The Effect of 17α-Ethynilestradiol and GPER1 Activation on Body and Muscle Growth, Muscle Composition and Growth-Related Gene Expression of Gilthead Seabream, Sparus aurata L
Abstract
1. Introduction
2. Results
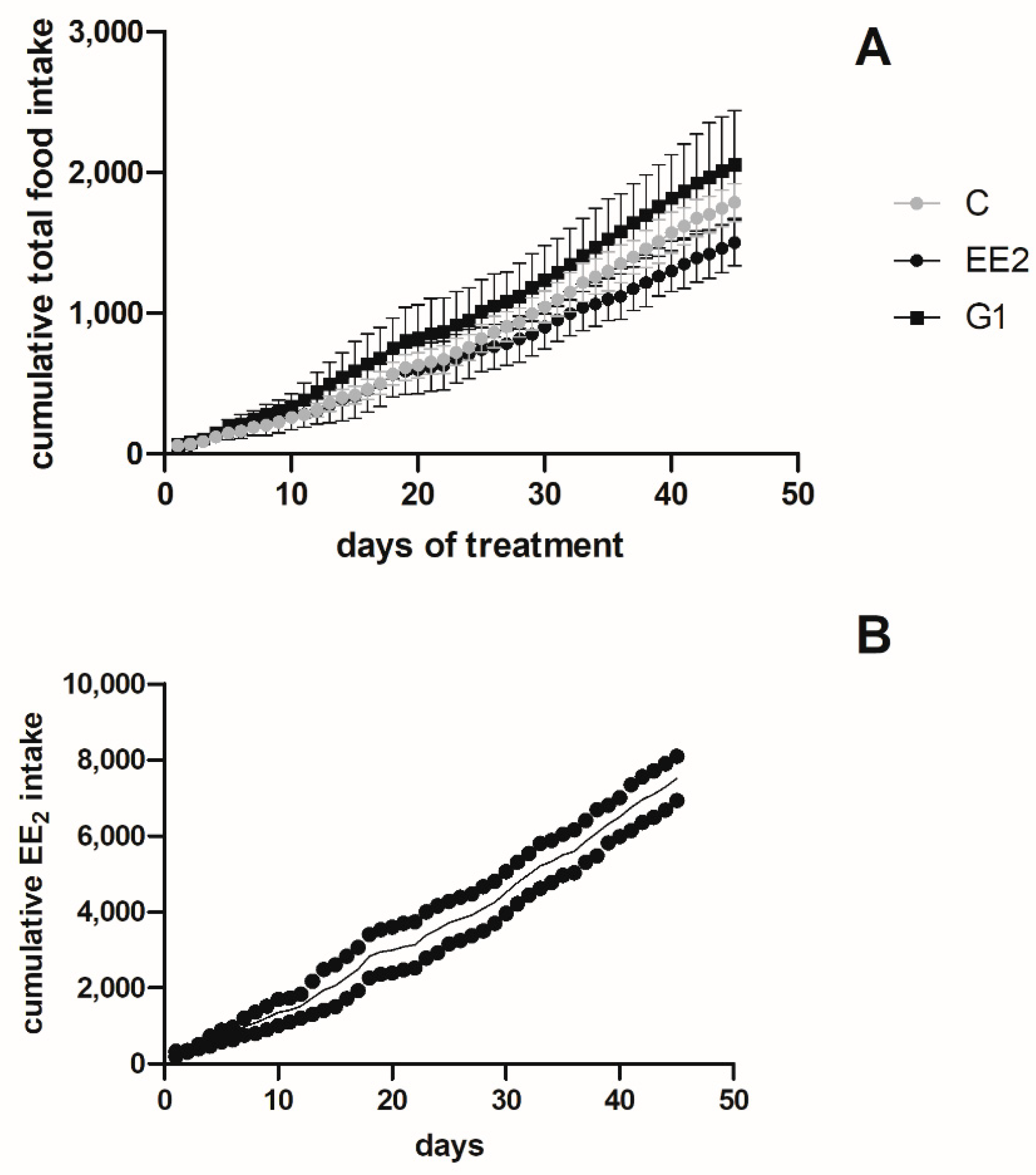
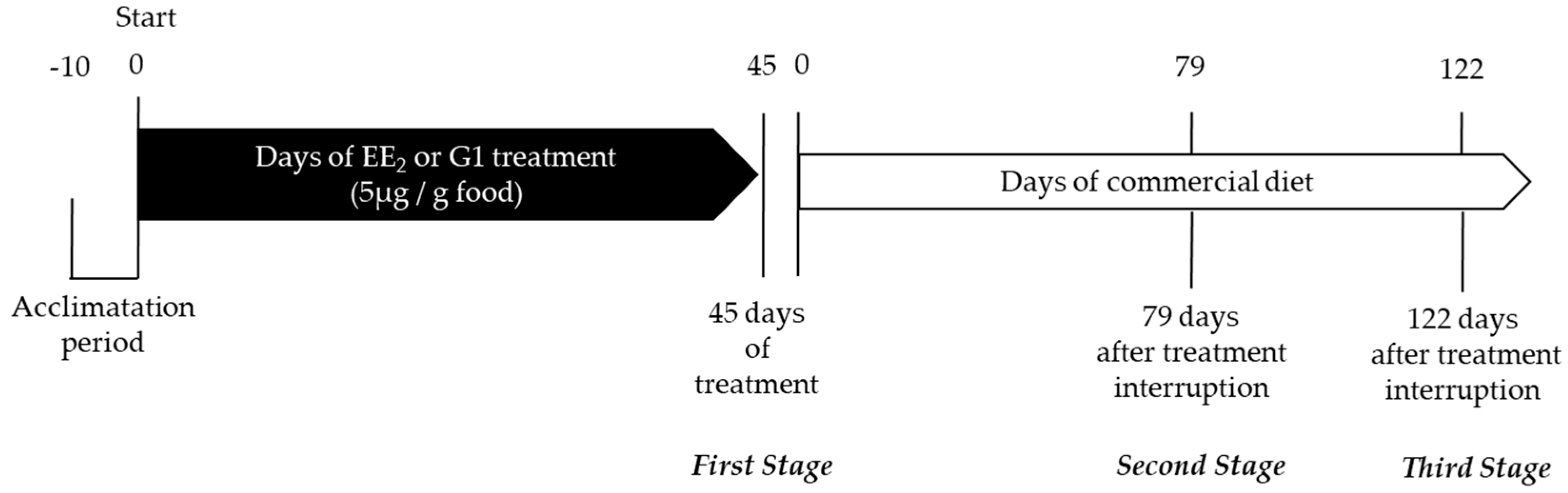
2.1. Body Parameters, Cumulative Intake and Survival
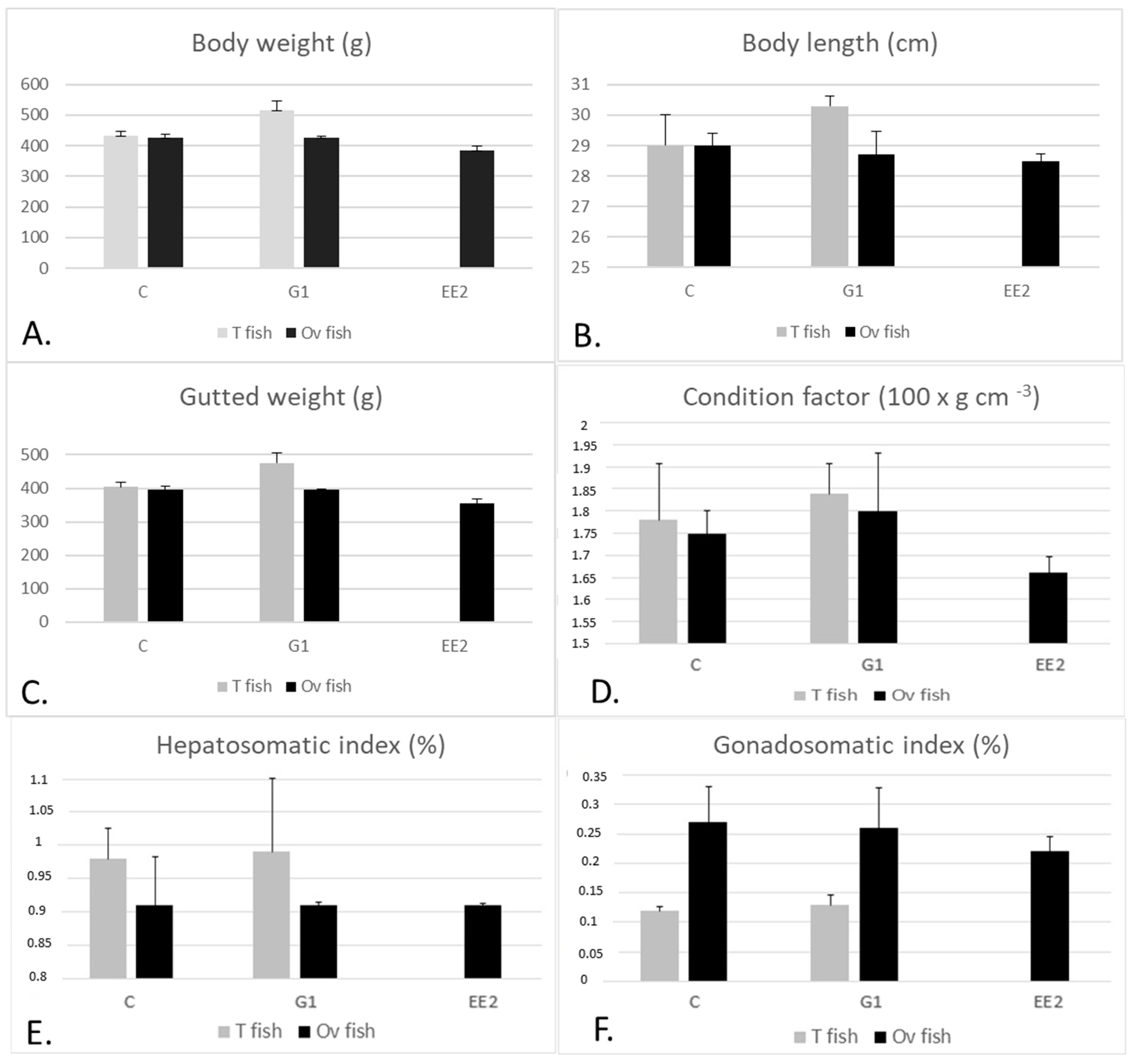
2.1.1. Body Parameters
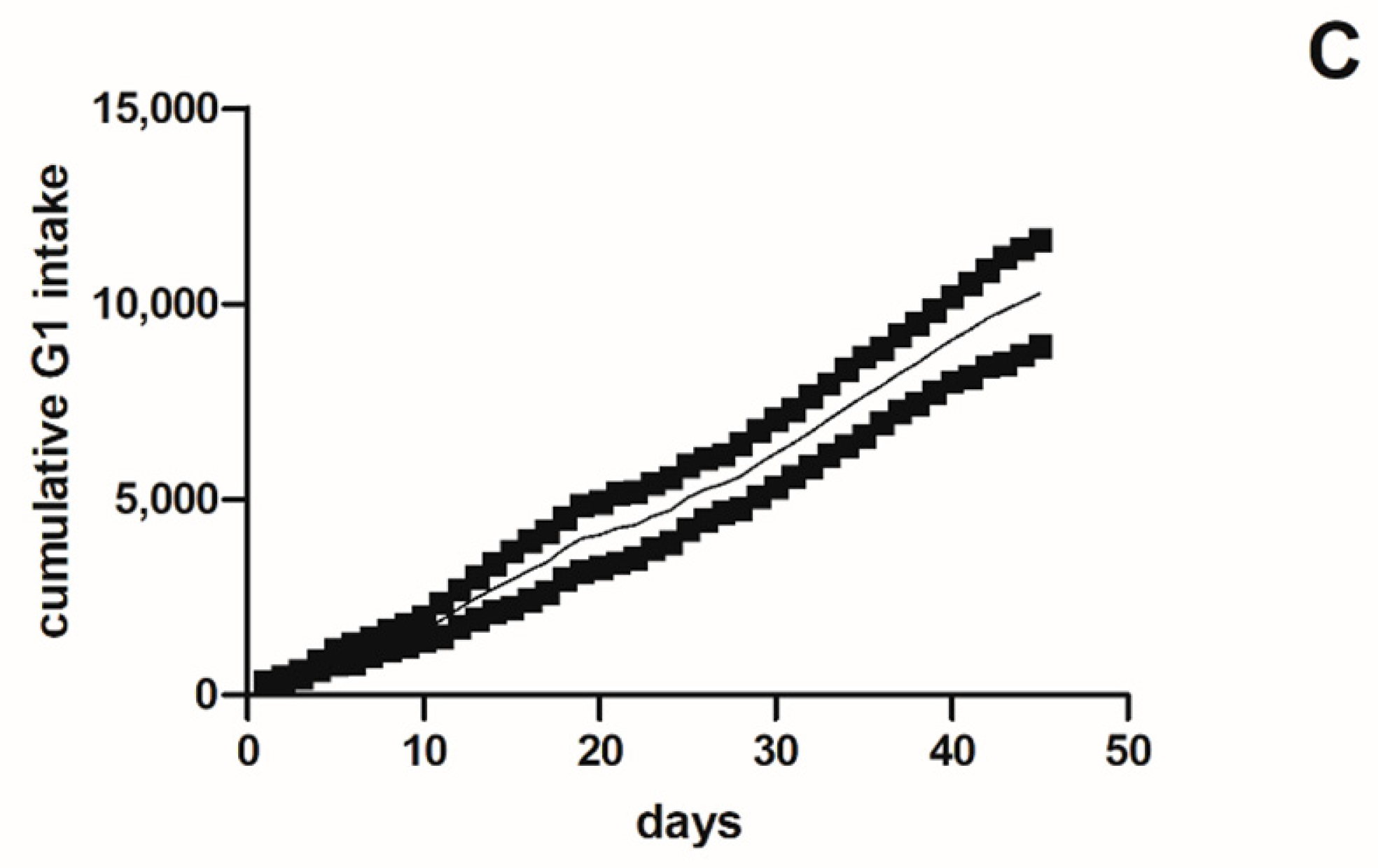
2.1.2. Cumulative Intake and Survival
2.1.3. Fish Gonad Structure
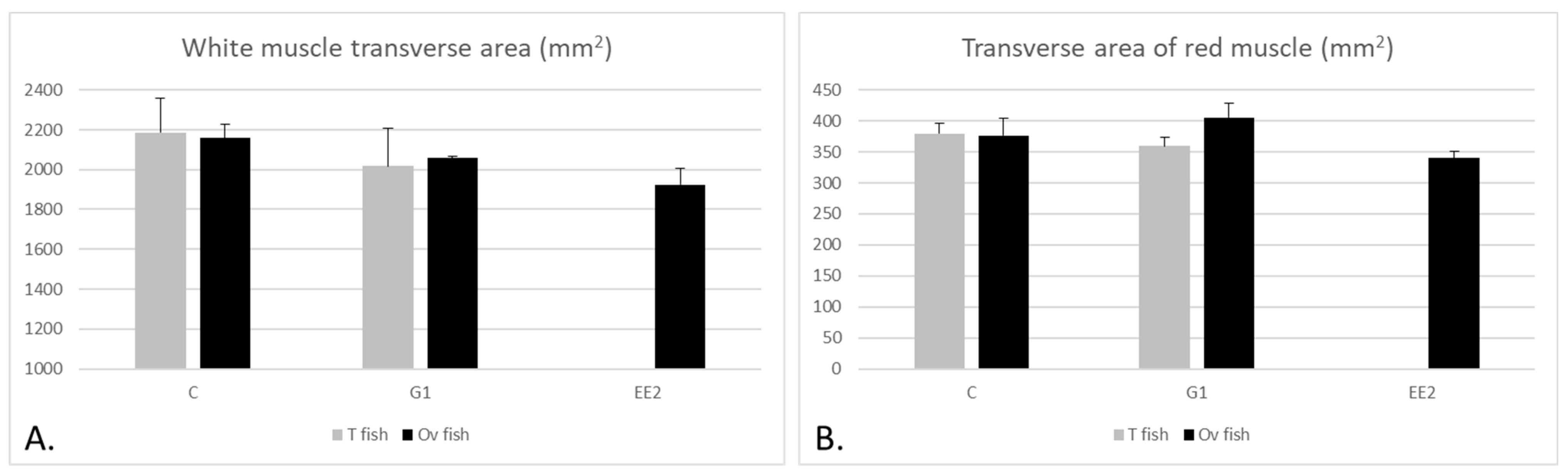
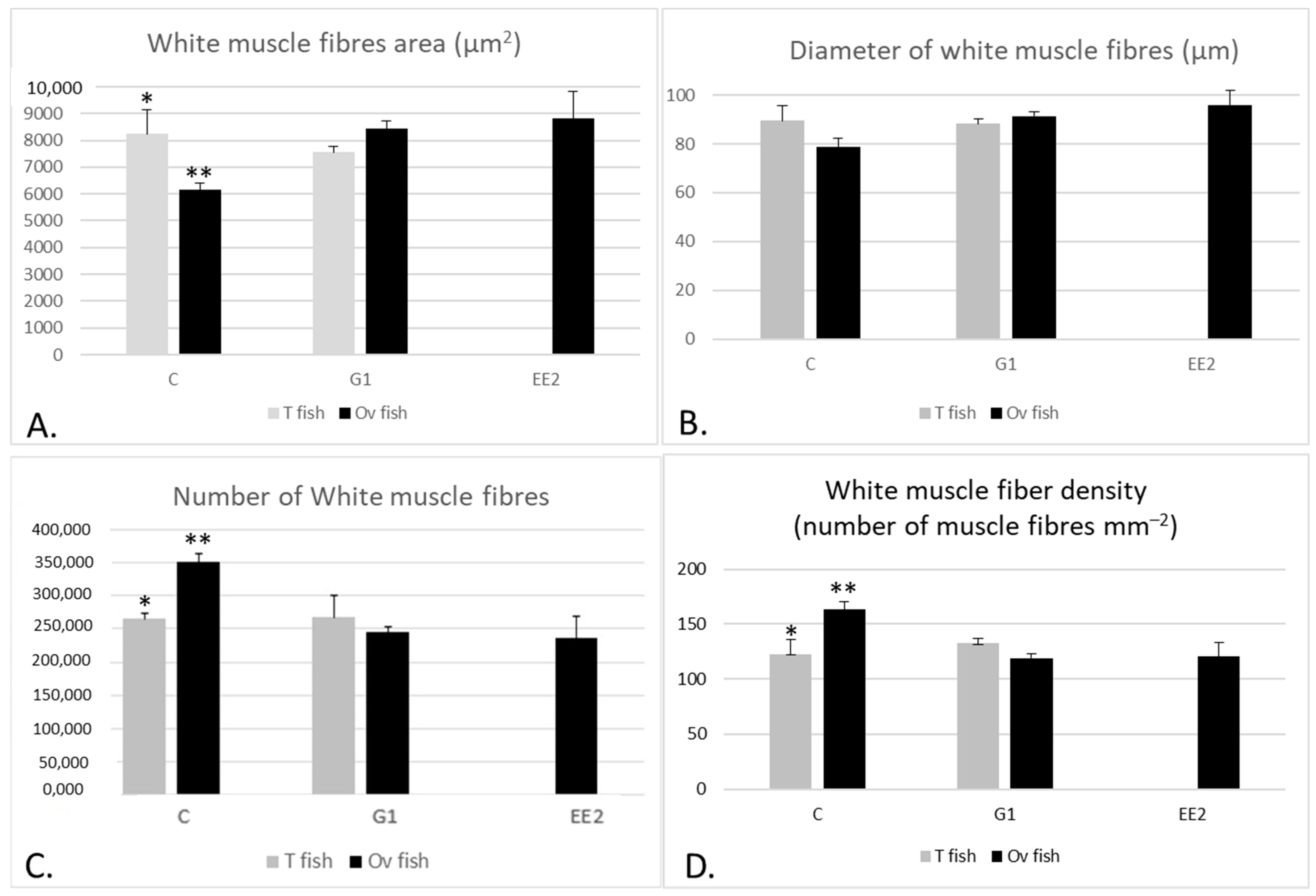
2.2. Muscle Parameters
