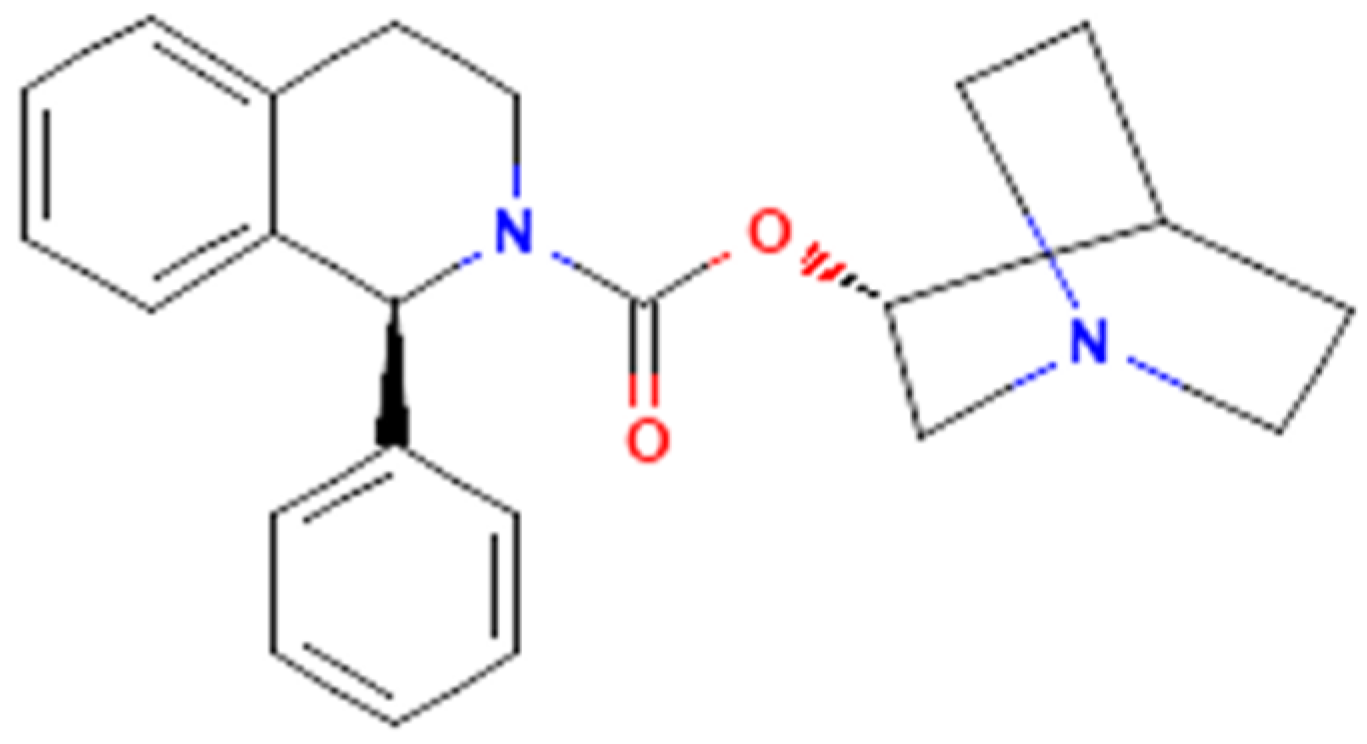
The Effectiveness in Activating M-Type K+ Current Produced by Solifenacin ([(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action
Abstract
1. Introduction
2. Results
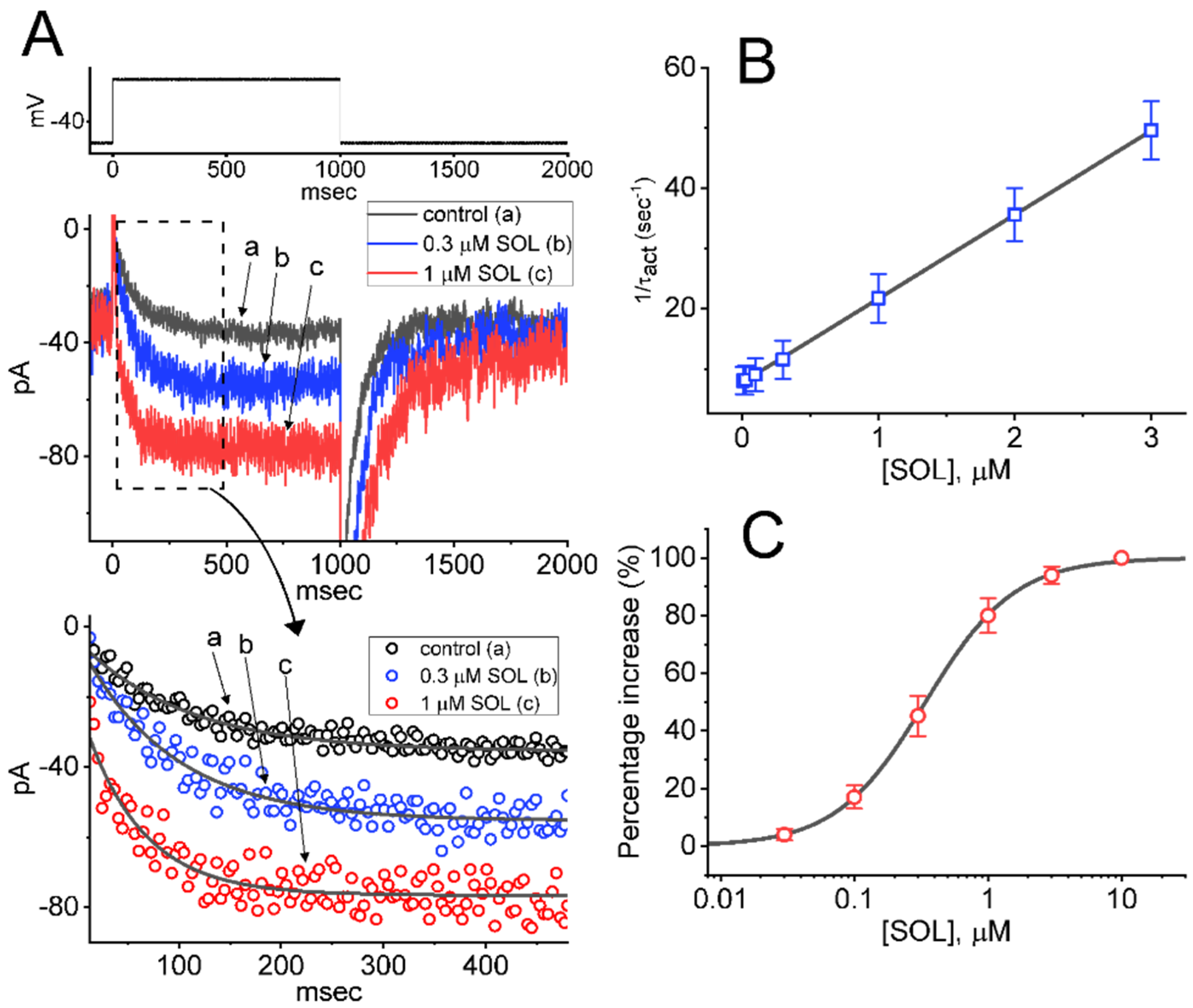
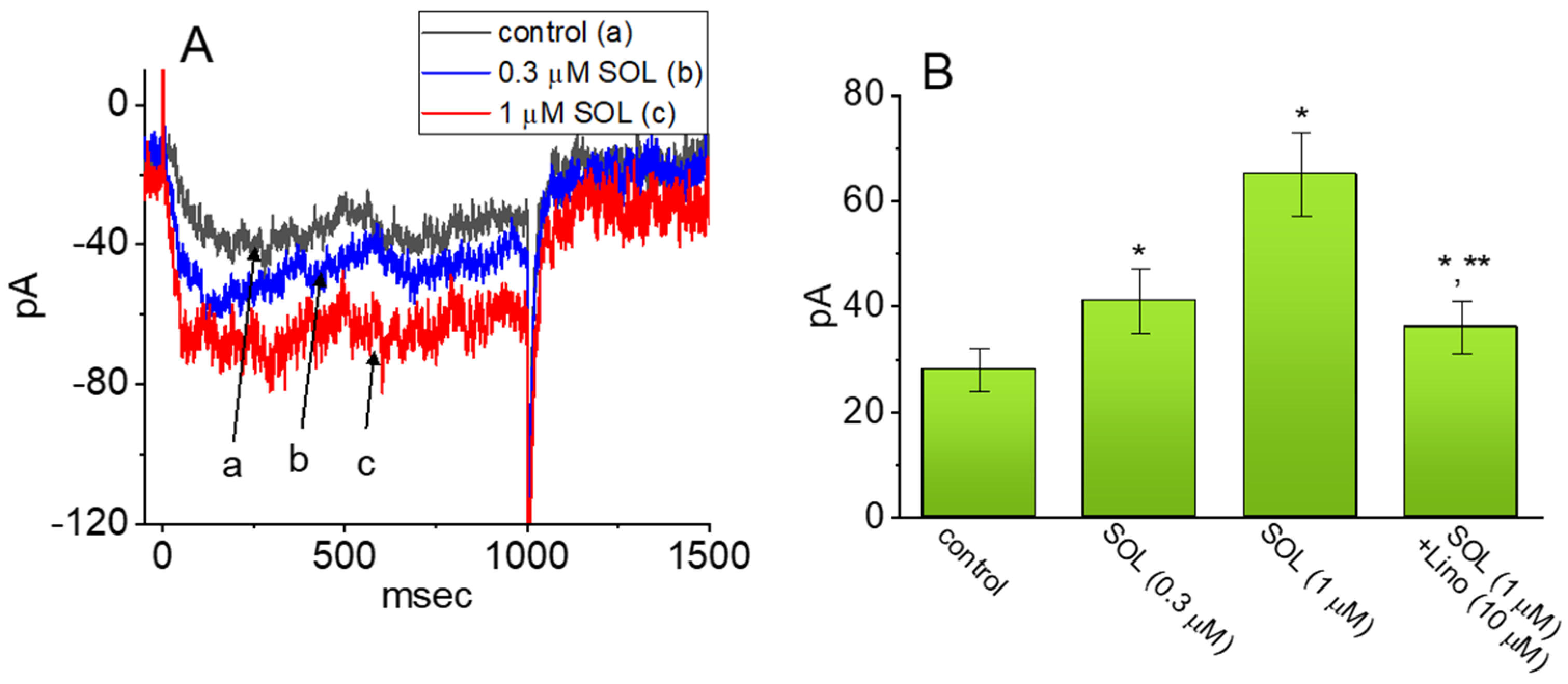
2.1. Effect of SOL on the M-Type K+ Current (IK(M)) Measured from GH3 Cells
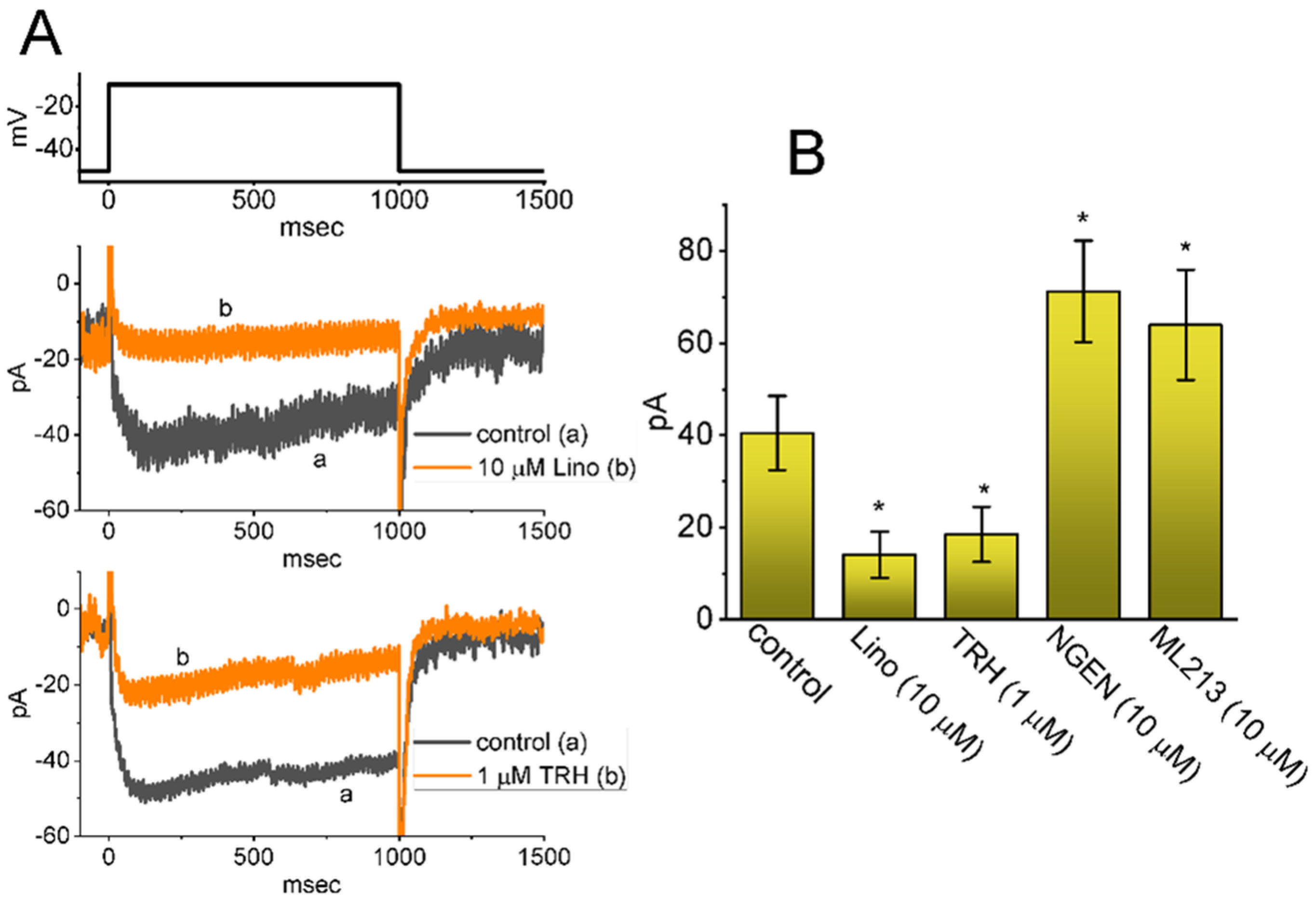
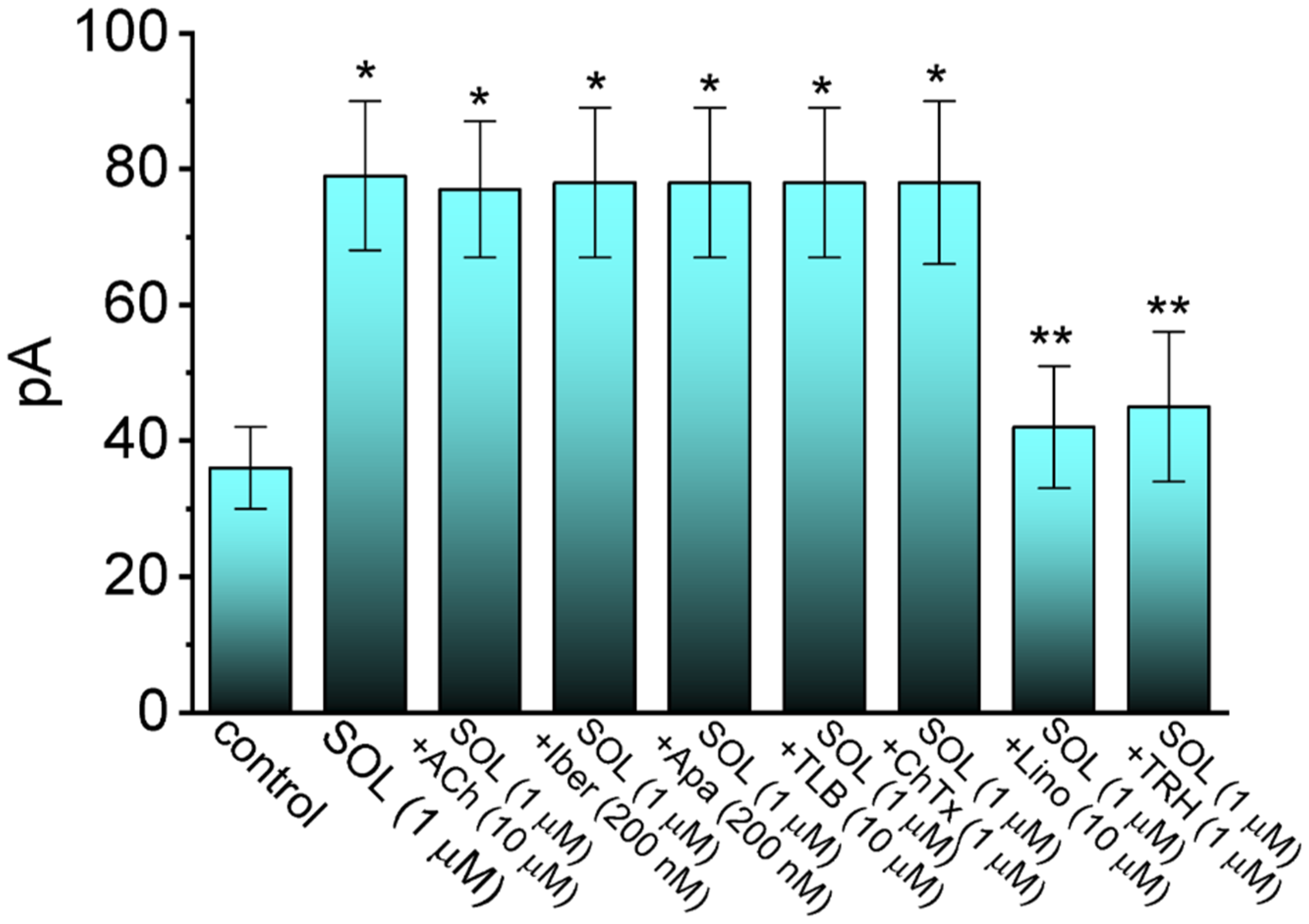
2.2. Comparison in IK(M) Amplitudes Caused by the Presence of SOL, SOL plus Acetylcholine (ACh), SOL plus Iberiotoxin (Iber), SOL plus Apamin (Apa), SOL plus Tolbutamide (TLB), SOL plus Chlorotoxin (ChTx), SOL plus Linopirdine (Lino), or SOL plus Thyrotropin Releasing Hormone (TRH)
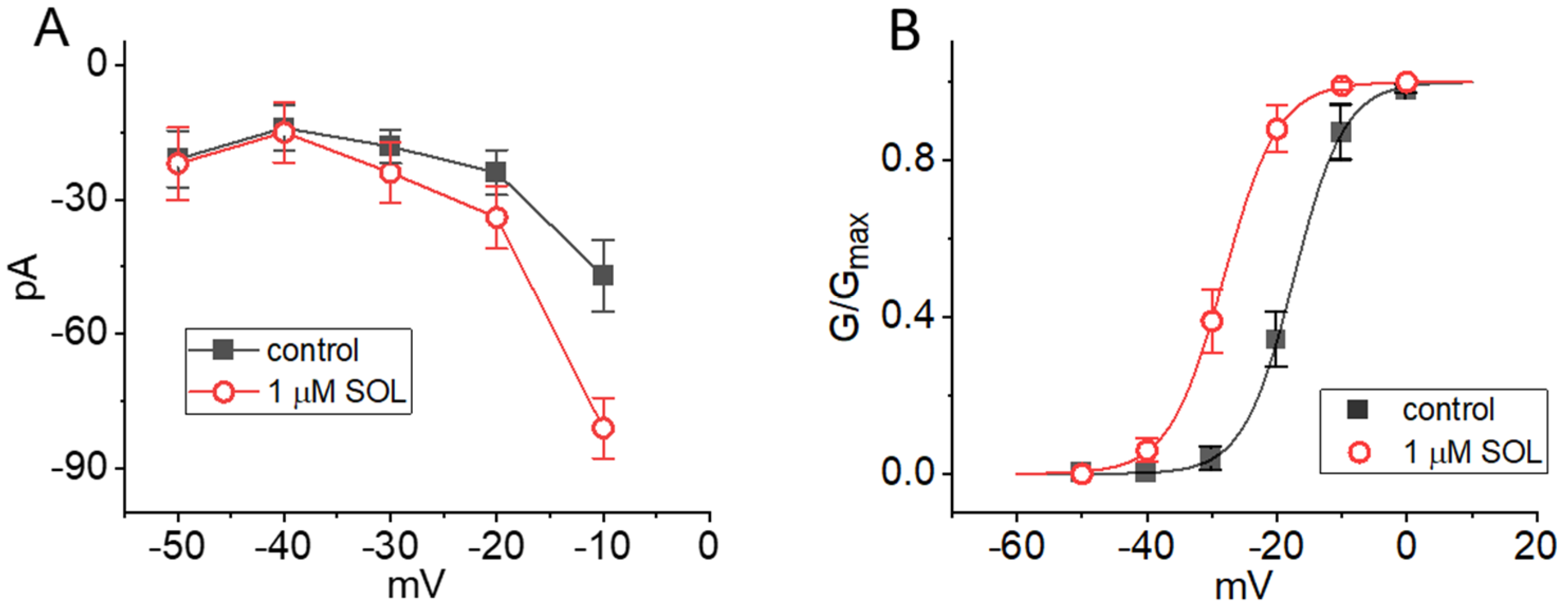
2.3. Current-Voltage (I-V) Relationship and Steady-State Activation Curve of IK(M) in the Absence and Presence of SOL
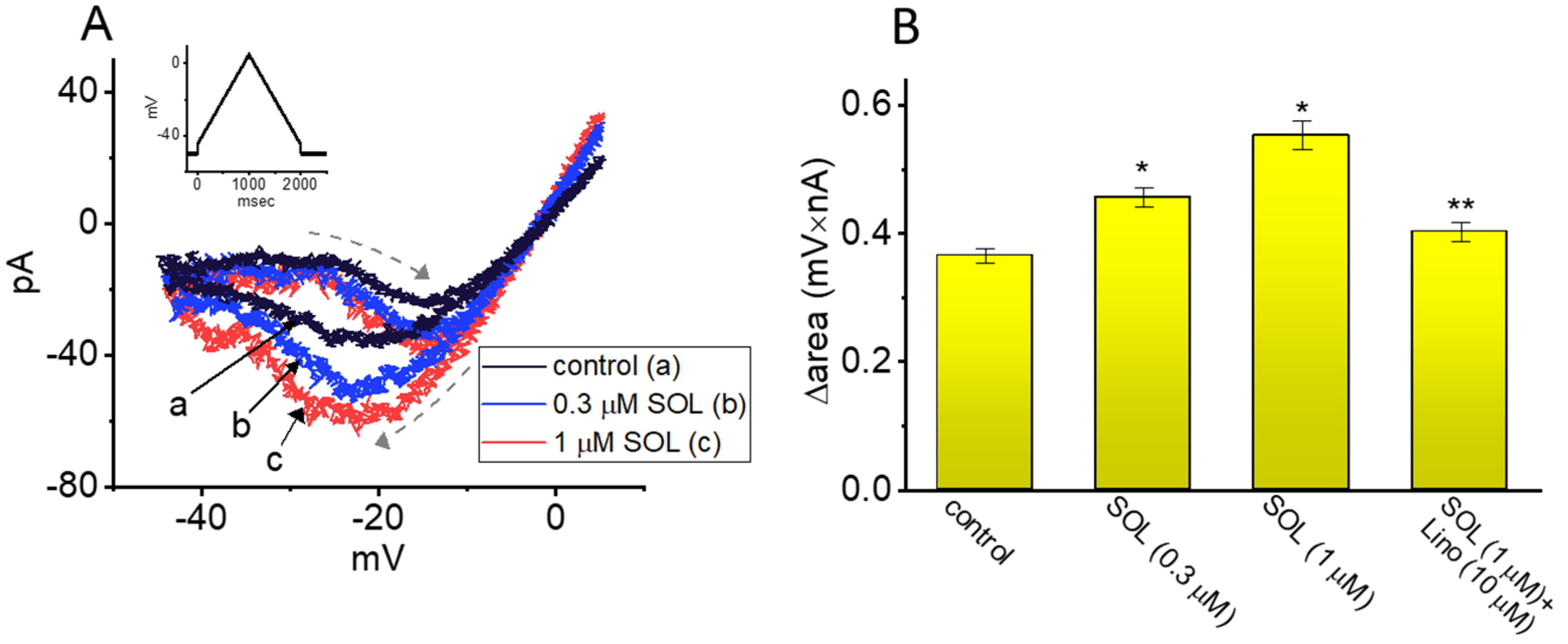
2.4. Effect of SOL on Voltage-Dependent Hysteresis (Vhys) of IK(M) Activated by Long Isosceles-Triangular Ramp Pulse
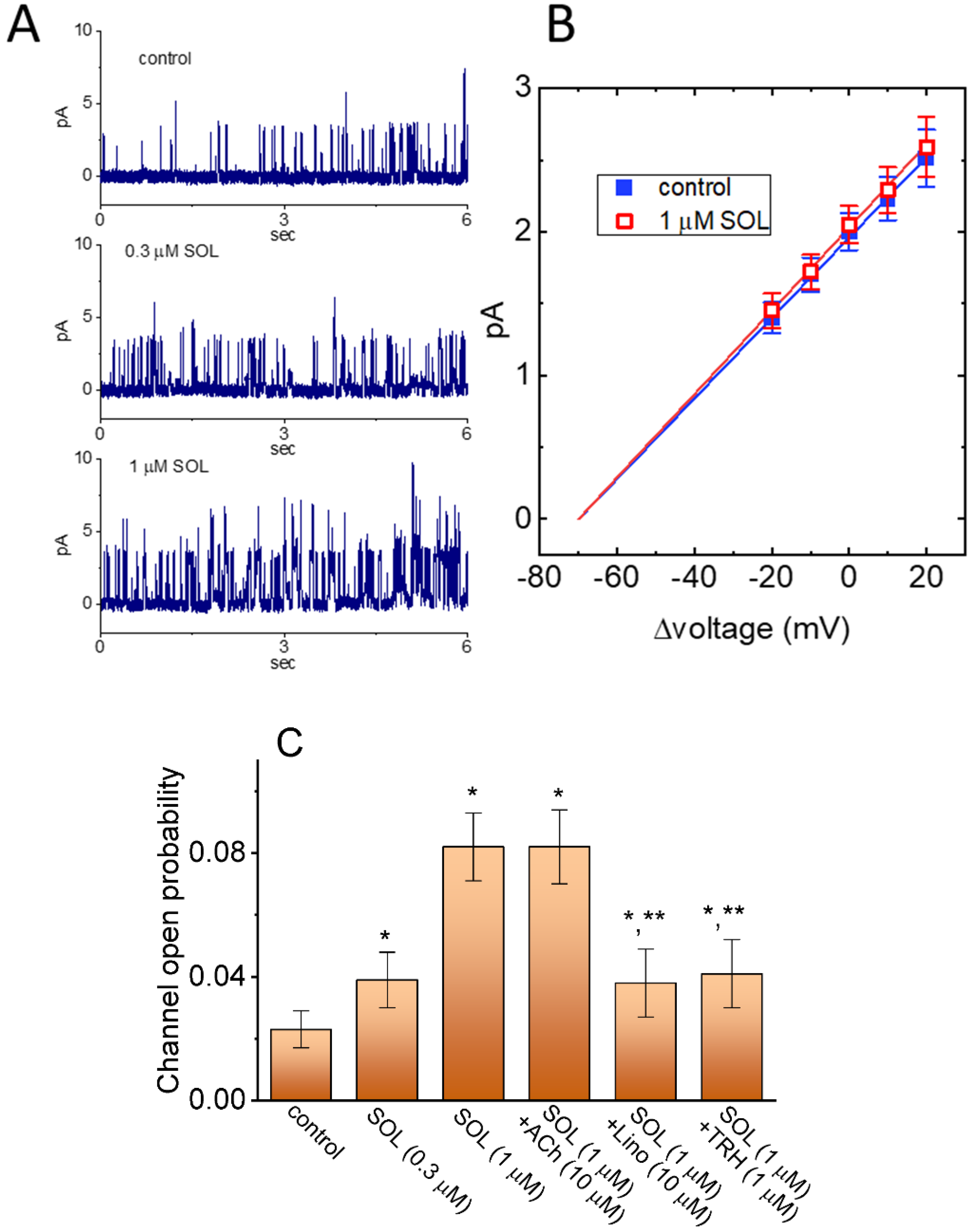
2.5. Stimulatory Effect of SOL on the Activity of M-Type K+ (KM) Channels in GH3 Cells
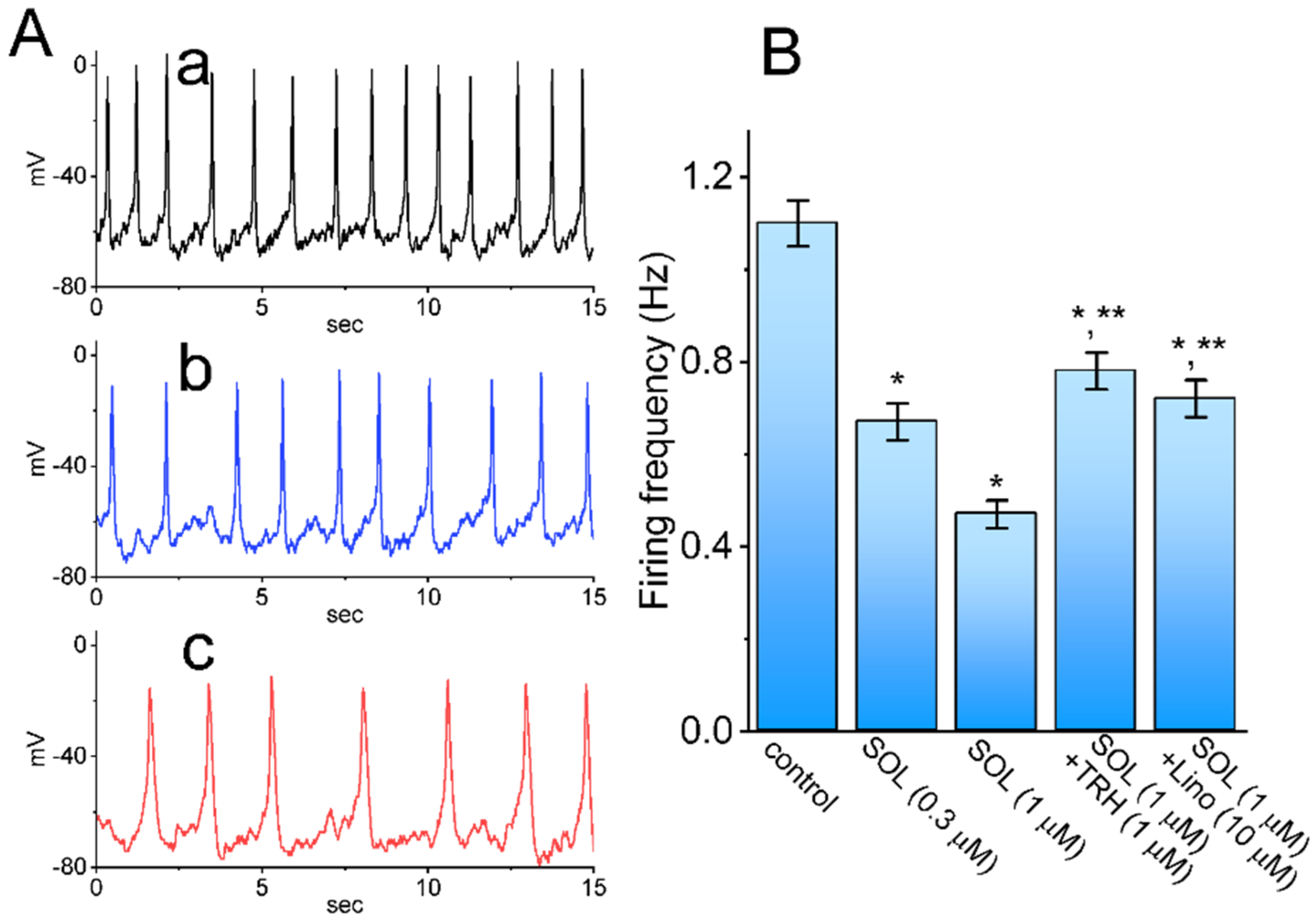
2.6. Effect of SOL on Spontaneous Action Potentials (APs) Recorded from GH3 Cells
2.7. Stimulatory Effect of SOL on IK(M) Present in mHippoE-14 Neurons
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs and Solutions Used in This Work
4.2. Cell Preparations
4.3. Electrophysiological Measurements
4.4. Data Recordings
4.5. Whole-Cell Current Analyses
4.6. Analyses of Single M-Type K+ (KM) Channels
4.7. Curve-Fitting Procedures and Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| ANOVA | analysis of variance |
| AP | action potential |
| Apa | apamin |
| ChTx | chlorotoxin |
| EC50 | the concentration required for 50% stimulation |
| I-V | current versus voltage |
| Iber | iberiotoxin |
| IK(M) | M-type K+ current |
| KD | dissociation constant |
| KM channel | M-type K+ channel |
| Lino | linopirdine |
| SEM | standard error of mean |
| SOL | solifenacin (Vesicare®) |
| TLB | tolbutamide |
| TRH | thyrotropin releasing hormone |
| τact | activation time constant |
| TTX | tetrodotoxin |
| Vhys | voltage-dependent hysteresis |
References
- Chilman-Blair, K.; Bosch, J.L.H.R. Solifenacin: Treatment of overactive bladder. Drugs Today 2004, 40, 343. [Google Scholar] [CrossRef]
- Simpson, D.; Wagstaff, A.J. Solifenacin in Overactive Bladder Syndrome. Drugs Aging 2005, 22, 1061–1069. [Google Scholar] [CrossRef]
- Kreder, K.J. Solifenacin. Urol. Clin. N. Am. 2006, 33, 483–490. [Google Scholar] [CrossRef]
- Payne, C.K. Solifenacin in Overactive Bladder Syndrome. Drugs 2006, 66, 175–190. [Google Scholar] [CrossRef]
- Kennelly, M.J.; DeVoe, W.B. Overactive Bladder: Pharmacologic Treatments in the Neurogenic Population. Rev. Urol. 2008, 10, 182–191. [Google Scholar]
- Pelman, R.S.; Capo, J.P., Jr.; Forero-Schwanhaeuser, S. Solifenacin at 3 years: A review of efficacy and safety. Postgrad. Med. 2008, 120, 85–91. [Google Scholar] [CrossRef]
- Doroshyenko, O.; Fuhr, U. Clinical Pharmacokinetics and Pharmacodynamics of Solifenacin. Clin. Pharmacokinet. 2009, 48, 281–302. [Google Scholar] [CrossRef]
