Circulating Microvesicle-Associated Inducible Nitric Oxide Synthase Is a Novel Therapeutic Target to Treat Sepsis: Current Status and Future Considerations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Radio-Labeling MV-A iNOS as Tracer
2.2. T½ determination for 125I-MV-A iNOS in Blood
2.3. Tissue Distribution of MV-A iNOS
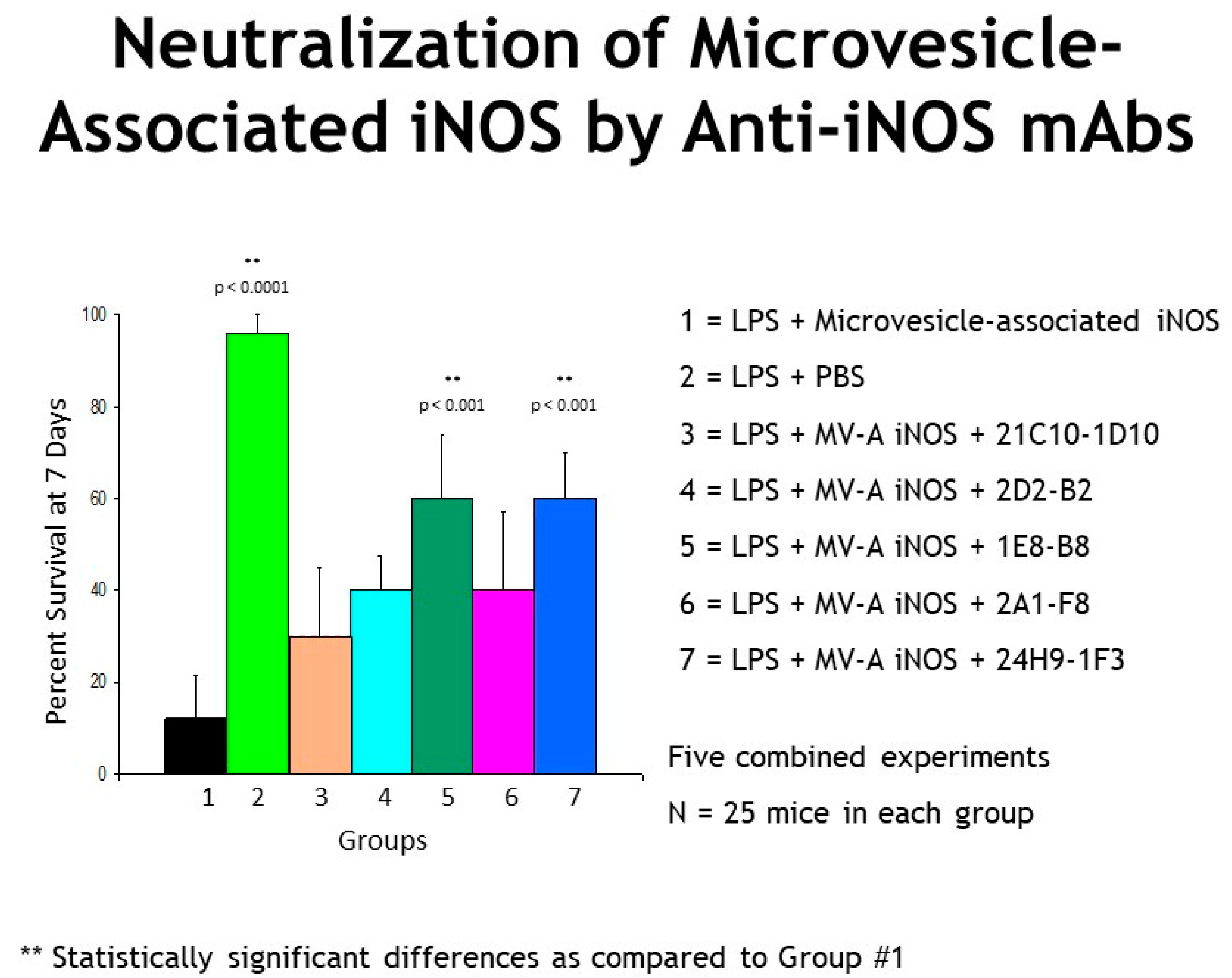
2.4. Genetically Engineered Recombinant Humanized Anti-MV-A iNOS Monoclonal Antibodies (mAbs)
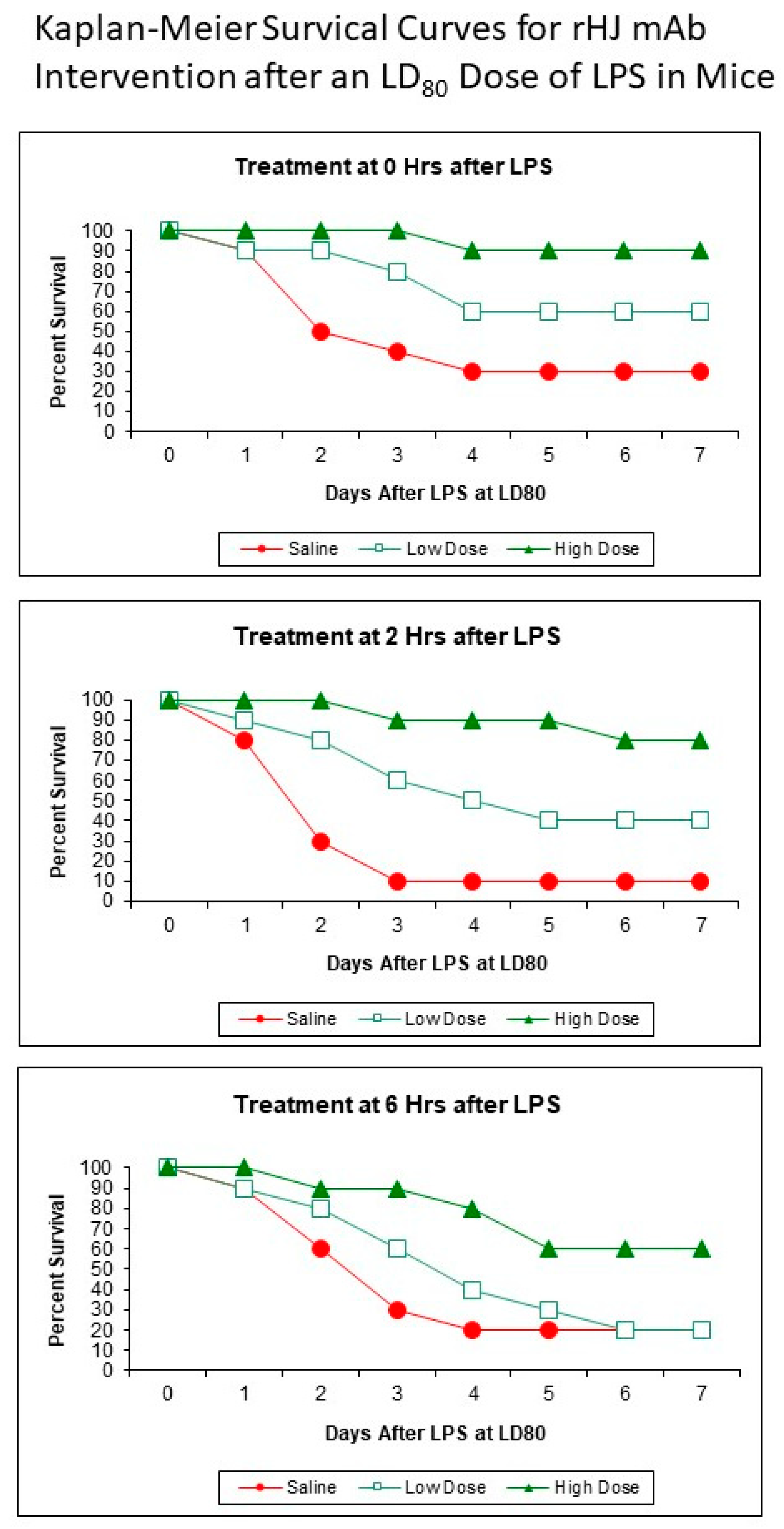
2.5. Kaplan–Meier Survival Curves
2.6. Potential Drug Interference
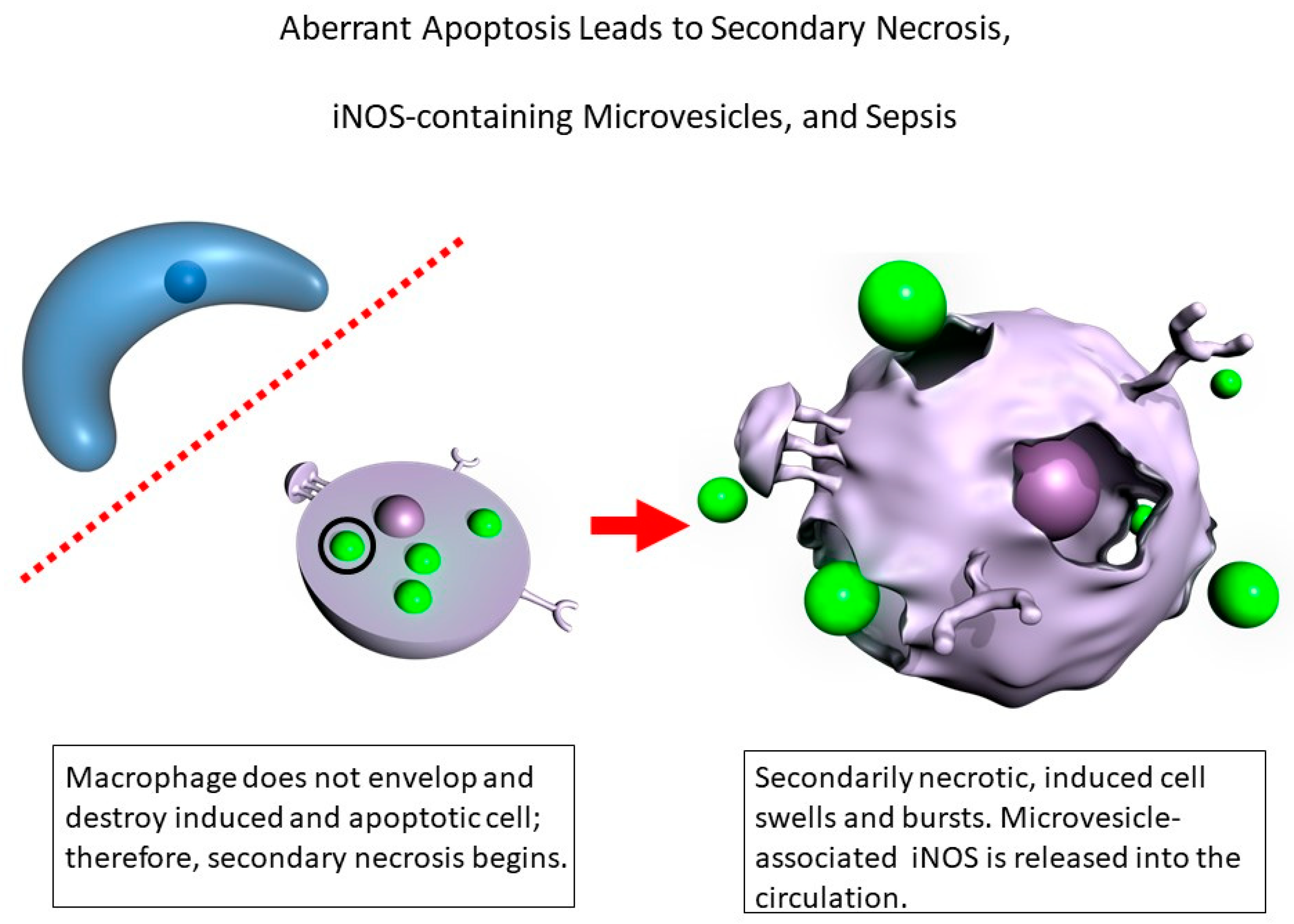
