrate diet is also associated with an increase in TXNIP levels in the aorta [13,52]. In fact, high glucose and high fat levels are important inducers of higher endothelial TXNIP expression [99,177]. As reviewed elsewhere, glucose-induced higher tissue TXNIP expression has become a relevant therapeutic target not only to improve insulin secretion and sensitivity, but also for ameliorating the long-term microvascular and macrovascular complications of diabetes [67,145,178,179,180,181]. As the first piece of evidence, commonly used antidiabetic therapies are associated with a decreased expression of TXNIP [91,182,183,184,185,186], in particular via ChREBP and FOXO1 inactivation [121]. Indeed, endothelial dysfunction induced by high levels of TXNIP may have profound effects on the vasculature, a characteristic feature of metabolic disorders [13,20].
2.6. TXNIP is a Target of MiRNA
MiRNAs are also involved in glucose-induced TXNIP regulation. Analysis of the gene of TXNIP reveals several potential miRNA binding sites. [187]. For example, miR-17 downregulation by high glucose stabilizes TXNIP and removes TRX inhibition on ASK, leading to apoptosis [122]. Moreover, miR-33/TXNIP is believed to be essential in cell adaptation to bioenergetic demands [129]. Additionally, in numerous cardiovascular diseases, TXNIP has recently been identified as a target of miRNAs. For example, studies demonstrated a regulatory effect of miRNAs on TXNIP, resulting in oxidative stress control. In fact, by downregulating TXNIP, miR-370 or miR-20a protect endothelial cells from induced ox-LDL [126,131], and miR-20b protects endothelial cells from senescence. The relationship between miR-146a and TXNIP is involved in enhanced ROS production and vascular smooth muscle cell calcification [130]. TXNIP is also a regulatory target of miRNAs in pyroptosis. While an axis miR-497/TXNIP has been described in diabetic nephropathy, other studies report the role of different miRNAs, including miR-17 in the TXNIP/NLRP3 signaling pathway in inflammation-induced kidney injury or brain ischemia [123,124,125,128,132].
3. TXNIP is a Novel Marker in Cardiovascular Diseases
TXNIP is a genetic, blood, peripheral blood cells, and tissue ischemia marker associated with cardiovascular diseases, as summarized in Figure 2 and in Table 2, thus making TXNIP an interesting target for prognostic and treatment.
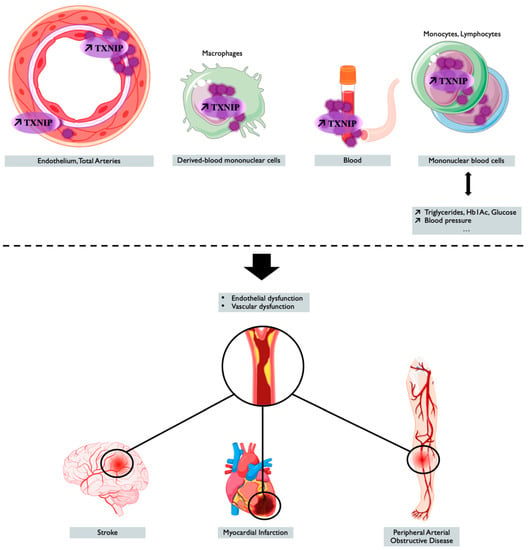
Figure 2.
TXNIP overexpression is associated with cardiovascular outcomes and diseases. Tissue, blood levels of TXNIP, and the genetic regulation of TXNIP make it a potential marker associated with cardiovascular risk factors or cardiovascular event or diseases. Created with BioRender.com.

Table 2.
TXNIP as a marker of cardiovascular risk and disease.
3.1. TXNIP as a Genetic Marker
3.1.1. Genetic Variants of TXNIP
Even though a recent genetic study in a family with homozygous nonsense mutations shows that the suppression of TXNIP expression is non-lethal in humans, functional variants are reportedly associated with disease in the literature. Two different genetic variants of TXNIP can be described as genetic markers for cardiovascular risk. First, TXNIP rs7211 and rs7212 variants were significantly associated with glucose and blood-pressure-related phenotypes in the Brazilian general population, and TXNIP rs7211 was linked to arterial stiffness [24]. A more recent study reports that the rs7211 variant of TXNIP is a protective factor against obesity in non-diabetic subjects and in women in Mexican patients [151]. However, TXNIP rs7211 has not been found to be associated with retinopathy or with diabetes in Caucasian patients with type 2 diabetes (T2D) [233]. Interestingly, the same variants of TXNIP rs7212 and rs7211 are significantly associated with increased coronary artery disease risk, and the cumulative effects of these two SNPs have been described on coronary artery disease risk and the severity of coronary atherosclerosis in a Chinese population [26]. Finally, in this population, coronary artery disease risk is also found associated with the TXNIP DNA methylation level independently of TXNIP rs7211 and 7212 variants [26].
3.1.2. Epigenetic Regulation of TXNIP
In addition to changes in the genomic sequence of TXNIP, epigenetic modifications, mainly influenced by environmental and lifestyle exposures, are also believed to contribute to cardiovascular disease risk. For instance, epigenetic regulations of the TXNIP gene via hyperglycemia have been described [188,189,234]. The most common epigenetic modification is DNA methylation, mainly found at cytosine-guanine dinucleotides sites within promoter regions and generally associated with gene silencing. The main DNA methylation described in the literature for the TXNIP gene locus is cg19693031, and this site is reported in numerous studies, in different tissues and in populations of different ethnic origin [27,235]. One of the 13 sites of methylation associated with blood pressure is cg19693031 at the TXNIP gene locus. In this study, decreased DNA methylation of TXNIP cg19693031 is related to increased blood pressure [28]. TXNIP methylation cg19693031 is also associated with lipid traits, and in particular with triglycerides levels [27,198]. In this two-stage epigenome-wide association study (EWAS), low TXNIP cg19693031 methylation is associated with high triglycerides levels independently of diabetes [27] in contrast to a previous study reporting an association of hypertriglyceridemia and genetic variation of TXNIP in diabetes patients [190]. In a Spanish EWAS, TXNIP cg19693031 is associated with prevalent T2D with TXNIP methylation inversely correlated with HbA1c levels in T2D [191], confirming the results obtained in the ESTHER cohort study [192], or more recently, in sub-Saharan African individuals with T2D [193]. The largest longitudinal study investigating DNA methylation in association with future risk of T2D in a multiethnic cohort reports that risk of future T2D was decreased per 1% increase in methylation at TXNIP cg19693031 site [RR = 0.92, 95%CI = 0.90–0.94]. Interestingly, this association surpasses further adjustment for non-genetic established risk factors for T2D [194]. A more recent study has been conducted in the British population to investigate the role of methylation in the etiology of T2D by investigating up to 11 years before T2D onset [195]. This study confirmed cg19693031 TXNIP methylation as a strong and consistent association with incident and prevalent T2D [195,196,197]. In fact, most epidemiological studies focus on T2D, probably related to the important role of TXNIP in glucose regulation by directly suppressing glucose uptake through binding to the glucose transporter Glut 1 [196,236,237,238,239,240]. In conclusion, the regulation of cg19693031 TXNIP methylation has been associated with cardiovascular diseases. However, today it is unknown whether cg19693031 TXNIP methylation plays a causal role in the development of diseases, remains a consequence of disease status, or is due to residual confounding. On the other hand, it will be important to consider the fact that the DNA methylation site is reversible, and therefore, aberrant DNA methylation modifications should in the future generate increased interest as drug targets.
3.2. TXNIP as a Blood Marker
Plasma levels of TXNIP may serve as a useful predictor of cardiovascular diseases. For example, carotid artery intima-media thickness is used as an indicator of atherosclerosis in patients with early-stage diabetes, and impaired glucose tolerance and is associated with increased plasma levels of TXNIP [30]. As a mechanical explanation, some works show, for example, that TXNIP overexpression induces endothelial dysfunction, vasoregulation disorders, and aortic stiffening via reduced levels of phosphorylated eNOS or NO bioavailability [12,21]. Moreover, TXNIP expression robustly correlates with the level of ROS production [241]. A study in diabetic rats also shows a correlation between circulating levels of TXNIP and the observation of aortic endothelial dysfunction with decreased levels of NO and VEGF and increased levels of ROS and VCAM-1 [17]. In contrast, lowering TXNIP blood levels by treating diabetic rats is associated with decreased aortic TXNIP levels, NO bioavailability, and arterial function [19]. In this study, TXNIP is also overexpressed at the aortic level, whether at the level of endothelial cells or VSMCs. Blood TXNIP levels are also found to be higher in stroke patients than in healthy controls [199]. Moreover, circulating TXNIP levels are also found to be increased after a heart attack induced by irradiation with 8Gy in rodents [55] and is associated with reduced TRX and TRX reductase levels, with increased cardiac TXNIP content and decreased cardiac antioxidant enzymes expression. In the literature, researchers are increasingly demonstrating a relationship between TXNIP blood levels and various bad outcomes beyond the cardiovascular field. For example, there is a correlation between TXNIP and peripheral nerve conduction velocity in patients with diabetes [242].
3.3. TXNIP as a Marker in Peripheral Blood Cells or Derived-Blood Cells
TXNIP levels can be altered in circulating blood cells, and it could be the signature of a risk factor for cardiovascular diseases. The expression of TXNIP is increased in the peripheral blood mononuclear cells of individuals with type 2 or type 1 diabetes and is correlated with the increase of inflammatory markers or endoplasmic reticulum stress [31,243,244]. TXNIP expression in peripheral blood mononuclear cells is increased in at-risk Takayasu arteritis patients. Interestingly, this TXNIP expression in peripheral blood mononuclear cells is associated with higher TXNIP expression in the aortic walls of these patients [200]. More specifically, TXNIP mRNA levels in leucocytes have also been investigated and are increased in patients with unstable angina pectoris [29] or with acute myocardial infarction [204], suggesting the role of TXNIP in atherosclerosis and the pathogenesis of cardiovascular diseases. Even if the endothelial levels of TXNIP are correlated with the adhesion of leucocytes, TXNIP levels in leucocytes also have an impact on the physiopathology of atherosclerosis [94,201]. For instance, TXNIP is one of the most significantly enriched genes in a subtype of macrophages resident in mouse atherosclerotic aortas [202]. In fact, TXNIP ablation has an atheroprotective effect through its regulation in the oxidative inflammatory response and atherosclerotic lesion development via a reduction in macrophage adhesion to VSMCs [110]. In addition, the deletion of TXNIP in leucocytes is reported to reduce leukostasis [80]. In vitro studies indicate that the mRNA expressions of NLRP3, IL-1, and IL-18 are up-regulated and positively correlated with the increased TXNIP mRNA in the peripheral blood leucocytes of coronary artery disease patients or THP-1 cells [203], which is consistent with the fact that TXNIP can promote vascular inflammatory responses and accelerate the process of atherosclerosis by activating the NLRP3 inflammasome [18,48,111]. TXNIP promotes inflammation and the activation of the monocytes in association with DNA demethylation, which orientates monocytes towards an inflammatory status through the NLRP3 inflammasome pathway [203]. Alternatively, one team tried to correlate TXNIP platelet content with platelet reactivity, atrial fibrillation, left atrial wall deformation, or tachycardia, but was not able to show any correlation [245,246,247,248]. Finally, OGA N-acetyl-glucosaminidase, which is the enzyme implicated in posttranslational modification of diabetic complications, has its mRNA levels correlated with TXNIP mRNA levels in the leukocytes of diabetic patients [205].
In the bone marrow, TXNIP could also have an impact in the mobilization of cells useful for post-injury or post-ischemic repair. TXNIP appears to be essential for maintaining the quiescence of hematopoietic stem cells and inhibits their mobilization. In fact, in the bone marrow, TXNIP decreases the Wnt signaling pathway and increases the interactions between hematopoietic stem cells and the niche [207]. In this study, the deletion of TXNIP in a transgenic mouse model promotes the proliferation of hematopoietic stem cells and their mobilization. Thus, TXNIP could inhibit the migration, differentiation, and mobilization of bone marrow cells via its antioxidant properties, but also inhibit the recruitment of these same cells to the ischemic site. Indeed, a more recent study from the same team suggests that TXNIP-p38 axis acts as a regulator mechanism in hematopoietic stem cell ageing; in particular, TXNIP increase the cell engraftment [208]. Additionally, a single-cell transcriptomic survey of aortas and coronary arteries in young and old primates reported, in the same way, FOXO3A loss (a transcription factor essential regulator of a pool of bone marrow cells and of oxidative stress level) as a key driver for arterial endothelial aging associated with the downregulation of TXNIP in smooth muscle cells [206]. However, the results have to be confirmed because the role of TXNIP in the mobilization of bone marrow cells is still poorly explored.
