Cyclophilin BcCyp2 Regulates Infection-Related Development to Facilitate Virulence of the Gray Mold Fungus Botrytis cinerea
Abstract
1. Introduction
2. Results
2.1. BcCYP2 Is a Virulence-Associated Gene in B. cinerea
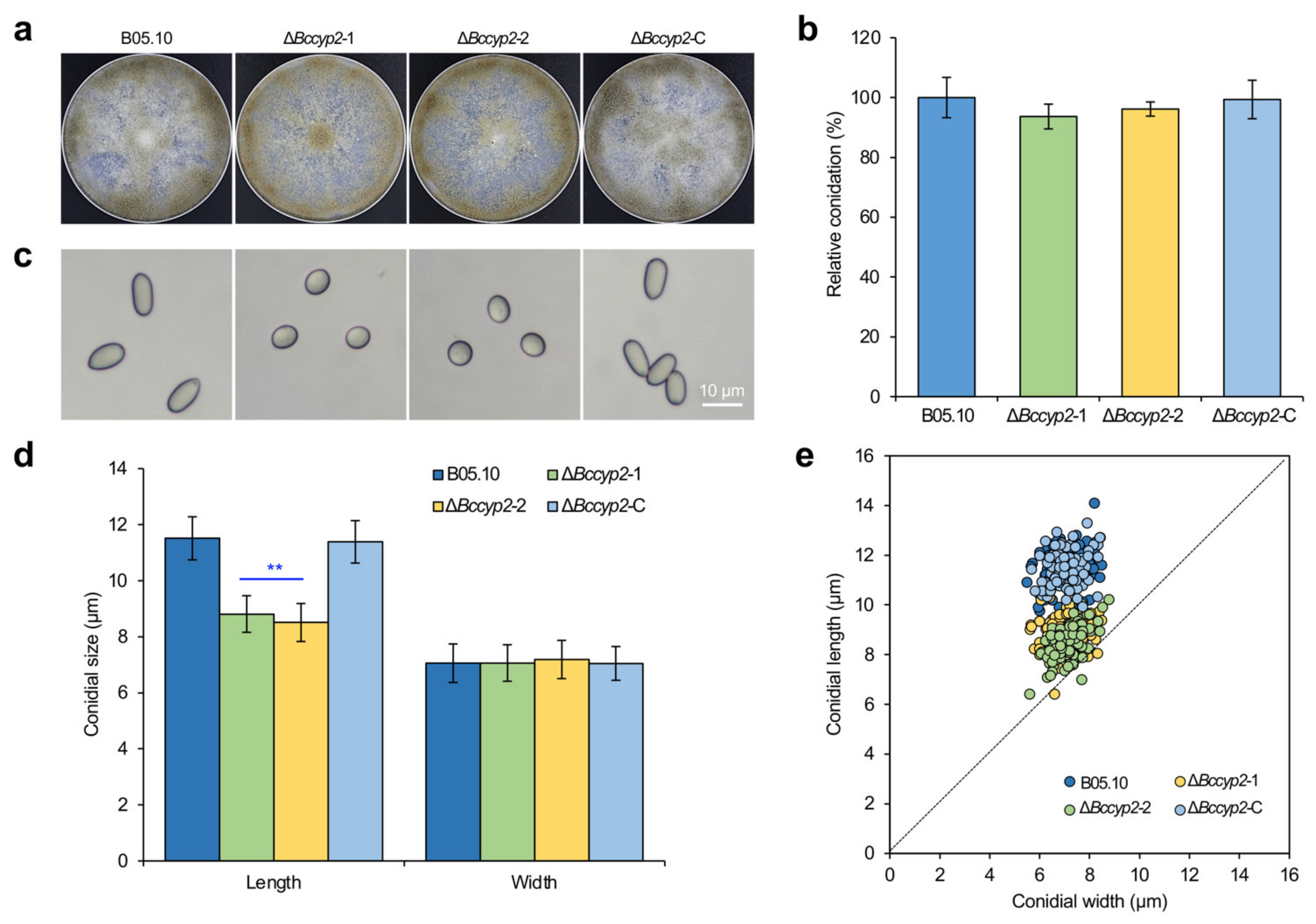
2.2. BcCYP2 Mediates B. cinerea Conidial Development and Morphogenesis but Is Dispensable for Conidiation and Radial Growth of Mycelia
2.3. BcCYP2 Is Dispensable for B. cinerea Cell Wall Integrity and Stress Adaptation
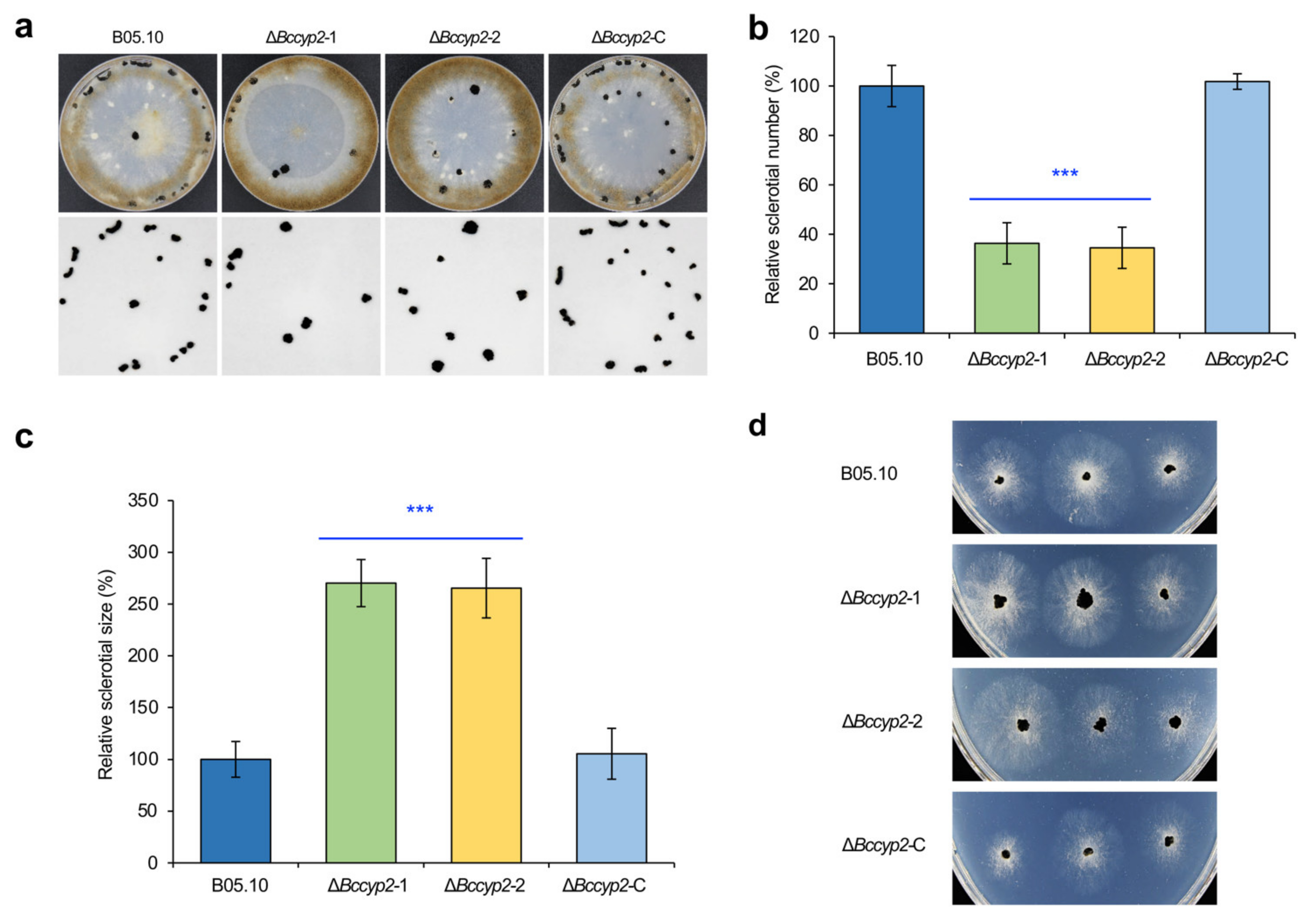
2.4. BcCYP2 Regulates B. cinerea Sclerotium Production and Morphogenesis
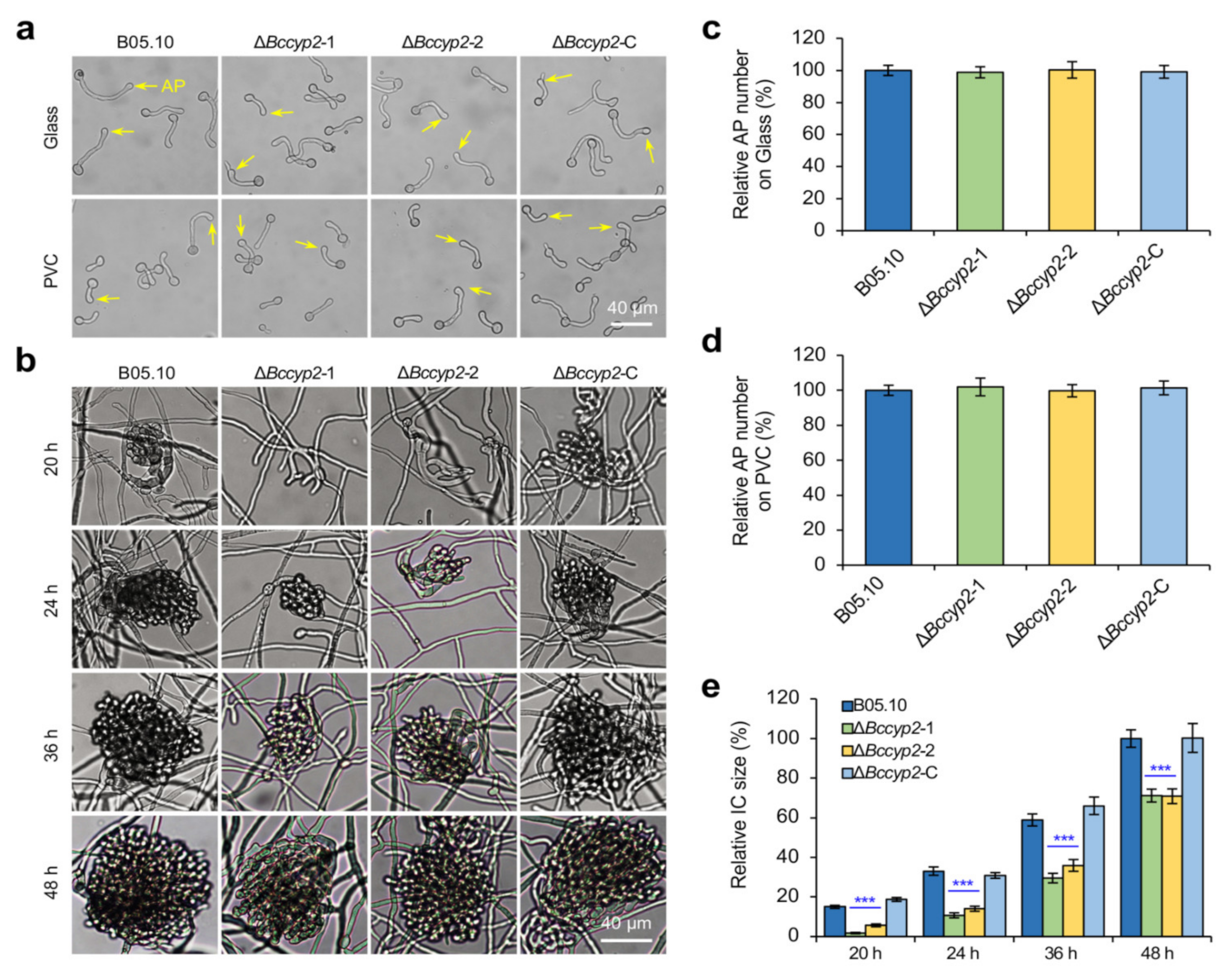
2.5. BcCYP2 Regulates IC Formation but Is Dispensable for Appressorium Formation
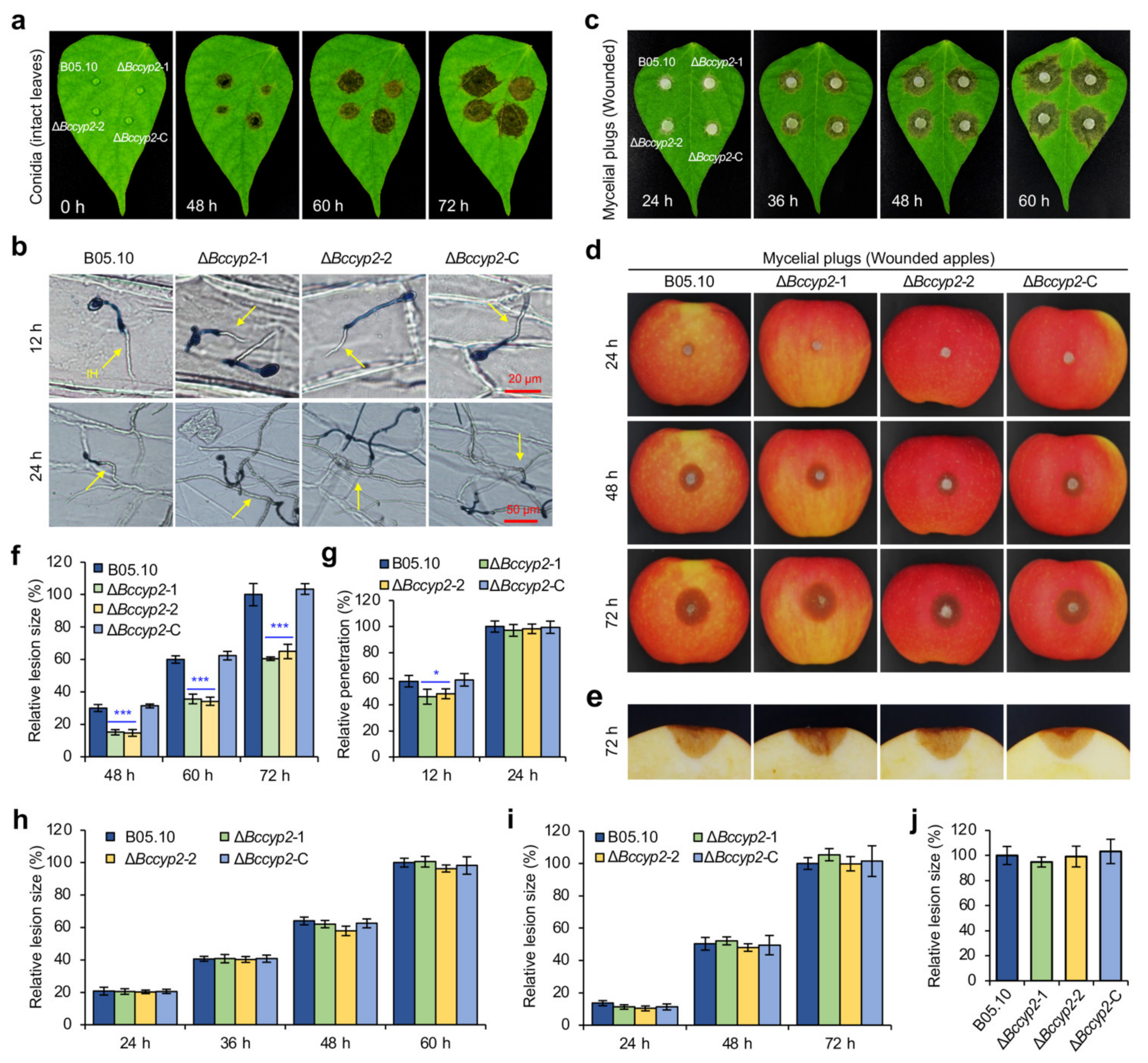
2.6. BcCYP2 Is Required for Full Virulence of B. cinerea
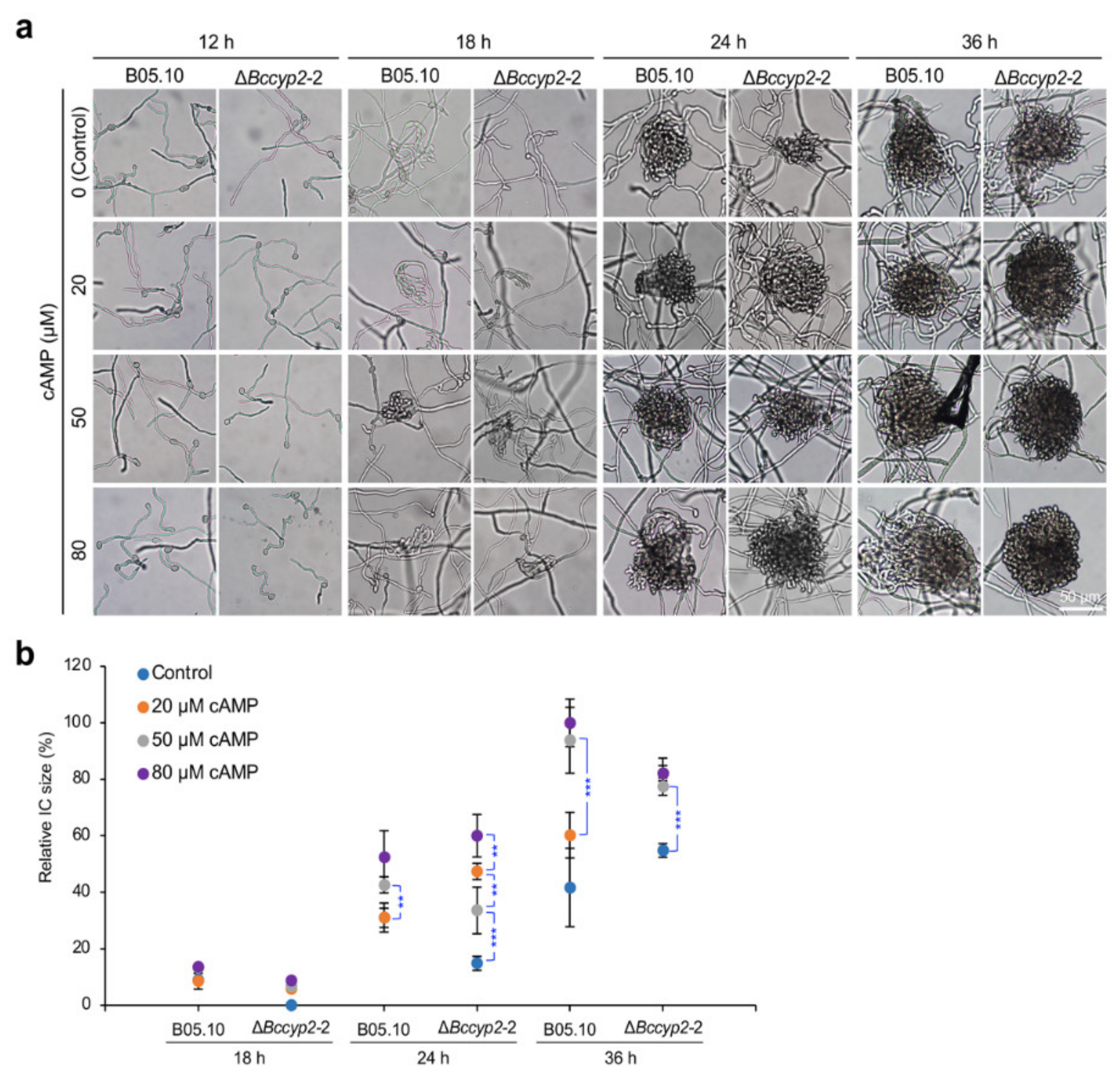
2.7. Exogenous cAMP Restores the Ability of BcCyp2-Deficient Mutants to Form ICs
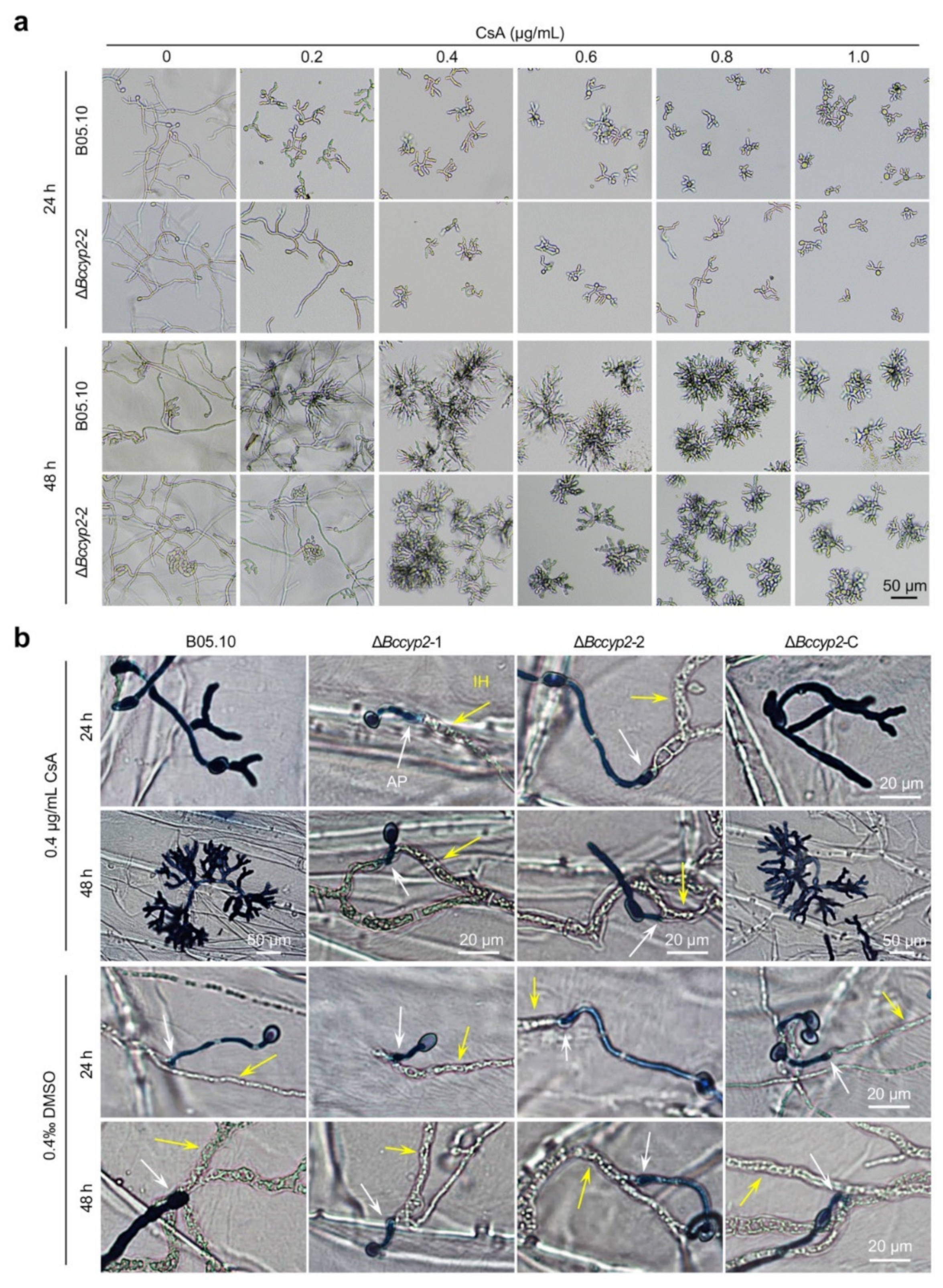
2.8. CsA Suppresses Conidial Germination, Hyphal Morphogenesis and Host Penetration of B. cinerea Strains Harboring CYP2
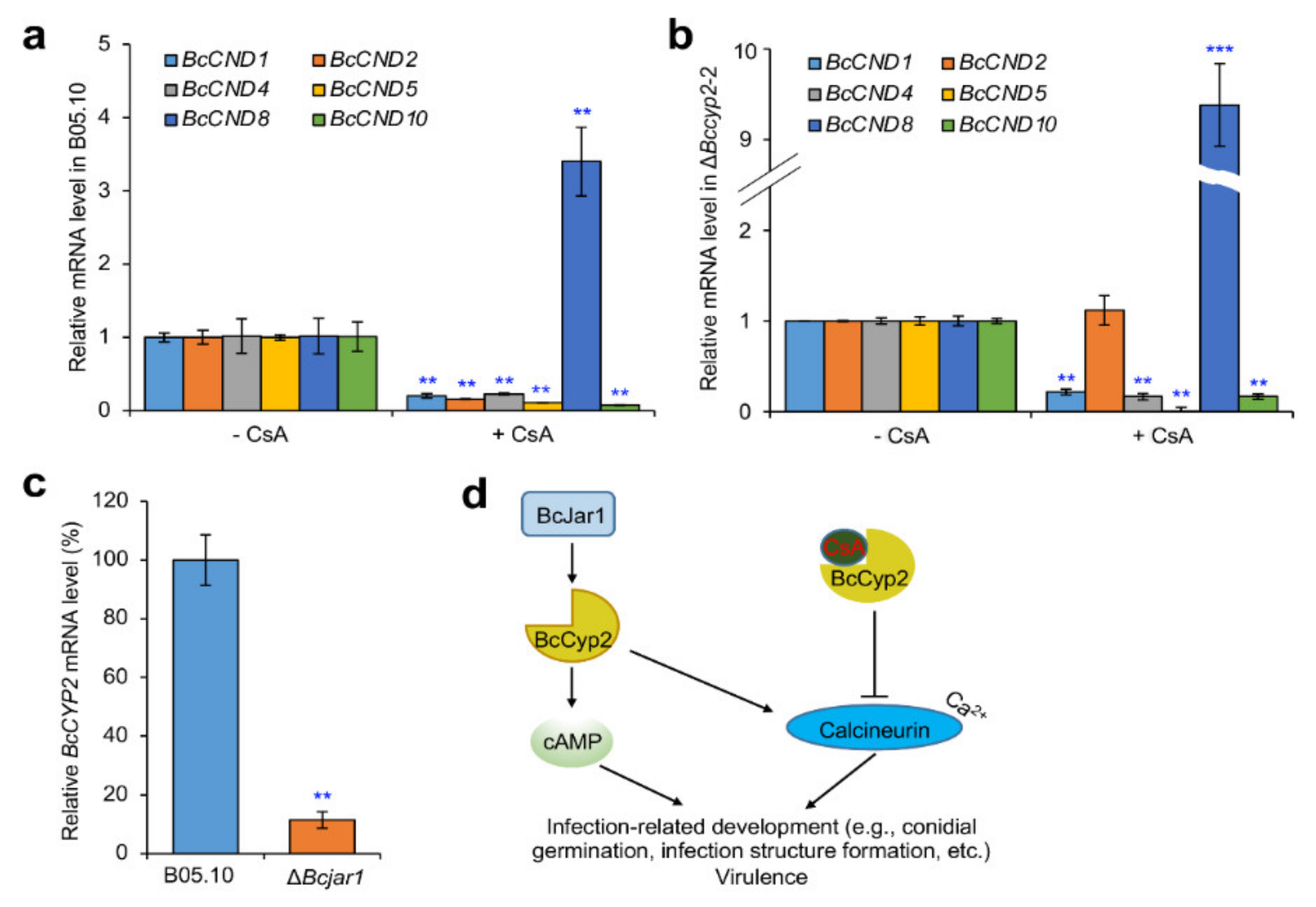
2.9. BcCyp2 Mediates CsA-Regulation of Calcineurin Dependent (CND) Genes
2.10. BcCyp2 Functions under the Control of Histone 3 Lysine 4 (H3K4) Demethylase BcJar1
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Culture Conditions
4.2. Identification of BcCYP2 as a Pathogenicity-Related Gene
4.3. Generation of Gene Deletion and the Corresponding Genetic Complemented Strains
4.4. Fungal Developmental Assays
4.5. Plant Infection and Cytological Assays
4.6. Stress Adaptation Assays
4.7. CsA Sensitivity Assay
4.8. cAMP Sensitivity Assay
4.9. Gene Expression Profiling Assays