- Morales-Olivas, F.J.; Estañ, L. Solifenacin pharmacology. Arch. Esp. Urol. 2010, 63, 43–52. [Google Scholar] [CrossRef][Green Version]
- Abrams, P.; Andersson, K.-E. Muscarinic receptor antagonists for overactive bladder. BJU Int. 2007, 100, 987–1006. [Google Scholar] [CrossRef]
- Luo, D.; Liu, L.; Han, P.; Wei, Q.; Shen, H. Solifenacin for overactive bladder: A systematic review and meta-analysis. Int. Urogynecol. J. 2012, 23, 983–991. [Google Scholar] [CrossRef]
- Abrams, P.; Kelleher, C.; Staskin, D.; Rechberger, T.; Kay, R.; Martina, R.; Newgreen, D.; Paireddy, A.; van Maanen, R.; Ridder, A. Combination Treatment with Mirabegron and Solifenacin in Patients with Overactive Bladder: Efficacy and Safety Results from a Randomised, Double-blind, Dose-ranging, Phase 2 Study (Symphony). Eur. Urol. 2015, 67, 577–588. [Google Scholar] [CrossRef]
- Drake, M.J.; Chapple, C.; Esen, A.A.; Athanasiou, S.; Cambronero, J.; Mitcheson, D.; Herschorn, S.; Saleem, T.; Huang, M.; Siddiqui, E.; et al. Efficacy and Safety of Mirabegron Add-on Therapy to Solifenacin in Incontinent Overactive Bladder Patients with an Inadequate Response to Initial 4-Week Solifenacin Monotherapy: A Randomised Double-blind Multicentre Phase 3B Study (BESIDE). Eur. Urol. 2016, 70, 136–145. [Google Scholar] [CrossRef]
- Herschorn, S.; Chapple, C.R.; Abrams, P.; Arlandis, S.; Mitcheson, D.; Lee, K.-S.; Ridder, A.; Stoelzel, M.; Paireddy, A.; Van Maanen, R.; et al. Efficacy and safety of combinations of mirabegron and solifenacin compared with monotherapy and placebo in patients with overactive bladder (SYNERGY study). BJU Int. 2017, 120, 562–575. [Google Scholar] [CrossRef]
- Gratzke, C.; van Maanen, R.; Chapple, C.; Abrams, P.; Herschorn, S.; Robinson, D.; Ridder, A.; Stoelzel, M.; Paireddy, A.; Yoon, S.J.; et al. Long-term Safety and Efficacy of Mirabegron and Solifenacin in Combination Compared with Monotherapy in Patients with Overactive Bladder: A Randomised, Multicentre Phase 3 Study (SYNERGY II). Eur. Urol. 2018, 74, 501–509. [Google Scholar] [CrossRef]
- Yamada, S.; Ito, Y.; Nishijima, S.; Kadekawa, K.; Sugaya, K. Basic and clinical aspects of antimuscarinic agents used to treat overactive bladder. Pharmacol. Ther. 2018, 189, 130–148. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Cui, Y.; Li, Y.; Yuan, H.; Gao, Z.; Zhu, Z.; Wu, J. Meta-analysis of the efficacy and safety of mirabegron and solifenacin monotherapy for overactive bladder. Neurourol. Urodyn. 2019, 38, 22–30. [Google Scholar] [CrossRef]
- Aschenbrenner, D.S. New Indication for Solifenacin. AJN Am. J. Nurs. 2020, 120, 24–25. [Google Scholar] [CrossRef]
- Mirzaei, M.; Daneshpajooh, A.; Anvari, S.O.; Dozchizadeh, S.; Teimorian, M. Evaluation of the Clinical Efficacy and Complications of Duloxetine in Comparison to Solifenacin in the Treatment of Overactive Bladder Disease in Women: A Randomized Clinical Trial. Urol. J. 2021, 6274. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Zu, S.; Zhang, C. Transcutaneous electrical nerve stimulation and solifenacin succinate versus solifenacin succinate alone for treatment of overactive bladder syndrome: A double-blind randomized controlled study. PLoS ONE 2021, 16, e0253040. [Google Scholar] [CrossRef]