2.2.1. Muscle Parameters According to the Gonad Structure
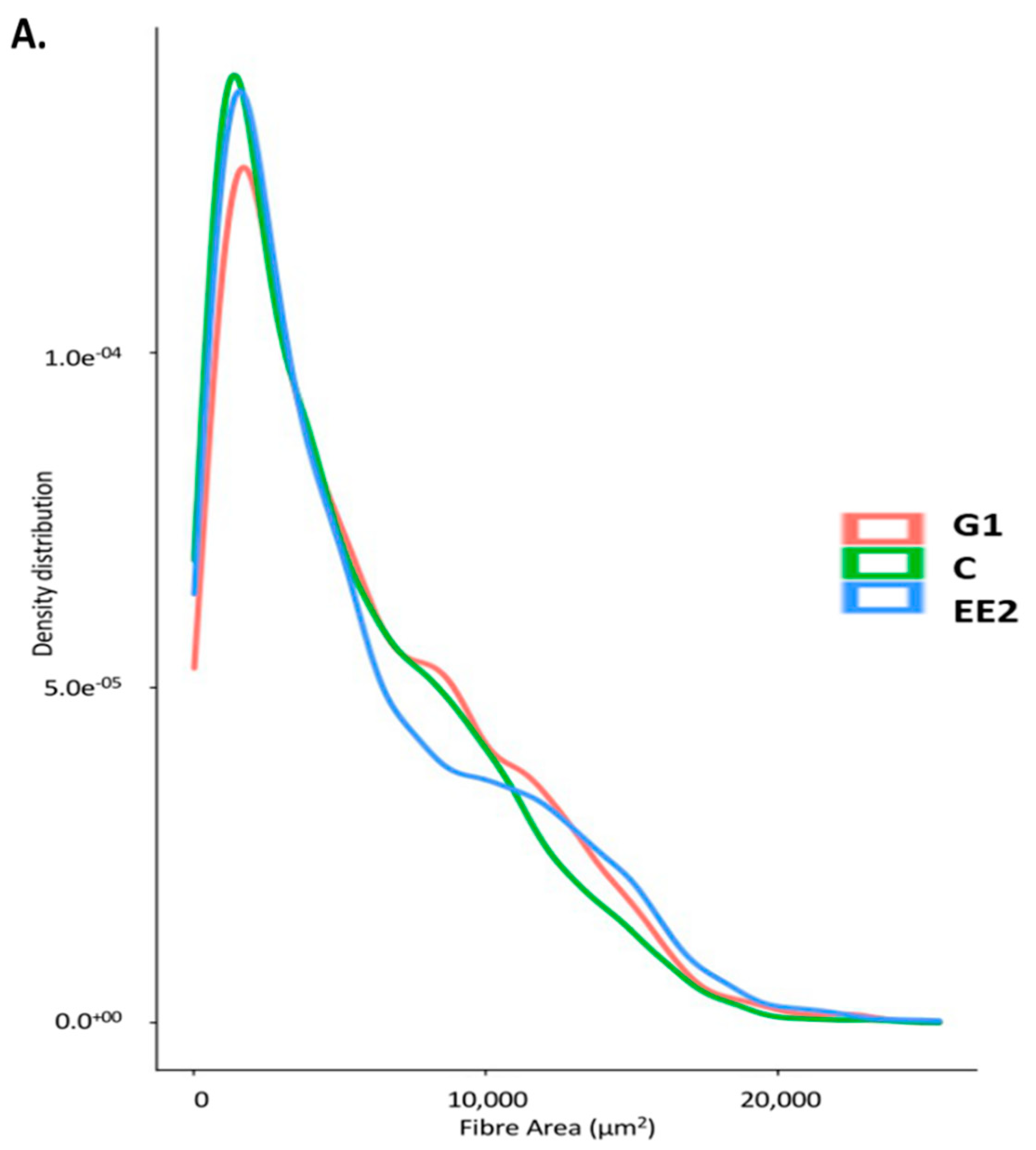
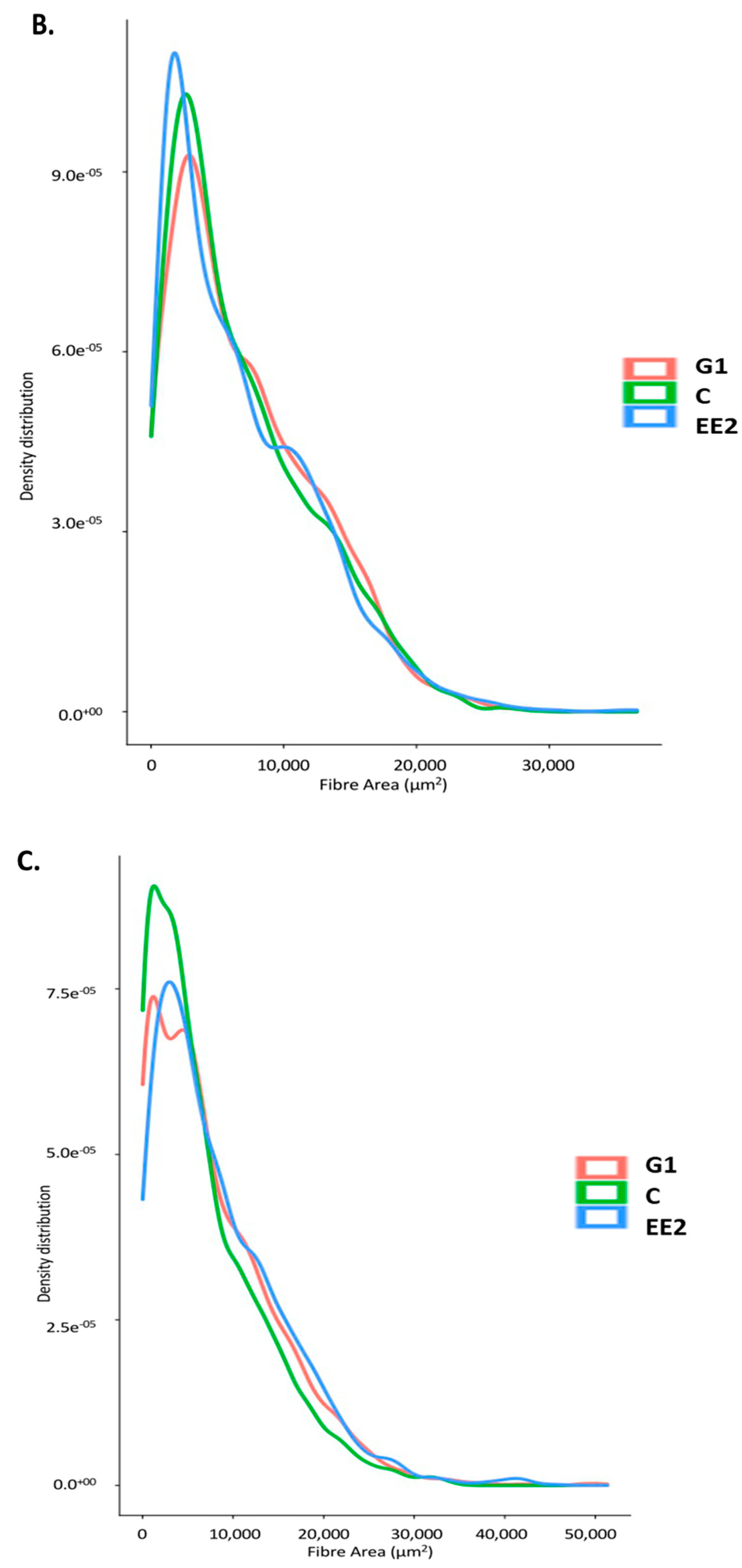
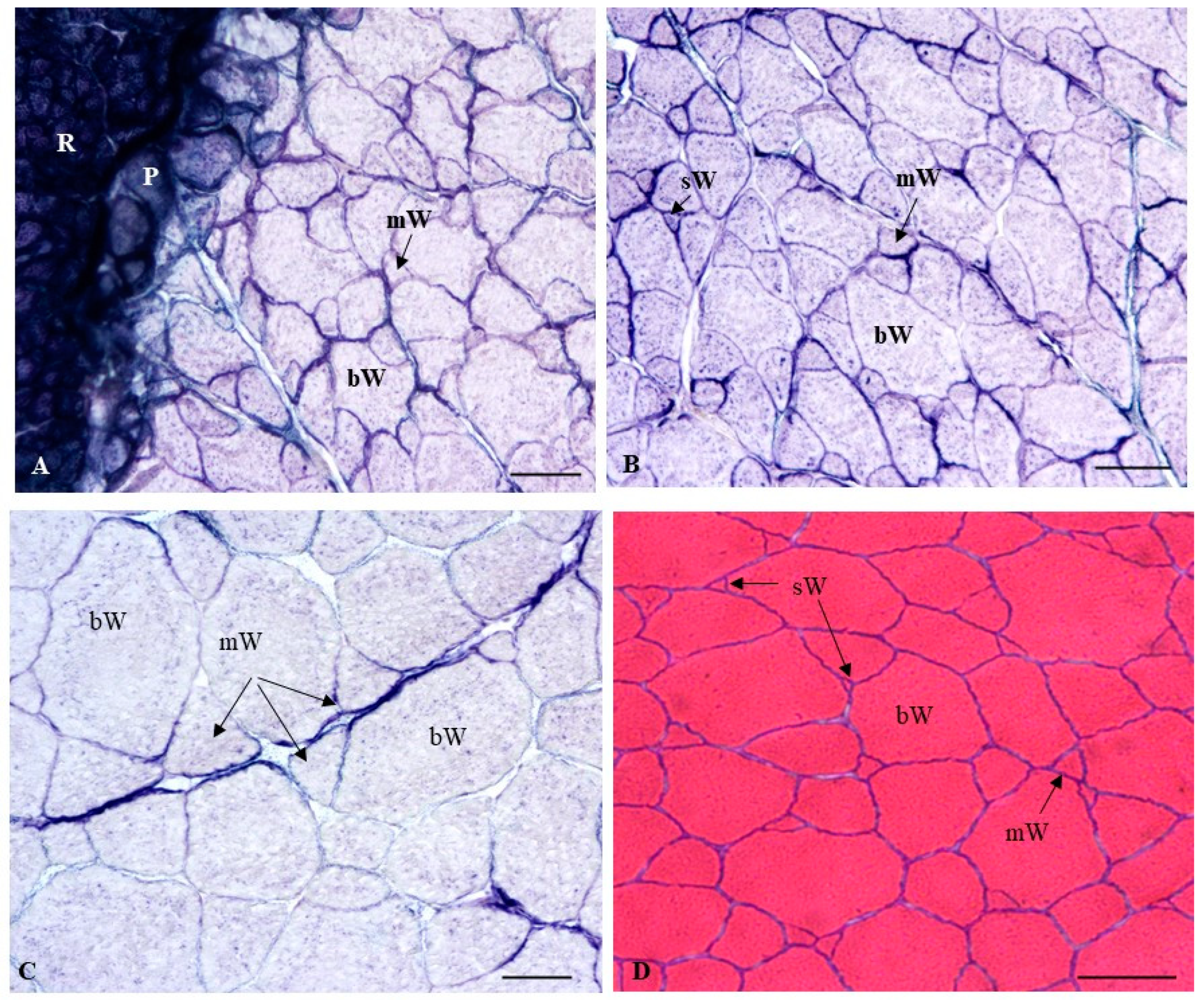
2.2.2. Histochemical Characterization of the Muscle Fibers
2.3. Muscle Composition and Textural Hardness
Muscle Composition and Textural Hardness According to Gonad Structure
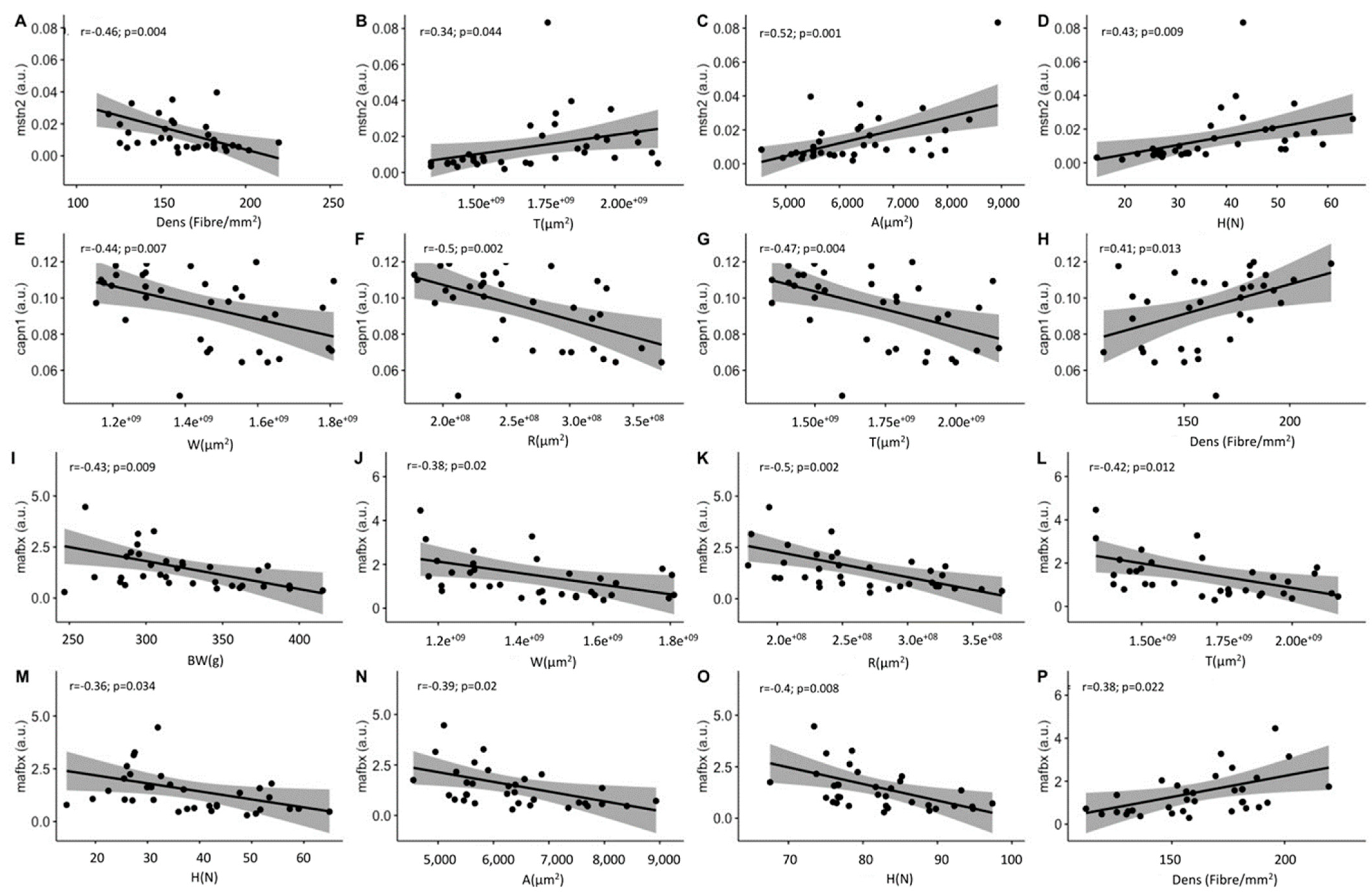
2.4. Growth-Related Gene Expression
3. Discussion
3.1. Body Growth and Survival
3.2. Muscle Growth Parameters, Muscle Enzymatic Activity and Textural Hardness
3.3. Muscle Protein and Fat
3.4. Growth-Related Gene Expression
4. Material and Methods
4.1. Animals and Management
4.2. Experimental Treatments and Quantification of Cumulative Intake and Final Survival
4.3. Sampling and Measurement of Body Parameters
4.4. Quantitative Analysis of Muscle Growth and Histochemical Techniques
4.5. Analysis of the Composition and Texture of the Fillet
4.6. Gene Expression Analysis
4.7. Statistical Analysis
5. Conclusions
- GPER1 activation and EE2 treatment reduced body growth in the short and long term, respectively.
- EE2 treatment, and to a lesser extent G1 treatment, reduced muscle growth (transverse area of the muscle) in the long term and was correlated with a high expression of key growth muscle-related genes in the EE2 group, such as mfbx (upregulated gene in catabolic signals) and mstn2 (negative regulator gene of the muscle growth).
- EE2 treatment and GPER1 activation increased the hypertrophy and glycolytic activity of the white muscle fibers, whereas fibrillary hyperplasia and muscle fiber density showed the contrary tendency, indicating immediate and persistent long-lasting effects on muscle cellularity.
- EE2 treatment and GPER1 activation reduced the textural firmness, showing a negative correlation with the size of the muscle fibers.
- EE2 exposure reduced muscle fat and was related with the predominance of ovarian area in the gonads of EE2-treated fish.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benotti, M.J.; Brownawell, B.J. Distributions of pharmaceuticals in an urban estuary during both dry- and wet-weather conditions. Environ. Sci. Technol. 2007, 41, 5795–5802. [Google Scholar] [CrossRef]
- Desbrow, C.; Routledge, E.J.; Brighty, G.C.; Sumpter, J.P.; Waldock, M. Identification of estrogenic chemicals in STW effluent. Chemical fractionation and in vitro biological screening. Environ. Sci. Technol. 1988, 32, 1549–1558. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Skopec, M.; Meyer, M.T.; Furlong, E.T.; Zaugg, S.D. Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci. Total Environ. 2004, 328, 119–130. [Google Scholar] [CrossRef]
- Sumpter, J.P.; Jobling, S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ. Health Perspect. 1995, 103, 173–178. [Google Scholar]
- Pawlowski, S.; van Aerle, R.; Tyler, C.R.; Braunbeck, T. Effects of 17α-ethinylestradiol in a fathead minnow (Pimephales promelas) gonadal recrudescence assay. Ecotoxicol. Environ. Saf. 2004, 57, 330–345. [Google Scholar] [CrossRef]
- Cabas, I.; Chaves-Pozo, E.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. Dietary intake of 17α-ethinylestradiol promotes leukocytes infiltration in the gonad of the hermaphrodite gilthead seabream. Mol. Immunol. 2011, 48, 2079–2086. [Google Scholar] [CrossRef]
- Cabas, I.; Chaves-Pozo, E.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. The effect of 17α-ethynylestradiol on steroidogenesis and gonadal cytokine gene expression is related to the reproductive stage in marine hermaphrodite fish. Mar. Drugs 2013, 11, 4973–4992. [Google Scholar] [CrossRef]
- Colli-Dula, R.C.; Martyniuk, C.J.; Kroll, K.J.; Prucha, M.S.; Kozuch, M.; Barber, D.S.; Denslow, N.D. Dietary exposure of 17-alpha ethinylestradiol modulates physiological endpoints and gene signaling pathways in female largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2014, 156, 148–160. [Google Scholar] [CrossRef]
- Reyhanian Caspillo, N.; Volkova, K.; Hallgren, S.; Olsson, P.E.; Porsch-Hällström, I. Short-term treatment of adult male zebrafish (Danio rerio) with 17α-ethinyl estradiol affects the transcription of genes involved in development and male sex differentiation. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 164, 35–42. [Google Scholar] [CrossRef][Green Version]
- Oropesa, A.L.; Martín-Hidalgo, D.; Fallola, C.; Gil, M.C. Effects of exposure to 17-alpha-ethynylestradiol on sperm quality of tench (Tinca tinca). Ecotoxicol. Environ. Saf. 2015, 120, 318–325. [Google Scholar] [CrossRef]
- Armstrong, B.M.; Lazorchak, J.M.; Jensen, K.M.; Haring, H.J.; Smith, M.E.; Flick, R.W.; Bencic, D.C.; Biales, A.D. Reproductive effects in fathead minnows (Pimphales promelas) following a 21 d exposure to 17α-ethinylestradiol. Chemosphere 2016, 144, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Gárriz, Á.; del Fresno, P.S.; Miranda, L.A. Exposure to E2 and EE2 environmental concentrations affect different components of the Brain––Pituitary–Gonadal axis in pejerrey fish (Odontesthes bonariensis). Ecotoxicol. Environ. Saf. 2017, 144, 45–53. [Google Scholar] [CrossRef]
- Jackson, L.M.; Felgenhauer, B.E.; Klerks, P.L. Feminization, altered gonadal development, and liver damage in least killifish (Heterandria formosa) exposed to sublethal concentrations of 17α-ethinylestradiol. Ecotoxicol. Environ. Saf. 2019, 170, 331–337. [Google Scholar] [CrossRef]
- Condeça, J.B.; Canario, A.V.M. The effect of estrogen on the gonads and on in vitro conversion of androstenedione to testosterone, 11-ketotestosterone, and estradiol-17β in Sparus aurata (Teleostei, Sparidae). Gen. Comp. Endocrinol. 1999, 116, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Porseryd, T.; Larsson, J.; Kellner, M.; Bollner, T.; Dinnétz, P.; Porsch Hällström, I. Altered non-reproductive behavior and feminization caused by developmental exposure to 17α-ethinylestradiol persist to adulthood in three-spined stickleback (Gasterosteus aculeatus). Aquat. Toxicol. 2019, 207, 142–152. [Google Scholar] [CrossRef]
- Baumann, L.; Knörr, S.; Keiter, S.; Rehberger, K.; Volz, S.; Schiller, V.; Fenske, M.; Holbech, H.; Segner, H.; Braunbeck, T. Reversibility of endocrine disruption in zebrafish (Danio rerio) after discontinued exposure to the estrogen 17α-ethinylestradiol. Toxicol. Appl. Pharmacol. 2014, 278, 230–237. [Google Scholar] [CrossRef]
- Bologa, C.G.; Revankar, C.M.; Young, S.M.; Edwards, B.S.; Arterburn, J.B.; Kiselyov, A.S.; Parker, M.A.; Tkachenko, S.E.; Savchuck, N.P.; Sklar, L.A.; et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006, 2, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.K.; Field, A.S.; Burai, R.; Ramesh, C.; Petrie, W.K.; Bologa, C.G.; Oprea, T.I.; Yamaguchi, Y.; Hayashi, S.; Sklar, L.A.; et al. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counter selectivity. J. Steroid Biochem. Mol. Biol. 2011, 127, 358–366. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Chaves-Pozo, E.; García-Alcázar, A.; Mulero, V.; Meseguer, J.; García-Ayala, A. Testicular involution prior to sex change in gilthead seabream is characterized by a decrease in DMRT1 gene expression and by massive leukocyte infiltration. Reprod. Biol. Endocrinol. 2007, 5, 20. [Google Scholar] [CrossRef]
- Chaves-Pozo, E.; Liarte-Lastra, S.; García-Ayala, A. Inmune and reproductive interaction: An essential clue for understanding gonad function in gilthead seabream. In Recent Advances in Fish Reproductive Biology; García-Ayala, A., Meseguer, J., Chaves-Pozo, E., Eds.; Research Signpost: Thiruvananthapuram, India, 2010; pp. 127–139. [Google Scholar]
- García Hernández, M.P.; Cabas, I.; Rodenas, M.C.; Arizcun, M.; Chaves-Pozo, E.; Power, D.M.; García Ayala, A. 17α-ethynylestradiol prevents the natural male-to-female sex in gilthead seabream (Sparus aurata L.). Sci. Rep. 2020, 10, 20067. [Google Scholar] [CrossRef] [PubMed]
- Rodenas, M.C.; Cabas, I.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. Selective estrogen receptor modulators differentially alter the immune response of gilthead seabream juveniles. Fish Shellfish Immunol. 2016, 52, 189–197. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Weber, G.M. Effects of steroid treatment on growth, nutrient partitioning, and expression of genes related to growth and nutrient metabolism in adult triploid rainbow trout (Oncorhynchus mykiss). Domest. Anim. Endocrinol. 2016, 56, 1–12. [Google Scholar] [CrossRef]
- Ko, K.; Malison, J.A.; Reed, J.D. Effect of genistein on the growth and reproductive function of male and female yellow perch Perca flavescens. J. World Aquacult. Soc. 1999, 30, 73–79. [Google Scholar]
- Santandreu, I.A.; Diaz, N.F. Effect of 17alpha-methyltestosterone on growth and nitrogen excretion in masu salmon (Oncorhynchus masau Brevoort). Aquaculture 1994, 124, 321–333. [Google Scholar] [CrossRef]
- Thorarensen, H.; Young, G.; Davie, P.S. 11-Ketotestosterone stimulates growth of heart and red muscle in rainbow trout. Can. J. Zool. 1996, 74, 912–917. [Google Scholar] [CrossRef]
- Ando, S.; Yamazaki, F.; Hatano, M.; Zama, K. Deterioration of chum salmon (Oncorhynchus keta) muscle during spawning migration—III. Changes in protein composition and protease activity of juvenile chum salmon muscle upon treatment with sex steroids. Comp. Biochem. Physiol. 1986, 83B, 325–330. [Google Scholar] [CrossRef]
- Toyohara, H.; Ito, K.; Hori, N.; Touhata, K.; Kinoshita, M.; Kutoba, S.; Sato, K.; Ohtsuki, K.; Sakaguchi, M. Effect of steroid hormone administration on the breakdown of muscle proteins in ayu. Fish. Sci. 1998, 64, 419–422. [Google Scholar] [CrossRef][Green Version]
- Fajardo-Zapata, A.L.; Méndez-Casallas, F.J.; Molina, L.H. Residuos de fármacos anabolizantes en carnes destinadas al consumo humano. Univ. Sci. 2011, 16, 77–91. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A. Environment and plasticity of myogenesis in teleost fish. J. Exp. Biol. 2006, 209, 2249–2264. [Google Scholar] [CrossRef]
- Bower, N.I.; García de la serrana, D.; Johnston, I.A. Characterisation and differential regulation of MAFbx/Atrogin-1 a and b transcripts in skeletal muscle of Atlantic salmon (Salmo salar). Biochem. Biophys. Res. Comm. 2010, 396, 265–271. [Google Scholar] [CrossRef]
- García de la serrana, D.; Vieira, V.L.A.; Andree, K.B.; Darias, M.; Estévez, A.; Gisbert, E.; Johnston, I.A. Development temperature has persistent effects on muscle growth responses in gilthead sea bream. PLoS ONE 2012, 7, e51884. [Google Scholar]
- García de la serrana, D.; Johnston, I.A. Expression of Heat Shock Protein (Hsp90) Paralogues is Regulated by Amino Acids in Skeletal Muscle of Atlantic Salmon. PLoS ONE 2013, 6, e74295. [Google Scholar] [CrossRef]
- Hatae, K.; Yoshimatsu, F.; Matsumoto, J.J. Discriminative characterization of different texture profiles of various cooked fish muscles. J. Food Sci. 1984, 49, 721–726. [Google Scholar] [CrossRef]
- Hatae, K.; Yoshimatsu, F.; Matsumoto, J.J. Role of muscle fibers in contributing firmness of cooked fish. J. Food Sci. 1990, 55, 693–696. [Google Scholar] [CrossRef]
- Periago, M.J.; Ayala, M.D.; López-Albors, O.; Abdel, I.; Martínez, C.; García-Alcázar, A.; Ros, G.; Gil, F. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aquaculture 2005, 249, 175–188. [Google Scholar] [CrossRef]
- Cabas, I.; Chaves-Pozo, E.; Mulero, V.; García-Ayala, A. Role of estrogens in fish immunity with special emphasis on GPER1. Dev. Comp. Immunol. 2018, 89, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Shved, N.; Berishvili, G.; Baroiller, J.F.; Segner, H.; Reinecke, M. Environmentally relevant concentrations of 17alpha-ethinylestradiol (EE2) interfere with the growth hormone (GH)/insulin-like growth factor (IGF)-I system in developing bony fish. Toxicol. Sci. 2008, 106, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, B.M.; Weber, G.M. 2011. Effects of sex steroids on indices of protein turnover in rainbow trout (Oncorhynchus mykiss) white muscle. Gen. Comp. Endocrinol. 2011, 174, 132–142. [Google Scholar] [CrossRef]
- Nassour, I.; Leger, C.L. Deposition and mobilisation of body fat during sexual maturation in female trout (Salmo gairdneri richardson). Aqua Liv. Res. 1989, 2, 153–159. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Kenney, P.B.; Manor, M.L.; Weber, G.M. Effects of feeding level and sexual maturation on carcass and fillet characteristics and indices of protein degradation in rainbow trout (Oncorhynchus mykiss). Aquaculture 2012, 338, 228–236. [Google Scholar] [CrossRef]
- Haux, C.; Norberg, B. The influence of estradiol 17-beta on the liver content of protein, lipids, glycogen and nucleic acids in juvenile rainbow trout. Salmo gairdneri. Comp. Biochem. Physiol. B 1985, 81, 275–279. [Google Scholar] [CrossRef]
- Reinboth, R. The peculiarities of gonad transformation in teleosts. Differentiation 1983, 23, S82–S86. [Google Scholar]
- Happe, A.; Zohar, Y. Self-fertilization in the protandrous hermaphrodite Sparus aurata: Development of the technology. In Reproduction in Fish-Basic and Applied Aspects in Endocrinology and Genetics; Zohar, Y., Breton, B., Eds.; Les Colloques de l´INRA, nº 44; INRA: Paris, France, 1988; pp. 177–180. [Google Scholar]
- Mommsen, T.P. Salmon spawning migration and muscle protein metabolism: The August Krogh principle at work. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 383–400. [Google Scholar] [CrossRef]
- Nazar, D.S.; Persson, G.; Olin, T.; Waters, S.; von der Decken, A. Sarcoplasmic and myofibrillar proteins in white truck muscle of salmon (Salmo salar) after estradiol treatment. Comp. Biochem. Physiol. B 1991, 98B, 109–114. [Google Scholar] [CrossRef]
- Olin, T.; Nazar, D.S.; von der Decken, A. Response of epaxial muscle and liver to 17-b estradiol in fed and starved Atlantic salmon Salmo salar. Aquaculture 1991, 99, 179–191. [Google Scholar] [CrossRef]
- Riley, L.G.; Hirano, T.; Grau, E.G. Disparate effects of gonadal steroid hormones on plasma and liver mRNA levels of insulin-like growth factor-I and vitellogenin in the tilapia, Oreochromis mossambicus. Fish Phys. Biochem. 2002, 26, 223–230. [Google Scholar] [CrossRef]
- Castillo, J.; Codina, M.; Martinez, M.L.; Navarro, I.; Gutierrez, J. Metabolic and mitogenic effects of IGF-I and insulin on muscle cells of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R935–R941. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Weber, G.M. Effects of insulin-like growth factor-I, insulin, and leucine on protein turnover and ubiquitin ligase expression in rainbow trout primary myocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R341–R350. [Google Scholar] [CrossRef]
- McCormick, S.D.; Kelley, K.M.; Young, G.; Nishioka, R.S.; Bern, H.A. Stimulation of coho salmon growth by insulin-like growth factor I. Gen. Comp. Endocrinol. 1992, 86, 398–406. [Google Scholar] [CrossRef]
- Lappano, R.M.; De Marco, P.; De Francesco, E.M.; Chimento, A.; Pezzi, V.; Maggiolini, M. Cross-talk between GPER and growth factor signaling. J. Steroid Biochem. Mol. Biol. 2013, 137, 50–56. [Google Scholar] [CrossRef]
- Usher, M.L.; Stickland, N.C.; Thorpe, J.E. Muscle development in Atlantic salmon (Salmo salar) embryos and the effect of temperature on muscle cellularity. J Fish Biol. 1994, 44, 953–964. [Google Scholar] [CrossRef]
- Johnston, I.A. Physiology of muscle in hatchery raised fish. Comp. Biochem. Physiol. 1982, 73B, 105–124. [Google Scholar] [CrossRef]
- Weatherley, A.H.; Gill, H.S. The Biology of Fish Growth; Academic Press: London, UK, 1987; 443p. [Google Scholar]
- Gagnière, H.; Picard, B.; Jurie, C.; Geay, Y. Comparative study of metabolic differentiation of foetal muscle in normal and double-muscled cattle. Meat Sci. 1997, 45, 145–152. [Google Scholar] [CrossRef]
- Oliván, M.; Martínez, A.; Osoro, K.; Sañudo, C.; Panea, P.; Olleta, J.L.; Campo, M.M.; Olivér, M.A.; Serra, X.; Gil, M.; et al. Effect of muscular hypertrophy on physico-chemical, biochemical and texture traits of meat from yearling bulls. Meat Sci. 2004, 68, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Bouley, J.; Meunier, B.; Chambon, C.; De Smet, S.; Hocquette, J.F.; Picard, B. Proteomic analysis of bovine skeletal muscle hypertrophy. Proteomics 2005, 5, 490–500. [Google Scholar] [CrossRef]
- Caballero, B.; Sierra, V.; Olivan, M.; Vega-Naredo, I.; Tomás-Zapico, C.; Alvarez-García, O.; Tolivia, D.; Hardeland, R.; Rodríguez-Colunga, M.J.; Coto-Montes, A. Activity of cathepsins during beef aging related to mutations in the myostatin gene. J Sci. Food Agric. 2007, 87, 192–199. [Google Scholar] [CrossRef]
- Sierra, V.; Aldai, N.; Castro, P.; Osoro, K.; Coto-Montes, A.; Oliván, M. Prediction of the fatty acid composition of beef by near infrared transmittance spectroscopy. Meat Sci. 2008, 78, 248–255. [Google Scholar] [CrossRef]
- Sierra, V.; Guerrero, L.; Fernández-Suárez, V.; Martínez, A.; Castro, P.; Osoro, K.; Rodríguez-Colunga, M.J.; Coto-Montes, A.; Oliván, M. Eating quality of beef from biotypes included in the PGI “Ternera Asturiana” showing distinct physicochemical characteristics and tenderization pattern V. Meat Sci. 2010, 86, 343–351. [Google Scholar] [CrossRef]
- García de la serrana, D.; Fuentes, E.N.; Martin, S.A.M.; Johnston, I.A.; Macqueen, D.J. Divergent regulation of insulin-like growth factor binding protein genes in cultures Atlantic salmon myotubes under different models of catabolism and anabolism. Gen. Comp. Endocrin. 2017, 247, 53–65. [Google Scholar] [CrossRef]
- Amaral, I.P.G.; Johnston, I.A. Insulin-like growth factor (IGF) signaling and genome-wide transcriptional regulation in fast muscle of zebrafish following a single-satiation meal. J. Exp. Biol. 2011, 214, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Lavajoo, F.; Perelló-Amarós, M.; Vélez, E.J.; Sánchez-Moya, A.; Balbuena-Pecino, S.; Riera-Heredia, N.; Fernández-Borràs, J.; Blasco, J.; Navarro, I.; Capilla, E.; et al. Regulatory mechanisms involved in muscle and bone remodeling during refeeding in gilthead sea bream. Sci. Rep. 2020, 13, 184. [Google Scholar] [CrossRef]
- Salmerón, D.; Garcia de la serrana, D.; Jiménez-Amilburu, V.; Fontanillas, R.; Navarro, I.; Johnston, I.A.; Gutiérrez, J.; Capilla, E. Characterisation and expression of calpain family members in relation to nutritional status, diet composition and flesh texture in gilthead sea bream (Sparus aurata). PLoS ONE 2013, 8, e75349. [Google Scholar] [CrossRef] [PubMed]
- Shved, N.; Berishvili, G.; D’Cotta, H.; Baroiller, J.F.; Segner, H.; Eppler, E.; Reinecke, M. Ethinylestradiol differentially interferes with IGF-I in liver and extrahepatic sites during development of male and female bony fish. J. Endocrinol. 2007, 195, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.D.; Abellán, E.; Arizcun, M.; García-Alcázar, A.; Navarro, F.; Blanco, A.; López-Albors, O. Muscle development and body growth in larvae and early postlarvae of shi drum, Umbrina cirrosa L., reared under different larval photoperiod. Muscle structural and ultrastructural Study. Fish Physiol. Biochem. 2013, 39, 807–827. [Google Scholar] [CrossRef]
- Ayala, M.D.; Arizcun, M.; García-Alcázar, A.; Santaella, M.; Abellán, E. Long-term effects of the larval photoperiod on the subsequent growth of shi drum Umbrina cirrosa L. specimens and the fillet texture at commercial size. Turk. J. Fish Aquat. Sci. 2015, 15, 93–101. [Google Scholar] [CrossRef]
- Dubowitz, V.; Brooke, M.H. Muscle Biopsy: A Modern Approach; Saunders. W.B.: London, UK, 1973. [Google Scholar]
- Gil, F. Diferenciación Postnatal de los Tipos de Miofibrillas de Algunos Músculos del Perro. Ph.D. Thesis, University of Murcia, Murcia, Spain, 1986. [Google Scholar]
