Adsorption of 1,2-Dichlorobenzene from the Aqueous Phase onto Activated Carbons and Modified Carbon Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
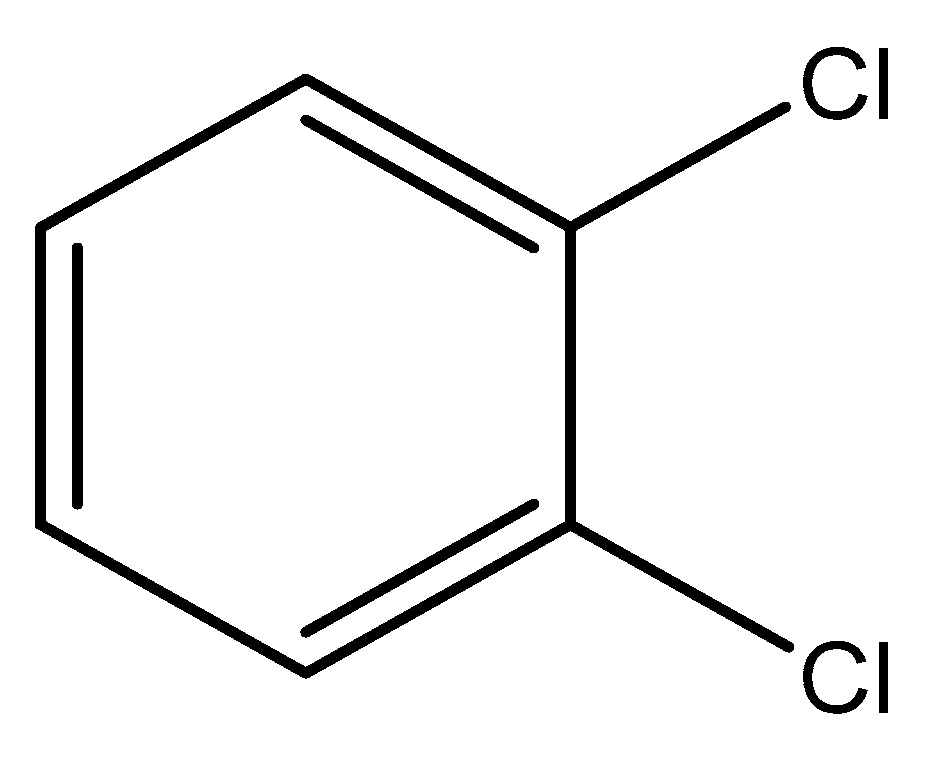
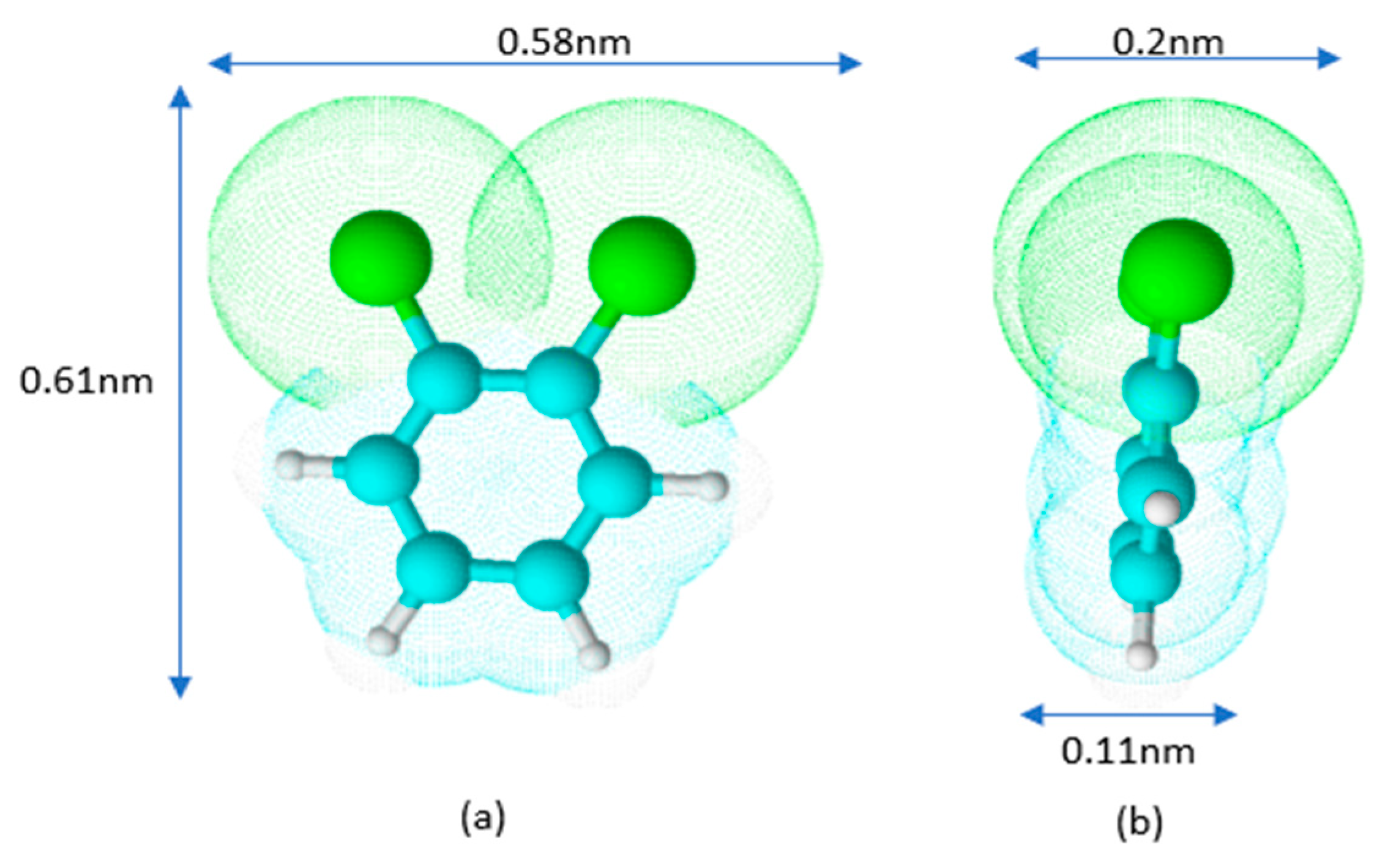
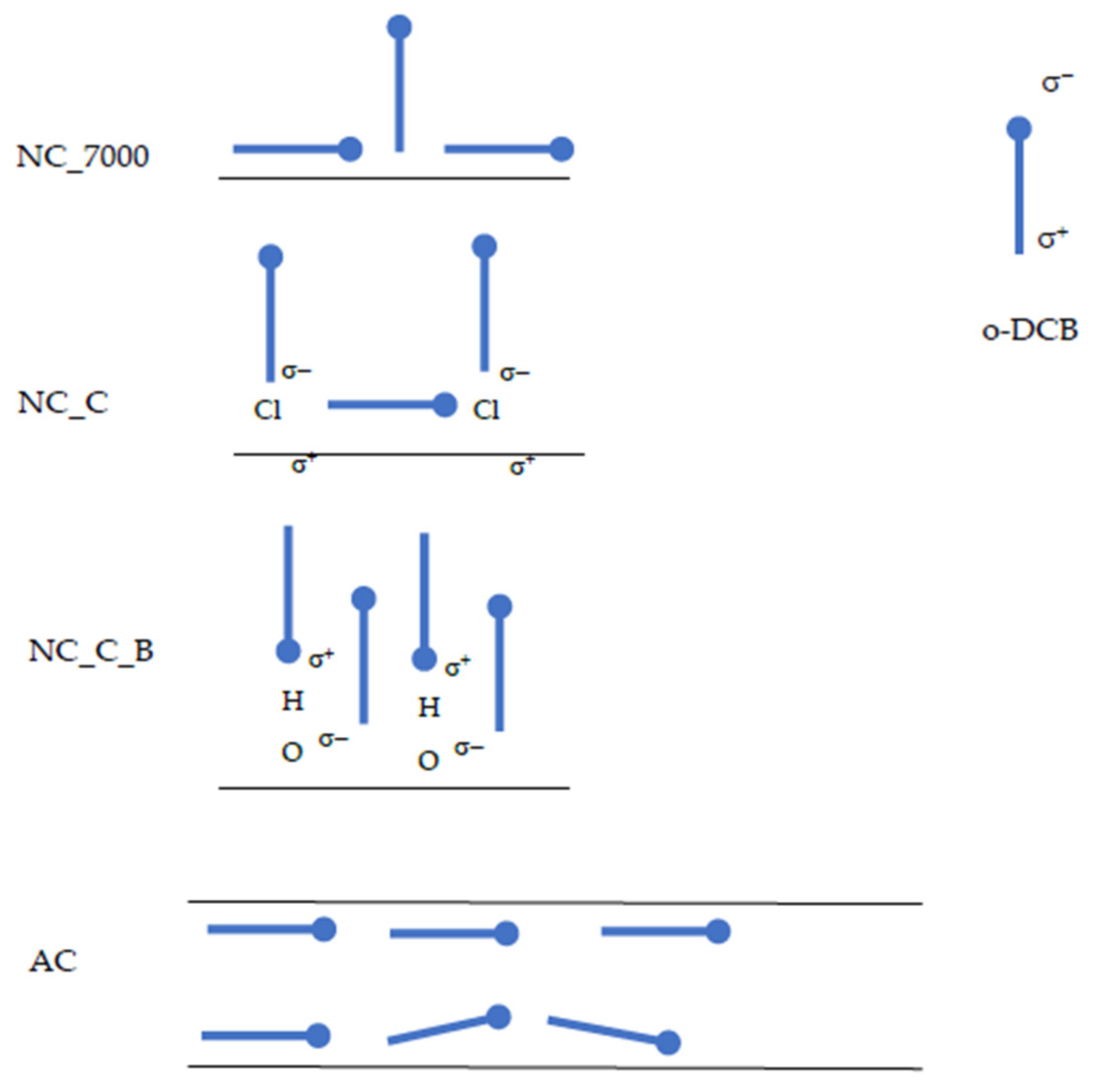
2.1. Equilibrium of o-DCB Adsorption
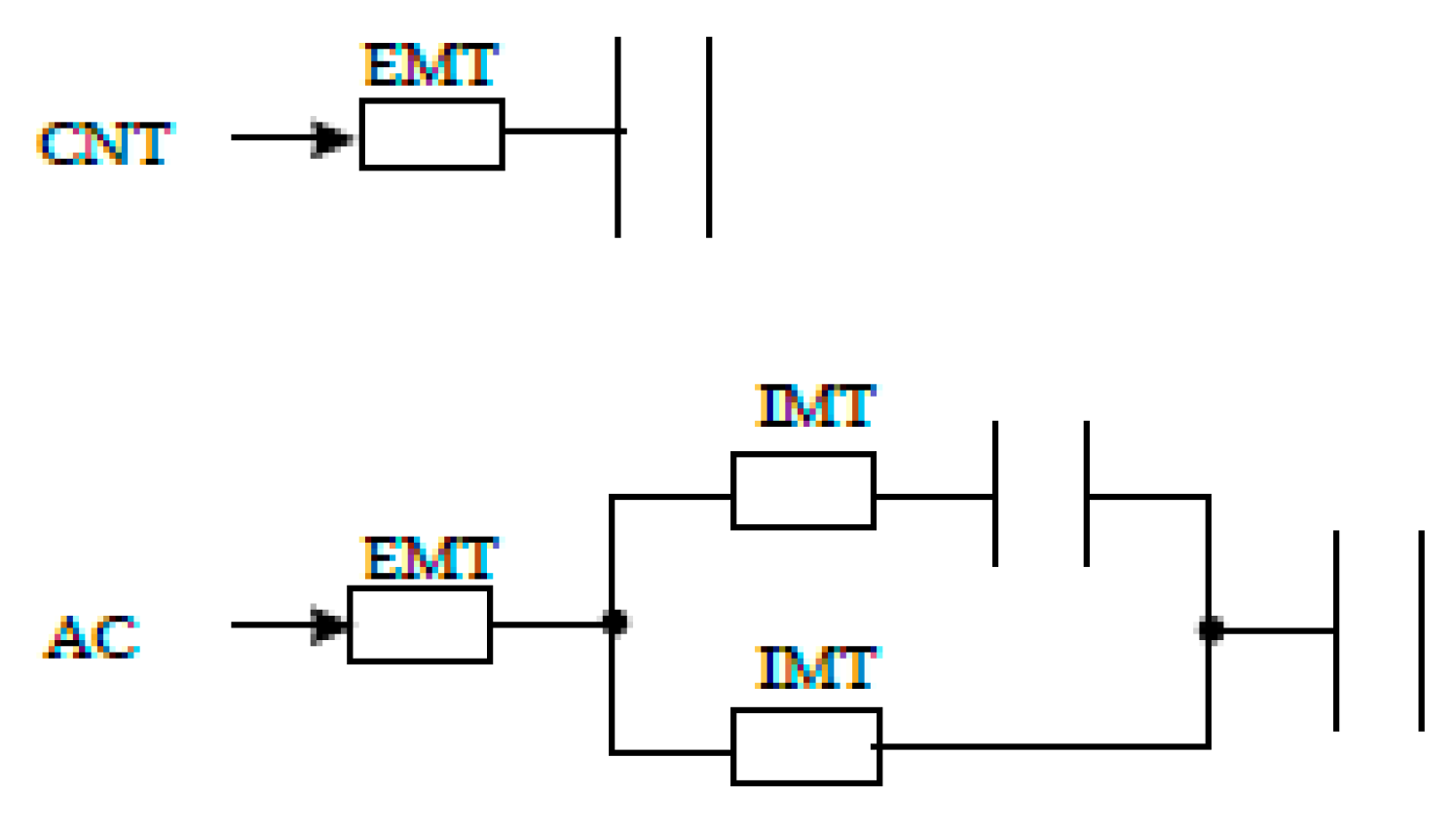
2.2. Kinetics of Adsorption of o-DCB onto ACs and CNTs
2.3. Regeneration of Adsorbents
3. Materials and Methods
3.1. Adsorbents
3.2. Adsorption Procedure
- −
- Hydrogen flow rate: 35 mL/min;
- −
- Air flow rate: 350 mL/min;
- −
- Analysis temperature: 240 °C;
- −
- Temperature increment: 15 °C/min;
- −
- Time per analysis: 25.33 min;
- −
- Partition coefficient: 15.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Zhang, Z.; Wu, H.; Li, J.; Yang, L. Adsorption of Volatile Organic Compounds at Medium-High Temperature Conditions by Activated Carbons. Energy Fuels 2020, 34, 3679–3690. [Google Scholar] [CrossRef]
- Alhooshani, K.R. Adsorption of chlorinated organic compounds from water with cerium oxide-activated carbon composite. Arab. J. Chem. 2019, 12, 2585–2596. [Google Scholar] [CrossRef] [Green Version]
- Pełech, R.; Milchert, E.; Wróbel, R. Adsorption dynamics of chlorinated hydrocarbons from multi-component aqueous solution onto activated carbon. J. Hazard. Mater. 