- Pagoria, D.; O’Connor, R.C.; Guralnick, M.L. Antimuscarinic Drugs: Review of the Cognitive Impact When Used to Treat Overactive Bladder in Elderly Patients. Curr. Urol. Rep. 2011, 12, 351–357. [Google Scholar] [CrossRef]
- Park, J.-W. The Effect of Solifenacin on Cognitive Function following Stroke. Dement. Geriatr. Cogn. Disord. Extra 2013, 3, 143–147. [Google Scholar] [CrossRef]
- Kachru, N.; Carnahan, R.; Johnson, M.L.; Aparasu, R.R. Potentially inappropriate anticholinergic medication use in older adults with dementia. J. Am. Pharm. Assoc. 2015, 55, 603–612. [Google Scholar] [CrossRef]
- Kachru, N.; Holmes, H.M.; Johnson, M.L.; Chen, H.; Aparasu, R.R. Risk of Mortality Associated with Non-selective Antimuscarinic medications in Older Adults with Dementia: A Retrospective Study. J. Gen. Intern. Med. 2020, 35, 2084–2093. [Google Scholar] [CrossRef]
- Kachru, N.; Holmes, H.M.; Johnson, M.L.; Chen, H.; Aparasu, R.R. Antimuscarinic use among older adults with dementia and overactive bladder: A Medicare beneficiaries study. Curr. Med. Res. Opin. 2021, 37, 1303–1313. [Google Scholar] [CrossRef]
- Kachru, N.; Holmes, H.M.; Johnson, M.L.; Chen, H.; Aparasu, R.R. Comparative risk of adverse outcomes associated with nonselective and selective antimuscarinic medications in older adults with dementia and overactive bladder. Int. J. Geriatr. Psychiatry 2021, 36, 684–696. [Google Scholar] [CrossRef]
- Orme, S.; Morris, V.; Gibson, W.; Wagg, A. Managing Urinary Incontinence in Patients with Dementia: Pharmacological Treatment Options and Considerations. Drugs Aging 2015, 32, 559–567. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Liu, H.-H.; Lin, T.-H.; Chuang, H.-Y.; Hsieh, T. Association between different anticholinergic drugs and subsequent dementia risk in patients with diabetes mellitus. PLoS ONE 2017, 12, e0175335. [Google Scholar] [CrossRef]
- Kosilov, K.; Kuzina, I.; Kuznetsov, V.; Gaynullina, Y.; Kosilova, L.; Prokofyeva, A.; Loparev, S. Cognitive functions and health-related quality of life in men with benign prostatic hyperplasia and symptoms of overactive bladder when treated with a combination of tamsulosin and solifenacin in a higher dosage. Aging Male 2017, 21, 121–129. [Google Scholar] [CrossRef]
- Barthold, D.; Marcum, Z.A.; Gray, S.L.; Zissimopoulos, J. Alzheimer’s disease and related dementias risk: Comparing users of non-selective and M3-selective bladder antimuscarinic drugs. Pharmacoepidemiol. Drug Saf. 2020, 29, 1650–1658. [Google Scholar] [CrossRef]
- Welk, B.; McArthur, E. Increased risk of dementia among patients with overactive bladder treated with an anticholinergic medication compared to a beta-3 agonist: A population-based cohort study. BJU Int. 2020, 126, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Harnod, T.; Yang, Y.-C.; Chiu, L.-T.; Wang, J.-H.; Lin, S.-Z.; Ding, D.-C. Use of bladder antimuscarinics is associated with an increased risk of dementia: A retrospective population-based case–control study. Sci. Rep. 2021, 11, 4827. [Google Scholar] [CrossRef]