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.W.; Zhang, Z.H.; Kaloshian, I.; Huang, H.D.; Jin, H.L. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2010, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Gourgues, M.; Brunet-Simon, A.; Lebrun, M.H.; Levis, C. The tetraspanin BcPls1 is required for appressorium-mediated penetration of Botrytis cinerea into host plant leaves. Mol. Microbiol. 2010, 51, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R.; Tudzynski, P. Reactive oxygen species in development and infection processes. Semin. Cell Dev. Biol. 2016, 57, 138–146. [Google Scholar] [CrossRef]
- Feng, H.Q.; Li, G.H.; Du, S.W.; Yang, S.; Li, X.Q.; de Figueiredo, P.; Qin, Q.M. The septin protein Sep4 facilitates host infection by plant fungal pathogens via mediating initiation of infection structure formation. Environ. Microbiol. 2017, 19, 1730–1749. [Google Scholar] [CrossRef]
- Heuvel, J.V.D.; Waterreus, L.P. Conidial concentration as an important factor determining the type of prepenetration structures formed by Botrytis cinerea on leaves of French bean (Phaseolus vulgaris). Plant Pathol. 1983, 32, 263–272. [Google Scholar] [CrossRef]
- González-Fernández, R.; Valero-Galván, J.; Gómez-Gálvez, F.J.; Jorrín-Novo, J.V. Unraveling thein vitrosecretome of the phytopathogenBotrytis cinereato understand the interaction with its hosts. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Huang, C.Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs–Big Players in Plant.-Microbe Interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef]
- Stamnes, A.M.; Rutherford, S.L.; Zuker, C.S. Cyclophilins: A new family of proteins involved in intracellular folding. Trends Cell Biol. 1992, 2, 272–276. [Google Scholar] [CrossRef]
- Idris, M.; Idris, M.; Adeola, F.; Mensah Sedzro, D. Cyclophilins: The Structure and Functions of an Important Peptidyl-prolyl Isomerase. Int. J. Biochem. Biophys. Mol. Biol. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Barbosa dos Santos, I.; Park, S.-W. Versatility of Cyclophilins in Plant. Growth and Survival: A Case Study in Arabidopsis. Biomolecules 2019, 9, 20. [Google Scholar] [CrossRef]
- Marks, A.R. Cellular functions of immunophilins. Physiol. Rev. 1996, 76, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Wittmann-Liebold, B.; Lang, K.; Kiefhaber, T.; Schmid, F.X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 1989, 337, 476–478. [Google Scholar] [CrossRef]
- Lodish, F.H.; Kong, N. Cyclosporin A inhibits an initial step in folding of transferrin within the endoplasmic reticulum. J. Biol. Chem. 1991, 266, 14835–14838. [Google Scholar] [CrossRef]
- Takahashi, N.; Hayano, T.; Suzuki, M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 1989, 337, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Viaud, M.; Brunet-Simon, A.; Brygoo, Y.; Pradier, J.M.; Levis, C. Cyclophilin A and calcineurin functions investigated by gene inactivation, cyclosporin A inhibition and cDNA arrays approaches in the phytopathogenic fungus Botrytis cinerea. Mol. Microbiol. 2003, 50, 1451–1465. [Google Scholar] [CrossRef]
- Fox, D.S.; Cruz, M.C.; Sia, R.A.L.; Ke, H.; Cox, G.M.; Cardenas, M.E.; Heitman, J. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12–FK506 in Cryptococcus neoformans. Mol. Microbiol. 2001, 39, 835–849. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.R.; Kuroki, Y.; Arioka, M.; Nakajima, H.; Kitamoto, K. Functional analysis of the calcineurin-encoding gene cnaA from Aspergillus oryzae: Evidence for its putative role in stress adaptation. Arch. Microbiol. 2003, 179, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.D.; Heitman, J. Good fungi gone bad: The corruption of calcineurin. Bioessays 2002, 24, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Harren, K.; Schumacher, J.; Tudzynski, B. The Ca2+/calcineurin-dependent signaling pathway in the gray mold Botrytis cinerea: The role of calcipressin in modulating calcineurin activity. PLoS ONE 2012, 7, e41761. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.K.; Yadav, S.; Vaid, N.; Tuteja, N. Genome wide analysis of Cyclophilin gene family from rice and Arabidopsis and its comparison with yeast. Plant Signal. Behav. 2012, 7, 1653–1666. [Google Scholar]
- Blair, L.J.; Baker, J.D.; Sabbagh, J.J.; Dickey, C.A. The emerging role of peptidyl-prolyl isomerase chaperones in tau oligomerization, amyloid processing, and Alzheimer’s disease. J. Neurochem. 2015, 133, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.L.; Walker, J.R.; Campagna-Slater, V.; Finerty, P.J.; Paramanathan, R.; Bernstein, G.; Mackenzie, F.; Tempel, W.; Ouyang, H.; Lee, W.H.; et al. Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases. PLoS Biol. 2010, 8, e1000439. [Google Scholar]
- Satoh, K.; Fukumoto, Y.; Sugimura, K.; Miura, Y.; Aoki, T.; Nochioka, K.; Tatebe, S.; Miyamichi Yamamoto, S.; Shimizu, T.; Osaki, S. Plasma cyclophilin A is a novel biomarker for coronary artery disease. Circ. J. 2013, 77, 447–455. [Google Scholar] [CrossRef]
- Schmidt, B.; Tradler, T.; Rahfeld, J.U.; Ludwig, B.; Jain, B.; Mann, K.; Rücknagel, K.P.; Janowski, B.; Schierhorn, A.; Küllertz, G. A cyclophilin-like peptidyl-prolyl cis/trans isomerase from Legionella pneumophila--characterization, molecular cloning and overexpression. Mol. Microbiol. 2010, 21, 1147–1160. [Google Scholar]
- Surichan, S.; Arroo, R.R.; Ruparelia, K.; Tsatsakis, A.M.; Androutsopoulos, V.P. Nobiletin bioactivation in MDA-MB-468 breast cancer cells by cytochrome P450 CYP1 enzymes. Food Chem. Toxicol. 2018, 113, 228–235. [Google Scholar] [CrossRef]
- Zhou, Y.; Keyhani, N.O.; Zhang, Y.; Luo, Z.; Fan, Y.; Li, Y.; Zhou, Q.; Chen, J.; Pei, Y. Dissection of the contributions of cyclophilin genes to development and virulence in a fungal insect pathogen. Environ. Microbiol. 2016, 18, 3812–3826. [Google Scholar] [CrossRef] [PubMed]
- Viaud, C.M.; Balhadère, P.V.; Talbot, N.J. A Magnaporthe grisea cyclophilin acts as a virulence determinant during plant infection. Plant Cell 2002, 14, 917–930. [Google Scholar] [PubMed]
- Chen, M.M.; Jiang, M.; Shang, J.; Lan, X.; Yang, F.; Huang, J.; Nuss, D.L.; Chen, B. CYP1, a hypovirus-regulated cyclophilin, is required for virulence in the chestnut blight fungus. Mol. Plant Pathol. 2011, 12, 239–246. [Google Scholar]
- Cao, S.N.; Yuan, Y.; Qin, Y.H.; Zhang, M.Z.; de Figueiredo, P.; Li, G.H.; Qin, Q.M. The pre-rRNA processing factor Nop53 regulates fungal development and pathogenesis via mediating production of reactive oxygen species. Environ. Microbiol. 2018, 20, 1531–1549. [Google Scholar]
- Liu, J.K.; Chang, H.W.; Liu, Y.; Qin, Y.; Ding, Y.H.; Wang, L.; Zhao, Y.; Zhang, M.Z.; Cao, S.N.; Li, L.T. The key gluconeogenic gene PCK1 is crucial for virulence of Botrytis cinerea via initiating its conidial germination and host penetration. Environ. Microbiol. 2018, 20, 1794–1814. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.K.; Li, G.H.; Zhang, M.Z.; Zhang, Y.Y.; Wang, Y.Y.; Hou, J.; Yang, S.; Sun, J.; Qin, Q.M. A novel Botrytis cinerea-specific gene BcHBF1 enhances virulence of the grey mould fungus via promoting host penetration and invasive hyphal development. Mol. Plant Pathol. 2019, 20, 731–747. [Google Scholar] [CrossRef]
- Van Kan, J.A.; Stassen, J.H.; Mosbach, A.; Van Der Lee, T.A.; Faino, L.; Farmer, A.D.; Papasotiriou, D.G.; Zhou, S.; Seidl, M.F.; Cottam, E. A gapless genome sequence of the fungus Botrytis cinerea. Mol. Plant Pathol. 2017, 18, 75–89. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Sun, C.H.; Liu, Y.; Feng, H.Q.; Chang, H.W.; Cao, S.N.; Li, G.H.; Yang, S.; Hou, J.; Zhu-Salzman, K.; et al. Transcriptome analysis and functional validation reveal a novel gene, BcCGF1, that enhances fungal virulence by promoting infection-related development and host penetration. Mol. Plant Pathol. 2020, 21, 834–853. [Google Scholar] [CrossRef]
- Hou, J.; Feng, H.Q.; Chang, H.W.; Liu, Y.; Li, G.H.; Yang, S.; Sun, C.H.; Zhang, M.Z.; Yuan, Y.; Sun, J.; et al. The H3K4 demethylase Jar1 orchestrates ROS production and expression of pathogenesis-related genes to facilitate Botrytis cinerea virulence. New Phytol. 2020, 225, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Zhang, H.F.; Li, G.T.; Shaw, B.; Xu, J.R. The Cyclase-associated protein Cap1 is important for proper regulation of infection-related morphogenesis in Magnaporthe oryzae. PLoS Pathog. 2012, 8, e1002911. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cardenas, M.E.; Cox, G.M.; Perfect, J.R.; Heitman, J. Two cyclophilin A homologs with shared and distinct functions important for growth and virulence of Cryptococcus neoformans. Embo Rep. 2001, 2, 511–518. [Google Scholar] [CrossRef]
- Xu, R.J.; Hamer, J.E. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 1996, 10, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.; Zhou, X.Y.; Zhao, X.H.; Xu, J.R. The PMK1 MAP Kinase Pathway and Infection-Related Morphogenesis. In Advances in Genetics, Genomics and Control of Rice Blast Disease; Springer: Dordrecht, The Netherlands, 2009; pp. 13–21. [Google Scholar]
- Leroch, M.; Mueller, N.; Hinsenkamp, I.; Hahn, M. The signalling mucin Msb2 regulates surface sensing and host penetration via BMP1 MAP kinase signalling in B otrytis cinerea. Mol. Plant Pathol. 2015, 16, 787–798. [Google Scholar] [CrossRef]
- Schumacher, J.; Kokkelink, L.; Huesmann, C.; Jimenez-Teja, D.; Collado, I.G.; Barakat, R.; Tudzynski, P.; Tudzynski, B. The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol. Plant-Microbe Interact. 2008, 21, 1443–1459. [Google Scholar] [CrossRef]
- Giesbert, S.; Schumacher, J.; Kupas, V.; Espino, J.; Segmüller, N.; Haeuser-Hahn, I.; Schreier, P.H.; Tudzynski, P. Identification of pathogenesis-associated genes by T-DNA–mediated insertional mutagenesis in Botrytis cinerea: A Type 2A phosphoprotein phosphatase and an SPT3 transcription factor have significant impact on virulence. Mol. Plant-Microbe Interact. 2012, 25, 481–495. [Google Scholar] [CrossRef]
- Mullins, E.D.; Chen, X.; Romaine, P.; Raina, R.; Geiser, D.M.; Kang, S. Agrobacterium-mediated transformation of Fusarium oxysporum: An efficient tool for insertional mutagenesis and gene transfer. Phytopathology 2001, 91, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cenis, J.L. Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res. 1992, 20, 2380. [Google Scholar] [CrossRef] [PubMed]