2006, 137, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Pelech, R. Isotherms, isosteres, and enthalpy of adsorption of 1,2-dichloroethane from aqueous solution onto activated carbons. Ind. Eng. Chem. Res. 2008, 47, 5615–5622. [Google Scholar] [CrossRef]
- Sharma, Y.C. Uma Optimization of parameters for adsorption of methylene blue on a low-cost activated carbon. J. Chem. Eng. Data 2010, 55, 435–439. [Google Scholar] [CrossRef]
- Brunchi, C.C.; Sanchez, J.M.C.; Stankiewicz, A.I.; Kramer, H.J.M.; Vlugt, T.J.H. Adsorption of volatile organic compounds. Experimental and theoretical study. Ind. Eng. Chem. Res. 2012, 51, 16697–16708. [Google Scholar] [CrossRef]
- Wibowo, N.; Setyadhi, L.; Wibowo, D.; Setiawan, J.; Ismadji, S. Adsorption of benzene and toluene from aqueous solutions onto activated carbon and its acid and heat treated forms: Influence of surface chemistry on adsorption. J. Hazard. Mater. 2007, 146, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Yang, Y.; Kong, X.M.; Li, P.; Yu, J.G.; Ribeiro, A.M.; Rodrigues, A.E. Adsorption of Pure and Binary CO2, CH4, and N2 Gas Components on Activated Carbon Beads. J. Chem. Eng. Data 2015, 60, 2684–2693. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Monser, L.; Adhoum, N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep. Purif. Technol. 2002, 26, 137–146. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, S.; Zhang, X.; Wei, J.; Xu, C.; Luan, Z.; Wu, D. Adsorption of fluoride from water by aligned carbon nanotubes. Mater. Res. Bull. 2003, 38, 469–476. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Carbon Nanotubes as Superior Sorbent for Dioxin Removal. J. Am. Chem. Soc. 2001, 123, 2058–2059. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, S. Lead adsorption on carbon nanotubes. A B. Ion. Exch. Adsorpt. Solvent Extr. 2002, 357, 263–266. [Google Scholar] [CrossRef]
- Milchert, E. Technologie Produkcji Chloropochodnych Organicznych, Utylizacja Odpadów; Wydawnictwo Uczelniane Politechniki Szczecińskiej: Szczecin, Poland, 1997. [Google Scholar]
- Soćko, R.; Czerczak, S. 1,2-Dichlorobenzen. Pod. i Metod. Oceny Środowiska Pr. 2004, 3, 81–101. [Google Scholar]
- WHO. Dichlorobenzenes in Drinking-Water, Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Peng, X.; Li, Y.; Luan, Z.; Di, Z.; Wang, H.; Tian, B.; Jia, Z. Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 2003, 376, 154–158. [Google Scholar] [CrossRef]
- Pelech, R.; Bembnowska, A.; Milchert, E. Adsorption of Hydrocarbon Chloro-Derivatives onto DTO Commercial Activated Carbon from Multi-Component Aqueous Solutions. Adsorpt. Sci. Technol. 2003, 21, 707–720. [Google Scholar] [CrossRef]
- Bembnowska, A.; Pełech, R.; Milchert, E. Adsorption from aqueous solutions of chlorinated organic compounds onto activated carbons. J. Colloid Interface Sci. 2003, 265, 276–282. [Google Scholar] [CrossRef]