- Soliman, M.G.; El-Abd, S.; El-Gamal, O.M.; Raheem, A.A.; Abou-Ramadan, A.R.; El-Abd, A.S. Mirabegron versus Solifenacin in Children with Overactive Bladder: Prospective Randomized Single-Blind Controlled Trial. Urol. Int. 2021, 105, 1011–1017. [Google Scholar] [CrossRef]
- Kushmerick, C.; Romano-Silva, M.; Gomez, M.V.; Prado, M.A.M. Muscarinic regulation of Ca2+ oscillation frequency in GH3 cells. Brain Res. 1999, 851, 39–45. [Google Scholar] [CrossRef]
- Wojcikiewicz, R.J.; Dobson, P.R.; Brown, B.L. Muscarinic acetylcholine receptor activation causes inhibition of cyclic AMP accumulation, prolactin and growth hormone secretion in GH3 rat anterior pituitary tumour cells. Biochim. Biophys. Acta 1984, 805, 25–29. [Google Scholar] [CrossRef]
- Schlegel, W.; Wuarin, F.; Zbaren, C.; Zahnd, G.R. Lowering of Cytosolic Free Ca2+ by Carbachol, a Muscarinic Cholinergic Agonist, in Clonal Pituitary Cells (GH 3 Cells). Endocrinology 1985, 117, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, S.E.; Brostrom, C.O.; Brostrom, M.A. Mechanisms of action of inhibitors of prolactin secretion in GH3 pituitary cells. I. Ca2+-dependent inhibition of amino acid incorporation. Mol. Pharmacol. 1986, 29, 411–419. [Google Scholar] [PubMed]
- Hedlund, B.; Barker, J.L. Carbachol changes spike-generation properties of GH3 pituitary cells. Brain Res. 1987, 402, 311–317. [Google Scholar] [CrossRef]
- Hitoshi, Y.; Simmonds, S.H.; Hawthorne, J.N. The muscarinic receptor of rat pituitary GH3 cells is coupled with adenylate cyclase inhibition, but not with phosphoinositide turnover. Biochem. Pharmacol. 1988, 37, 2675–2681. [Google Scholar] [CrossRef]
- Chou, Y.; Fong, J. Pretreatment with muscarinic receptor agonist activates a calcium-inhibitable adenylate cyclase in GH3 pituitary cells. Biochim. Biophys. Acta 2005, 1722, 148–155. [Google Scholar] [CrossRef]
- Secondo, A.; De Mizio, M.; Zirpoli, L.; Santillo, M.; Mondola, P. The Cu–Zn superoxide dismutase (SOD1) inhibits ERK phosphorylation by muscarinic receptor modulation in rat pituitary GH3 cells. Biochem. Biophys. Res. Commun. 2008, 376, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Aragay, A.M.; Katz, A.; Simon, M.I. The G alpha q and G alpha 11 proteins couple the thyrotropin-releasing hormone receptor to phospholipase C in GH3 rat pituitary cells. J. Biol. Chem. 1992, 267, 24983–24988. [Google Scholar] [CrossRef]
- Yatani, A.; Codina, J.; Sekura, R.D.; Birnbaumer, L.; Brown, A.M. Reconstitution of somatostatin and muscarinic receptor mediated stimulation of K+ channels by isolated GK protein in clonal rat anterior pituitary cell membranes. Mol. Endocrinol. 1987, 1, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Yatani, A.; Birnbaumer, L.; Brown, A. Direct coupling of the somatostatin receptor to potassium channels by a G protein. Metabolism 1990, 39 (Suppl. 2), 91–95. [Google Scholar] [CrossRef]
- Offermanns, S.; Gollasch, M.; Hescheler, J.; Spicher, K.; Schmidt, A.; Schultz, G.; Rosenthal, W. Inhibition of Voltage-Dependent Ca2+Currents and Activation of Pertussis Toxin-Sensitive G-Proteins via Muscarinic Receptors in GH3Cells. Mol. Endocrinol. 1991, 5, 995–1002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, H.-S.; Pan, Z.; Shi, W.; Brown, B.S.; Wymore, R.S.; Cohen, I.S.; Dixon, J.E.; McKinnon, D. KCNQ2 and KCNQ3 Potassium Channel Subunits: Molecular Correlates of the M-Channel. Science 1998, 282, 1890–1893. [Google Scholar] [CrossRef]