3.4. TXNIP as a Marker in the Context of Tissue Ischemia
The gene encoding TXNIP is induced by hypoxia in several cell types [249,250,251,252,253]. However, another group suggests that hypoxia induces a rapid decrease in the expression of mRNAs and proteins encoding TXNIP in an in vitro study [254]. The expression of TXNIP would therefore be regulated in a biphasic manner by hypoxia. First, TXNIP expression is rapidly reduced, and then its expression is increased under prolonged hypoxia. Based on these results, it appears that the expression of TXNIP can be upregulated or downregulated by ischemia, depending on the cell type and the pathological context. In vivo, ischemia is usually known to increase TXNIP levels [61,215,219,255,256,257]. Finally, the high sensitivity of TXNIP expression to a number of different stimuli suggests that TXNIP is a molecular switch that responds to various cellular stresses and regulates several molecular mechanisms in ischemic injuries such as oxidative stress and inflammation [253,258]. Moreover, knowing TXNIP involvement in ROS production and inflammation, which leads to endothelial dysfunction, TXNIP may also be implicated in vessel damage. Then, these vessel alterations can culminate in the occurrence of ischemic diseases.
3.4.1. TXNIP as a Marker in Myocardial Ischemia
In a study on cardiac ischemia-reperfusion, the authors showed that the NLRP3 inflammasome is increased in cardiac endothelial cells via TXNIP [209]. Although the rapid restoration of coronary flow is essential for the rescue of heart muscle, reperfusion is inevitably accompanied by sterile inflammation, which has been widely studied to be the primary cause of myocardial damage and dysfunction. Indeed, this inflammation can lead to ventricular remodeling and heart failure [210,211]. The increased activation of the inflammasome is evidenced by increased expression of NLRP3 and caspase-1 activity followed by the increased production of IL-1β and IL-18. The intramyocardial injection of anti-NLRP3 siRNA or the intraperitoneal injection of an inflammasome inhibitor results in an attenuated infiltration of macrophages and neutrophils and a decrease in ischemia-reperfusion damage as measured by apoptosis of cardiomyocytes and the size of the infarct. The intramyocardial injection of anti-TXNIP siRNA also decreases the size of the infarction and the activation of NLRP3, which suggests the value of targeting TXNIP to prevent deleterious effects of ischemia [209]. As a matter of fact, increased endothelial expression of TXNIP was found in diabetic hearts, which correlated well with the fact that insufficient angiogenesis aggravated cardiac remodeling and caused poor survival following myocardial infarction [173]. These results are in agreement with a previous study using intramyocardial injection of anti-TXNIP siRNA in the context of diabetes, which reduces oxidative stress, apoptosis and ischemia-induced myocardial damage [216]. A recent study shows that the interaction between TXNIP and NLRP3 is the key point in the damage-induced myocardial ischemia. Preventing the interaction between TXNIP and NLRP3 suppresses the ROS-TXNIP-NLRP3 pathway and alleviates myocardial ischemia/reperfusion injury [95,259]. In recent years, a growing body of research has begun to target TXNIP and thus suppress the ROS-TXNIP-NLRP3 pathway to hamper heart damage in myocardial ischemia [109]. A plethora of inhibitors proposed to hamper post-myocardial ischemia damage inhibit hypoxia-induced TXNIP and NLRP3 expressions [257]. This strategy is believed to improve cardiac function and reduce atrial fibrillation after myocardial infarction [100]. In addition, the administration of a vector-encoding TRX in diabetic rats increases capillary and arteriolar density, and therefore improves the restoration of cardiac function after myocardial infarction in the context of diabetes [260]. The balance of the TRX-TXNIP system is essential for the survival of cardiomyocytes in the context of ischemia [261]. In ischemic cardiomyopathy, the TRX-TXNIP system is impaired with reduced TRX and overexpressed TXNIP, whereas these features are not observed in dilatated cardiomyopathy [262]. TXNIP upregulation and the subsequently increased formation of the TRX-TXNIP complex is a proposed pathway by which diabetes induces insufficient angiogenesis and thereby exacerbates myocardial ischemia injury [215]. Moreover, in rats, treatment with resveratrol, a well-known inhibitor of TXNIP, is reported to be cardioprotective and to promote revascularization in a model of myocardial infarction. In this study, the authors show that resveratrol induces an increase in the expression of TRX and VEGF in a dose-dependent manner [263]. Again, novel strategies or several compounds proposed as treatments for myocardial infarction are regulators of the TRX-TXNIP system [64,264,265,266,267]. In addition to inflammation and dysregulation of the oxidative state trough the TRX-TXNIP balance, autophagy is also a mechanism where TXNIP plays a critical role in myocardial injury via the TXNIP/Redd1 pathway [64]. The heart responds to oxygen deprivation by increasing glucose uptake and glycolysis. Given the crucial role of glucose supply in the cardiac response to ischemia and the role of TXNIP in glucose uptake via GLUT1, it is likely that the resulting increase in glucose supply is due to TXNIP deficiency, and it provides cardioprotection to the ischemic heart. Indeed, the suppression of TXNIP in cardiomyocytes in mice was found to confer a protective advantage on the ischemic heart [212] as well as on left ventricular hypertrophy and heart failure [213,214].
3.4.2. TXNIP as a Marker in Hind Limb Ischemia
The role of the TRX-TXNIP system in the occurrence of pathologies such as arteriopathy of the lower limbs, and therefore ischemia of the lower limbs, has been poorly studied. Transgenic mice overexpressing endothelial TRX promote angiogenesis and post-ischemic arteriogenesis [268]. Indeed, in this study, the overexpression of TRX improves endothelial function via a decrease in ROS and an increase in the NO bioavailability. This study is interested in mitochondrial TRX, but an improvement in post-ischemic revascularization in the same model of lower limb ischemia is also observed with endothelial overexpression of cytosolic TRX [217]. The role of TXNIP, using paw-level anti-TXNIP siRNA injections in a mouse model of lower limb ischemia and diabetes, provides some insight. The team shows that the angiogenesis defect attributed to diabetes is dependent on TXNIP. Indeed, targeting TXNIP helps to counter the deleterious effects of diabetes through improved reperfusion of the ischemic limb, reduction of tissue damage, and increased capillary density [154]. In fact, the inhibition of TXNIP expression using fenofibrate treatment also helps to counter the post-ischemic revascularization defect of the lower limbs associated with diabetes [218]. Thanks to a transgenic model, TXNIP deletion is also involved in reducing the deleterious effects of a fatty diet in the revascularization of the lower limbs after ischemia [269]. Moreover, targeting specifically endothelial TXNIP protects from metabolic-disorder-related impairment in post-ischemic revascularization and tissue recovery [21].
3.4.3. TXNIP as a Marker in Cerebral Ischemia
TXNIP is also of growing interest to those who study neurological diseases, including cerebral ischemia, as previously reviewed [270]. In cerebral ischemia, four major pathologies are described: ischemic stroke, subarachnoid hemorrhage, neonatal hypoxic-ischemia, and vascular dementia. A number of studies have investigated the role of the TRX-TXNIP system in cerebral ischemia. In fact, a high serum TRX level is a good prognostic marker in ischemic stroke [271]. Cerebral ischemia induces activation of the inflammasome and is characterized by an increase in NLRP3 and TXNIP. When TXNIP and NLRP3 are decreased, tissue damage associated with cerebral ischemia is reduced [219,220]. Many other original compounds with antioxidant properties are able to decrease the expression of TXNIP and attenuate brain damage and neurotoxicity following ischemia by suppressing the activation of the inflammasome [103,127,221,222]. Nrf2 is reported to inhibit the NLRP3 inflammasome by regulating the TRX/TXNIP complex and consequently hampers cerebral ischemia reperfusion injury [102]. Increasing the expression of Nrf2 subsequently decreases the expression of TXNIP, NLRP3, Cleaved Caspase-1, and IL-1β, and reduces the infarction volume and improved neurological outcomes after middle cerebral artery occlusion [60]. Other authors support the idea that TXNIP silencing alleviates oxidative stress injury by regulating the MAPK-Nrf2 axis in ischemic stroke [61]. In addition to regulating Nrf2 expression with different compounds, a critical mechanism associated with the downregulation of TXNIP is the nuclear translocation of Nrf2 promoted by AMPK and GSK-3β [108]. This signaling pathway is in line with the improvement of the oxidative defense with augmentation of TRX and the diminution of TXNIP expression [225]. TXNIP upregulation is associated with blood–brain barrier (BBB) disruption in response to experimental hyperglycemic stroke with an increase of BBB permeability trough the TXNIP / NLRP3 inflammasome axis [223]. In a metabolic stress context, TXNIP is also upregulated and associated with the loss of mural cells since targeting TXNIP with different strategies hampered the signalization cascade of the NRLP3 inflammasome and induced protection against the loss of cells [13,21]. Moreover, TXNIP expression increases in the cytoplasm of neurons with significant brain damage due to focal cerebral ischemia in mice. The inhibition of TXNIP using anti-TXNIP siRNA protects neuronal cells and increases cell viability [224]. TXNIP, with its pro-inflammatory and pro-apoptotic effects, widely participates in early brain injury after subarachnoid hemorrhage [228,229]. In addition, NADPH oxidase-dependent inflammasome activation appears to contribute to traumatic brain injury pathology via a mechanism associated with TXNIP [58]. Again, the overexpression of TXNIP is linked to inflammasome activation or reticulum endoplasmic stress [62,230]. TXNIP is also involved in neonatal hypoxic-ischemia, which occurs in the youngest neonates. In rat models of the disease, PPAR-β/δ agonist mitigates apoptosis and reduces NLRP3-related neuroinflammation by increasing the miR-17-5p level and decreasing TXNIP expression [124,125]. The PPAR-β/δ/miR-17/TXNIP pathway is able to control TXNIP expression and subsequently inhibits NLRP3 activation [231]. Moreover, the ROS/TXNIP/NLRP3 pathway plays an important role in hemorrhagic transformation [226]. Finally, targeting TXNIP seems to also be an interesting target in vascular dementia since acupuncture shows neuroprotective effects by decreasing TXNIP and NLRP3 expression-associated oxidative stress and inflammation in a rat model of the disease [232]. These neuroprotective properties have also been reported in ischemic stroke [227].
4. Conclusions
Although larger-scale clinical studies need to be performed to use TXNIP as a biomarker in the clinic, TXNIP appears to be a preferred therapeutic target in endothelial and vascular dysfunction to prevent cardiovascular complications associated with age, metabolic disorders, and oxidative stress-related disorders. Indeed, the overexpression TXNIP is now well-recognized as being deleterious and an emergent new suspect for cardiovascular risk and diseases, as summarized in the diagram (Figure 2). Moreover, the action of drugs used in the treatment and prevention of cardiovascular pathologies with effects on the expression of TXNIP indirectly suggests the therapeutic benefit of targeting TXNIP. Therapies targeting endothelial TXNIP could thus delay endothelial dysfunction and the onset of cardiovascular complications induced by aging and its comorbidities.
Funding
This research received no external funding.
Acknowledgments
The figures were created using BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collins, A.R.; Lyon, C.J.; Xia, X.; Liu, J.Z.; Tangirala, R.K.; Yin, F.; Boyadjian, R.; Bikineyeva, A.; Praticò, D.; Harrison, D.G.; et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ. Res. 2009, 104, e42–e54. [Google Scholar] [CrossRef]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: New perspectives. J Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef]
- Hebert-Schuster, M.; Cottart, C.H.; Laguillier-Morizot, C.; Raynaud-Simon, A.; Golmard, J.L.; Cynober, L.; Beaudeux, J.L.; Fabre, E.E.; Nivet-Antoine, V. Catalase rs769214 SNP in elderly malnutrition and during renutrition: Is glucagon to blame? Free Radic. Biol. Med. 2011, 51, 1583–1588. [Google Scholar] [CrossRef]
- Hebert-Schuster, M.; Fabre, E.E.; Nivet-Antoine, V. Catalase polymorphisms and metabolic diseases. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Fabre, E.E.; Raynaud-Simon, A.; Golmard, J.-L.; Hebert, M.; Dulcire, X.; Succari, M.; Myara, J.; Durand, D.; Nivet-Antoine, V. Gene polymorphisms of oxidative stress enzymes: Prediction of elderly renutrition. Am. J. Clin. Nutr. 2008, 87, 1504–1512. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Nivet-Antoine, V.; Labat, C.; El Shamieh, S.; Dulcire, X.; Cottart, C.-H.; Beaudeux, J.-L.; Zannad, F.; Visvikis-Siest, S.; Benetos, A. Relationship between catalase haplotype and arterial aging. Atherosclerosis 2013, 227, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer Iv, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Corkey, B.E.; Deeney, J.T. The Redox Communication Network as a Regulator of Metabolism. Front. Physiol. 2020, 11, 567796. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Tian, D.; Dong, J.; Jin, S.; Teng, X.; Wu, Y. Endogenous hydrogen sulfide-mediated MAPK inhibition preserves endothelial function through TXNIP signaling. Free Radic. Biol. Med. 2017, 110, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Bedarida, T.; Domingues, A.; Baron, S.; Ferreira, C.; Vibert, F.; Cottart, C.-H.; Paul, J.-L.; Escriou, V.; Bigey, P.; Gaussem, P.; et al. Reduced endothelial thioredoxin-interacting protein protects arteries from damage induced by metabolic stress in vivo. FASEB J. 2018, 32, 3108–3118. [Google Scholar] [CrossRef]
- Alhawiti, N.M.; Al Mahri, S.; Aziz, M.A.; Malik, S.S.; Mohammad, S. TXNIP in Metabolic Regulation: Physiological Role and Therapeutic Outlook. Curr. Drug Targets 2017, 18, 1095–1103. [Google Scholar] [CrossRef]
- Chaves, A.B.; Haus, J.M.; Houmard, J.A. Role of TXNIP Biology in Glucose Metabolism. Int. J. Diabetes Clin. Res. 2018, 5. [Google Scholar] [CrossRef]
- Yoshihara, E. TXNIP/TBP-2: A Master Regulator for Glucose Homeostasis. Antioxidants 2020, 9, 765. [Google Scholar] [CrossRef]
- Li, X.; Kover, K.L.; Heruth, D.P.; Watkins, D.J.; Guo, Y.; Moore, W.V.; He, L.G.; Zang, M.; Clements, M.A.; Yan, Y. Thioredoxin-interacting protein promotes high-glucose-induced macrovascular endothelial dysfunction. Biochem. Biophys. Res. Commun. 2017, 493, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.M.; Abdelaziz, R.R.; Hamed, M.F.; Nader, M.A.; Shehatou, G.S.G. Dimethyl fumarate ameliorates diabetes-associated vascular complications through ROS-TXNIP-NLRP3 inflammasome pathway. Life Sci. 2020, 256, 117887. [Google Scholar] [CrossRef]
- Dunn, L.L.; Buckle, A.M.; Cooke, J.P.; Ng, M.K.C. The emerging role of the thioredoxin system in angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Domingues, A.; Boisson-Vidal, C.; Marquet de Rouge, P.; Dizier, B.; Sadoine, J.; Mignon, V.; Vessières, E.; Henrion, D.; Escriou, V.; Bigey, P.; et al. Targeting endothelial thioredoxin-interacting protein (TXNIP) protects from metabolic disorder-related impairment of vascular function and post-ischemic revascularisation. Angiogenesis 2020, 23, 249–264. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Lee, K.-N.; Hwang, C.Y.; Kwon, K.-S.; You, K.-H.; Choi, I. Tumor suppressor VDUP1 increases p27(kip1) stability by inhibiting JAB1. Cancer Res. 2005, 65, 4485–4489. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, Y.; Tu, Y.; Guo, Y.; Sun, X.; Xu, X.; Liu, X.; Wang, L.; Qin, X.; Zhu, M.; et al. A prodrug of epigallocatechin-3-gallate alleviates high glucose-induced pro-angiogenic factor production by inhibiting the ROS/TXNIP/NLRP3 inflammasome axis in retinal Müller cells. Exp. Eye Res. 2020, 196, 108065. [Google Scholar] [CrossRef] [PubMed]
- Alvim, R.O.; Santos, P.C.J.L.; Ferreira, N.E.; Mill, J.G.; Krieger, J.E.; Pereira, A.C. Thioredoxin interacting protein (TXNIP) rs7212 polymorphism is associated with arterial stiffness in the Brazilian general population. J. Hum. Hypertens. 2012, 26, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.E.; Omae, S.; Pereira, A.; Rodrigues, M.V.; Miyakawa, A.A.; Campos, L.C.G.; Santos, P.C.J.L.; Dallan, L.A.; Martinez, T.L.; Santos, R.D.; et al. Thioredoxin interacting protein genetic variation is associated with diabetes and hypertension in the Brazilian general population. Atherosclerosis 2012, 221, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Han, Y.-D.; Zhang, S.; Cui, N.-H.; Liu, Z.-J.; Huang, Z.-L.; Li, C.; Zheng, F. Associations of polymorphisms in TXNIP and gene-environment interactions with the risk of coronary artery disease in a Chinese Han population. J. Cell. Mol. Med. 2016, 20, 2362–2373. [Google Scholar] [CrossRef]
- Sayols-Baixeras, S.; Subirana, I.; Lluis-Ganella, C.; Civeira, F.; Roquer, J.; Do, A.N.; Absher, D.; Cenarro, A.; Muñoz, D.; Soriano-Tárraga, C.; et al. Identification and validation of seven new loci showing differential DNA methylation related to serum lipid profile: An epigenome-wide approach. The REGICOR study. Hum. Mol. Genet. 2016, 25, 4556–4565. [Google Scholar] [CrossRef]
- Richard, M.A.; Huan, T.; Ligthart, S.; Gondalia, R.; Jhun, M.A.; Brody, J.A.; Irvin, M.R.; Marioni, R.; Shen, J.; Tsai, P.-C.; et al. DNA Methylation Analysis Identifies Loci for Blood Pressure Regulation. Am. J. Hum. Genet. 2017, 101, 888–902. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Yang, X.; Sun, X.; Xu, Q.; Wang, B.; Zhong, P.; Wei, Z. Altered Expression of TXNIP in the peripheral leukocytes of patients with coronary atherosclerotic heart disease. Medicine 2017, 96, e9108. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Tang, S. Retrospective analysis of the relationship between elevated plasma levels of TXNIP and carotid intima-media thickness in subjects with impaired glucose tolerance and early Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2015, 109, 372–377. [Google Scholar] [CrossRef]
- Szpigel, A.; Hainault, I.; Carlier, A.; Venteclef, N.; Batto, A.-F.; Hajduch, E.; Bernard, C.; Ktorza, A.; Gautier, J.-F.; Ferré, P.; et al. Lipid environment induces ER stress, TXNIP expression and inflammation in immune cells of individuals with type 2 diabetes. Diabetologia 2018, 61, 399–412. [Google Scholar] [CrossRef]
- Spindel, O.N.; World, C.; Berk, B.C. Thioredoxin Interacting Protein: Redox Dependent and Independent Regulatory Mechanisms. Antioxid. Redox Signal. 2012, 16, 587–596. [Google Scholar] [CrossRef]
- Elgort, M.G.; O’Shea, J.M.; Jiang, Y.; Ayer, D.E. Transcriptional and Translational Downregulation of Thioredoxin Interacting Protein Is Required for Metabolic Reprogramming during G(1). Genes Cancer 2010, 1, 893–907. [Google Scholar] [CrossRef]
- Held, M.A.; Greenfest-Allen, E.; Jachimowicz, E.; Stoeckert, C.J.; Stokes, M.P.; Wood, A.W.; Wojchowski, D.M. Phospho-proteomic discovery of novel signal transducers including thioredoxin-interacting protein as mediators of erythropoietin-dependent human erythropoiesis. Exp. Hematol. 2020, 84, 29–44. [Google Scholar] [CrossRef]
- Hirata, C.L.; Ito, S.; Masutani, H. Thioredoxin interacting protein (Txnip) forms redox sensitive high molecular weight nucleoprotein complexes. Arch. Biochem. Biophys. 2019, 677, 108159. [Google Scholar] [CrossRef] [PubMed]
- World, C.; Spindel, O.N.; Berk, B.C. Thioredoxin-interacting protein mediates TRX1 translocation to the plasma membrane in response to tumor necrosis factor-α: A key mechanism for vascular endothelial growth factor receptor-2 transactivation by reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1890–1897. [Google Scholar] [CrossRef]
- Hwang, J.; Suh, H.-W.; Jeon, Y.H.; Hwang, E.; Nguyen, L.T.; Yeom, J.; Lee, S.-G.; Lee, C.; Kim, K.J.; Kang, B.S.; et al. The structural basis for the negative regulation of thioredoxin by thioredoxin-interacting protein. Nat. Commun. 2014, 5, 2958. [Google Scholar] [CrossRef]
- Shin, D.; Jeon, J.-H.; Jeong, M.; Suh, H.-W.; Kim, S.; Kim, H.-C.; Moon, O.-S.; Kim, Y.-S.; Chung, J.W.; Yoon, S.R.; et al. VDUP1 mediates nuclear export of HIF1alpha via CRM1-dependent pathway. Biochim. Biophys. Acta 2008, 1783, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Devi, T.S.; Hosoya, K.-I.; Terasaki, T.; Singh, L.P. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J. Cell. Physiol. 2009, 221, 262–272. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, W.S.; Kim, D.O.; Byun, J.-E.; Huy, H.; Lee, S.Y.; Song, H.Y.; Park, Y.-J.; Kim, T.-D.; Yoon, S.R.; et al. Macrophage migration inhibitory factor interacts with thioredoxin-interacting protein and induces NF-κB activity. Cell. Signal. 2017, 34, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, Z.T.; Wang, C.; Forrester, M.T.; Foster, M.W.; Marshall, H.E. ERK-dependent proteasome degradation of Txnip regulates thioredoxin oxidoreductase activity. J. Biol. Chem. 2019, 294, 13336–13343. [Google Scholar] [CrossRef]
- Otaki, Y.; Takahashi, H.; Watanabe, T.; Funayama, A.; Netsu, S.; Honda, Y.; Narumi, T.; Kadowaki, S.; Hasegawa, H.; Honda, S.; et al. HECT-Type Ubiquitin E3 Ligase ITCH Interacts with Thioredoxin-Interacting Protein and Ameliorates Reactive Oxygen Species-Induced Cardiotoxicity. J. Am. Heart Assoc. 2016, 5, e002485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lau, J.; Li, W.; Tempel, W.; Li, L.; Dong, A.; Narula, A.; Qin, S.; Min, J. Structural basis for the regulatory role of the PPxY motifs in the thioredoxin-interacting protein TXNIP. Biochem. J. 2016, 473, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Patwari, P.; Higgins, L.J.; Chutkow, W.A.; Yoshioka, J.; Lee, R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006, 281, 21884–21891. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 1979, 254, 9627–9632. [Google Scholar] [CrossRef]
- Nakamura, H. Thioredoxin and its related molecules: Update 2005. Antioxid. Redox Signal. 2005, 7, 823–828. [Google Scholar] [CrossRef]
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef]
- Junn, E.; Han, S.H.; Im, J.Y.; Yang, Y.; Cho, E.W.; Um, H.D.; Kim, D.K.; Lee, K.W.; Han, P.L.; Rhee, S.G.; et al. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J. Immunol. 2000, 164, 6287–6295. [Google Scholar] [CrossRef]
- Chen, K.-S.; DeLuca, H.F. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1994, 1219, 26–32. [Google Scholar] [CrossRef]
- Katakam, P.V.G.; Tulbert, C.D.; Snipes, J.A.; Erdös, B.; Miller, A.W.; Busija, D.W. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H854–H860. [Google Scholar] [CrossRef]