- Chen, W.; Kan, A.T.; Tomson, M.B. Irreversible Adsorption of Chlorinated Benzenes to Natural Sediments: Implications for Sediment Quality Criteria. Environ. Sci. Technol. 2000, 34, 385–392. [Google Scholar] [CrossRef]
- Peköz, R.; Johnston, K.; Donadio, D. Adsorption of dichlorobenzene on Au and Pt stepped surfaces using van der waals density functional theory. J. Phys. Chem. C 2012, 116, 20409–20416. [Google Scholar] [CrossRef]
- Deitsch, J.J.; Smith, J.A.; Arnold, M.B.; Bolus, J. Sorption and desorption rates of carbon tetrachloride and 1,2- dichlorobenzene to three organobentonites and a natural peat soil. Environ. Sci. Technol. 1998, 32, 3169–3177. [Google Scholar] [CrossRef]
- Deitsch, J.J.; Smith, J.A.; Culver, T.B.; Brown, R.A.; Riddle, S.A. Distributed-rate model analysis of 1,2-dichlorobenzene batch sorption and desorption rates for five natural sorbents. Environ. Sci. Technol. 2000, 34, 1469–1476. [Google Scholar] [CrossRef]
- Bullot, L.; Vieira-Sellaï, L.; Chaplais, G.; Simon-Masseron, A.; Daou, T.J.; Patarin, J.; Fiani, E. Adsorption of 1,2-dichlorobenzene and 1,2,4-trichlorobenzene in nano- and microsized crystals of MIL-101(Cr): Static and dynamic gravimetric studies. Environ. Sci. Pollut. Res. 2017, 24, 26562–26573. [Google Scholar] [CrossRef] [PubMed]
- Pełech, R. Equilibrium of trichloroethylene adsorption from aqueous solution onto activated carbons. Adsorpt. Sci. Technol. 2008, 26, 449–462. [Google Scholar] [CrossRef]
- Pełech, I.; Pełech, R.; Jedrzejewska, A.; Moszyński, D. Selective introduction of hydroxyl groups onto the surface of carbon nanotubes via chlorination and hydrolytic dechlorination. Sci. Adv. Mater. 2016, 8, 1208–1215. [Google Scholar] [CrossRef]




| Adsorbent | Coefficients of Langmuir Equation | |
|---|---|---|
| am | b | |
| DT0 | 357 | 0.61 |
| AG-5 | 417 | 0.29 |
| NC_7000 | 275 | 3.2 |
| NC_C | 320 | 2.6 |
| NC_C_B | 370 | 1.7 |
| Adsorbent | am1 [mmol/g] | am2 Amount of o-DCB per nm2 [molecules/nm2] | Sc1 for A1 = 0.35 nm2 | Sc2 for A2 = 0.06 nm2 |
|---|---|---|---|---|
| DT0 | 2.4 | 1.54 | 0.55 | 0.09 |
| AG-5 | 2.8 | 1.99 | 0.72 | 0.12 |
| NC_7000 | 1.9 | 6.16 | 2.22 | 0.37 |
| NC_C | 2.2 | 7.12 | 2.56 | 0.43 |
| NC_C_B | 2.5 | 9.13 | 3.29 | 0.55 |
| Adsorbent | Kinetic Constants k at Initial Concentration C0 [mg/L] | ||||||
|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 70 | 100 | |
| DT0 | 0.040 | 0.038 | 0.035 | 0.032 | 0.028 | 0.027 | 0.025 |
| AG-5 | 0.025 | 0.022 | 0.020 | 0.018 | 0.017 | 0.015 | 0.015 |
| NC_7000 | 0.180 | 0.180 | 0.180 | 0.180 | 0.180 | 0.180 | 0.180 |
| NC_C | 0.120 | 0.120 | 0.120 | 0.120 | 0.120 | 0.120 | 0.120 |
| NC_C_B | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 |