- Chen, H.; Smith, P.A. M-currents in frog sympathetic ganglion cells: Manipulation of membrane phosphorylation. Br. J. Pharmacol. 1992, 105, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Chen, H.; Kurenny, D.E.; Selyanko, A.A.; Zidichouski, J.A. Regulation of the M current: Transduction mechanism and role in ganglionic transmission. Can. J. Physiol. Pharmacol. 1992, 70, S12–S18. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Simasko, S. Characterization of an M-like current modulated by thyrotropin- releasing hormone in normal rat lactotrophs. J. Neurosci. 1996, 16, 1668–1678. [Google Scholar] [CrossRef]
- Selyanko, A.A.; Hadley, J.K.; Wood, I.C.; Abogadie, F.C.; Delmas, P.; Buckley, N.J.; London, B.; Brown, D.A. Two Types of K+ Channel Subunit, Erg1 and KCNQ2/3, Contribute to the M-Like Current in a Mammalian Neuronal Cell. J. Neurosci. 1999, 19, 7742–7756. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009, 156, 1185–1195. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Lin, C.-L.; Fang, W.-Y.; Lőrinczi, B.; Szatmári, I.; Chang, W.-H.; Fülöp, F.; Wu, S.-N. Effective Activation by Kynurenic Acid and Its Aminoalkylated Derivatives on M-Type K+ Current. Int. J. Mol. Sci. 2021, 22, 1300. [Google Scholar] [CrossRef]
- Afeli, S.A.Y.; Malysz, J.; Petkov, G.V. Molecular Expression and Pharmacological Evidence for a Functional Role of Kv7 Channel Subtypes in Guinea Pig Urinary Bladder Smooth Muscle. PLoS ONE 2013, 8, e75875. [Google Scholar] [CrossRef]
- Anderson, U.A.; Carson, C.; Johnston, L.; Joshi, S.; Gurney, A.M.; McCloskey, K.D. Functional expression of KCNQ (Kv7) channels in guinea pig bladder smooth muscle and their contribution to spontaneous activity. Br. J. Pharmacol. 2013, 169, 1290–1304. [Google Scholar] [CrossRef]
- Haick, J.M.; Byron, K.L. Novel treatment strategies for smooth muscle disorders: Targeting Kv7 potassium channels. Pharmacol. Ther. 2016, 165, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Vanhoof-Villalba, S.L.; Gautier, N.M.; Mishra, V.; Glasscock, E. Pharmacogenetics of KCNQ channel activation in 2 potassium channelopathy mouse models of epilepsy. Epilepsia 2018, 59, 358–368. [Google Scholar] [CrossRef] [PubMed]
- So, E.C.; Foo, N.P.; Ko, S.Y.; Wu, S.-H. Bisoprolol, Known to Be a Selective β₁-Receptor Antagonist, Differentially but Directly Suppresses I(K(M)) and I(K(erg)) in Pituitary Cells and Hippocampal Neurons. Int. J. Mol. Sci. 2019, 20, 657. [Google Scholar] [CrossRef]
- Ebihara, S.; Akaike, N. Potassium currents operated by thyrotrophin-releasing hormone in dissociated CA1 pyramidal neurones of rat hippocampus. J. Physiol. 1993, 472, 689–710. [Google Scholar] [CrossRef]
- Yu, H.; Wu, M.; Townsend, S.D.; Zou, B.; Long, S.; Daniels, J.S.; McManus, O.B.; Li, M.; Lindsley, C.W.; Hopkins, C.R. Discovery, Synthesis, and Structure–Activity Relationship of a Series of N-Aryl-bicyclo[2.2.1]heptane-2-carboxamides: Characterization of ML213 as a Novel KCNQ2 and KCNQ4 Potassium Channel Opener. ACS Chem. Neurosci. 2011, 2, 572–577. [Google Scholar] [CrossRef]