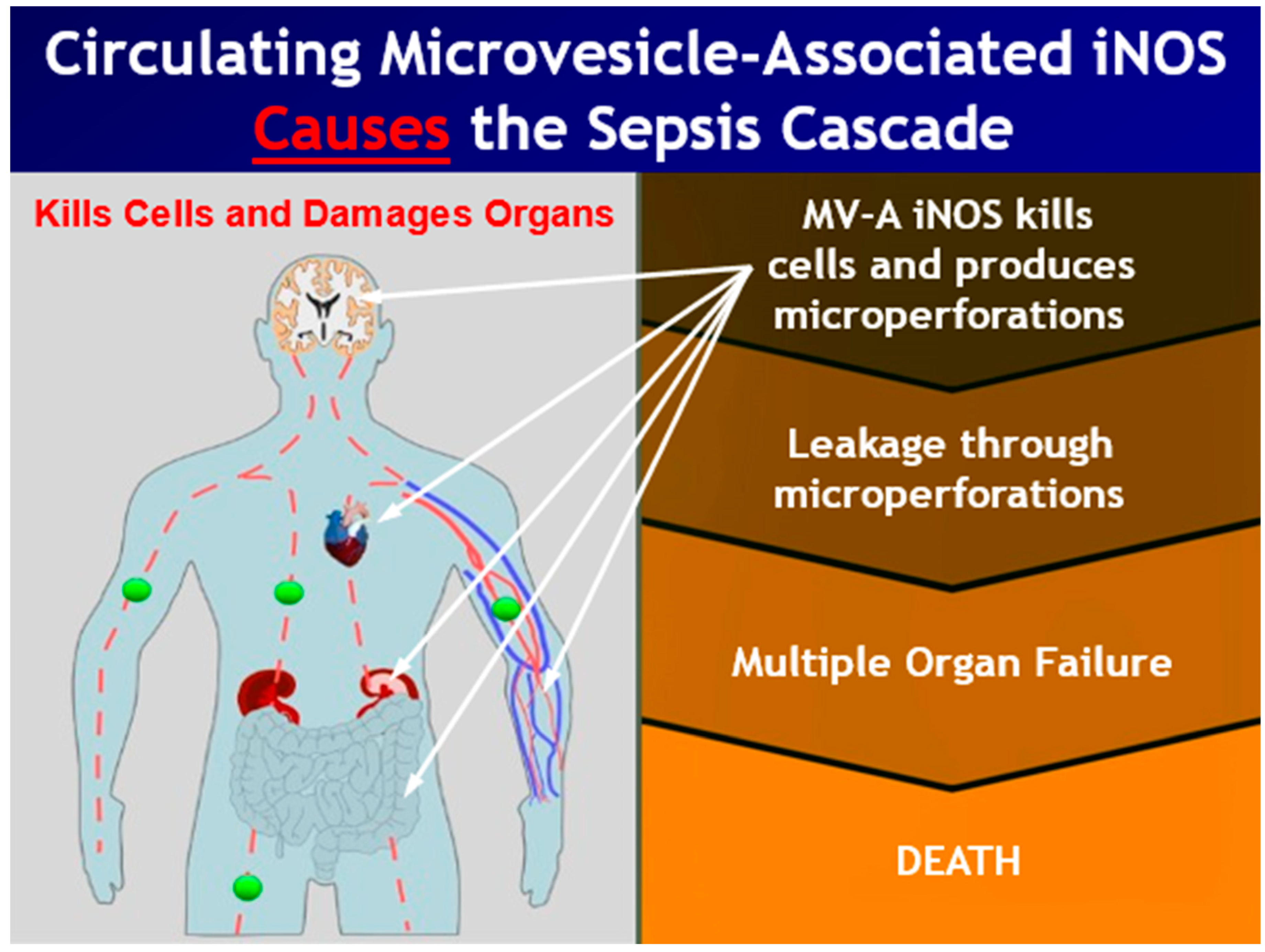
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webber, R.J.; Rodriguez, J.G.; Webber, D.S.; Dunnebacke, T.H. Development, Characterization, and Epitope Mapping of a Panel of Twenty-Four Monoclonal Antibodies Specific for Human Inducible Nitric Oxide Synthase. Hybridoma 2005, 24, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Marshall, J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014, 20, 195–203. [Google Scholar] [CrossRef]
- Cavaillon, J.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128. [Google Scholar] [CrossRef] [PubMed]