- Cunniff, P.; AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; The Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1999. [Google Scholar]
- Friedman, H.H.; Whitney, J.E.; Szczesniak, A.S. The texturometer: A new instrument for objective texture measurement. J. Food Sci. 1962, 28, 390–396. [Google Scholar] [CrossRef]
- Wickham, H. Programming with ggplot2. In ggplot2. Use R! Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
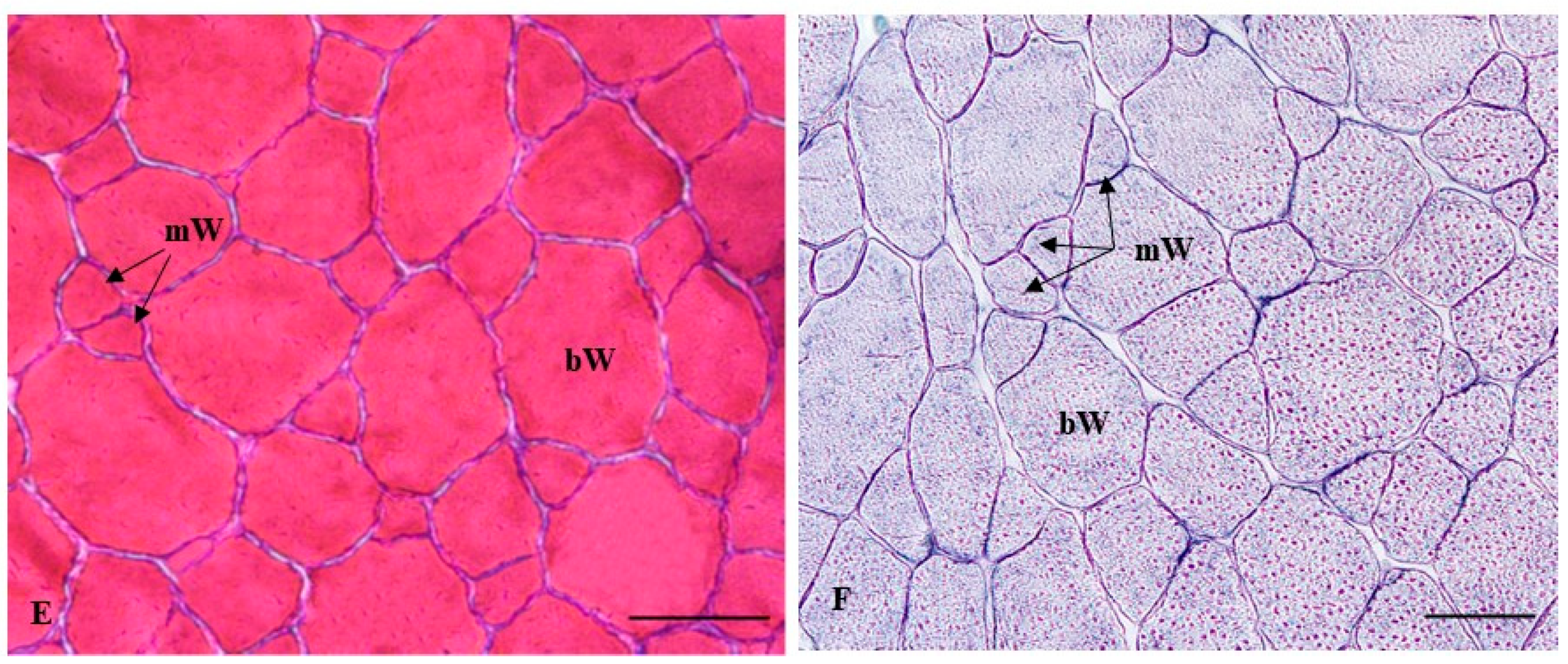
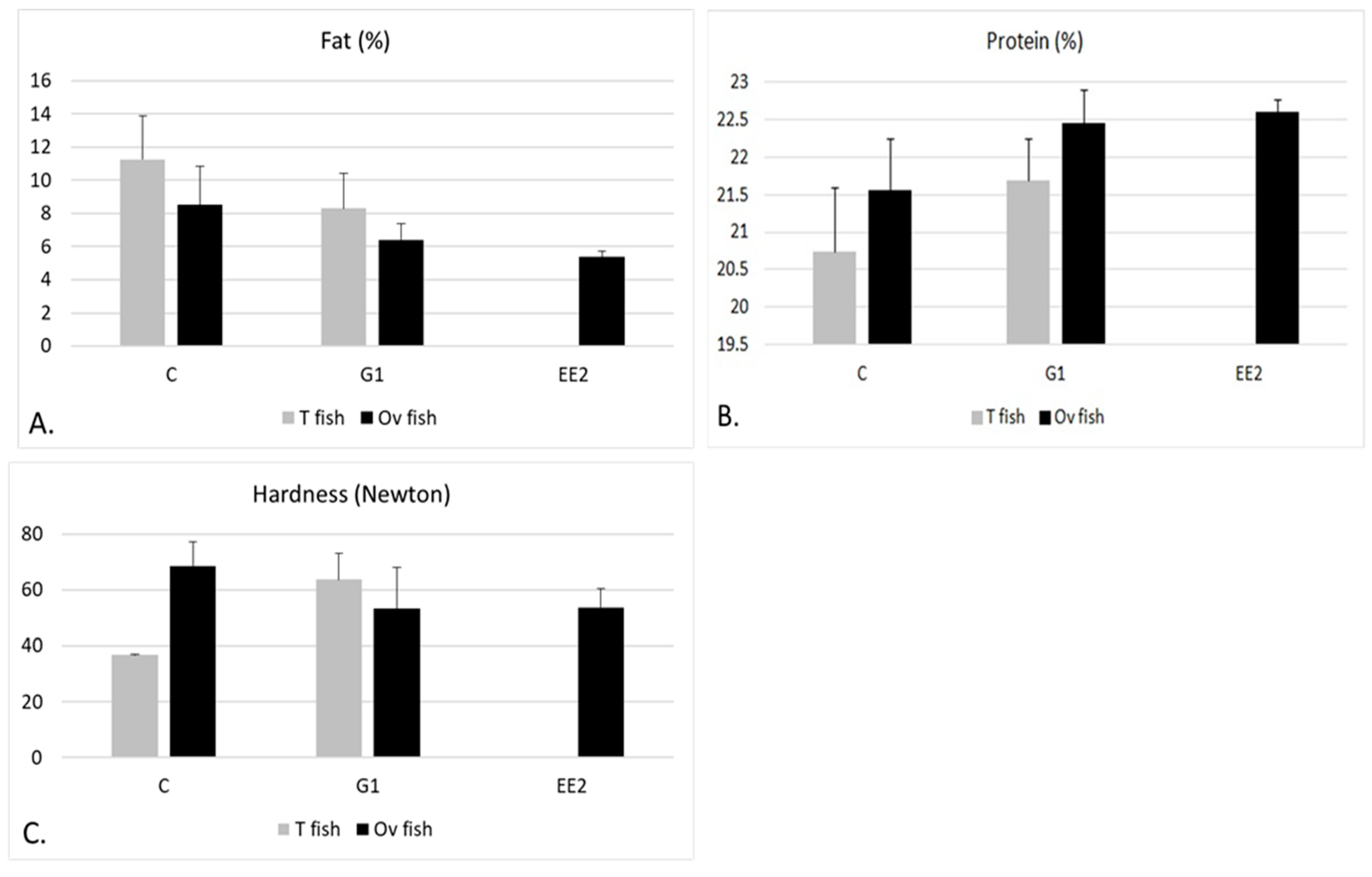
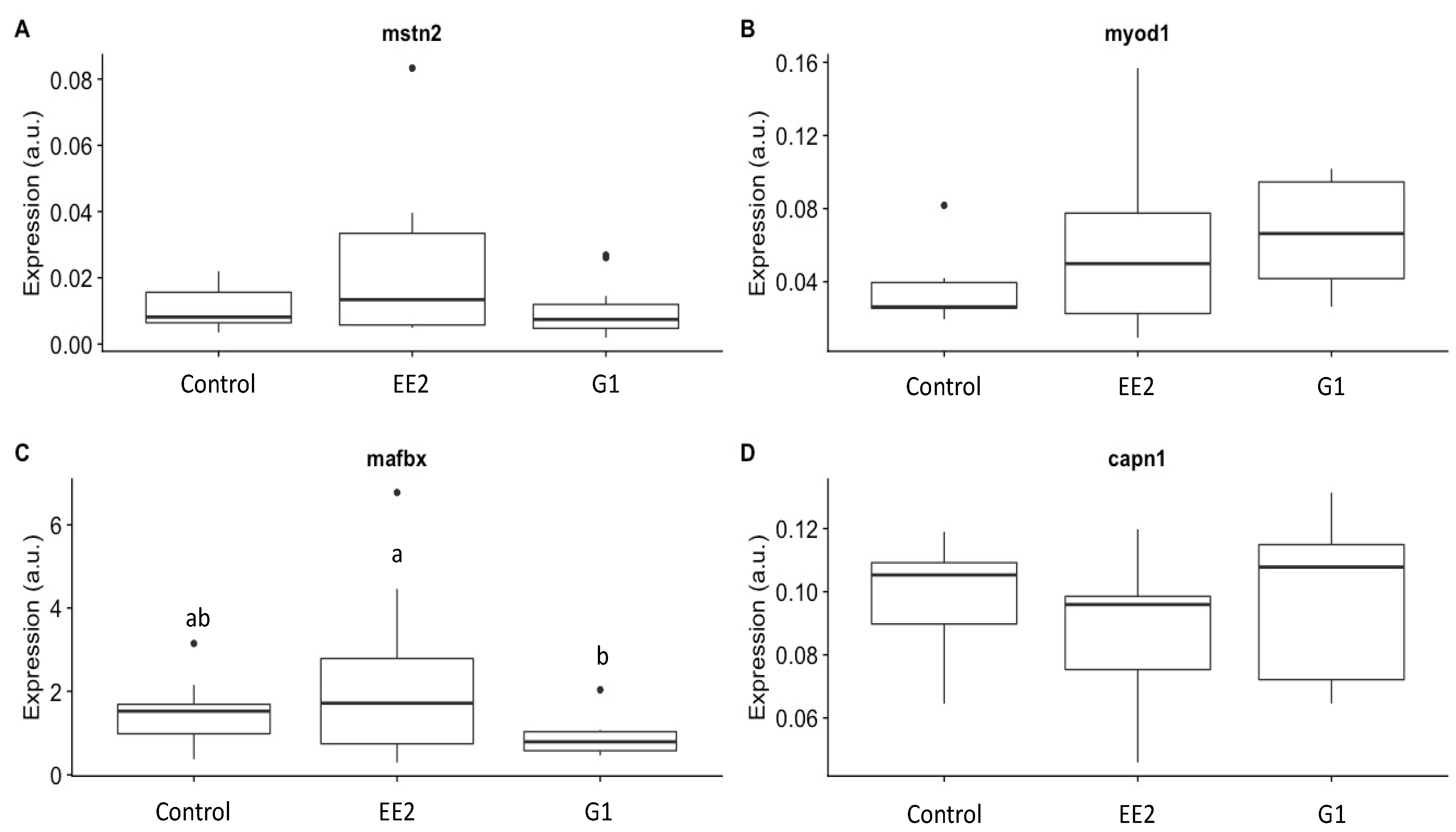
| Stage | Groups | BW (g) | GW (g) | BL (cm) | CF (100 × g cm−3) | HSI (%) | GSI (%) |
|---|---|---|---|---|---|---|---|
| First (on day 45 of treatment) | C | 314.4 a ± 8.5 | 286.86 a ± 7.41 | 26.6 a ± 0.2 | 1.68 ab ± 0.1 | 2.01 a ± 0.08 | 0.29 a ± 0.06 |
| EE2 | 292.7 a ± 8.76 | 265.8 a ± 7.8 | 26.75 a ± 0.2 | 1.53 b ± 0.01 | 2.53 a ± 0.22 | 0.26 a ± 0.1 | |
| G1 | 288.6 a ± 6.41 | 262.8 a ± 5.98 | 25.67 b ± 0.3 | 1.71 a ± 0.05 | 2.4 a ± 0.06 | 0.19 a ± 0.04 | |
| Second (79 days after treatment cessation) | C | 374.9 a ± 9.85 | 344.2 a ± 9.54 | 28.42 a ± 0.47 | 1.6 a ± 0.06 | 1.5 a ± 0.3 | 0.22 a ± 0.04 |
| EE2 | 300.23 b ± 12.16 | 292.6 b ± 7.12 | 27.17 b ± 0.21 | 1.5 a ± 0.08 | 1.87 a ± 0.57 | 0.2 a ± 0.03 | |
| G1 | 366.03 a ± 9.09 | 336.2 a ± 9.18 | 27.92 ab ± 0.2 | 1.7 a ± 0.03 | 1.28 a ± 0.06 | 0.23 a ± 0.03 | |
| Third (122 days after treatment cessation) | C | 428.23 ab ± 8.85 | 397.5 ab ± 8.24 | 29.0 a ± 0.36 | 1.76 a ± 0.05 | 0.93 a ± 0.05 | 0.22 a ± 0.05 |
| EE2 | 384.38 b ± 13.66 | 355.47 b ± 12.99 | 28.5 a ± 0.22 | 1.66 a ± 0.04 | 0.91 a ± 0.004 | 0.22 a ± 0.03 | |
| G1 | 471.57 a ± 23.7 | 436.9 a ± 21.91 | 29.6 a ± 0.42 | 1.82 a ± 0.05 | 0.95 a ± 0.05 | 0.18 a ± 0.02 |
| Stage | Groups | W (mm2) | R (mm2) | A (μm2) | D (μm) | N | Dens |
|---|---|---|---|---|---|---|---|
| First (on day 45 of treatment) | C | 1251.2 a ± 35.6 | 204.5 a ± 9.6 | 5246.52 a ± 248.5 | 74.5 a ± 2.28 | 241,808.4 a ± 15,472.2 | 192.64 a ± 8.6 |
| EE2 | 1327.1 a ± 49.2 | 224.75 a ± 9.4 | 5676.8 a ± 137.5 | 77.2 a ± 0.85 | 233,501.89 a ± 4373.5 | 176.69 a ± 4.46 | |
| G1 | 1281.9 a ± 25.5 | 223.96 a ± 8.4 | 5773.6 a ± 265.4 | 78.8 a ± 1.73 | 223,892.97 a ± 9215.5 | 174.9 a ± 7.43 | |
| Second (79 days after treatment cessation) | C | 1632.32 a ± 38.46 | 307.69 a ± 20.04 | 6825.29 a ± 440.66 | 85.40 a ± 3.2 | 244,515.14 a ± 17,359.1 | 149.7 a ± 9.8 |
| EE2 | 1580.99 a ± 50.36 | 287.15 a ± 11.44 | 6871.26 a ± 492.99 | 85.43 a ± 2.9 | 236,680.22 a ± 19,501.24 | 149.03 a ± 9.9 | |
| G1 | 1608.37 a ± 67.2 | 318.86 a ± 10.75 | 7266.59 a ± 314.0 | 88.3 a ± 1.95 | 223,948.69 a ± 14,958.87 | 138.87 a ± 5.85 | |
| Third (122 days after treatment cessation) | C | 2166.78 a ± 62.8 | 377.6 a ± 18.5 | 6853.4 a ± 526.3 | 82.4 a ± 3.4 | 323,080.1 a ± 20,136.3 | 149.8 a ± 10.2 |
| EE2 | 1924.18 a ± 80.9 | 340.3 a ± 10.7 | 8813.9 a ± 994.3 | 96.0 a ± 5.7 | 235,558.5 a ± 34,306.7 | 120.4 a ± 12.78 | |
| G1 | 2063.9 a ± 90.1 | 374.6 a ± 12.9 | 7916.96 a ± 216.8 | 89.5 a ± 1.19 | 262,117.11 a ± 15,718.8 | 126.8 a ± 3.4 |
| Stage | Groups | Protein (%) | Fat (%) | Hardness (N) |
|---|---|---|---|---|
| First (on day 45 of treatment) | C | 22.77 a ± 0.29 | 1.63 ab ± 0.1 | 29.42 a ± 1.7 |
| EE2 | 22.50 a ± 0.33 | 1.28 b ± 0.04 | 29.21 a ± 1.14 | |
| G1 | 22.30 a ± 0.42 | 1.67 a ± 0.12 | 23.87 a ± 2.43 | |
| Second (79 days after treatment cessation) | C | 22.45 a ± 0.14 | 3.52 a ± 0.59 | 49.29 a ± 2.78 |
| EE2 | 21.87 a ± 0.2 | 2.93 a ± 0.36 | 46.7 a ± 2.55 | |
| G1 | 22.38 a ± 0.34 | 3.47 a ± 0.33 | 47.14 a ± 4.87 | |
| Third (122 days after treatment cessation) | C | 21.3 a ± 0.51 | 9.43 a ± 1.73 | 58.06 a ± 8.61 |
| EE2 | 22.6 a ± 0.17 | 5.38 a ± 0.37 | 53.8 a ± 6.64 | |
| G1 | 22.03 a ± 0.31 | 7.18 a ± 1.1 | 56.16 a ± 6.93 |
| Genes | BW | BL | W | R | T | Fat | Prot | H | A | D | N | Dens |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mstn2 | 0.1 | 0.1 | 0.3 · | 0.4 | 0.3 * | 0.3 · | −0.3 | 0.4 ** | 0.5 ** | 0.4 ** | −0.2 | −0.5 ** |
| capn1 | −0.3 · | −0.3 · | −0.4 ** | −0.5 ** | −0.5 ** | −0.1 | −0.1 | −0.2 | −0.3 * | −0.3 * | 0 | 0.4 * |
| mafbx | −0.5 ** | −0.3 · | −0.4 * | −0.5 ** | −0.4 * | −0.2 | −0.2 | −0.4 * | −0.4 * | −0.4 ** | −0.4 * | 0.4 * |
| myod | −0.2 | −0.2 | −0.3 · | −0.2 | −0.3 · | −0.2 | 0.1 | −0.2 | −0.1 | −0.1 | −0.2 | 0.1 |
| Gene | ID | Primers (5′–3′) | Tm (°C) | E (%) | Size (bp) |
|---|---|---|---|---|---|
| myod2 | ENSSAUG00010004893 | F:CACTACAGCGGGGATTCAGAC | 83.3 | 99 | 149 |
| R:CGTTTGCTTCTCCTGGACTC | |||||
| mstn2 | ENSSAUG00010004791 | F:ACCTGGTGAACAAAGCCAAC | 84.8 | 99 | 201 |
| R:TGCGGTTGAAGTAGAGCATG | |||||
| capn1 | ENSSAUG00010025995 | F:CCTACGAGATGAGGATGGCT | 87 | 95 | 114 |
| R:AGTTGTCAAAGTCGGCGGT | |||||
| mafbx | ENSG00000156804 | F:GGTGCAACTTTCTGGGTTGT | 85 | 97 | 105 |
| R:GGTCACCTGGAGTGGAAGAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, M.D.; Gómez, V.; Cabas, I.; García Hernández, M.P.; Chaves-Pozo, E.; Arizcun, M.; Garcia de la Serrana, D.; Gil, F.; García-Ayala, A. The Effect of 17α-Ethynilestradiol and GPER1 Activation on Body and Muscle Growth, Muscle Composition and Growth-Related Gene Expression of Gilthead Seabream, Sparus aurata L. Int. J. Mol. Sci. 2021, 22, 13118. https://doi.org/10.3390/ijms222313118
Ayala MD, Gómez V, Cabas I, García Hernández MP, Chaves-Pozo E, Arizcun M, Garcia de la Serrana D, Gil F, García-Ayala A. The Effect of 17α-Ethynilestradiol and GPER1 Activation on Body and Muscle Growth, Muscle Composition and Growth-Related Gene Expression of Gilthead Seabream, Sparus aurata L. International Journal of Molecular Sciences. 2021; 22(23):13118. https://doi.org/10.3390/ijms222313118
Chicago/Turabian StyleAyala, Maria D., Victoria Gómez, Isabel Cabas, María P. García Hernández, Elena Chaves-Pozo, Marta Arizcun, