AS1411 Nucleolin-Specific Binding Aptamers Reduce Pathological Angiogenesis through Inhibition of Nucleolin Phosphorylation
Abstract
:1. Introduction
2. Results
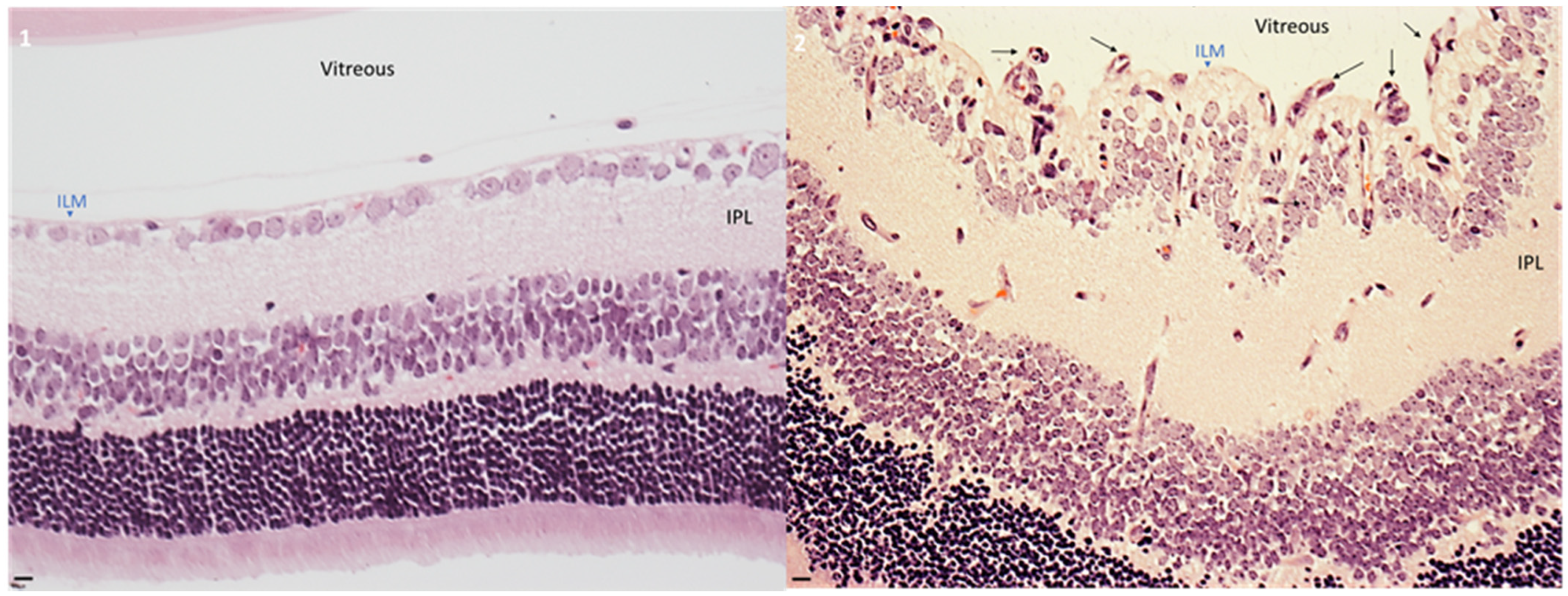
2.1. Hyperoxia Developed Pathological Angiogenesis in Retinas from Neonatal Mice
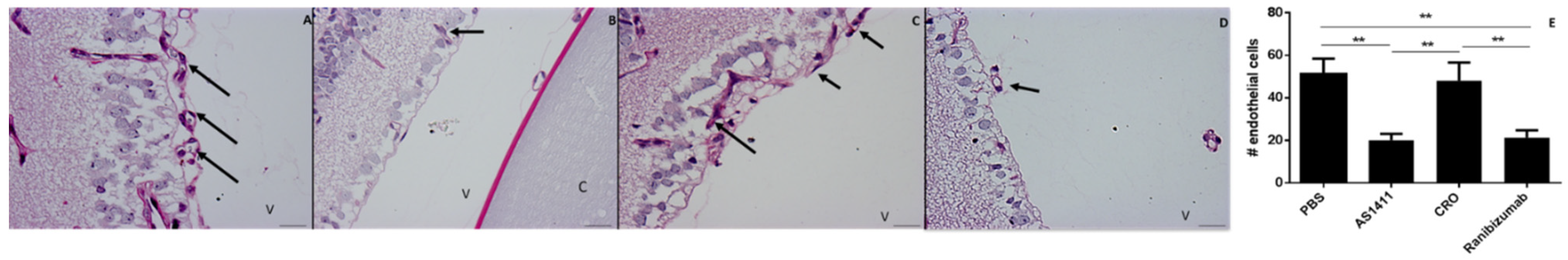
2.2. Intravitreal Injection of AS1411 Aptamers Reduced Pathological Angiogenesis in the Oxygen Induced Retinopathy (OIR) Mouse Model
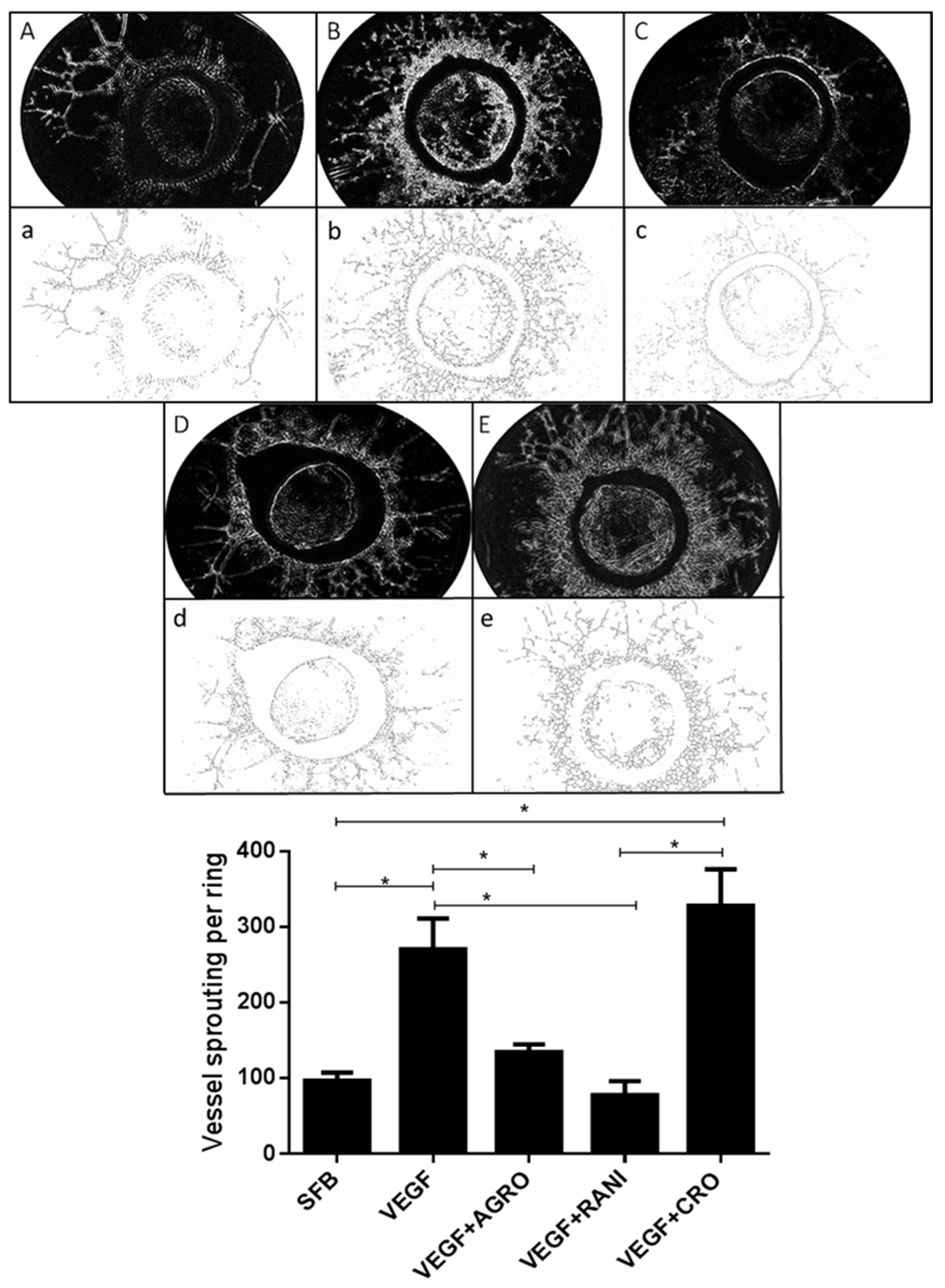
2.3. AS1411 Reduces Angiogenesis in Rat Aortic Ring Assay
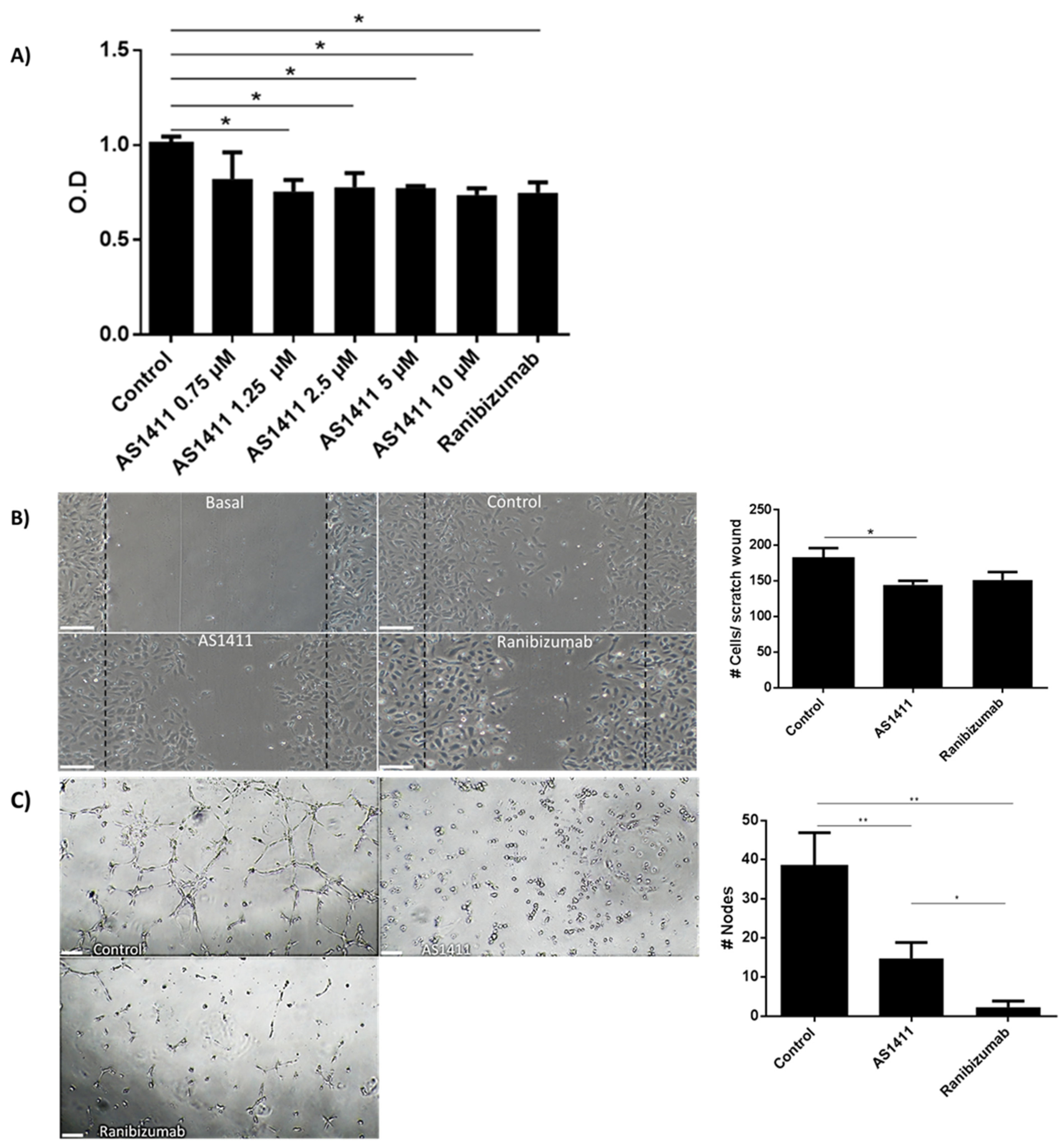
2.4. AS1411 Reduced Cell Proliferation, Migration and Tube Formation on Human Umbilical Endothelial Cells (HUVEC)
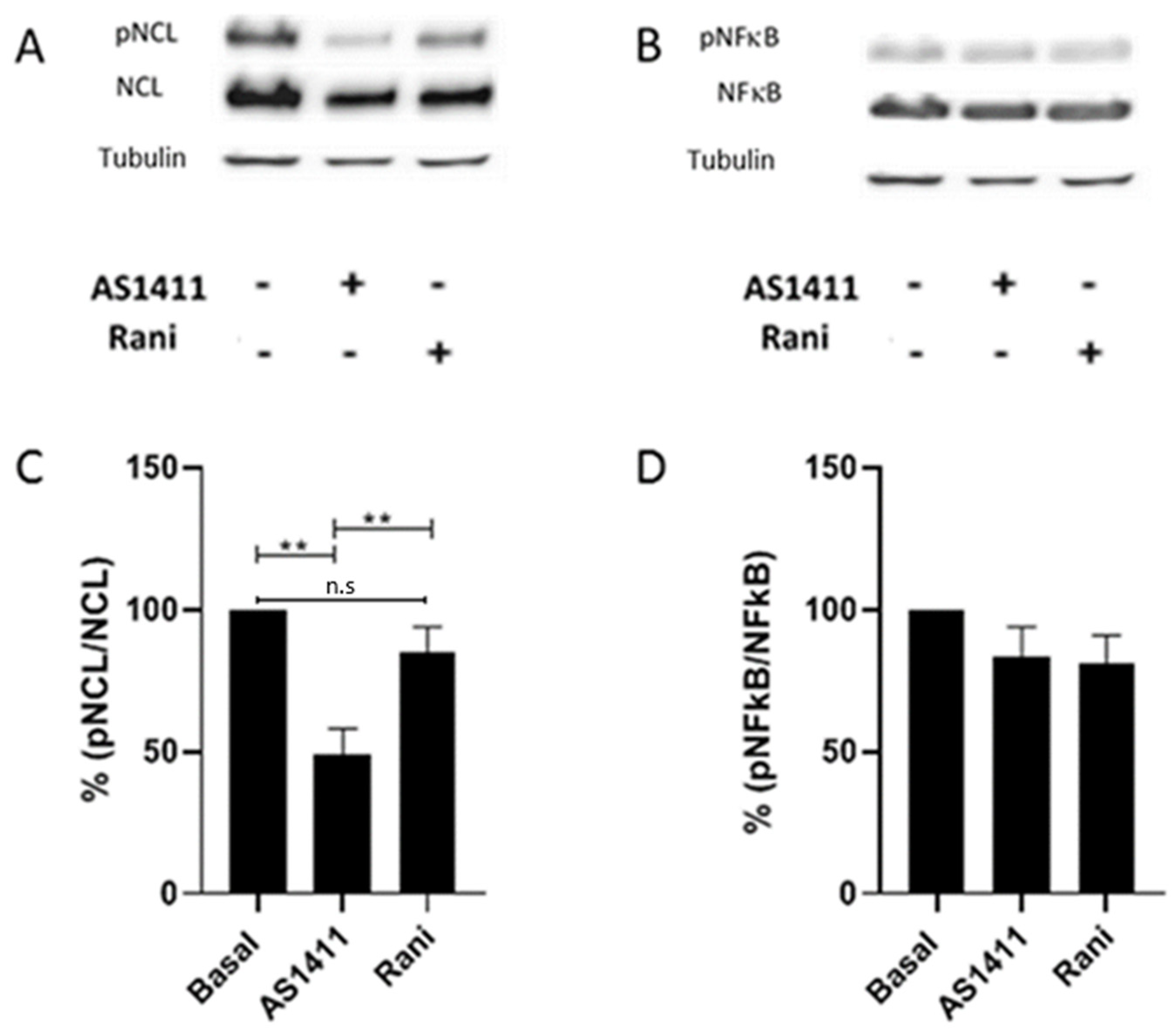
2.5. AS1411 Inhibited Nucleolin Phosphorylation in HUVEC
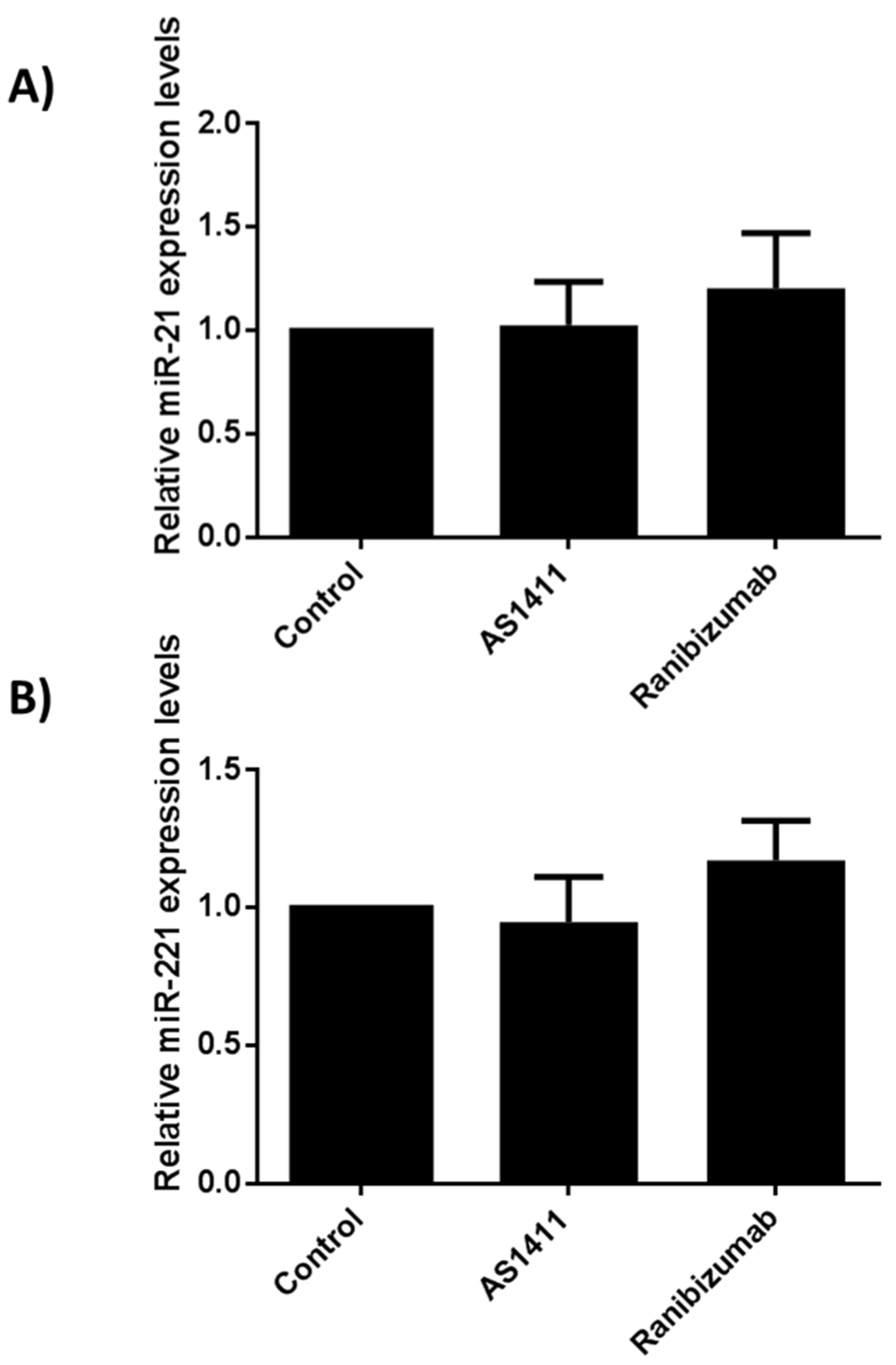
2.6. AS14111 Does Not Affect miR-21 and -221 Expression in HUVEC
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animal Model
4.3. Intravitreal Injection of Nucleolin-Binding Aptamer and Controls
4.4. Quantification of Neovascular Proliferative Retinopathy
4.5. Immunofluorescence
4.6. Rat Aortic Ring Assay
4.7. Aptamer Effects on Human Umbilical Vein Endothelial Cells (HUVEC)
4.8. Nucleolin Immunoblotting
4.9. MiRNA Arrays
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dreyfuss, J.L.; Giordano, R.J.; Regatieri, C.V. Ocular Angiogenesis. J. Ophthalmol. 2015, 2015, 892043. [Google Scholar] [CrossRef]
- Sapieha, P.; Hamel, D.; Shao, Z.; Rivera, J.C.; Zaniolo, K.; Joyal, J.S.; Chemtob, S. Proliferative retinopathies: Angiogenesis that blinds. Int. J. Biochem. Cell Biol. 2010, 42, 5–12. [Google Scholar] [CrossRef]
- Harhaj, N.S.; Felinski, E.A.; Wolpert, E.B.; Sundstrom, J.M.; Gardner, T.W.; Antonetti, D.A. VEGF Activation of Protein Kinase C Stimulates Occludin Phosphorylation and Contributes to Endothelial Permeability. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5106–5115. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; McGuire, P.G.; Franco Nitta, C.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine mediated monocyte trafficking into the retina: Role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS ONE 2014, 9, e108508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, J.; Fortmann, S.D.; Wang, J.; Vestweber, D.; Campochiaro, P.A. Reversible retinal vessel closure from VEGF-induced leukocyte plugging. JCI Insight 2017, 2, e95530. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee for the Diabetic Retinopathy Clinical Research Network; Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA 2015, 314, 2137–2146. [Google Scholar] [CrossRef] [Green Version]
- Wilgus, T.A.; Matthies, A.M.; Radek, K.A.; Dovi, J.V.; Burns, A.L.; Shankar, R.; DiPietro, L.A. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am. J. Pathol. 2005, 167, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Knöfler, M.; Pollheimer, J. Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling. Front. Genet. 2013, 4, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graubert, M.D.; Ortega, M.A.; Kessel, B.; Mortola, J.F.; Iruela-Arispe, M.L. Vascular repair after menstruation involves regulation of vascular endothelial growth factor-receptor phosphorylation by sFLT-1. Am. J. Pathol. 2001, 158, 1399–1410. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Zhao, W.; Chen, Y.; Ahokas, R.A.; Sun, Y. Vascular endothelial growth factor (VEGF)-A: Role on cardiac angiogenesis following myocardial infarction. Microvasc. Res. 2010, 80, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kilic, E.; Kilic, Ü.; Weber, B.; Bassetti, C.L.; Marti, H.H.; Hermann, D.M. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain 2004, 128, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, X.; Elliott, M.H.; Zhu, M.; Le, Y.-Z. Müller Cell-Derived VEGF Is Essential for Diabetes-Induced Retinal Inflammation and Vascular Leakage. Diabetes 2010, 59, 2297. [Google Scholar] [CrossRef] [Green Version]
- Saint-Geniez, M.; Kurihara, T.; Sekiyama, E.; Maldonado, A.E.; Amore, P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA 2009, 106, 18751. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.; Powner, M.B.; Gandhi, P.; Clarkin, C.; Gutmann, D.H.; Johnson, R.S.; Ferrara, N.; Fruttiger, M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS ONE 2010, 5, e11863. [Google Scholar] [CrossRef] [Green Version]
- Alon, T.; Hemo, I.; Itin, A.; Pe’er, J.; Stone, J.; Keshet, E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1995, 1, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Munk, M.R.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. Retinal Ganglion Cell Layer Change in Patients Treated With Anti–Vascular Endothelial Growth Factor for Neovascular Age-related Macular Degeneration. Am. J. Ophthalmol. 2016, 167, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Julien, S.; Biesemeier, A.; Taubitz, T.; Schraermeyer, U. Different effects of intravitreally injected ranibizumab and aflibercept on retinal and choroidal tissues of monkey eyes. Br. J. Ophthalmol. 2014, 98, 813. [Google Scholar] [CrossRef] [PubMed]
- Mccannel, C.A. Meta-Analysis of Endophthalmitis after Intravitreal Injection of Anti–Vascular Endothelial Growth Factor Agents: Causative Organisms and Possible Prevention Strategies. RETINA 2011, 31, 654–661. [Google Scholar] [CrossRef]
- Hoang, Q.V.; Mendonca, L.S.; Della Torre, K.E.; Jung, J.J.; Tsuang, A.J.; Freund, K.B. Effect on Intraocular Pressure in Patients Receiving Unilateral Intravitreal Anti-Vascular Endothelial Growth Factor Injections. Ophthalmology 2012, 119, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.H.; Michels, S.; Rodrigues, E.B.; Hager, A.; Mennel, S.; Schmidt, J.C.; Helb, H.-M.; Farah, M.E. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 2011, 89, 70–75. [Google Scholar] [CrossRef]
- Karagiannis, D.A.; Mitropoulos, P.; Ladas, I.D. Large Subretinal Haemorrhage following Change from Intravitreal Bevacizumab to Ranibizumab. Ophthalmologica 2009, 223, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, A.; Gandhi, P.; Amoaku, W.; Kelkar, J.; Kelkar, S.; Raut, P.; Shah, R. Hemorrhagic macular infarction after intravitreal bevacizumab for chronic multifocal central serous chorioretinopathy. Case Rep. Ophthalmol. 2014, 5, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Bynoe, L.A.; Welch, J.C.; Pesavento, R.; Mahendradas, P.; Ziemssen, F.; Pai, S.A. Retinal vascular events after intravitreal bevacizumab. Acta Ophthalmol. 2010, 88, 730–735. [Google Scholar] [CrossRef]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.A.; Rabena, M.D.; Le, K.; et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 2014, 98, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Hanhart, J.; Tiosano, L.; Averbukh, E.; Banin, E.; Hemo, I.; Chowers, I. Fellow eye effect of unilateral intravitreal bevacizumab injection in eyes with diabetic macular edema. Eye 2014, 28, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Peyman, M.; Peyman, A.; Lansingh, V.C.; Orandi, A.; Subrayan, V. Intravitreal bevacizumab versus ranibizumab: Effects on the vessels of the fellow non-treated eye. J. Curr. Ophthalmol. 2018, 31, 55–60. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, C.F.; Mongan, A.-M.; Chetty, S.; McAteer, D.M.J.; Quinn, S.M. Aflibercept in Diabetic Macular Oedema Previously Refractory to Standard Intravitreal Therapy: An Irish Retrospective Study. Ophthalmol. Ther. 2018, 7, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Eghøj, M.S.; Sørensen, T.L. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br. J. Ophthalmol. 2012, 96, 21. [Google Scholar] [CrossRef]
- Shah, K.; Gandhi, A.; Natarajan, S. Diabetic Retinopathy Awareness and Associations with Multiple Comorbidities: Insights from DIAMOND Study. Indian J. Endocrinol. Metab. 2018, 22, 30–35. [Google Scholar] [CrossRef]
- Schmid, M.K.; Bachmann, L.M.; Fäs, L.; Kessels, A.G.; Job, O.M.; Thiel, M.A. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age-related macular degeneration: A trade-off analysis. Br. J. Ophthalmol. 2015, 99, 141. [Google Scholar] [CrossRef]
- Tschulakow, A.; Christner, S.; Julien, S.; Ludinsky, M.; van der Giet, M.; Schraermeyer, U. Effects of a single intravitreal injection of aflibercept and ranibizumab on glomeruli of monkeys. PLoS ONE 2014, 9, e113701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.; Rundqvist, H.; Branco, C.; Johnson, R.S. Autocrine VEGF Isoforms Differentially Regulate Endothelial Cell Behavior. Front. Cell Dev. Biol. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, I.M.; Kennedy, S.A.; Paliashvili, K.; Santra, T.; Yamaji, M.; Lovering, R.C.; Britton, G.; Frankel, P.; Kolch, W.; Zachary, I.C. Vascular Endothelial Growth Factor (VEGF) Promotes Assembly of the p130Cas Interactome to Drive Endothelial Chemotactic Signaling and Angiogenesis. Mol. Cell Proteom. 2017, 16, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Hombrebueno, J.R.; Ali, I.H.A.; Xu, H.; Chen, M. Sustained intraocular VEGF neutralization results in retinal neurodegeneration in the Ins2(Akita) diabetic mouse. Sci. Rep. 2015, 5, 18316. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, H.; Zhou, H.; Song, X.; Yuan, S.; Luo, Y.J.B. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood 2006, 107, 3564–3571. [Google Scholar] [CrossRef]
- Wu, D.M.; Zhang, P.; Liu, R.Y.; Sang, Y.X.; Zhou, C.; Xu, G.C.; Yang, J.L.; Tong, A.P.; Wang, C.T. Phosphorylation and changes in the distribution of nucleolin promote tumor metastasis via the PI3K/Akt pathway in colorectal carcinoma. FEBS Lett. 2014, 588, 1921–1929. [Google Scholar] [CrossRef] [Green Version]
- Gilles, M.E.; Maione, F.; Cossutta, M.; Carpentier, G.; Caruana, L.; Di Maria, S.; Houppe, C.; Destouches, D.; Shchors, K.; Prochasson, C.; et al. Nucleolin Targeting Impairs the Progression of Pancreatic Cancer and Promotes the Normalization of Tumor Vasculature. Cancer Res. 2016, 76, 7181–7193. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Zhou, F.; Zhang, Q.; Sun, X.; Shi, X.; Liang, Y.; Wang, X.; Yue, L. Overexpression of nucleolin and different expression sites both related to the prognosis of gastric cancer. APMIS 2013, 121, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Galzio, R.; Rosati, F.; Benedetti, E.; Cristiano, L.; Aldi, S.; Mei, S.; D’Angelo, B.; Gentile, R.; Laurenti, G.; Cifone, M.G.; et al. Glycosilated nucleolin as marker for human gliomas. J. Cell. Biochem. 2012, 113, 571–579. [Google Scholar] [CrossRef]
- Huang, F.; Wu, Y.; Tan, H.; Guo, T.; Zhang, K.; Li, D.; Tong, Z. Phosphorylation of nucleolin is indispensable to its involvement in the proliferation and migration of non-small cell lung cancer cells. Oncol. Rep. 2019, 41, 590–598. [Google Scholar] [CrossRef]
- Dhez, A.C.; Benedetti, E.; Antonosante, A.; Panella, G.; Ranieri, B.; Florio, T.M.; Cristiano, L.; Angelucci, F.; Giansanti, F.; Di Leandro, L.; et al. Targeted therapy of human glioblastoma via delivery of a toxin through a peptide directed to cell surface nucleolin. J. Cell. Physiol. 2018, 233, 4091–4105. [Google Scholar] [CrossRef]
- Ferrara, B.; Belbekhouche, S.; Habert, D.; Houppe, C.; Vallee, B.; Bourgoin-Voillard, S.; Cohen, J.L.; Cascone, I.; Courty, J. Cell surface nucleolin as active bait for nanomedicine in cancer therapy: A promising option. Nanotechnology 2021, 32, 322001. [Google Scholar] [CrossRef]
- Quiroz-Mercado, J.; Ramírez-Velázquez, N.; Partido, G.; Zenteno, E.; Chávez, R.; Agundis-Mata, C.; Jiménez-Martínez, M.C.; Garfias, Y. Tissue and cellular characterisation of nucleolin in a murine model of corneal angiogenesis. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1753–1763. [Google Scholar] [CrossRef]
- Vivanco-Rojas, O.; García-Bermúdez, M.Y.; Iturriaga-Goyon, E.; Rebollo, W.; Buentello-Volante, B.; Magaña-Guerrero, F.S.; Bates, P.; Pérez-Torres, A.; Garfias, Y. Corneal neovascularization is inhibited with nucleolin-binding aptamer, AS1411. Exp. Eye Res. 2020, 193, 107977. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga-Goyon, E.; Buentello-Volante, B.; Magana-Guerrero, F.S.; Garfias, Y. Future Perspectives of Therapeutic, Diagnostic and Prognostic Aptamers in Eye Pathological Angiogenesis. Cells 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Calzi, S.L.; Shaw, L.C.; Moldovan, L.; Shelley, W.C.; Qi, X.; Racette, L.; Quigley, J.L.; Fortmann, S.D.; Boulton, M.E.; Yoder, M.C.J.J.i. Progenitor cell combination normalizes retinal vascular development in the oxygen-induced retinopathy (OIR) model. JCI Insight 2019, 4, e129224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.-H.; MacIntyre, A.; Nicosia, R.F. Regulation of Angiogenesis by Vascular Endothelial Growth Factor and Angiopoietin-1 in the Rat Aorta Model: Distinct Temporal Patterns of Intracellular Signaling Correlate with Induction of Angiogenic Sprouting. Am. J. Pathol. 2002, 161, 823–830. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Eng, V.A.; Rayess, N.; Nguyen, H.V.; Leng, T. Complete RPE and outer retinal atrophy in patients receiving anti-VEGF treatment for neovascular age-related macular degeneration. PLoS ONE 2020, 15, e0232353. [Google Scholar] [CrossRef]
- Sato, T.; Ooto, S.; Suzuki, M.; Spaide, R.F. Retinal pigment epithelial tear after intravitreal aflibercept for neovascular age-related macular degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.L.; Zhu, G.; Xiao, X.; Puszyk, W.; Sefah, K.; Wu, Q.; Tan, W.; Liu, C. A Synthetic Aptamer-Drug Adduct for Targeted Liver Cancer Therapy. PLoS ONE 2015, 10, e0136673. [Google Scholar] [CrossRef] [Green Version]
- Leaderer, D.; Cashman, S.M.; Kumar-Singh, R. Topical application of a G-Quartet aptamer targeting nucleolin attenuates choroidal neovascularization in a model of age-related macular degeneration. Exp. Eye Res. 2015, 140, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Vähätupa, M.; Nättinen, J.; Jylhä, A.; Aapola, U.; Kataja, M.; Kööbi, P.; Järvinen, T.A.H.; Uusitalo, H.; Uusitalo-Järvinen, H. SWATH-MS Proteomic Analysis of Oxygen-Induced Retinopathy Reveals Novel Potential Therapeutic Targets. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3294–3306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, A.R.; Yarovinsky, T.O.; Young, B.D.; Moraes, F.; Ross, T.D.; Ceneri, N.; Zhang, J.; Zhuang, Z.W.; Sinusas, A.J.; Pardi, R.; et al. Chemokine-coupled β2 integrin-induced macrophage Rac2-Myosin IIA interaction regulates VEGF-A mRNA stability and arteriogenesis. J. Exp. Med. 2014, 211, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Darche, M.; Cossutta, M.; Caruana, L.; Houppe, C.; Gilles, M.-E.; Habert, D.; Guilloneau, X.; Vignaud, L.; Paques, M.; Courty, J.; et al. Antagonist of nucleolin, N6L, inhibits neovascularization in mouse models of retinopathies. FASEB J. 2020, 34, 5851–5862. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xiong, S.Q.; Shang, L.; Tian, X.F.; Yang, J.; Xia, X.B. Expression of netrin-1 receptors in retina of oxygen-induced retinopathy in mice. BMC Ophthalmol. 2014, 14, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, S.; Pilch, J.; Akerman, M.E.; Porkka, K.; Laakkonen, P.; Ruoslahti, E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 2003, 163, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.; Fruttiger, M. Oxygen-induced retinopathy: A model for vascular pathology in the retina. Eye 2010, 24, 416–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.-H.; Iurlaro, M.; MacIntyre, A.; Fogel, E.; Nicosia, R.F. The Mouse Aorta Model: Influence of Genetic Background and Aging on bFGF- and VEGF-Induced Angiogenic Sprouting. Angiogenesis 2003, 6, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.P.; Wang, X.; Xie, X.L.; Zhang, G.P.; Lv, F.J.; Weng, W.T.; Qiu, F.; Li, Z.F.; Lin, J.S.; Diao, Y. Cell surface expression of nucleolin mediates the antiangiogenic and antitumor activities of kallistatin. Oncotarget 2018, 9, 2220–2235. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Liu, H.; Bi, Y.; Cheng, C.; Li, G.; Kong, P.; Zhang, L.; Shi, R.; Zhang, Y.; Zhang, R.; et al. MYH9 promotes cell metastasis via inducing Angiogenesis and Epithelial Mesenchymal Transition in Esophageal Squamous Cell Carcinoma. Int. J. Med. Sci. 2020, 17, 2013–2023. [Google Scholar] [CrossRef]
- Birmpas, C.; Briand, J.P.; Courty, J.; Katsoris, P. Nucleolin mediates the antiangiogenesis effect of the pseudopeptide N6L. BMC Cell Biol. 2012, 13, 32. [Google Scholar] [CrossRef] [Green Version]
- Birmpas, C.; Briand, J.P.; Courty, J.; Katsoris, P. The pseudopeptide HB-19 binds to cell surface nucleolin and inhibits angiogenesis. Vasc. Cell 2012, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsioumpa, M.; Polytarchou, C.; Courty, J.; Zhang, Y.; Kieffer, N.; Mikelis, C.; Skandalis, S.S.; Hellman, U.; Iliopoulos, D.; Papadimitriou, E. Interplay between αvβ3 integrin and nucleolin regulates human endothelial and glioma cell migration. J. Biol. Chem. 2013, 288, 343–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girvan, A.C.; Teng, Y.; Casson, L.K.; Thomas, S.D.; Jüliger, S.; Ball, M.W.; Klein, J.B.; Pierce, W.M., Jr.; Barve, S.S.; Bates, P.J. AGRO100 inhibits activation of nuclear factor-kappaB (NF-kappaB) by forming a complex with NF-kappaB essential modulator (NEMO) and nucleolin. Mol. Cancer Ther. 2006, 5, 1790–1799. [Google Scholar] [CrossRef] [Green Version]
- Sabatel, C.; Malvaux, L.; Bovy, N.; Deroanne, C.; Lambert, V.; Gonzalez, M.-L.A.; Colige, A.; Rakic, J.-M.; Noël, A.; Martial, J.A.; et al. MicroRNA-21 Exhibits Antiangiogenic Function by Targeting RhoB Expression in Endothelial Cells. PLoS ONE 2011, 6, e16979. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Tuccoli, A.; Mariani, L.; Evangelista, M.; Citti, L.; Woods, K.; Mercatanti, A.; Hammond, S.; Rainaldi, G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef]
- Hu, J.; Ni, S.; Cao, Y.; Zhang, T.; Wu, T.; Yin, X.; Lang, Y.; Lu, H.J.P.O. The angiogenic effect of microRNA-21 targeting TIMP3 through the regulation of MMP2 and MMP9. PLoS ONE 2016, 11, e0149537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Z.; Tang, Y.; Jiang, B.; Wu, Y.; Liu, Y.; Li, Y.; Xiao, X. Phosphorylation of nucleolin is indispensable to upregulate miR-21 and inhibit apoptosis in cardiomyocytes. J. Cell. Physiol. 2019, 234, 4044–4053. [Google Scholar] [CrossRef]
- Montassar, F.; Darche, M.; Blaizot, A.; Augustin, S.; Conart, J.-B.; Millet, A.; Elayeb, M.; Sahel, J.-A.; Goazigo, A.R.-L.; Sennlaub, F.; et al. Lebecetin, a C-type lectin, inhibits choroidal and retinal neovascularization. FASEB J. 2017, 31, 1107–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, C.; Ehlken, C.; Stahl, A.; Martin, G.; Hansen, L.; Agostini, H.T. Kinetics of retinal vaso-obliteration and neovascularisation in the oxygen-induced retinopathy (OIR) mouse model. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Rymo, S.F.; Gerhardt, H.; Wolfhagen Sand, F.; Lang, R.; Uv, A.; Betsholtz, C. A Two-Way Communication between Microglial Cells and Angiogenic Sprouts Regulates Angiogenesis in Aortic Ring Cultures. PLoS ONE 2011, 6, e15846. [Google Scholar] [CrossRef]
- Aravind, A.; Jeyamohan, P.; Nair, R.; Veeranarayanan, S.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. AS1411 aptamer tagged PLGA-lecithin-PEG nanoparticles for tumor cell targeting and drug delivery. Biotechnol. Bioeng. 2012, 109, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Yazdian-Robati, R.; Abnous, K. A novel AS1411 aptamer-based three-way junction pocket DNA nanostructure loaded with doxorubicin for targeting cancer cells in vitro and in vivo. Mol. Pharm. 2018, 15, 1972–1978. [Google Scholar] [CrossRef]
- Futami, K.; Kimoto, M.; Lim, Y.W.S.; Hirao, I. Genetic Alphabet Expansion Provides Versatile Specificities and Activities of Unnatural-Base DNA Aptamers Targeting Cancer Cells. Mol. Ther.-Nucleic. Acids 2019, 14, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Reyes, E.M.; Šalipur, F.R.; Shams, M.; Forsthoefel, M.K.; Bates, P.J. Mechanistic studies of anticancer aptamer AS1411 reveal a novel role for nucleolin in regulating Rac1 activation. Mol. Oncol. 2015, 9, 1392–1405. [Google Scholar] [CrossRef] [Green Version]
- Liang, P.; Jiang, B.; Lv, C.; Huang, X.; Sun, L.; Zhang, P.; Huang, X. The expression and proangiogenic effect of nucleolin during the recovery of heat-denatured HUVECs. Biochim. Biophys. Acta 2013, 1830, 4500–4512. [Google Scholar] [CrossRef]
- Ali, M.H.; Elsherbiny, M.E.; Emara, M. Updates on Aptamer Research. Int. J. Mol. Sci. 2019, 20, 2511. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iturriaga-Goyon, E.; Vivanco-Rojas, O.; Magaña-Guerrero, F.S.; Buentello-Volante, B.; Castro-Salas, I.; Aguayo-Flores, J.E.; Gracia-Mora, I.; Rivera-Huerta, M.; Sánchez-Bartés, F.; Garfias, Y. AS1411 Nucleolin-Specific Binding Aptamers Reduce Pathological Angiogenesis through Inhibition of Nucleolin Phosphorylation. Int. J. Mol. Sci. 2021, 22, 13150. https://doi.org/10.3390/ijms222313150
Iturriaga-Goyon E, Vivanco-Rojas O, Magaña-Guerrero FS, Buentello-Volante B, Castro-Salas I, Aguayo-Flores JE, Gracia-Mora I, Rivera-Huerta M, Sánchez-Bartés F, Garfias Y. AS1411 Nucleolin-Specific Binding Aptamers Reduce Pathological Angiogenesis through Inhibition of Nucleolin Phosphorylation. International Journal of Molecular Sciences. 2021; 22(23):13150. https://doi.org/10.3390/ijms222313150
Chicago/Turabian StyleIturriaga-Goyon, Emilio, Oscar Vivanco-Rojas, Fátima Sofía Magaña-Guerrero, Beatriz Buentello-Volante, Ilse Castro-Salas, José Eduardo Aguayo-Flores, Isabel Gracia-Mora, Marisol Rivera-Huerta, Francisco Sánchez-Bartés, and Yonathan Garfias. 2021. "AS1411 Nucleolin-Specific Binding Aptamers Reduce Pathological Angiogenesis through Inhibition of Nucleolin Phosphorylation" International Journal of Molecular Sciences 22, no. 23: 13150. https://doi.org/10.3390/ijms222313150
APA StyleIturriaga-Goyon, E., Vivanco-Rojas, O., Magaña-Guerrero, F. S., Buentello-Volante, B., Castro-Salas, I., Aguayo-Flores, J. E., Gracia-Mora, I., Rivera-Huerta, M., Sánchez-Bartés, F., & Garfias, Y. (2021). AS1411 Nucleolin-Specific Binding Aptamers Reduce Pathological Angiogenesis through Inhibition of Nucleolin Phosphorylation. International Journal of Molecular Sciences, 22(23), 13150. https://doi.org/10.3390/ijms222313150