- Hsu, H.-T.; Tseng, Y.-T.; Lo, Y.-C.; Wu, S.-N. Ability of naringenin, a bioflavonoid, to activate M-type potassium current in motor neuron-like cells and to increase BKCa-channel activity in HEK293T cells transfected with α-hSlo subunit. BMC Neurosci. 2014, 15, 135. [Google Scholar] [CrossRef]
- Delmar, M.; Ibarra, J.; Davidenko, J.; Lorente, P.; Jalife, J. Dynamics of the background outward current of single guinea pig ventricular myocytes. Ionic mechanisms of hysteresis in cardiac cells. Circ. Res. 1991, 69, 1316–1326. [Google Scholar] [CrossRef]
- Boucher, M.; Chassaing, C.; Chapuy, E.; Lorente, P. Effects of Quinidine, Verapamil, Nifedipine and Ouabain on Hysteresis in Atrial Refractoriness in the Conscious Dog: An Approach to Ionic Mechanisms. Gen. Pharmacol. Vasc. Syst. 1999, 32, 47–50. [Google Scholar] [CrossRef]
- Bennekou, P.; Barksmann, T.L.; Jensen, L.R.; Kristensen, B.I.; Christophersen, P. Voltage activation and hysteresis of the non-selective voltage-dependent channel in the intact human red cell. Bioelectrochemistry 2004, 62, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Teijido, O.; Hoogerheide, D.; Rostovtseva, T.K.; Berezhkovskii, A.M.; Bezrukov, S.M. Conductance hysteresis in the voltage-dependent anion channel. Eur. Biophys. J. 2015, 44, 465–472. [Google Scholar] [CrossRef]
- Meloni, S.; Moehl, T.; Tress, W.; Franckevicius, M.; Saliba, M.; Lee, Y.H.; Gao, P.; Nazeeruddin, W.T.M.S.Y.H.L.P.G.M.K.; Zakeeruddin, S.M.; Rothlisberger, S.M.U.; et al. Ionic polarization-induced current–voltage hysteresis in CH3NH3PbX3 perovskite solar cells. Nat. Commun. 2016, 7, 10334. [Google Scholar] [CrossRef]
- Noskov, S.Y.; Rostovtseva, T.K.; Chamberlin, A.C.; Teijido, O.; Jiang, W.; Bezrukov, S.M. Current state of theoretical and experimental studies of the voltage-dependent anion channel (VDAC). Biochim. Biophys. Acta 2016, 1858 Pt B, 1778–1790. [Google Scholar] [CrossRef]
- Wu, S.-N.; Huang, C.-W. Editorial to the Special Issue “Electrophysiology”. Int. J. Mol. Sci. 2021, 22, 2956. [Google Scholar] [CrossRef]
- Maruyama, S.; Tsukada, H.; Nishiyama, S.; Kakiuchi, T.; Fukumoto, D.; Oku, N.; Yamada, S. In Vivo Quantitative Autoradiographic Analysis of Brain Muscarinic Receptor Occupancy by Antimuscarinic Agents for Overactive Bladder Treatment. J. Pharmacol. Exp. Ther. 2008, 325, 774–781. [Google Scholar] [CrossRef]
- Anisuzzaman, A.S.M.; Nishimune, A.; Yoshiki, H.; Uwada, J.; Muramatsu, I. Influence of Tissue Integrity on Pharmacological Phenotypes of Muscarinic Acetylcholine Receptors in the Rat Cerebral Cortex. J. Pharmacol. Exp. Ther. 2011, 339, 186–193. [Google Scholar] [CrossRef]
- Gingerich, S.; Kim, G.L.; Chalmers, J.A.; Koletar, M.M.; Wang, X.; Wang, Y.; Belsham, D.D. Estrogen receptor α and G-protein coupled receptor 30 mediate the neuroprotective effects of 17β-estradiol in novel murine hippocampal cell models. Neuroscience 2010, 170, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Lin, K.-M.; Hung, T.-Y.; Wu, S.-N. Multiple Actions of Rotenone, an Inhibitor of Mitochondrial Respiratory Chain, on Ionic Currents and Miniature End-Plate Potential in Mouse Hippocampal (mHippoE-14) Neurons. Cell. Physiol. Biochem. 2018, 47, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-C.; Wu, S.-N.; Huang, C.-W. The Specific Effects of OD-1, a Peptide Activator, on Voltage-Gated Sodium Current and Seizure Susceptibility. Int. J. Mol. Sci. 2020, 21, 8254. [Google Scholar] [CrossRef] [PubMed]
- Castle, N.A. Pharmacological modulation of voltage-gated potassium channels as a therapeutic strategy. Expert Opin. Ther. Patents 2010, 20, 1471–1503. [Google Scholar] [CrossRef]
- Chang, W.-T.; Liu, P.-Y.; Gao, Z.-H.; Lee, S.-W.; Lee, W.-K.; Wu, S.-N. Evidence for the Effectiveness of Remdesivir (GS-5734), a Nucleoside-Analog Antiviral Drug in the Inhibition of I (K(M)) or I (K(DR)) and in the Stimulation of I (MEP). Front. Pharmacol. 2020, 11, 1091. [Google Scholar] [PubMed]
- Mistri, H.N.; Jangid, A.G.; Pudage, A.; Rathod, D.M.; Shrivastav, P.S. Highly sensitive and rapid LC–ESI-MS/MS method for the simultaneous quantification of uroselective α1-blocker, alfuzosin and an antimuscarinic agent, solifenacin in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 876, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Macek, J.; Ptáček, P.; Klíma, J. Determination of solifenacin in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 3327–3330. [Google Scholar] [CrossRef]
- Tannenbaum, S.; Adel, M.D.; Krauwinkel, W.; Meijer, J.; Hollestein-Havelaar, A.; Verheggen, F.; Newgreen, D. Pharmacokinetics of solifenacin in pediatric populations with overactive bladder or neurogenic detrusor overactivity. Pharmacol. Res. Perspect. 2020, 8, e00684. [Google Scholar] [CrossRef]
- Smulders, R.A.; Smith, N.N.; Krauwinkel, W.J.; Hoon, T.J. Pharmacokinetics, safety, and tolerability of solifenacin in patients with renal insufficiency. J. Pharmacol. Sci. 2007, 103, 67–74. [Google Scholar] [CrossRef]
- Gopalakrishnan, M.; Shieh, C.-C. Potassium channel subtypes as molecular targets for overactive bladder and other urological disorders. Expert Opin. Ther. Targets 2004, 8, 437–458. [Google Scholar] [CrossRef]
- Anderson, U.A.; Carson, C.; McCloskey, K.D. KCNQ Currents and Their Contribution to Resting Membrane Potential and the Excitability of Interstitial Cells of Cajal From the Guinea Pig Bladder. J. Urol. 2009, 182, 330–336. [Google Scholar] [CrossRef]
- Rode, F.; Svalø, J.; Sheykhzade, M.; Rønn, L.C.B. Functional effects of the KCNQ modulators retigabine and XE991 in the rat urinary bladder. Eur. J. Pharmacol. 2010, 638, 121–127. [Google Scholar] [CrossRef]
- Brickel, N.; Gandhi, P.; VanLandingham, K.; Hammond, J.; DeRossett, S. The urinary safety profile and secondary renal effects of retigabine (ezogabine): A first-in-class antiepileptic drug that targets KCNQ (K(v)7) potassium channels. Epilepsia 2012, 53, 606–612. [Google Scholar] [CrossRef]
- Svalø, J.; Hansen, H.H.; Rønn, L.C.B.; Sheykhzade, M.; Munro, G.; Rode, F. Kv7 Positive Modulators Reduce Detrusor Overactivity and Increase Bladder Capacity in Rats. Basic Clin. Pharmacol. Toxicol. 2011, 110, 145–153. [Google Scholar] [CrossRef]
- Svalø, J.; Bille, M.; Theepakaran, N.P.; Sheykhzade, M.; Nordling, J.; Bouchelouche, P. Bladder contractility is modulated by Kv7 channels in pig detrusor. Eur. J. Pharmacol. 2013, 715, 312–320. [Google Scholar] [CrossRef]
- Svalø, J.; Sheykhzade, M.; Nordling, J.; Matras, C.; Bouchelouche, P. Functional and Molecular Evidence for Kv7 Channel Subtypes in Human Detrusor from Patients with and without Bladder Outflow Obstruction. PLoS ONE 2015, 10, e0117350. [Google Scholar] [CrossRef]
- Takagi, H.; Hashitani, H. Effects of K+ channel openers on spontaneous action potentials in detrusor smooth muscle of the guinea-pig urinary bladder. Eur. J. Pharmacol. 2016, 789, 179–186. [Google Scholar] [CrossRef]
- Bientinesi, R.; Mancuso, C.; Martire, M.; Bassi, P.F.; Sacco, E.; Currò, D. K(V)7 channels in the human detrusor: Channel modulator effects and gene and protein expression. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Provence, A.; Angoli, D.; Petkov, G.V. K(V)7 Channel Pharmacological Activation by the Novel Activator ML213: Role for Heteromeric K(V)7.4/K(V)7.5 Channels in Guinea Pig Detrusor Smooth Muscle Function. J. Pharmacol. Exp. Ther. 2018, 364, 131–144. [Google Scholar] [CrossRef]
- Seefeld, M.A.; Lin, H.; Holenz, J.; Downie, D.; Donovan, B.; Fu, T.; Pasikanti, K.; Zhen, W.; Cato, M.; Chaudhary, K.W.; et al. Novel K(V)7 ion channel openers for the treatment of epilepsy and implications for detrusor tissue contraction. Bioorg. Med. Chem. Lett. 2018, 28, 3793–3797. [Google Scholar] [CrossRef] [PubMed]
- Malysz, J.; Petkov, G.V. Detrusor Smooth Muscle K(V)7 Channels: Emerging New Regulators of Urinary Bladder Function. Front. Physiol. 2020, 11, 1004. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.; Robinson, D.; Cardozo, L. Darifenacin in the treatment of overactive bladder. Int. J. Clin. Pract. 2005, 59, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Hsu, M.-C.; Liao, Y.-K.; Wu, F.-T.; Jong, Y.-J.; Lo, Y.-C. Evidence for Inhibitory Effects of Flupirtine, a Centrally Acting Analgesic, on Delayed Rectifier K+ Currents in Motor Neuron-Like Cells. Evid. Based Complement. Altern. Med. 2012, 2012, 148403. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-Y.; Chuang, T.-H.; Wu, S.-N. The Effectiveness in Activating M-Type K+ Current Produced by Solifenacin ([(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action. Int. J. Mol. Sci. 2021, 22, 12399. https://doi.org/10.3390/ijms222212399
Cho H-Y, Chuang T-H, Wu S-N. The Effectiveness in Activating M-Type K+ Current Produced by Solifenacin ([(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action. International Journal of Molecular Sciences. 2021; 22(22):12399. https://doi.org/10.3390/ijms222212399
Chicago/Turabian StyleCho, Hsin-Yen, Tzu-Hsien Chuang, and Sheng-Nan Wu. 2021. "The Effectiveness in Activating M-Type K+ Current Produced by Solifenacin ([(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action" International Journal of Molecular Sciences 22, no. 22: 12399. https://doi.org/10.3390/ijms222212399
APA StyleCho, H.-Y., Chuang, T.-H., & Wu, S.-N. (2021). The Effectiveness in Activating M-Type K+ Current Produced by Solifenacin ([(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate): Independent of Its Antimuscarinic Action. International Journal of Molecular Sciences, 22(22), 12399. https://doi.org/10.3390/ijms222212399