- Bedarida, T.; Baron, S.; Vibert, F.; Ayer, A.; Henrion, D.; Thioulouse, E.; Marchiol, C.; Beaudeux, J.-L.; Cottart, C.-H.; Nivet-Antoine, V. Resveratrol Decreases TXNIP mRNA and Protein Nuclear Expressions with an Arterial Function Improvement in Old Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 720–729. [Google Scholar] [CrossRef]
- Bedarida, T.; Baron, S.; Vessières, E.; Vibert, F.; Ayer, A.; Marchiol-Fournigault, C.; Henrion, D.; Paul, J.-L.; Noble, F.; Golmard, J.-L.; et al. High-protein-low-carbohydrate diet: Deleterious metabolic and cardiovascular effects depend on age. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H649–H657. [Google Scholar] [CrossRef] [PubMed]
- Nivet-Antoine, V.; Cottart, C.-H.; Lemaréchal, H.; Vamy, M.; Margaill, I.; Beaudeux, J.-L.; Bonnefont-Rousselot, D.; Borderie, D. trans-Resveratrol downregulates Txnip overexpression occurring during liver ischemia-reperfusion. Biochimie 2010, 92, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; Yoshioka, J.; Takahashi, T.; He, Z.; King, G.L.; Lee, R.T. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J. Biol. Chem. 2004, 279, 30369–30374. [Google Scholar] [CrossRef]
- Abdel Magied, N.; Shedid, S.M. Impact of zinc oxide nanoparticles on thioredoxin-interacting protein and asymmetric dimethylarginine as biochemical indicators of cardiovascular disorders in gamma-irradiated rats. Environ. Toxicol. 2020, 35, 430–442. [Google Scholar] [CrossRef]
- Shah, A.; Xia, L.; Goldberg, H.; Lee, K.W.; Quaggin, S.E.; Fantus, I.G. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J. Biol. Chem. 2013, 288, 6835–6848. [Google Scholar] [CrossRef]
- Zhang, C.; Abdukerim, M.; Abilailieti, M.; Tang, L.; Ling, Y.; Pan, S. The protective effects of orexin a against high glucose-induced activation of NLRP3 inflammasome in human vascular endothelial cells. Arch. Biochem. Biophys. 2019, 672, 108052. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.W.; Wang, J.; Dhandapani, K.M.; Brann, D.W. NADPH Oxidase 2 Regulates NLRP3 Inflammasome Activation in the Brain after Traumatic Brain Injury. Oxid. Med. Cell. Longev. 2017, 2017, 6057609. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, L.; Li, J.; Cai, Y.; Xue, Y. TXNIP mediated the oxidative stress response in glomerular mesangial cells partially through AMPK pathway. Biomed. Pharmacother. 2018, 107, 785–792. [Google Scholar] [CrossRef]
- Yu, J.; Wang, W.-N.; Matei, N.; Li, X.; Pang, J.-W.; Mo, J.; Chen, S.-P.; Tang, J.-P.; Yan, M.; Zhang, J.H. Ezetimibe Attenuates Oxidative Stress and Neuroinflammation via the AMPK/Nrf2/TXNIP Pathway after MCAO in Rats. Oxid. Med. Cell. Longev. 2020, 2020, 4717258. [Google Scholar] [CrossRef]
- Tian, Y.; Su, Y.; Ye, Q.; Chen, L.; Yuan, F.; Wang, Z. Silencing of TXNIP Alleviated Oxidative Stress Injury by Regulating MAPK-Nrf2 Axis in Ischemic Stroke. Neurochem. Res. 2020, 45, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, T.; Gao, L.; Zheng, J.; Yan, J.; Zhang, J.; Shao, A. Apelin-13/APJ system attenuates early brain injury via suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation and oxidative stress in a AMPK-dependent manner after subarachnoid hemorrhage in rats. J. Neuroinflamm. 2019, 16, 247. [Google Scholar] [CrossRef]
- Hou, X.; Yang, S.; Yin, J. Blocking the REDD1/TXNIP axis ameliorates LPS-induced vascular endothelial cell injury through repressing oxidative stress and apoptosis. Am. J. Physiol. Cell Physiol. 2019, 316, C104–C110. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, R.; Li, B.; Guo, Y.; Yin, T.; Xia, Y.; Zhang, F.; Lian, K.; Liu, Y.; Wang, H.; et al. TXNIP/Redd1 signalling and excessive autophagy: A novel mechanism of myocardial ischaemia/reperfusion injury in mice. Cardiovasc. Res. 2020, 116, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, Y.; Song, J.; Hou, F.; Ma, X.; Liu, B.; Huang, F. Mangiferin suppresses endoplasmic reticulum stress in perivascular adipose tissue and prevents insulin resistance in the endothelium. Eur. J. Nutr. 2018, 57, 1563–1575. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhou, H.; Wu, H.; Wu, Q.; Duan, M.; Deng, W.; Tang, Q. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019, 24, 101215. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Chen, Z.; Yu, S.; Xu, H. Fenofibrate Ameliorates Oxidative Stress-Induced Retinal Microvascular Dysfunction in Diabetic Rats. Curr. Eye Res. 2018, 43, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Yeh, W.-J.; Yang, H.-Y.; Pai, M.-H.; Wu, C.-H.; Chen, J.-R. Long-term administration of advanced glycation end-product stimulates the activation of NLRP3 inflammasome and sparking the development of renal injury. J. Nutr. Biochem. 2017, 39, 68–76. [Google Scholar] [CrossRef]
- Liu, D.; Yang, P.; Gao, M.; Yu, T.; Shi, Y.; Zhang, M.; Yao, M.; Liu, Y.; Zhang, X. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin. Sci. 2019, 133, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Samra, Y.A.; Said, H.S.; Elsherbiny, N.M.; Liou, G.I.; El-Shishtawy, M.M.; Eissa, L.A. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: Role of NF-κB and NLRP3 inflammasome. Life Sci. 2016, 157, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Koka, S.; Xia, M.; Chen, Y.; Bhat, O.M.; Yuan, X.; Boini, K.M.; Li, P.-L. Endothelial NLRP3 inflammasome activation and arterial neointima formation associated with acid sphingomyelinase during hypercholesterolemia. Redox Biol. 2017, 13, 336–344. [Google Scholar] [CrossRef]
- Deng, Y.; Han, X.; Yao, Z.; Sun, Y.; Yu, J.; Cai, J.; Ren, G.; Jiang, G.; Han, F. PPARα Agonist Stimulated Angiogenesis by Improving Endothelial Precursor Cell Function Via a NLRP3 Inflammasome Pathway. Cell. Physiol. Biochem. 2017, 42, 2255–2266. [Google Scholar] [CrossRef]
- Luo, B.; Huang, F.; Liu, Y.; Liang, Y.; Wei, Z.; Ke, H.; Zeng, Z.; Huang, W.; He, Y. NLRP3 Inflammasome as a Molecular Marker in Diabetic Cardiomyopathy. Front. Physiol. 2017, 8, 519. [Google Scholar] [CrossRef]
- Feng, H.; Gu, J.; Gou, F.; Huang, W.; Gao, C.; Chen, G.; Long, Y.; Zhou, X.; Yang, M.; Liu, S.; et al. High Glucose and Lipopolysaccharide Prime NLRP3 Inflammasome via ROS/TXNIP Pathway in Mesangial Cells. J. Diabetes Res. 2016, 2016, 6973175. [Google Scholar] [CrossRef]
- Wu, M.; Han, W.; Song, S.; Du, Y.; Liu, C.; Chen, N.; Wu, H.; Shi, Y.; Duan, H. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol. Cell. Endocrinol. 2018, 478, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-P.; Fu, J.; Liu, L.-H.; Wu, K.-F.; Liu, H.-X.; Qi, B.-M.; Liu, Y.; Qi, B.-L. Honokiol antagonizes doxorubicin-induced cardiomyocyte senescence by inhibiting TXNIP-mediated NLRP3 inflammasome activation. Int. J. Mol. Med. 2020, 45, 186–194. [Google Scholar] [CrossRef]
- An, X.; Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. Punicalagin Protects Diabetic Nephropathy by Inhibiting Pyroptosis Based on TXNIP/NLRP3 Pathway. Nutrients 2020, 12, 1516. [Google Scholar] [CrossRef]
- Mohamed, I.N.; Sheibani, N.; El-Remessy, A.B. Deletion of Thioredoxin-Interacting Protein (TXNIP) Abrogates High Fat Diet-induced Retinal Leukostasis, Barrier Dysfunction and Microvascular Degeneration in a Mouse Obesity Model. Int. J. Mol. Sci. 2020, 21, 3983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, X.; Wang, P.; Peng, W.; Zhang, B.; Guo, L. Inhibitor of RAGE and glucose-induced inflammation in bone marrow mesenchymal stem cells: Effect and mechanism of action. Mol. Med. Rep. 2020, 22, 3255–3262. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; He, X.; Zhang, B.; Peng, W.; Guo, L. N-acetyl cysteine inhibits the lipopolysaccharide-induced inflammatory response in bone marrow mesenchymal stem cells by suppressing the TXNIP/NLRP3/IL-1β signaling pathway. Mol. Med. Rep. 2020, 22, 3299–3306. [Google Scholar] [CrossRef]
- Wang, W.; Mao, S.; Yu, H.; Wu, H.; Shan, X.; Zhang, X.; Cui, G.; Liu, X. Pinellia pedatisecta lectin exerts a proinflammatory activity correlated with ROS-MAPKs/NF-κB pathways and the NLRP3 inflammasome in RAW264.7 cells accompanied by cell pyroptosis. Int. Immunopharmacol. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Davis, B.K.; Ting, J.P.-Y. NLRP3 has a sweet tooth. Nat. Immunol. 2010, 11, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Hara, T.; O’Sullivan-Murphy, B.; Kanekura, K.; Lu, S.; Hara, M.; Ishigaki, S.; Zhu, L.J.; Hayashi, E.; Hui, S.T.; et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012, 16, 265–273. [Google Scholar] [CrossRef]
- Abais, J.M.; Xia, M.; Li, G.; Chen, Y.; Conley, S.M.; Gehr, T.W.B.; Boini, K.M.; Li, P.-L. Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein (TXNIP) during hyperhomocysteinemia. J. Biol. Chem. 2014, 289, 27159–27168. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Ye, L.; Wang, Y.; Zhang, K.; Wang, L.; Huang, Y.; Wang, L.; Xian, S.; Zhang, Y.; et al. Aspirin alleviates endothelial gap junction dysfunction through inhibition of NLRP3 inflammasome activation in LPS-induced vascular injury. Acta Pharm. Sin. B 2019, 9, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deng, H.; Zhou, Z.; Xiong, X.; Gao, L. Endothelium as a Potential Target for Treatment of Abdominal Aortic Aneurysm. Oxid. Med. Cell. Longev. 2018, 2018, 6306542. [Google Scholar] [CrossRef]
- Nyandwi, J.B.; Ko, Y.S.; Jin, H.; Yun, S.P.; Park, S.W.; Kim, H.J. Rosmarinic acid inhibits oxLDL-induced inflammasome activation under high-glucose conditions through downregulating the p38-FOXO1-TXNIP pathway. Biochem. Pharmacol. 2020, 182, 114246. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, J.; Chen, M.-H.; Wang, Q.; Qin, M.-J.; Zhang, T.; Chen, X.-Q.; Liu, B.-L.; Wen, X.-D. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol. Res. 2015, 99, 101–115. [Google Scholar] [CrossRef]
- Tang, G.; Duan, F.; Li, W.; Wang, Y.; Zeng, C.; Hu, J.; Li, H.; Zhang, X.; Chen, Y.; Tan, H. Metformin inhibited Nod-like receptor protein 3 inflammasomes activation and suppressed diabetes-accelerated atherosclerosis in apoE-/- mice. Biomed. Pharmacother. 2019, 119, 109410. [Google Scholar] [CrossRef]
- Wei, H.; Bu, R.; Yang, Q.; Jia, J.; Li, T.; Wang, Q.; Chen, Y. Exendin-4 Protects against Hyperglycemia-Induced Cardiomyocyte Pyroptosis via the AMPK-TXNIP Pathway. J. Diabetes Res. 2019, 2019, 8905917. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Xu, Y.; Xu, J.; Zhao, D.; Ye, L.; Yu, G.; Wang, Z.; Lu, Q.; Lin, J.; Yang, T.; et al. Berberine Inhibits Nod-Like Receptor Family Pyrin Domain Containing 3 Inflammasome Activation and Pyroptosis in Nonalcoholic Steatohepatitis via the ROS/TXNIP Axis. Front. Pharmacol. 2020, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Hashiguchi, K.; Yamamoto, H.; Tagami, M. Dietary Apigenin Reduces Induction of LOX-1 and NLRP3 Expression, Leukocyte Adhesion, and Acetylated Low-Density Lipoprotein Uptake in Human Endothelial Cells Exposed to Trimethylamine-N-Oxide. J. Cardiovasc. Pharmacol. 2019, 74, 558–565. [Google Scholar] [CrossRef]
- Wang, D.-S.; Yan, L.-Y.; Yang, D.-Z.; Lyu, Y.; Fang, L.-H.; Wang, S.-B.; Du, G.-H. Formononetin ameliorates myocardial ischemia/reperfusion injury in rats by suppressing the ROS-TXNIP-NLRP3 pathway. Biochem. Biophys. Res. Commun. 2020, 525, 759–766. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Su, C.; Zhong, Q.; Wu, G. Cilostazol ameliorates high free fatty acid (FFA)-induced activation of NLRP3 inflammasome in human vascular endothelial cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3704–3710. [Google Scholar] [CrossRef]
- Luo, X.; Hu, Y.; He, S.; Ye, Q.; Lv, Z.; Liu, J.; Chen, X. Dulaglutide inhibits high glucose-induced endothelial dysfunction and NLRP3 inflammasome activation. Arch. Biochem. Biophys. 2019, 671, 203–209. [Google Scholar] [CrossRef]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D3 Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef]
- Lian, D.; Yuan, H.; Yin, X.; Wu, Y.; He, R.; Huang, Y.; Chen, Y. Puerarin inhibits hyperglycemia-induced inter-endothelial junction through suppressing endothelial Nlrp3 inflammasome activation via ROS-dependent oxidative pathway. Phytomedicine 2019, 55, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liu, W.; Lan, T.; Pan, W.; Chen, X.; Wu, H.; Xu, D. Salvianolate reduces atrial fibrillation through suppressing atrial interstitial fibrosis by inhibiting TGF-β1/Smad2/3 and TXNIP/NLRP3 inflammasome signaling pathways in post-MI rats. Phytomedicine 2018, 51, 255–265. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.-H.; Sui, Y.; Wang, Y.; Qiu, X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed. Pharmacother. 2017, 86, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, Y.; He, Q.; Li, L.; Xie, H.; Zhao, Y.; Zhao, J. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 2018, 336, 32–39. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Gao, X.; Yuan, Z.; Ma, T.; Li, G.; Zhang, X. Tilianin Protects Diabetic Retina through the Modulation of Nrf2/TXNIP/NLRP3 Inflammasome Pathways. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhou, Z.; Liu, W.; Chang, Q.; Sun, G.; Dai, Y. Vascular endothelial cells senescence is associated with NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation via reactive oxygen species (ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int. J. Biochem. Cell Biol. 2017, 84, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, P.; Rasool, M. Berberine, an isoquinoline alkaloid suppresses TXNIP mediated NLRP3 inflammasome activation in MSU crystal stimulated RAW 264.7 macrophages through the upregulation of Nrf2 transcription factor and alleviates MSU crystal induced inflammation in rats. Int. Immunopharmacol. 2017, 44, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Koska, J.; Lopez, L.; D’Souza, K.; Osredkar, T.; Deer, J.; Kurtz, J.; Salbe, A.D.; Harman, S.M.; Reaven, P.D. Effect of liraglutide on dietary lipid-induced insulin resistance in humans. Diabetes Obes. Metab. 2018, 20, 69–76. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Luo, J.; Zhao, L.; Li, X.; Guo, H.; Bai, H.; Cui, W.; Guo, W.; Feng, D.; et al. Adiponectin peptide alleviates oxidative stress and NLRP3 inflammasome activation after cerebral ischemia-reperfusion injury by regulating AMPK/GSK-3β. Exp. Neurol. 2020, 329, 113302. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, S.; Chang, S.; Ren, D.; Shali, S.; Li, C.; Yang, H.; Huang, Z.; Ge, J. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-κB/NLRP3 inflammasome signaling pathway. J. Mol. Cell. Cardiol. 2020, 142, 65–79. [Google Scholar] [CrossRef]
- Byon, C.H.; Han, T.; Wu, J.; Hui, S.T. Txnip ablation reduces vascular smooth muscle cell inflammation and ameliorates atherosclerosis in apolipoprotein E knockout mice. Atherosclerosis 2015, 241, 313–321. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Nigro, P.; World, C.; Fujiwara, K.; Yan, C.; Berk, B.C. Thioredoxin interacting protein promotes endothelial cell inflammation in response to disturbed flow by increasing leukocyte adhesion and repressing Kruppel-like factor 2. Circ. Res. 2012, 110, 560–568. [Google Scholar] [CrossRef]
- Andres, A.M.; Ratliff, E.P.; Sachithanantham, S.; Hui, S.T. Diminished AMPK signaling response to fasting in thioredoxin-interacting protein knockout mice. FEBS Lett. 2011, 585, 1223–1230. [Google Scholar] [CrossRef]
- Wu, N.; Zheng, B.; Shaywitz, A.; Dagon, Y.; Tower, C.; Bellinger, G.; Shen, C.-H.; Wen, J.; Asara, J.; McGraw, T.E.; et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol. Cell 2013, 49, 1167–1175. [Google Scholar] [CrossRef]
- Wilde, B.R.; Ye, Z.; Lim, T.-Y.; Ayer, D.E. Cellular acidosis triggers human MondoA transcriptional activity by driving mitochondrial ATP production. Elife 2019, 8, e40199. [Google Scholar] [CrossRef]
- Parikh, H.; Carlsson, E.; Chutkow, W.A.; Johansson, L.E.; Storgaard, H.; Poulsen, P.; Saxena, R.; Ladd, C.; Schulze, P.C.; Mazzini, M.J.; et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007, 4, e158. [Google Scholar] [CrossRef]
- Nagaraj, K.; Lapkina-Gendler, L.; Sarfstein, R.; Gurwitz, D.; Pasmanik-Chor, M.; Laron, Z.; Yakar, S.; Werner, H. Identification of thioredoxin-interacting protein (TXNIP) as a downstream target for IGF1 action. Proc. Natl. Acad. Sci. USA 2018, 115, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Patwari, P.; Chutkow, W.A.; Cummings, K.; Verstraeten, V.L.R.M.; Lammerding, J.; Schreiter, E.R.; Lee, R.T. Thioredoxin-independent regulation of metabolism by the alpha-arrestin proteins. J. Biol. Chem. 2009, 284, 24996–25003. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.; Peñaloza, H.; Wang, Z.; Wickersham, M.; Parker, D.; Patel, P.; Koller, A.; Chen, E.I.; Bueno, S.M.; Uhlemann, A.-C.; et al. Acquired resistance to innate immune clearance promotes Klebsiella pneumoniae ST258 pulmonary infection. JCI Insight 2016, 1, e89704. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, W.J.; Mullen, P.J.; Schmid, E.W.; Flores, A.; Momcilovic, M.; Sharpley, M.S.; Jelinek, D.; Whiteley, A.E.; Maxwell, M.B.; Wilde, B.R.; et al. Extracellular Matrix Remodeling Regulates Glucose Metabolism through TXNIP Destabilization. Cell 2018, 175, 117–132.e21. [Google Scholar] [CrossRef]
- Waldhart, A.N.; Dykstra, H.; Peck, A.S.; Boguslawski, E.A.; Madaj, Z.B.; Wen, J.; Veldkamp, K.; Hollowell, M.; Zheng, B.; Cantley, L.C.; et al. Phosphorylation of TXNIP by AKT Mediates Acute Influx of Glucose in Response to Insulin. Cell Rep. 2017, 19, 2005–2013. [Google Scholar] [CrossRef]
- Li, X.; Kover, K.L.; Heruth, D.P.; Watkins, D.J.; Moore, W.V.; Jackson, K.; Zang, M.; Clements, M.A.; Yan, Y. New Insight into Metformin Action: Regulation of ChREBP and FOXO1 Activities in Endothelial Cells. Mol. Endocrinol. 2015, 29, 1184–1194. [Google Scholar]
- Dong, D.; Fu, N.; Yang, P. MiR-17 Downregulation by High Glucose Stabilizes Thioredoxin-Interacting Protein and Removes Thioredoxin Inhibition on ASK1 Leading to Apoptosis. Toxicol. Sci. 2016, 150, 84–96. [Google Scholar] [PubMed]
- Liu, Y.; Lou, G.; Li, A.; Zhang, T.; Qi, J.; Ye, D.; Zheng, M.; Chen, Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine 2018, 36, 140–150. [Google Scholar]
- Gamdzyk, M.; Doycheva, D.M.; Kang, R.; Tang, H.; Travis, Z.D.; Tang, J.; Zhang, J.H. GW0742 activates miR-17-5p and inhibits TXNIP/NLRP3-mediated inflammation after hypoxic-ischaemic injury in rats and in PC12 cells. J. Cell. Mol. Med. 2020, 24, 12318–12330. [Google Scholar] [CrossRef] [PubMed]
- Gamdzyk, M.; Doycheva, D.M.; Malaguit, J.; Enkhjargal, B.; Tang, J.; Zhang, J.H. Role of PPAR-β/δ/miR-17/TXNIP pathway in neuronal apoptosis after neonatal hypoxic-ischemic injury in rats. Neuropharmacology 2018, 140, 150–161. [Google Scholar]
- Chen, M.; Li, W.; Zhang, Y.; Yang, J. MicroRNA-20a protects human aortic endothelial cells from Ox-LDL-induced inflammation through targeting TLR4 and TXNIP signaling. Biomed. Pharmacother. 2018, 103, 191–197. [Google Scholar] [PubMed]
- Zhang, H.-S.; Liu, M.-F.; Ji, X.-Y.; Jiang, C.-R.; Li, Z.-L.; OuYang, B. Gastrodin combined with rhynchophylline inhibits cerebral ischaemia-induced inflammasome activation via upregulating miR-21-5p and miR-331-5p. Life Sci. 2019, 239, 116935. [Google Scholar]
- Li, X.; Yao, L.; Zeng, X.; Hu, B.; Zhang, X.; Wang, J.; Zhu, R.; Yu, Q. miR-30c-5p Alleviated Pyroptosis During Sepsis-Induced Acute Kidney Injury via Targeting TXNIP. Inflammation 2021, 44, 217–228. [Google Scholar] [CrossRef]
- Klein Geltink, R.I.; O’Sullivan, D.; Corrado, M.; Bremser, A.; Buck, M.D.; Buescher, J.M.; Firat, E.; Zhu, X.; Niedermann, G.; Caputa, G.; et al. Mitochondrial Priming by CD28. Cell 2017, 171, 385–397.e11. [Google Scholar]
- Wang, Y.; Ma, W.-Q.; Zhu, Y.; Han, X.-Q.; Liu, N. Exosomes Derived from Mesenchymal Stromal Cells Pretreated With Advanced Glycation End Product-Bovine Serum Albumin Inhibit Calcification of Vascular Smooth Muscle Cells. Front. Endocrinol. 2018, 9, 524. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Zhang, A.; Gao, L. Circular RNA circ-BANP regulates ox-LDL-induced endothelial cell injury through targeting the miR-370/TXNIP axis. J. Cardiovasc. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.-M. LncRNA-antisense non-coding RNA in the INK4 locus promotes pyroptosis via miR-497/thioredoxin-interacting protein axis in diabetic nephropathy. Life Sci. 2021, 264, 118728. [Google Scholar] [CrossRef]
- Nishinaka, Y.; Masutani, H.; Oka, S.-I.; Matsuo, Y.; Yamaguchi, Y.; Nishio, K.; Ishii, Y.; Yodoi, J. Importin alpha1 (Rch1) mediates nuclear translocation of thioredoxin-binding protein-2/vitamin D(3)-up-regulated protein 1. J. Biol. Chem. 2004, 279, 37559–37565. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.-Y.; Kim, S.-K. Quercetin and Ascorbic Acid Suppress Fructose-Induced NLRP3 Inflammasome Activation by Blocking Intracellular Shuttling of TXNIP in Human Macrophage Cell Lines. Inflammation 2017, 40, 980–994. [Google Scholar]
- Schulze, P.C.; De Keulenaer, G.W.; Yoshioka, J.; Kassik, K.A.; Lee, R.T. Vitamin D3-upregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ. Res. 2002, 91, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Addinsall, A.B.; Wright, C.R.; Shaw, C.S.; McRae, N.L.; Forgan, L.G.; Weng, C.-H.; Conlan, X.A.; Francis, P.S.; Smith, Z.M.; Andrikopoulos, S.; et al. Deficiency of selenoprotein S, an endoplasmic reticulum resident oxidoreductase, impairs the contractile function of fast-twitch hindlimb muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R380–R396. [Google Scholar] [CrossRef]
- Wei, J.; Wu, H.; Zhang, H.; Li, F.; Chen, S.; Hou, B.; Shi, Y.; Zhao, L.; Duan, H. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in db/db mice. Int. J. Mol. Med. 2018, 41, 1608–1618. [Google Scholar] [CrossRef]
- Oberacker, T.; Bajorat, J.; Ziola, S.; Schroeder, A.; Röth, D.; Kastl, L.; Edgar, B.A.; Wagner, W.; Gülow, K.; Krammer, P.H. Enhanced expression of thioredoxin-interacting-protein regulates oxidative DNA damage and aging. FEBS Lett. 2018, 592, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.T.; Seth, D.; Hausladen, A.; Eyler, C.E.; Foster, M.W.; Matsumoto, A.; Benhar, M.; Marshall, H.E.; Stamler, J.S. Thioredoxin-interacting protein (Txnip) is a feedback regulator of S-nitrosylation. J. Biol. Chem. 2009, 284, 36160–36166. [Google Scholar] [CrossRef]
- Matsuo, Y.; Yodoi, J. Extracellular thioredoxin: A therapeutic tool to combat inflammation. Cytokine Growth Factor Rev. 2013, 24, 345–353. [Google Scholar] [CrossRef]
- Nakamura, T.; Nakamura, H.; Hoshino, T.; Ueda, S.; Wada, H.; Yodoi, J. Redox regulation of lung inflammation by thioredoxin. Antioxid. Redox Signal. 2005, 7, 60–71. [Google Scholar] [CrossRef]
- Duan, M.; Yuan, Y.; Liu, C.; Cai, Z.; Xie, Q.; Hu, T.; Tang, Q.; Wu, Q. Indigo Fruits Ingredient, Aucubin, Protects against LPS-Induced Cardiac Dysfunction in Mice. J. Pharmacol. Exp. Ther. 2019, 371, 348–359. [Google Scholar] [CrossRef]
- Myers, R.B.; Fomovsky, G.M.; Lee, S.; Tan, M.; Wang, B.F.; Patwari, P.; Yoshioka, J. Deletion of thioredoxin-interacting protein improves cardiac inotropic reserve in the streptozotocin-induced diabetic heart. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1748–H1759. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Draga, D.; Zhou, C.; Su, T.; Zou, C.; Gu, Q.; Lahm, T.; Zheng, Z.; Qiu, Q. miR-590-3p Inhibits Pyroptosis in Diabetic Retinopathy by Targeting NLRP1 and Inactivating the NOX4 Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4215–4223. [Google Scholar] [CrossRef] [PubMed]
- Forred, B.J.; Neuharth, S.; Kim, D.I.; Amolins, M.W.; Motamedchaboki, K.; Roux, K.J.; Vitiello, P.F. Identification of Redox and Glucose-Dependent Txnip Protein Interactions. Oxid. Med. Cell. Longev. 2016, 2016, 5829063. [Google Scholar] [CrossRef]
- Cao, X.; He, W.; Pang, Y.; Cao, Y.; Qin, A. Redox-dependent and independent effects of thioredoxin interacting protein. Biol. Chem. 2020, 401, 1215–1231. [Google Scholar] [CrossRef]
- Sheth, S.S.; Castellani, L.W.; Chari, S.; Wagg, C.; Thipphavong, C.K.; Bodnar, J.S.; Tontonoz, P.; Attie, A.D.; Lopaschuk, G.D.; Lusis, A.J. Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J. Lipid Res. 2005, 46, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Chutkow, W.A.; Patwari, P.; Yoshioka, J.; Lee, R.T. Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J. Biol. Chem. 2008, 283, 2397–2406. [Google Scholar] [CrossRef]
- Oka, S.-I.; Liu, W.; Masutani, H.; Hirata, H.; Shinkai, Y.; Yamada, S.I.; Yoshida, T.; Nakamura, H.; Yodoi, J. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: A unique animal model of Reye syndrome. FASEB J. 2006, 20, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Katsu-Jiménez, Y.; Vázquez-Calvo, C.; Maffezzini, C.; Halldin, M.; Peng, X.; Freyer, C.; Wredenberg, A.; Giménez-Cassina, A.; Wedell, A.; Arnér, E.S.J. Absence of TXNIP in Humans Leads to Lactic Acidosis and Low Serum Methionine Linked to Deficient Respiration on Pyruvate. Diabetes 2019, 68, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.H.L.; Vong, C.T.; Kwan, Y.W.; Lee, S.M.-Y.; Hoi, M.P.M. TRPM2 regulates TXNIP-mediated NLRP3 inflammasome activation via interaction with p47 phox under high glucose in human monocytic cells. Sci. Rep. 2016, 6, 35016. [Google Scholar] [CrossRef]
- Johnson, M.L.; Distelmaier, K.; Lanza, I.R.; Irving, B.A.; Robinson, M.M.; Konopka, A.R.; Shulman, G.I.; Nair, K.S. Mechanism by Which Caloric Restriction Improves Insulin Sensitivity in Sedentary Obese Adults. Diabetes 2016, 65, 74–84. [Google Scholar] [CrossRef]
- Dunn, L.L.; Simpson, P.J.L.; Prosser, H.C.; Lecce, L.; Yuen, G.S.C.; Buckle, A.; Sieveking, D.P.; Vanags, L.Z.; Lim, P.R.; Chow, R.W.Y.; et al. A critical role for thioredoxin-interacting protein in diabetes-related impairment of angiogenesis. Diabetes 2014, 63, 675–687. [Google Scholar] [CrossRef]
- Song, S.; Qiu, D.; Shi, Y.; Wang, S.; Zhou, X.; Chen, N.; Wei, J.; Wu, M.; Wu, H.; Duan, H. Thioredoxin-interacting protein deficiency alleviates phenotypic alterations of podocytes via inhibition of mTOR activation in diabetic nephropathy. J. Cell Physiol. 2019, 234, 16485–16502. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Li, B.; Zhu, X.; Lai, X. Klotho improves diabetic cardiomyopathy by suppressing the NLRP3 inflammasome pathway. Life Sci. 2019, 234, 116773. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, M.; Zhao, S.; Lu, Q.; Ni, L.; Zou, C.; Lu, L.; Xu, X.; Guan, H.; Zheng, Z.; et al. Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: A novel inhibitory effect of minocycline. Inflamm. Res. 2017, 66, 157–166. [Google Scholar] [CrossRef]
- Jia, Q.; Mehmood, S.; Liu, X.; Ma, S.; Yang, R. Hydrogen sulfide mitigates myocardial inflammation by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation in diabetic rats. Exp. Biol. Med. 2020, 245, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Xia, L.; Masson, E.A.Y.; Gui, C.; Momen, A.; Shikatani, E.A.; Husain, M.; Quaggin, S.; John, R.; Fantus, I.G. Thioredoxin-Interacting Protein Deficiency Protects against Diabetic Nephropathy. J. Am. Soc. Nephrol. 2015, 26, 2963–2977. [Google Scholar] [CrossRef]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-L.; Gao, W.; Chen, Z.-M.; Shao, K.-K.; Wang, Y.-G.; Cui, L.; Guo, N.-Z. Relationships between diabetic nephropathy and insulin resistance, inflammation, Trx, Txnip, CysC and serum complement levels. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11700–11706. [Google Scholar]
- Selvaraju, V.; Suresh, S.C.; Thirunavukkarasu, M.; Mannu, J.; Foye, J.L.C.; Mathur, P.P.; Palesty, J.A.; Sanchez, J.A.; McFadden, D.W.; Maulik, N. Regulation of A-Kinase-Anchoring Protein 12 by Heat Shock Protein A12B to Prevent Ventricular Dysfunction Following Acute Myocardial Infarction in Diabetic Rats. J. Cardiovasc. Transl. Res. 2017, 10, 209–220. [Google Scholar] [CrossRef]
- Devi, T.S.; Yumnamcha, T.; Yao, F.; Somayajulu, M.; Kowluru, R.A.; Singh, L.P. TXNIP mediates high glucose-induced mitophagic flux and lysosome enlargement in human retinal pigment epithelial cells. Biol. Open 2019, 8, bio038521. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Qi, X.; Xu, X.; Wang, K.; Wu, Y.; Xia, L. TGP attenuates endoplasmic reticulum stress and regulates the expression of thioredoxin-interacting protein in the kidneys of diabetic rats. Biosci. Trends 2017, 10, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Buyandelger, U.; Walker, D.G.; Taguchi, H.; Yanagisawa, D.; Tooyama, I. Novel fluorinated derivative of curcumin negatively regulates thioredoxin-interacting protein expression in retinal pigment epithelial and macrophage cells. Biochem. Biophys. Res. Commun. 2020, 532, 668–674. [Google Scholar] [CrossRef]
- Singh, L.P.; Yumnamcha, T.; Swornalata Devi, T. Mitophagic Flux Deregulation, Lysosomal Destabilization and NLRP3 Inflammasome Activation in Diabetic Retinopathy: Potentials of Gene Therapy Targeting TXNIP and The Redox System. Ophthalmol. Res. Rep. 2018, 3, ORRT-126. [Google Scholar]
- Singh, L.P.; Devi, T.S.; Yumnamcha, T. The Role of Txnip in Mitophagy Dysregulation and Inflammasome Activation in Diabetic Retinopathy: A New Perspective. JOJ Ophthalmol. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S.; et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Kumar, A.; Mittal, R. Mapping Txnip: Key connexions in progression of diabetic nephropathy. Pharmacol. Rep. 2018, 70, 614–622. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Kelly, D.J.; Tan, C.Y.R.; Gill, A.; Cheng, D.; Braet, F.; Park, J.-S.; Sue, C.M.; Pollock, C.A.; et al. Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci. Rep. 2016, 6, 29196. [Google Scholar] [CrossRef]
- Duan, J.; Du, C.; Shi, Y.; Liu, D.; Ma, J. Thioredoxin-interacting protein deficiency ameliorates diabetic retinal angiogenesis. Int. J. Biochem. Cell Biol. 2018, 94, 61–70. [Google Scholar] [CrossRef]
- Parveen, A.; Jin, M.; Kim, S.Y. Bioactive phytochemicals that regulate the cellular processes involved in diabetic nephropathy. Phytomedicine 2018, 39, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Bai, D.; Liu, B.; Lu, X.; Hou, R.; Zeng, C.; Li, N.; Fu, Z.; Li, C.; Tao, L.; et al. Dysregulated Txnip-ROS-Wnt axis contributes to the impaired ischemic heart repair in diabetic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3735–3745. [Google Scholar] [CrossRef]
- Yeda, X.; Shaoqing, L.; Yayi, H.; Bo, Z.; Huaxin, W.; Hong, C.; Zhongyuan, X. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the P38-MAPK/TXNIP signaling activation in streptozotocin induced diabetic rats. Acta Cir. Bras. 2017, 32, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Wu, M.; Liu, H.; Ren, Y.; Du, Y.; Wu, H.; Wei, J.; Liu, C.; Yao, F.; Wang, H.; et al. Thioredoxin-interacting protein regulates lipid metabolism via Akt/mTOR pathway in diabetic kidney disease. Int. J. Biochem. Cell Biol. 2016, 79, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Almomen, S.M.K.; Guan, Q.; Liang, P.; Yang, K.; Sidiqi, A.M.; Levin, A.; Du, C. Daily Intake of Grape Powder Prevents the Progression of Kidney Disease in Obese Type 2 Diabetic ZSF1 Rats. Nutrients 2017, 9, 345. [Google Scholar] [CrossRef]
- Zhai, J.; Tao, L.; Zhang, Y.; Gao, H.; Qu, X.; Song, Y.; Zhang, S. Salvianolic Acid B Attenuates Apoptosis of HUVEC Cells Treated with High Glucose or High Fat via Sirt1 Activation. Evid. Based Complement. Alternat. Med. 2019, 2019, 9846325. [Google Scholar] [CrossRef]
- Wondafrash, D.Z.; Nire’a, A.T.; Tafere, G.G.; Desta, D.M.; Berhe, D.A.; Zewdie, K.A. Thioredoxin-Interacting Protein as a Novel Potential Therapeutic Target in Diabetes Mellitus and Its Underlying Complications. Diabetes Metab. Syndr. Obes. 2020, 13, 43–51. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, C.; Yang, H.; Deng, J.; Fan, D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol. Res. 2020, 155, 104746. [Google Scholar] [CrossRef]
- Wang, S.; Du, S.; Lv, Y.; Wang, W.; Zhang, F. Elevated microRNA-20b-3p and reduced thioredoxin-interacting protein ameliorate diabetic retinopathy progression by suppressing the NLRP3 inflammasomes. IUBMB Life 2020, 72, 1433–1448. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Yokoyama, A.; Noro, E.; Aoki, S.; Shimizu, K.; Shimada, H.; Sugawara, A. Expression and pathophysiological significance of carbohydrate response element binding protein (ChREBP) in the renal tubules of diabetic kidney. Endocr. J. 2020, 67, 335–345. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, D.; Zhang, L.; Ding, M.; Zhou, H. Anagliptin ameliorates high glucose- induced endothelial dysfunction via suppression of NLRP3 inflammasome activation mediated by SIRT1. Mol. Immunol. 2019, 107, 54–60. [Google Scholar] [CrossRef]
- Panse, M.; Kluth, O.; Lorza-Gil, E.; Kaiser, G.; Mühlbauer, E.; Schürmann, A.; Häring, H.-U.; Ullrich, S.; Gerst, F. Palmitate and insulin counteract glucose-induced thioredoxin interacting protein (TXNIP) expression in insulin secreting cells via distinct mechanisms. PLoS ONE 2018, 13, e0198016. [Google Scholar] [CrossRef] [PubMed]
- Koska, J.; Osredkar, T.; D’Souza, K.; Sands, M.; Sinha, S.; Zhang, W.; Meyer, C.; Reaven, P.D. Effects of saxagliptin on adipose tissue inflammation and vascular function in overweight and obese people: A placebo-controlled study. Diabet. Med. 2019, 36, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Pujadas, G.; De Nigris, V.; Prattichizzo, F.; La Sala, L.; Testa, R.; Ceriello, A. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin functions as antioxidant on human endothelial cells exposed to chronic hyperglycemia and metabolic high-glucose memory. Endocrine 2017, 56, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Zhang, C.; Liu, D.; Wu, J.; Tian, H.; Lu, L.; Xu, G.-T.; Liu, F.; Zhang, J. Metformin Protects ARPE-19 Cells from Glyoxal-Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 1740943. [Google Scholar]
- Dong, F.; Dong, S.; Liang, Y.; Wang, K.; Qin, Y.; Zhao, X. miR-20b inhibits the senescence of human umbilical vein endothelial cells through regulating the Wnt/β-catenin pathway via the TXNIP/NLRP3 axis. Int. J. Mol. Med. 2020, 45, 847–857. [Google Scholar] [CrossRef]
- De Marinis, Y.; Cai, M.; Bompada, P.; Atac, D.; Kotova, O.; Johansson, M.E.; Garcia-Vaz, E.; Gomez, M.F.; Laakso, M.; Groop, L. Epigenetic regulation of the thioredoxin-interacting protein (TXNIP) gene by hyperglycemia in kidney. Kidney Int. 2016, 89, 342–353. [Google Scholar] [CrossRef]
- Albao, D.S.; Cutiongco-de la Paz, E.M.; Mercado, M.E.; Lirio, A.; Mariano, M.; Kim, S.; Yangco, A.; Melegrito, J.; Wad-asen, K.; Gauran, I.I.; et al. Methylation changes in the peripheral blood of Filipinos with type 2 diabetes suggest spurious transcription initiation at TXNIP. Hum. Mol. Genet. 2019, 28, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Van Greevenbroek, M.M.J.; Vermeulen, V.M.M.-J.; Feskens, E.J.M.; Evelo, C.T.; Kruijshoop, M.; Hoebee, B.; van der Kallen, C.J.H.; de Bruin, T.W.A. Genetic variation in thioredoxin interacting protein (TXNIP) is associated with hypertriglyceridaemia and blood pressure in diabetes mellitus. Diabet. Med. 2007, 24, 498–504. [Google Scholar] [CrossRef]
- Soriano-Tárraga, C.; Jiménez-Conde, J.; Giralt-Steinhauer, E.; Mola-Caminal, M.; Vivanco-Hidalgo, R.M.; Ois, A.; Rodríguez-Campello, A.; Cuadrado-Godia, E.; Sayols-Baixeras, S.; Elosua, R.; et al. Epigenome-wide association study identifies TXNIP gene associated with type 2 diabetes mellitus and sustained hyperglycemia. Hum. Mol. Genet. 2016, 25, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Florath, I.; Butterbach, K.; Heiss, J.; Bewerunge-Hudler, M.; Zhang, Y.; Schöttker, B.; Brenner, H. Type 2 diabetes and leucocyte DNA methylation: An epigenome-wide association study in over 1,500 older adults. Diabetologia 2016, 59, 130–138. [Google Scholar] [CrossRef]
- Meeks, K.A.C.; Henneman, P.; Venema, A.; Addo, J.; Bahendeka, S.; Burr, T.; Danquah, I.; Galbete, C.; Mannens, M.M.A.M.; Mockenhaupt, F.P.; et al. Epigenome-wide association study in whole blood on type 2 diabetes among sub-Saharan African individuals: Findings from the RODAM study. Int. J. Epidemiol. 2019, 48, 58–70. [Google Scholar] [CrossRef]
- Chambers, J.C.; Loh, M.; Lehne, B.; Drong, A.; Kriebel, J.; Motta, V.; Wahl, S.; Elliott, H.R.; Rota, F.; Scott, W.R.; et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: A nested case-control study. Lancet Diabetes Endocrinol. 2015, 3, 526–534. [Google Scholar] [CrossRef]
- Cardona, A.; Day, F.R.; Perry, J.R.B.; Loh, M.; Chu, A.Y.; Lehne, B.; Paul, D.S.; Lotta, L.A.; Stewart, I.D.; Kerrison, N.D.; et al. Epigenome-Wide Association Study of Incident Type 2 Diabetes in a British Population: EPIC-Norfolk Study. Diabetes 2019, 68, 2315–2326. [Google Scholar] [CrossRef]
- Walaszczyk, E.; Luijten, M.; Spijkerman, A.M.W.; Bonder, M.J.; Lutgers, H.L.; Snieder, H.; Wolffenbuttel, B.H.R.; van Vliet-Ostaptchouk, J.V. DNA methylation markers associated with type 2 diabetes, fasting glucose and HbA1c levels: A systematic review and replication in a case-control sample of the Lifelines study. Diabetologia 2018, 61, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Gateva, A.T.; Assyov, Y.S.; Velikova, T.; Kamenov, Z.A. Higher levels of thioredoxin interacting protein (TXNIP) in patients with prediabetes compared to obese normoglycemic subjects. Diabetes Metab. Syndr. 2019, 13, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, L.; Wahl, S.; Pilling, L.C.; Reischl, E.; Sandling, J.K.; Kunze, S.; Holdt, L.M.; Kretschmer, A.; Schramm, K.; Adamski, J.; et al. DNA methylation of lipid-related genes affects blood lipid levels. Circ. Cardiovasc. Genet. 2015, 8, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussain, F.; Iqbal, M.; Al-Quwayee, M.; Jurays, A.B.; Al-Wabel, M.; Dayes, S.; Bashir, S. The role of serum levels of thioredoxin and thioredoxin-interacting protein in stroke. J. Nat. Sci. Med. 2018, 1, 55–58. [Google Scholar]
- Tamura, N.; Maejima, Y.; Matsumura, T.; Vega, R.B.; Amiya, E.; Ito, Y.; Shiheido-Watanabe, Y.; Ashikaga, T.; Komuro, I.; Kelly, D.P.; et al. Single-Nucleotide Polymorphism of the MLX Gene Is Associated with Takayasu Arteritis. Circ. Genom. Precis. Med. 2018, 11, e002296. [Google Scholar] [CrossRef] [PubMed]
- Riahi, Y.; Kaiser, N.; Cohen, G.; Abd Elrahman, I.; Blum, G.; Shapira, O.M.; Koler, T.; Simionescu, M.; Sima, A.V.; Zarkovic, N.; et al. Foam cell-derived 4-hydroxynonenal induces endothelial cell senescence in a TXNIP-dependent manner. J. Cell. Mol. Med. 2015, 19, 1887–1899. [Google Scholar] [CrossRef]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Pelisek, J.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.-E.; Zernecke, A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018, 122, 1661–1674. [Google Scholar] [CrossRef]
- Rong, J.; Xu, X.; Xiang, Y.; Yang, G.; Ming, X.; He, S.; Liang, B.; Zhang, X.; Zheng, F. Thioredoxin-interacting protein promotes activation and inflammation of monocytes with DNA demethylation in coronary artery disease. J. Cell. Mol. Med. 2020, 24, 3560–3571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhong, P.; Xu, Y.; Wang, B.; Zhu, T.; Zhang, W.; Wang, H.; Wei, Z.; Huang, J. Differential Expression of TXNIP Isoforms in the Peripheral Leukocytes of Patients with Acute Myocardial Infarction. Dis. Markers 2018, 2018, 9051481. [Google Scholar] [CrossRef]
- Pagesy, P.; Tachet, C.; Mostefa-Kara, A.; Larger, E.; Issad, T. Increased OGA Expression and Activity in Leukocytes from Patients with Diabetes: Correlation with Inflammation Markers. Exp. Clin. Endocrinol. Diabetes 2019, 127, 517–523. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Yan, P.; Ren, J.; Song, M.; Li, J.; Lei, J.; Pan, H.; Wang, S.; Ma, X.; et al. A single-cell transcriptomic landscape of primate arterial aging. Nat. Commun. 2020, 11, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Piao, Z.-H.; Kim, M.S.; Lee, S.H.; Yun, S.; Sun, H.-N.; Yoon, S.R.; Chung, J.W.; Kim, T.-D.; Jeon, J.-H.; et al. Thioredoxin-interacting protein regulates hematopoietic stem cell quiescence and mobilization under stress conditions. J. Immunol. 2009, 183, 2495–2505. [Google Scholar] [CrossRef]
- Jung, H.; Kim, D.O.; Byun, J.-E.; Kim, W.S.; Kim, M.J.; Song, H.Y.; Kim, Y.K.; Kang, D.-K.; Park, Y.-J.; Kim, T.-D.; et al. Thioredoxin-interacting protein regulates haematopoietic stem cell ageing and rejuvenation by inhibiting p38 kinase activity. Nat. Commun. 2016, 7, 13674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lian, K.; Zhang, L.; Wang, R.; Yi, F.; Gao, C.; Xin, C.; Zhu, D.; Li, Y.; Yan, W.; et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2014, 109, 415. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Yoshioka, J.; Chutkow, W.A.; Lee, S.; Kim, J.B.; Yan, J.; Tian, R.; Lindsey, M.L.; Feener, E.P.; Seidman, C.E.; Seidman, J.G.; et al. Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J. Clin. Investig. 2012, 122, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, J.; Schulze, P.C.; Cupesi, M.; Sylvan, J.D.; MacGillivray, C.; Gannon, J.; Huang, H.; Lee, R.T. Thioredoxin-interacting protein controls cardiac hypertrophy through regulation of thioredoxin activity. Circulation 2004, 109, 2581–2586. [Google Scholar] [CrossRef]
- Yoshioka, J.; Imahashi, K.; Gabel, S.A.; Chutkow, W.A.; Burds, A.A.; Gannon, J.; Schulze, P.C.; MacGillivray, C.; London, R.E.; Murphy, E.; et al. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ. Res. 2007, 101, 1328–1338. [Google Scholar] [CrossRef]
- Hou, R.; Shen, M.; Wang, R.; Liu, H.; Gao, C.; Xu, J.; Tao, L.; Yin, Z.; Yin, T. Thioredoxin1 Inactivation Mediates the Impairment of Ischemia-Induced Angiogenesis and Further Injury in Diabetic Myocardium. J. Vasc. Res. 2020, 57, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Ji, L.; Xing, W.; Zhang, W.; Zhou, H.; Qian, X.; Wang, X.; Gao, F.; Sun, X.; Zhang, H. Acute hyperglycaemia enhances oxidative stress and aggravates myocardial ischaemia/reperfusion injury: Role of thioredoxin-interacting protein. J. Cell. Mol. Med. 2013, 17, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, I.A.; Rishi, M.T.; Youssef, M.; Selvaraju, V.; Thirunavukkarasu, M.; Ukani, G.; Lakshmanan, R.; Palesty, J.A.; Maulik, N. Overexpression of Thioredoxin1 enhances functional recovery in a mouse model of hind limb ischemia. J. Surg. Res. 2017, 216, 158–168. [Google Scholar] [CrossRef]
- Yuan, J.; Tan, J.T.M.; Rajamani, K.; Solly, E.L.; King, E.J.; Lecce, L.; Simpson, P.J.L.; Lam, Y.T.; Jenkins, A.J.; Bursill, C.A.; et al. Fenofibrate Rescues Diabetes-Related Impairment of Ischemia-Mediated Angiogenesis by PPARα-Independent Modulation of Thioredoxin-Interacting Protein. Diabetes 2019, 68, 1040–1053. [Google Scholar] [CrossRef]
- Ishrat, T.; Mohamed, I.N.; Pillai, B.; Soliman, S.; Fouda, A.Y.; Ergul, A.; El-Remessy, A.B.; Fagan, S.C. Thioredoxin-interacting protein: A novel target for neuroprotection in experimental thromboembolic stroke in mice. Mol. Neurobiol. 2015, 51, 766–778. [Google Scholar] [CrossRef]
- Guo, M.; Wang, X.; Zhao, Y.; Yang, Q.; Ding, H.; Dong, Q.; Chen, X.; Cui, M. Ketogenic Diet Improves Brain Ischemic Tolerance and Inhibits NLRP3 Inflammasome Activation by Preventing Drp1-Mediated Mitochondrial Fission and Endoplasmic Reticulum Stress. Front. Mol. Neurosci. 2018, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, R.; Wang, X.; Fu, Q.; Ma, S. Umbelliferone ameliorates cerebral ischemia-reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci. Lett. 2015, 600, 182–187. [Google Scholar] [CrossRef]
- Cao, G.; Jiang, N.; Hu, Y.; Zhang, Y.; Wang, G.; Yin, M.; Ma, X.; Zhou, K.; Qi, J.; Yu, B.; et al. Ruscogenin Attenuates Cerebral Ischemia-Induced Blood-Brain Barrier Dysfunction by Suppressing TXNIP/NLRP3 Inflammasome Activation and the MAPK Pathway. Int. J. Mol. Sci. 2016, 17, 1418. [Google Scholar] [CrossRef] [PubMed]
- Ismael, S.; Nasoohi, S.; Yoo, A.; Ahmed, H.A.; Ishrat, T. Tissue Plasminogen Activator Promotes TXNIP-NLRP3 Inflammasome Activation after Hyperglycemic Stroke in Mice. Mol. Neurobiol. 2020, 57, 2495–2508. [Google Scholar] [CrossRef]
- Kim, G.S.; Jung, J.E.; Narasimhan, P.; Sakata, H.; Chan, P.H. Induction of thioredoxin-interacting protein is mediated by oxidative stress, calcium, and glucose after brain injury in mice. Neurobiol. Dis. 2012, 46, 440–449. [Google Scholar] [CrossRef]
- Hua, K.; Sheng, X.; Li, T.-T.; Wang, L.-N.; Zhang, Y.-H.; Huang, Z.-J.; Ji, H. The edaravone and 3-n-butylphthalide ring-opening derivative 10b effectively attenuates cerebral ischemia injury in rats. Acta Pharmacol. Sin. 2015, 36, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-N.; Xu, L.; Hu, Q.; Matei, N.; Yang, P.; Tong, L.-S.; He, Y.; Guo, Z.; Tang, J.; Yang, Y.; et al. Hyperbaric Oxygen Preconditioning Attenuates Hemorrhagic Transformation Through Reactive Oxygen Species/Thioredoxin-Interacting Protein/Nod-Like Receptor Protein 3 Pathway in Hyperglycemic Middle Cerebral Artery Occlusion Rats. Crit. Care Med. 2016, 44, e403–e411. [Google Scholar] [CrossRef]
- Liu, T.; Wang, W.; Liu, M.; Ma, Y.; Mu, F.; Feng, X.; Zhang, Y.; Guo, C.; Ding, Y.; Wen, A. Z-Guggulsterone alleviated oxidative stress and inflammation through inhibiting the TXNIP/NLRP3 axis in ischemic stroke. Int. Immunopharmacol. 2020, 89, 107094. [Google Scholar] [CrossRef]
- Zhao, Q.; Che, X.; Zhang, H.; Tan, G.; Liu, L.; Jiang, D.; Zhao, J.; Xiang, X.; Sun, X.; He, Z. Thioredoxin-Interacting Protein Mediates Apoptosis in Early Brain Injury after Subarachnoid Haemorrhage. Int. J. Mol. Sci. 2017, 18, 854. [Google Scholar] [CrossRef]