- Lecellier, G.; Silar, P. Rapid methods for nucleic acids extraction from Petri dish-grown mycelia. Curr. Genet. 1994, 25, 122–123. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Sun, C.-H.; Chang, H.-W.; Yang, S.; Liu, Y.; Zhang, M.-Z.; Hou, J.; Zhang, H.; Li, G.-H.; Qin, Q.-M. Cyclophilin BcCyp2 Regulates Infection-Related Development to Facilitate Virulence of the Gray Mold Fungus Botrytis cinerea. Int. J. Mol. Sci. 2021, 22, 1694. https://doi.org/10.3390/ijms22041694
Sun J, Sun C-H, Chang H-W, Yang S, Liu Y, Zhang M-Z, Hou J, Zhang H, Li G-H, Qin Q-M. Cyclophilin BcCyp2 Regulates Infection-Related Development to Facilitate Virulence of the Gray Mold Fungus Botrytis cinerea. International Journal of Molecular Sciences. 2021; 22(4):1694. https://doi.org/10.3390/ijms22041694
Chicago/Turabian StyleSun, Jiao, Chen-Hao Sun, Hao-Wu Chang, Song Yang, Yue Liu, Ming-Zhe Zhang, Jie Hou, Hao Zhang, Gui-Hua Li, and Qing-Ming Qin. 2021. "Cyclophilin BcCyp2 Regulates Infection-Related Development to Facilitate Virulence of the Gray Mold Fungus Botrytis cinerea" International Journal of Molecular Sciences 22, no. 4: 1694. https://doi.org/10.3390/ijms22041694
APA StyleSun, J., Sun, C.-H., Chang, H.-W., Yang, S., Liu, Y., Zhang, M.-Z., Hou, J., Zhang, H., Li, G.-H., & Qin, Q.-M. (2021). Cyclophilin BcCyp2 Regulates Infection-Related Development to Facilitate Virulence of the Gray Mold Fungus Botrytis cinerea. International Journal of Molecular Sciences, 22(4), 1694. https://doi.org/10.3390/ijms22041694