- Webber, R.J.; Dunnebacke, T.H. Inducible nitric oxide synthase is an early plasma biomarker for the onset of sepsis. In Proceedings of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 14–17 September 2003; ISBN 1555812848. [Google Scholar]
- Webber, R.J.; Sweet, R.M.; Webber, D.S. Inducible Nitric Oxide Synthase in Circulating Microvesicles: Discovery, Evolution, and Evidence as a Novel Biomarker and the Probable Causative Agent for Sepsis. J. Appl. Lab. Med. 2019, 3, 698–711. [Google Scholar] [CrossRef] [Green Version]
- Webber, R.J.; Dunnebacke, T.H.; Webber, D.S. Neutralization in vivo of particulate iNOS with humanized anti-iNOS mAbs rescues mice from death by sepsis. In Proceedings of the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 27–30 September 2006; ISBN 1555814093. [Google Scholar]
- Yataco, A.O.C.; Simpson, S.Q. Coronavirus Disease 2019 Sepsis. Chest 2020, 158, 1833–1834. [Google Scholar] [CrossRef]
- Three Major Scientific Societies Confirm Link between SARSCoV-2 and Sepsis, Which Causes Vast Majority of COVID-19 Deaths. ESICM Joint Statement. July 2021. Available online: https://www.esicm.org/wp-content/uploads/2021/06/PressRelease_SepsisandCOVID-19_JointStatement.pdf (accessed on 15 July 2021).
- European Society of Intensive Care Medicine (ESICM); Global Sepsis Alliance (GSA); Society of Critical Care Medicine (SCCM). Reducing the global burden of sepsis: A positive legacy for the COVID-19 pandemic? Intensiv. Care Med. 2021, 47, 733–736. [Google Scholar] [CrossRef]
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.; Sowers, N.; Collier, M.; Chavan, S.; Oke, I.; Pennini, M.E.; Santhosh, A.; et al. Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012–2018. Crit. Care Med. 2020, 48, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.; Sowers, N.; Collier, M.; Chavan, S.; Oke, I.; Pennini, M.E.; Santhosh, A.; et al. Sepsis Among Medicare Beneficiaries: 2. The Trajectories of Sepsis, 2012–2018. Crit. Care Med. 2020, 48, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.; Sowers, N.; Collier, M.; Chavan, S.; Oke, I.; Pennini, M.E.; Santhosh, A.; et al. Sepsis Among Medicare Beneficiaries: 3. The Methods, Models, and Forecasts of Sepsis, 2012–2018. Crit. Care Med. 2020, 48, 302–318. [Google Scholar] [CrossRef] [Green Version]
- Schmid, A.; Burchardi, H.; Clouth, J.; Schneider, H. Burden of illness imposed by severe sepsis in Germany. Eur. J. Health Econ. 2002, 3, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J.; Ely, E.W.; Smith, D.M.; Langa, K. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA 2010, 304, 1787–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, F.; Pike, F.; Alvarez, K.; Angus, D.; Newman, A.B.; Lopez, O.; Tate, J.; Kapur, V.; Wilsdon, A.; Krishnan, J.A.; et al. Bidirectional Relationship between Cognitive Function and Pneumonia. Am. J. Respir. Crit. Care Med. 2013, 188, 586–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angus, D.C.; Van Der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Gesten, F.; Prescott, H.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [Green Version]
- Liu, V.; Escobar, G.J.; Greene, J.D.; Soule, J.; Whippy, A.; Angus, D.C.; Iwashyna, T.J. Hospital Deaths in Patients with Sepsis From 2 Independent Cohorts. JAMA 2014, 312, 90–92. [Google Scholar] [CrossRef] [Green Version]
- Rhee, C.; Dantes, R.; Epstein, L.; Murphy, D.J.; Seymour, C.W.; Iwashyna, T.J.; Kadri, S.S.; Angus, D.C.; Danner, R.L.; Fiore, A.E.; et al. Incidence and Trends of Sepsis in US Hospitals Using Clinical vs. Claims Data, 2009–2014. JAMA 2017, 318, 1241–1249. [Google Scholar] [CrossRef]
- Donovan, K.; Shah, A.; Day, J.; McKechnie, S.R. Adjunctive treatments for the management of septic shock—A narrative review of the current evidence. Anaesthesia 2021, 76, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Szychowski, J.M.; Griffin, R.; Safford, M.M.; Shapiro, N.I.; Howard, G. Long-term mortality after community-acquired sepsis: A longitudinal population-based cohort study. BMJ Open 2014, 4, e004283. [Google Scholar] [CrossRef] [Green Version]
- Perl, T.M. Long-Term Survival and Function after Suspected Gram-Negative Sepsis. JAMA 1995, 274, 338–345. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 2003, 31, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.Q. SIRS in the Time of Sepsis-3. Chest 2018, 153, 34–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takauji, S.; Hayakawa, M.; Fujita, S. A Nationwide Comparison between Sepsis-2 and Sepsis-3 Definition in Japan. J. Intensiv. Care Med. 2020, 35, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; Deutschman, C.S.; Escobar, G.J.; Angus, D.C.; Iwashyna, T.J.; Brunkhorst, F.M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [Green Version]
- Gambim, M.H.; de Oliveira do Carmo, A.; Marti, L.; Veríssimo-Filho, S.; Lopes, L.R.; Janiszewski, M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: Experimental evidence for a novel mechanism of septic vascular dysfunction. Crit. Care 2007, 11, R107. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, L.C.; Janiszewski, M.; Pontieri, V.; Pedro, M.A.; Bassi, E.; Tucci, P.J.; Laurindo, F.R. Platelet-derived exosomes from septic shock patients induce myocardial dysfunction. Crit. Care 2007, 11, R120. [Google Scholar] [CrossRef] [Green Version]
- Mortaza, S.; Martinez, M.C.; Baron-Menguy, C.; Burban, M.; de la Bourdonnaye, M.; Fizanne, L.; Pierrot, M.; Calès, P.; Henrion, D.; Andriantsitohaina, R.; et al. Detrimental hemodynamic and inflammatory effects of microparticles originating from septic rats. Crit. Care Med. 2009, 37, 2045–2050. [Google Scholar] [CrossRef] [Green Version]
- Webber, R.J.; Webber, D.S. Method of Effecting a Mammalian Model of Sepsis, Severe Sepsis, or Septic Shock. PCT Application #WO 2007/024568, 16 August 2006. [Google Scholar]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, F.; Nickenig, G.; Werner, N. Extracellular Vesicles in Cardiovascular Disease. Circ. Res. 2017, 120, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.K.; Greening, D.W.; Rai, A.; Chen, M.; Xu, R.; Shafiq, A.; Mathias, R.A.; Zhu, H.-J.; Simpson, R.J. Extracellular vesicles: Their role in cancer biology and epithelial–mesenchymal transition. Biochem. J. 2017, 474, 21–45. [Google Scholar] [CrossRef] [PubMed]
| Normalized DPM 125I/mg Tissue | |||
|---|---|---|---|
| Organ | Saline Only | Saline + LPS | p Value * |
| Heart | 100.0% | 186.4% | p < 0.05 |
| Spinal Cord | 100.0% | 187.1% | p < 0.05 |
| Intestines | 100.0% | 201.1% | p < 0.05 |
| Liver | 100.0% | 103.5% | No Significant Difference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webber, R.J.; Sweet, R.M.; Webber, D.S. Circulating Microvesicle-Associated Inducible Nitric Oxide Synthase Is a Novel Therapeutic Target to Treat Sepsis: Current Status and Future Considerations. Int. J. Mol. Sci. 2021, 22, 13371. https://doi.org/10.3390/ijms222413371
Webber RJ, Sweet RM, Webber DS. Circulating Microvesicle-Associated Inducible Nitric Oxide Synthase Is a Novel Therapeutic Target to Treat Sepsis: Current Status and Future Considerations. International Journal of Molecular Sciences. 2021; 22(24):13371. https://doi.org/10.3390/ijms222413371
Chicago/Turabian StyleWebber, Robert J., Richard M. Sweet, and Douglas S. Webber. 2021. "Circulating Microvesicle-Associated Inducible Nitric Oxide Synthase Is a Novel Therapeutic Target to Treat Sepsis: Current Status and Future Considerations" International Journal of Molecular Sciences 22, no. 24: 13371. https://doi.org/10.3390/ijms222413371
APA StyleWebber, R. J., Sweet, R. M., & Webber, D. S. (2021). Circulating Microvesicle-Associated Inducible Nitric Oxide Synthase Is a Novel Therapeutic Target to Treat Sepsis: Current Status and Future Considerations. International Journal of Molecular Sciences, 22(24), 13371. https://doi.org/10.3390/ijms222413371