| Solvent | Recovery of o-DCB R [%] from | ||||
|---|---|---|---|---|---|
| DTO | AG-5 | NC_7000 | NC_C | NC_C_B | |
| acetone | 76 | 79 | |||
| methanol | 80 | 85 | 46 | 23 | 26 |
| ethanol | 78 | 75 | |||
| 1-propanol | 77 | 80 | |||
| Adsorbent | SBET [m2/g] |
|---|---|
| DT0 | 950 |
| AG-5 | 860 |
| NC_7000 | 183 |
| NC_C | 184 |
| NC_C_B | 166 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkiewicz, M.; Pełech, R. Adsorption of 1,2-Dichlorobenzene from the Aqueous Phase onto Activated Carbons and Modified Carbon Nanotubes. Int. J. Mol. Sci. 2021, 22, 13152. https://doi.org/10.3390/ijms222313152
Jurkiewicz M, Pełech R. Adsorption of 1,2-Dichlorobenzene from the Aqueous Phase onto Activated Carbons and Modified Carbon Nanotubes. International Journal of Molecular Sciences. 2021; 22(23):13152. https://doi.org/10.3390/ijms222313152
Chicago/Turabian StyleJurkiewicz, Martyna, and Robert Pełech. 2021. "Adsorption of 1,2-Dichlorobenzene from the Aqueous Phase onto Activated Carbons and Modified Carbon Nanotubes" International Journal of Molecular Sciences 22, no. 23: 13152. https://doi.org/10.3390/ijms222313152
APA StyleJurkiewicz, M., & Pełech, R. (2021). Adsorption of 1,2-Dichlorobenzene from the Aqueous Phase onto Activated Carbons and Modified Carbon Nanotubes. International Journal of Molecular Sciences, 22(23), 13152. https://doi.org/10.3390/ijms222313152