- Zhao, Q.; Che, X.; Zhang, H.; Fan, P.; Tan, G.; Liu, L.; Jiang, D.; Zhao, J.; Xiang, X.; Liang, Y.; et al. Thioredoxin-interacting protein links endoplasmic reticulum stress to inflammatory brain injury and apoptosis after subarachnoid haemorrhage. J. Neuroinflamm. 2017, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Che, X.; Zhao, Q.; Darwazeh, R.; Zhang, H.; Jiang, D.; Zhao, J.; Xiang, X.; Qin, W.; Liu, L.; et al. Thioredoxin-interacting protein mediates mitochondrion-dependent apoptosis in early brain injury after subarachnoid hemorrhage. Mol. Cell. Biochem. 2019, 450, 149–158. [Google Scholar] [CrossRef]
- Chen, D.; Dixon, B.J.; Doycheva, D.M.; Li, B.; Zhang, Y.; Hu, Q.; He, Y.; Guo, Z.; Nowrangi, D.; Flores, J.; et al. IRE1α inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic-ischemic brain injury in rats. J. Neuroinflamm. 2018, 15, 32. [Google Scholar] [CrossRef]
- Du, S.-Q.; Wang, X.-R.; Zhu, W.; Ye, Y.; Yang, J.-W.; Ma, S.-M.; Ji, C.-S.; Liu, C.-Z. Acupuncture inhibits TXNIP-associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats. CNS Neurosci. Ther. 2018, 24, 39–46. [Google Scholar] [CrossRef]
- Ramus, S.M.; Cilensek, I.; Petrovic, M.G.; Soucek, M.; Kruzliak, P.; Petrovic, D. Single nucleotide polymorphisms in the Trx2/TXNIP and TrxR2 genes of the mitochondrial thioredoxin antioxidant system and the risk of diabetic retinopathy in patients with Type 2 diabetes mellitus. J. Diabetes Complicat. 2016, 30, 192–198. [Google Scholar] [CrossRef]
- Pradhan, P.; Upadhyay, N.; Tiwari, A.; Singh, L.P. Genetic and epigenetic modifications in the pathogenesis of diabetic retinopathy: A molecular link to regulate gene expression. New Front. Ophthalmol. 2016, 2, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Zaghlool, S.B.; Mook-Kanamori, D.O.; Kader, S.; Stephan, N.; Halama, A.; Engelke, R.; Sarwath, H.; Al-Dous, E.K.; Mohamoud, Y.A.; Roemisch-Margl, W.; et al. Deep molecular phenotypes link complex disorders and physiological insult to CpG methylation. Hum. Mol. Genet. 2018, 27, 1106–1121. [Google Scholar] [CrossRef]
- Juvinao-Quintero, D.L.; Hivert, M.-F.; Sharp, G.C.; Relton, C.L.; Elliott, H.R. DNA Methylation and Type 2 Diabetes: The Use of Mendelian Randomization to Assess Causality. Curr. Genet. Med. Rep. 2019, 7, 191–207. [Google Scholar] [CrossRef]
- Rani, J.; Mittal, I.; Pramanik, A.; Singh, N.; Dube, N.; Sharma, S.; Puniya, B.L.; Raghunandanan, M.V.; Mobeen, A.; Ramachandran, S. T2DiACoD: A Gene Atlas of Type 2 Diabetes Mellitus Associated Complex Disorders. Sci. Rep. 2017, 7, 6892. [Google Scholar] [CrossRef] [PubMed]
- Willmer, T.; Johnson, R.; Louw, J.; Pheiffer, C. Blood-Based DNA Methylation Biomarkers for Type 2 Diabetes: Potential for Clinical Applications. Front. Endocrinol. 2018, 9, 744. [Google Scholar] [CrossRef]
- Kulkarni, H.; Kos, M.Z.; Neary, J.; Dyer, T.D.; Kent, J.W.; Göring, H.H.H.; Cole, S.A.; Comuzzie, A.G.; Almasy, L.; Mahaney, M.C.; et al. Novel epigenetic determinants of type 2 diabetes in Mexican-American families. Hum. Mol. Genet. 2015, 24, 5330–5344. [Google Scholar] [CrossRef] [PubMed]
- Al Muftah, W.A.; Al-Shafai, M.; Zaghlool, S.B.; Visconti, A.; Tsai, P.-C.; Kumar, P.; Spector, T.; Bell, J.; Falchi, M.; Suhre, K. Epigenetic associations of type 2 diabetes and BMI in an Arab population. Clin. Epigenetics 2016, 8, 13. [Google Scholar] [CrossRef]
- Koshy, N.V.; Friday, E.; Turturro, F. Thioredoxin Interacting Protein (TXNIP) Correlates with Production of Reactive Oxygen Species (ROS) and Underlines Differences in Stress Response Gene Profile in Patients with Chronic Lymphocytic Leukemia (CLL). Blood 2009, 114, 4375. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.; Peng, M.; Wang, Z.; Ren, L.; Mu, S.; Zheng, M. Correlation Between Thioredoxin-Interacting Protein and Nerve Conduction Velocity in Patients with Type 2 Diabetes Mellitus. Front. Neurol. 2020, 11, 733. [Google Scholar] [CrossRef]
- Monteiro, M.B.; Santos-Bezerra, D.P.; Thieme, K.; Admoni, S.N.; Perez, R.V.; Machado, C.G.; Queiroz, M.S.; Nery, M.; Oliveira-Souza, M.; Woronik, V.; et al. Thioredoxin interacting protein expression in the urinary sediment associates with renal function decline in type 1 diabetes. Free Radic. Res. 2015, 50, 101–110. [Google Scholar] [CrossRef]
- Lenin, R.; Sankaramoorthy, A.; Mohan, V.; Balasubramanyam, M. Altered immunometabolism at the interface of increased endoplasmic reticulum (ER) stress in patients with type 2 diabetes. J. Leukoc. Biol. 2015, 98, 615–622. [Google Scholar] [CrossRef]
- Procter, N.E.; Ball, J.; Ngo, D.T.; Isenberg, J.S.; Hylek, E.M.; Chirkov, Y.Y.; Stewart, S.; Horowitz, J.D. Gender and tachycardia: Independent modulation of platelet reactivity in patients with atrial fibrillation. J. Geriatr. Cardiol. 2016, 13, 202–208. [Google Scholar]
- Procter, N.; Goh, V.; Mahadevan, G.; Stewart, S.; Horowitz, J. Platelet Reactivity Is Independent of Left Atrial Wall Deformation in Patients with Atrial Fibrillation. Mediat. Inflamm. 2016, 2016, 9754808. [Google Scholar] [CrossRef]
- Chong, C.-R.; Liu, S.; Licari, G.; Heresztyn, T.; Chirkov, Y.Y.; Ngo, D.T.; Horowitz, J.D. Reversal of Hyperglycemia: Effects on Nitric Oxide Signaling. Am. J. Med. 2015, 128, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Ajaero, C.N.; Chong, C.-R.; Procter, N.E.K.; Liu, S.; Chirkov, Y.Y.; Heresztyn, T.; Chan, W.P.A.; Arstall, M.A.; McGavigan, A.D.; Frenneaux, M.P.; et al. Does cardiac resynchronization therapy restore peripheral circulatory homeostasis? ESC Heart Fail 2018, 5, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Le Jan, S.; Le Meur, N.; Cazes, A.; Philippe, J.; Le Cunff, M.; Léger, J.; Corvol, P.; Germain, S. Characterization of the expression of the hypoxia-induced genes neuritin, TXNIP and IGFBP3 in cancer. FEBS Lett. 2006, 580, 3395–3400. [Google Scholar] [CrossRef]
- Karar, J.; Dolt, K.S.; Mishra, M.K.; Arif, E.; Javed, S.; Pasha, M.A.Q. Expression and functional activity of pro-oxidants and antioxidants in murine heart exposed to acute hypobaric hypoxia. FEBS Lett. 2007, 581, 4577–4582. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.F.; Koh, M.Y.; Williams, R.R.; James, B.; Wang, H.; Tate, W.R.; Gallegos, A.; Von Hoff, D.D.; Han, H.; Powis, G. Identification of thioredoxin-interacting protein 1 as a hypoxia-inducible factor 1alpha-induced gene in pancreatic cancer. Pancreas 2008, 36, 178–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, L.-S.; Cai, Y.-M.; He, J.-X.; Zhang, Z.-N.; Wu, G.; Zheng, J. TXNIP knockdown alleviates hepatocyte ischemia reperfusion injury through preventing p38/JNK pathway activation. Biochem. Biophys. Res. Commun. 2018, 502, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, Y.-R.; Tang, T.-T.; Pan, M.-M.; Xu, S.-C.; Ma, K.-L.; Lv, L.-L.; Liu, H.; Liu, B.-C. mROS-TXNIP axis activates NLRP3 inflammasome to mediate renal injury during ischemic AKI. Int. J. Biochem. Cell Biol. 2018, 98, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.F.; Leck, Y.C.; He, H.; Yu, F.-X.; Luo, Y.; Hagen, T. Hypoxia-inducible factor independent down-regulation of thioredoxin-interacting protein in hypoxia. FEBS Lett. 2011, 585, 492–498. [Google Scholar] [CrossRef][Green Version]
- Coucha, M.; Shanab, A.Y.; Sayed, M.; Vazdarjanova, A.; El-Remessy, A.B. Modulating Expression of Thioredoxin Interacting Protein (TXNIP) Prevents Secondary Damage and Preserves Visual Function in a Mouse Model of Ischemia/Reperfusion. Int. J. Mol. Sci. 2019, 20, 3969. [Google Scholar] [CrossRef]
- Lan, X.F.; Zhang, X.J.; Lin, Y.N.; Wang, Q.; Xu, H.J.; Zhou, L.N.; Chen, P.L.; Li, Q.Y. Estradiol Regulates Txnip and Prevents Intermittent Hypoxia-Induced Vascular Injury. Sci. Rep. 2017, 7, 10318. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.H.; Shim, J.-K.; Oh, J.E.; Shin, E.-J.; Shin, E.; Kwak, Y.-L. Protective effect of ethyl pyruvate against myocardial ischemia reperfusion injury through regulations of ROS-related NLRP3 inflammasome activation. Oxid. Med. Cell. Longev. 2019, 2019, 4264580. [Google Scholar] [CrossRef]
- Wang, B.F.; Yoshioka, J. The Emerging Role of Thioredoxin-Interacting Protein in Myocardial Ischemia/Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 219–229. [Google Scholar] [CrossRef]
- Yang, C.; Xia, W.; Liu, X.; Lin, J.; Wu, A. Role of TXNIP/NLRP3 in sepsis-induced myocardial dysfunction. Int. J. Mol. Med. 2019, 44, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.M.; Thirunavukkarasu, M.; Penumathsa, S.V.; Koneru, S.; Zhan, L.; Maulik, G.; Sudhakaran, P.R.; Maulik, N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation 2010, 121, 1244–1255. [Google Scholar] [CrossRef]
- Wang, Y.; De Keulenaer, G.W.; Lee, R.T. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J. Biol. Chem. 2002, 277, 26496–26500. [Google Scholar] [CrossRef] [PubMed]
- Neidhardt, S.; Garbade, J.; Emrich, F.; Klaeske, K.; Borger, M.A.; Lehmann, S.; Jawad, K.; Dieterlen, M.-T. Ischemic Cardiomyopathy Affects the Thioredoxin System in the Human Myocardium. J. Card. Fail 2019, 25, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kaga, S.; Zhan, L.; Matsumoto, M.; Maulik, N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J. Mol. Cell. Cardiol. 2005, 39, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Danzl, K.; Messner, B.; Doppler, C.; Nebert, C.; Abfalterer, A.; Sakic, A.; Temml, V.; Heinz, K.; Streitwieser, R.; Edelmann, T.; et al. Early inhibition of endothelial retinoid uptake upon myocardial infarction restores cardiac function and prevents cell, tissue, and animal death. J. Mol. Cell. Cardiol. 2019, 126, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fan, C.; Li, Z.; Zhang, J.; Xue, X.; Xu, Y.; Zhao, G.; Yang, Y.; Wang, H. Melatonin rescues cardiac thioredoxin system during ischemia-reperfusion injury in acute hyperglycemic state by restoring Notch1/Hes1/Akt signaling in a membrane receptor-dependent manner. J. Pineal Res. 2017, 62, e12375. [Google Scholar] [CrossRef]
- Ma, M.; Li, R.; Sun, W.; Wang, Q.; Yu, H.; Yu, H. Sevoflurane preconditioning inhibits cardiomyocyte injury induced by oxygen-glucose deprivation by modulating TXNIP. Int. J. Mol. Med. 2020, 46, 889–897. [Google Scholar] [CrossRef]
- He, Y.; Yang, Z.; Li, J.; Li, E. Dexmedetomidine reduces the inflammation and apoptosis of doxorubicin-induced myocardial cells. Exp. Mol. Pathol. 2020, 113, 104371. [Google Scholar] [CrossRef]
- Dai, S.; He, Y.; Zhang, H.; Yu, L.; Wan, T.; Xu, Z.; Jones, D.; Chen, H.; Min, W. Endothelial-specific expression of mitochondrial thioredoxin promotes ischemia-mediated arteriogenesis and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 495–502. [Google Scholar] [CrossRef]
- Elshaer, S.L.; Mohamed, I.N.; Coucha, M.; Altantawi, S.; Eldahshan, W.; Bartasi, M.L.; Shanab, A.Y.; Lorys, R.; El-Remessy, A.B. Deletion of TXNIP Mitigates High-Fat Diet-Impaired Angiogenesis and Prevents Inflammation in a Mouse Model of Critical Limb Ischemia. Antioxidants 2017, 6, 47. [Google Scholar] [CrossRef]
- Nasoohi, S.; Ismael, S.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) in Cerebrovascular and Neurodegenerative Diseases: Regulation and Implication. Mol. Neurobiol. 2018, 55, 7900–7920. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, W.; Lin, Y.; Li, Q.; Xue, J.; Cai, Z.; Cheng, Y.; Shao, B. Prognostic value of serum thioredoxin levels in ischemic stroke. Neurol. Res. 2017, 39, 988–995. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).