Pharmacological Inhibition of Sonic Hedgehog Signaling Suppresses Tumor Development in a Murine Model of Intrahepatic Cholangiocarcinoma
Abstract
:1. Introduction
2. Results
2.1. Activation of SHH Signaling in Human Intrahepatic CCC
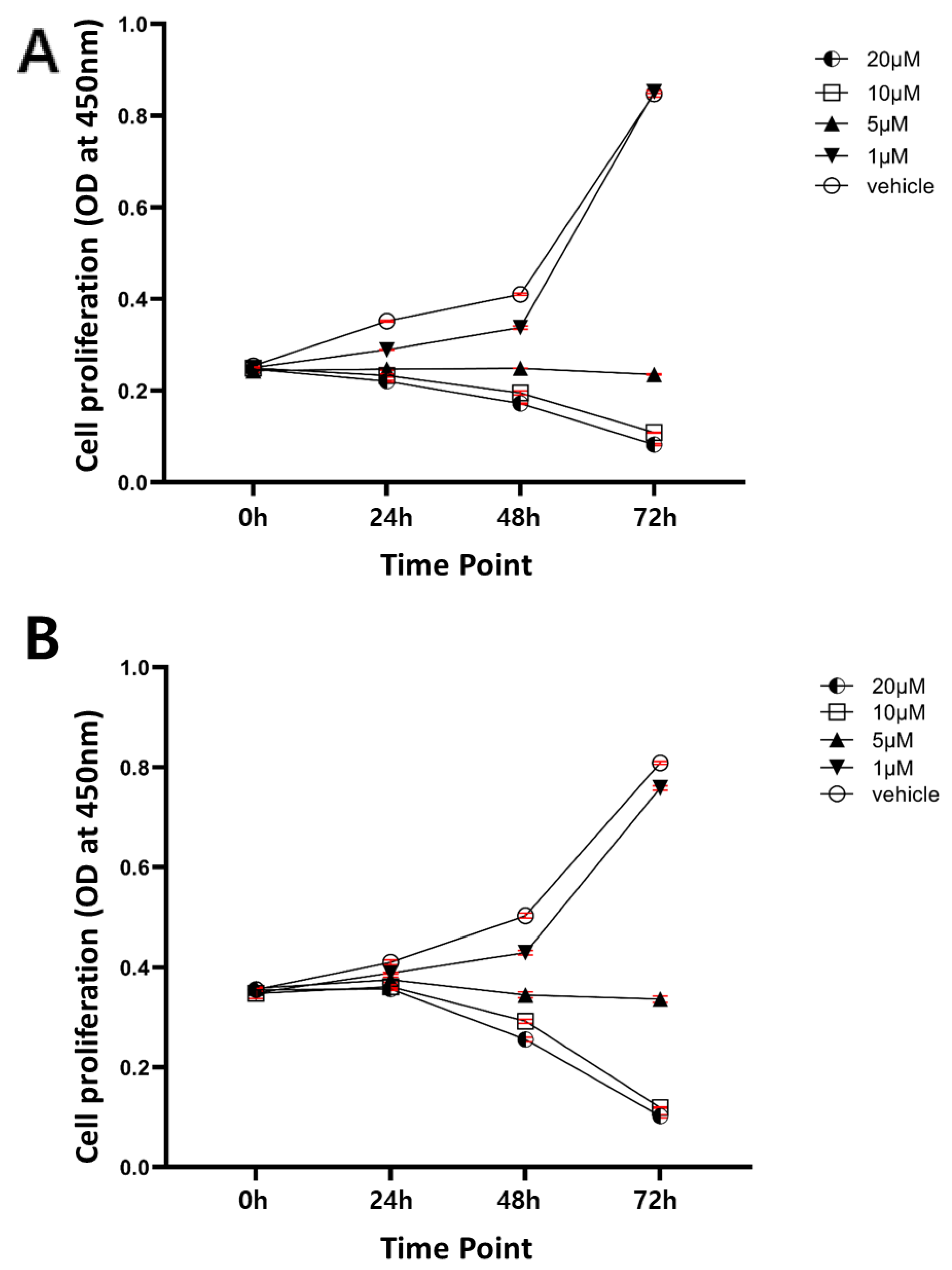
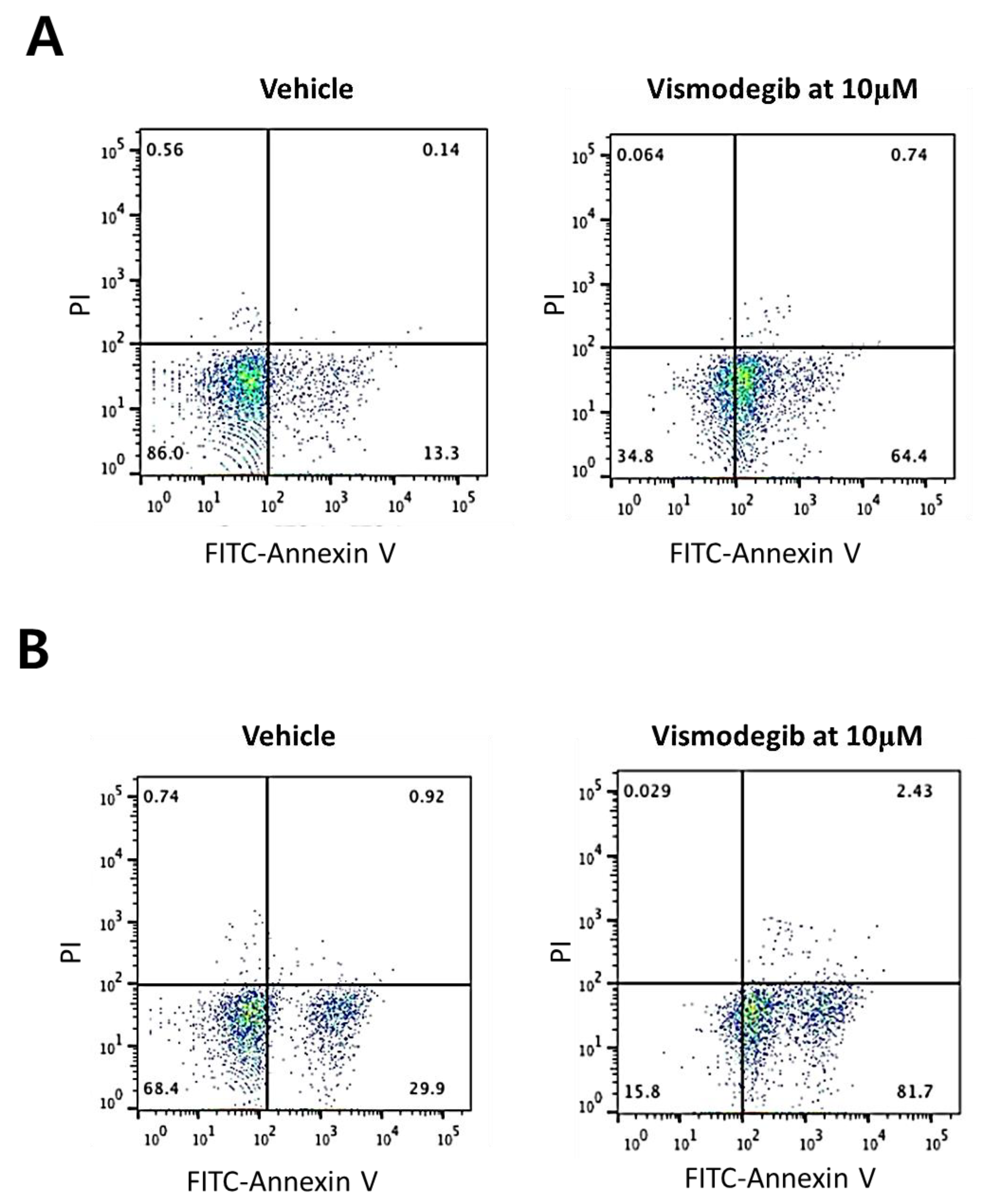
2.2. Sensitivity of Human Intrahepatic CCC Cells to a Chemical Inhibitor of SHH Signaling
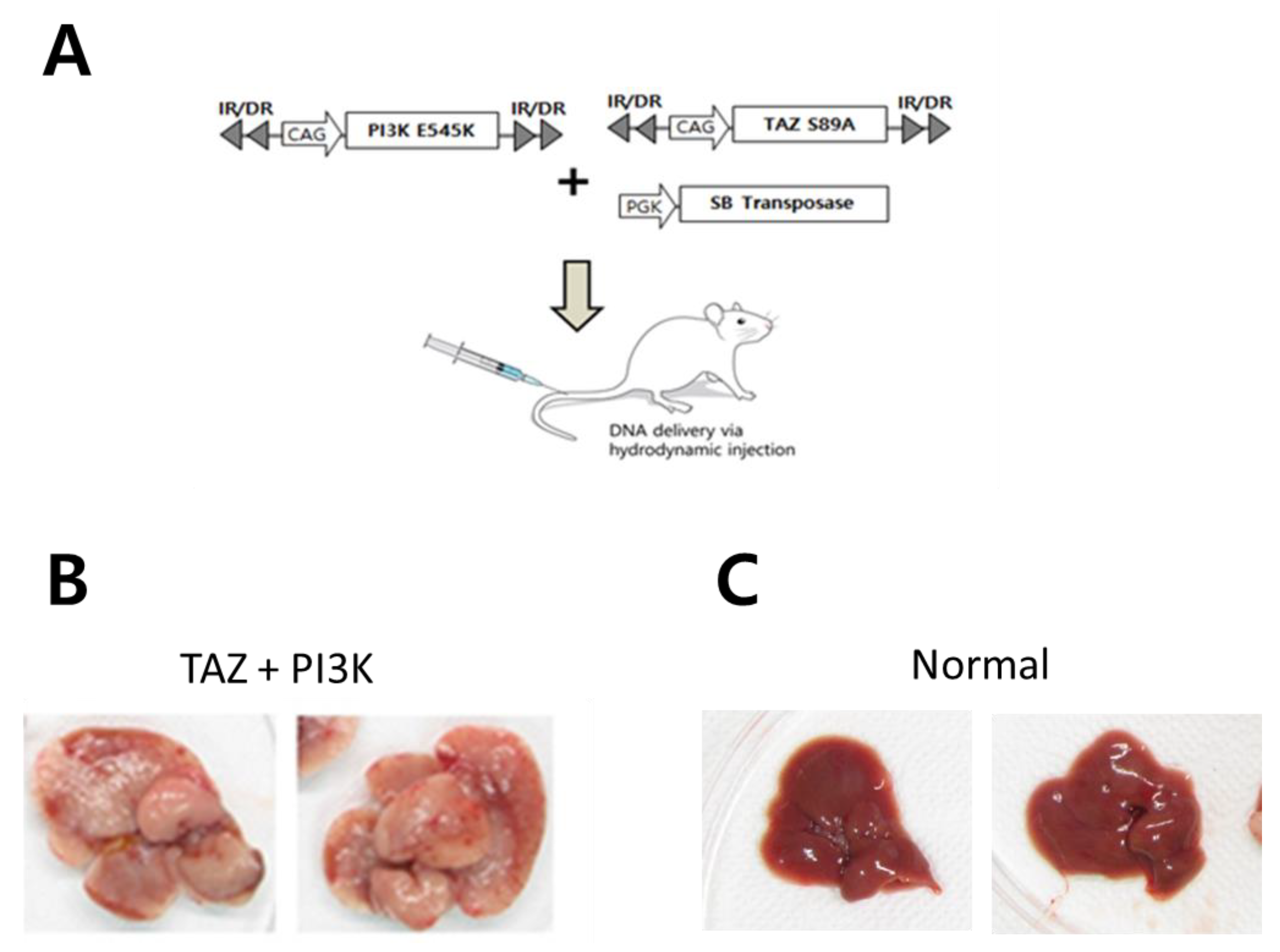
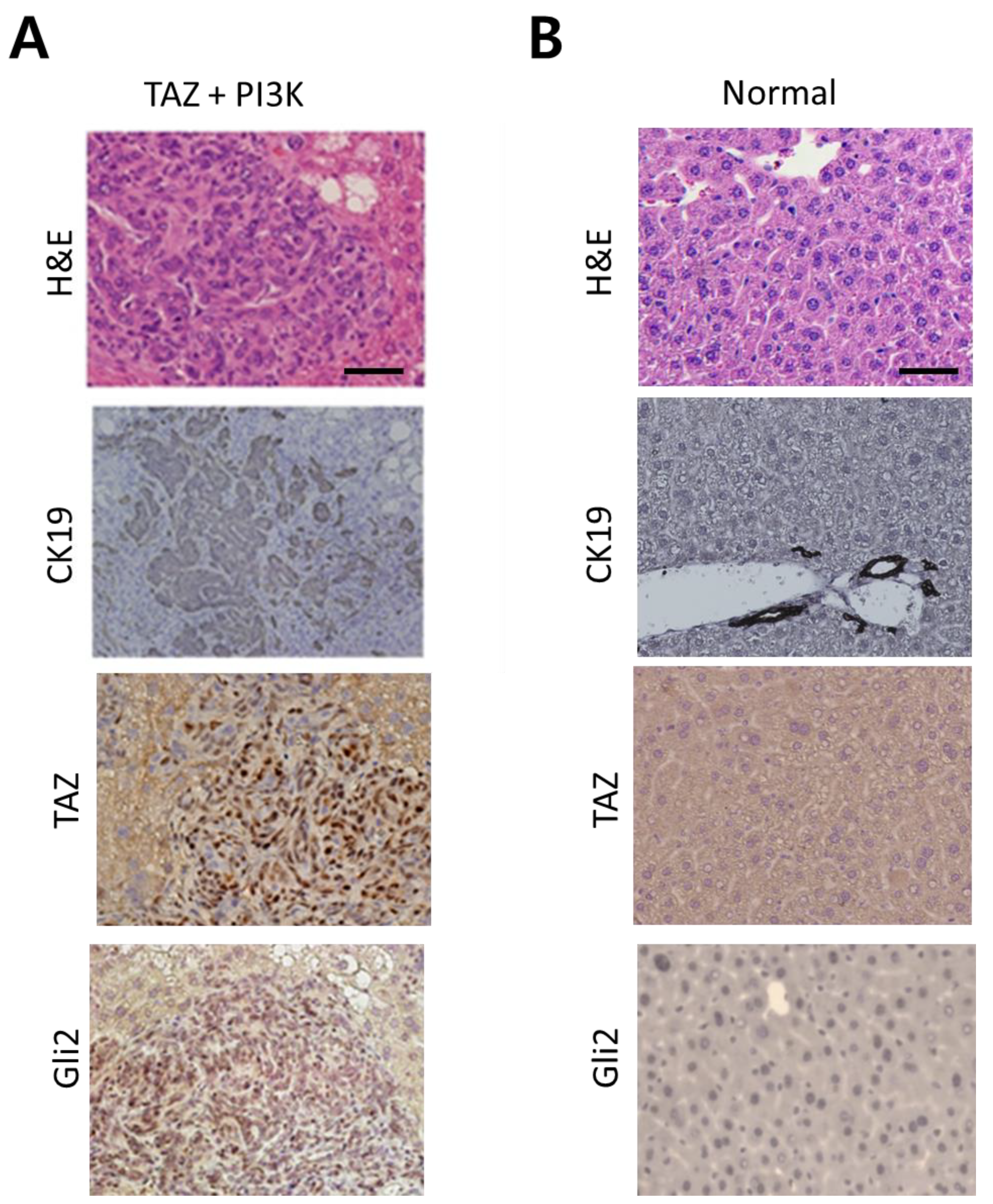
2.3. Activation of SHH Signaling in a Murine Model of CCC Induced by Activated Forms of TAZ and PI3K
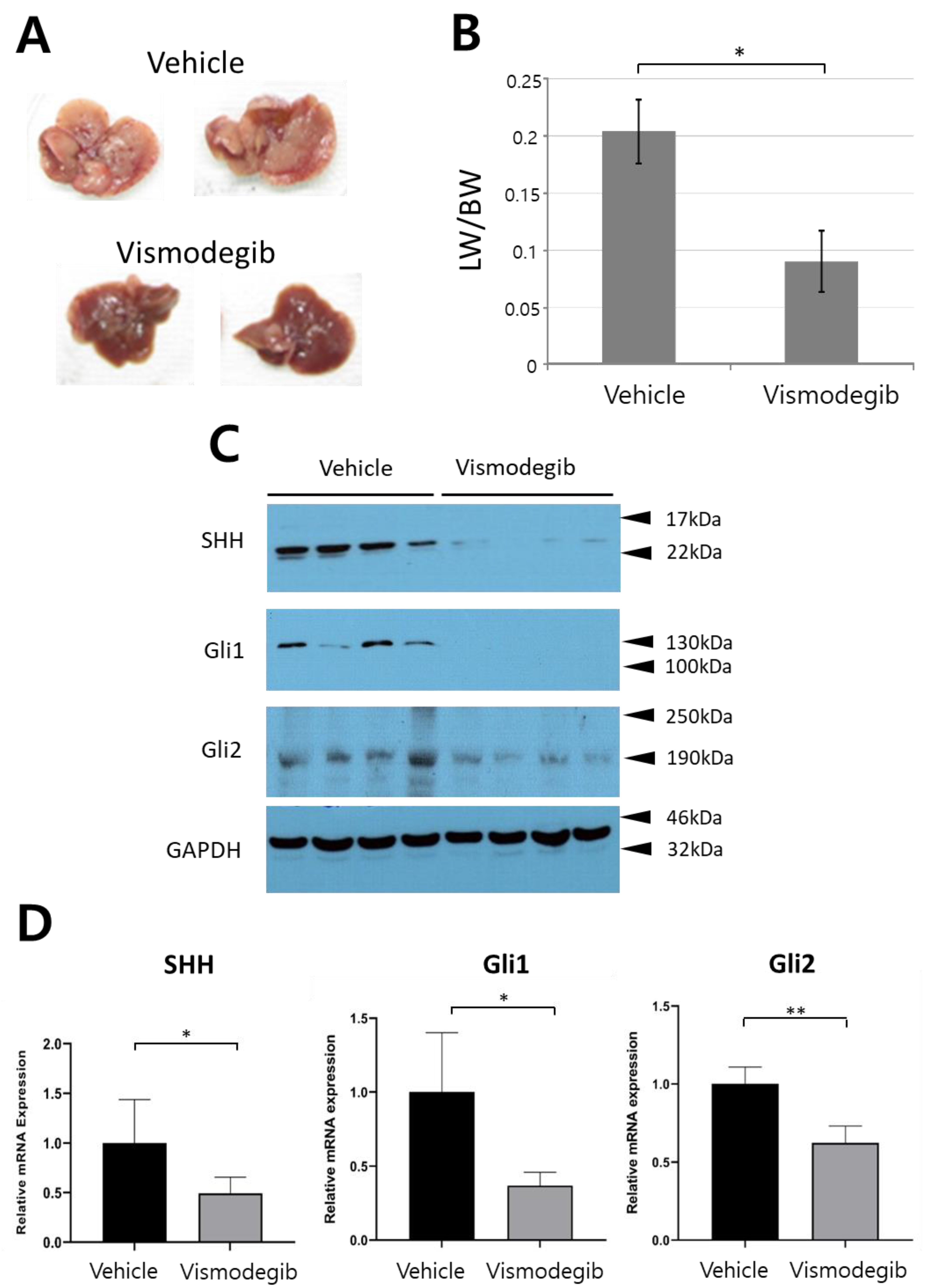
2.4. Vismodegib Suppresses Tumor Development in the Murine Model of CCC
3. Discussion
4. Materials and Methods
4.1. Publicly Available Genomic Data Analyses
4.2. Cell Culture and Treatment
4.3. Animal Models
4.4. Hydrodynamic Transfection and Drug Treatment
4.5. Liver Harvest and Tissue Processing
4.6. Immunohistochemical Analyses of Mouse Tissue Samples
4.7. RNA Purification, Reverse Transcription and Real-Time PCR Amplification
4.8. Protein Etraction and Western Blotting
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, W.K.; Jamshidi, N.; Felker, E.R.; Raman, S.S.; Lu, D.S. Radiomics and radiogenomics of primary liver cancers. Clin. Mol. Hepatol. 2019, 25, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Qian, H.; Wang, S.; Fulte, S.; Ding, W.X. Autophagy and liver cancer. Clin. Mol. Hepatol. 2020, 26, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Anichini, G.; Carrassa, L.; Stecca, B.; Marra, F.; Raggi, C. The Role of the Hedgehog Pathway in Cholangiocarcinoma. Cancers 2021, 13, 4774. [Google Scholar] [CrossRef]
- Chiang, C.; Litingtung, Y.; Lee, E.; Young, K.E.; Corden, J.L.; Westphal, H.; Beachy, P.A. Cyclopia and defective axial pat-terning in mice lacking sonic hedgehog gene function. Nature 1996, 383, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Basset-Séguin, N.; Hauschild, A.; Kunstfeld, R.; Grob, J.; Dréno, B.; Mortier, L.; Ascierto, P.; Licitra, L.; Dutriaux, C.; Thomas, L.; et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. Eur. J. Cancer 2017, 86, 334–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danial, C.; Sarin, K.Y.; Oro, A.E.; Chang, A.L.S. An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib. Clin. Cancer Res. 2015, 22, 1325–1329. [Google Scholar] [CrossRef] [Green Version]
- Riedlinger, D.; Bahra, M.; Boas-Knoop, S.; Lippert, S.; Bradtmöller, M.; Guse, K.; Seehofer, D.; Bova, R.; Sauer, I.; Neuhaus, P.; et al. Hedgehog pathway as a potential treatment target in human cholangiocarcinoma. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 607–615. [Google Scholar] [CrossRef]
- Che, L.; Yuan, Y.H.; Jia, J.; Ren, J. Activation of sonic hedgehog signaling pathway is an independent potential prognosis predictor in human hepatocellular carcinoma patients. Chin. J. Cancer Res. 2012, 24, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Feuerstein, M.; Chleilat, E.; Khakipoor, S.; Michailidis, K.; Ophoven, C.; Roussa, E. Expression patterns of key Sonic Hedgehog signaling pathway components in the developing and adult mouse midbrain and in the MN9D cell line. Cell Tissue Res. 2017, 370, 211–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Tao, J.; Cigliano, A.; Sini, M.; Calderaro, J.; Azoulay, D.; Wang, C.; Liu, Y.; Jiang, L.; Evert, K.; et al. Co-activation of PIK3CA and Yap promotes development of hepatocellular and cholangiocellular tumors in mouse and human liver. Oncotarget 2015, 6, 10102–10115. [Google Scholar] [CrossRef] [Green Version]
- Katoh, Y.; Katoh, M. Hedgehog Target Genes: Mechanisms of Carcinogenesis Induced by Aberrant Hedgehog Signaling Activation. Curr. Mol. Med. 2009, 9, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Omenetti, A.; Choi, S.; Michelotti, G.; Diehl, A.M. Hedgehog signaling in the liver. J. Hepatol. 2011, 54, 366–373. [Google Scholar] [CrossRef]
- Omenetti, A.; Diehl, A.M. The Adventures of Sonic Hedgehog in Development and Repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am. J. Physiol. Liver Physiol. 2008, 294, G595–G598. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, C.A.; Martelotto, L.G.; Sigglekow, N.D.; Patkunanathan, B.; Ajami, K.; Calabro, S.R.; Dwyer, B.; Tirnitz-Parker, J.; Watkins, D.N.; Warner, F.J.; et al. The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury. J. Hepatol. 2014, 60, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Clapéron, A.; Mergey, M.; Aoudjehane, L.; Ho-Bouldoires, T.H.N.; Wendum, D.; Prignon, A.; Merabtene, F.; Firrincieli, D.; Desbois-Mouthon, C.; Scatton, O.; et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology 2013, 58, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Campbell, J.S. Role of desmoplasia in cholangiocarcinoma and hepatocellular carcinoma. J. Hepatol. 2014, 61, 432–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, M.V.; Diehl, A.M. Hedgehog signalling in liver pathophysiology. J. Hepatol. 2018, 68, 550–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Zhang, Z.; Zhang, P.; Yu, M.; Yang, T. Role of canonical Hedgehog signaling pathway in liver. Int. J. Biol. Sci. 2018, 14, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.-L.; Ahn, S.H.; Kim, Y.; Baek, S.; Chung, S.I.; Seong, J.; Han, K.-H.; Ro, S.W. Investigation of Oncogenic Cooperation in Simple Liver-Specific Transgenic Mouse Models Using Noninvasive In Vivo Imaging. PLoS ONE 2013, 8, e59869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.; Ju, H.-L.; Chung, S.I.; Cho, K.J.; Eun, J.W.; Nam, S.W.; Han, K.-H.; Calvisi, D.F.; Ro, S.W. Transforming Growth Factor-β Promotes Liver Tumorigenesis in Mice via Up-regulation of Snail. Gastroenterology 2017, 153, 1378–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.; Moon, H.; Seo, S.H.; Ro, S.W.; Kim, B.K. Pharmacological Inhibition of Sonic Hedgehog Signaling Suppresses Tumor Development in a Murine Model of Intrahepatic Cholangiocarcinoma. Int. J. Mol. Sci. 2021, 22, 13214. https://doi.org/10.3390/ijms222413214
Cho K, Moon H, Seo SH, Ro SW, Kim BK. Pharmacological Inhibition of Sonic Hedgehog Signaling Suppresses Tumor Development in a Murine Model of Intrahepatic Cholangiocarcinoma. International Journal of Molecular Sciences. 2021; 22(24):13214. https://doi.org/10.3390/ijms222413214
Chicago/Turabian StyleCho, Kyungjoo, Hyuk Moon, Sang Hyun Seo, Simon Weonsang Ro, and Beom Kyung Kim. 2021. "Pharmacological Inhibition of Sonic Hedgehog Signaling Suppresses Tumor Development in a Murine Model of Intrahepatic Cholangiocarcinoma" International Journal of Molecular Sciences 22, no. 24: 13214. https://doi.org/10.3390/ijms222413214
APA StyleCho, K., Moon, H., Seo, S. H., Ro, S. W., & Kim, B. K. (2021). Pharmacological Inhibition of Sonic Hedgehog Signaling Suppresses Tumor Development in a Murine Model of Intrahepatic Cholangiocarcinoma. International Journal of Molecular Sciences, 22(24), 13214. https://doi.org/10.3390/ijms222413214





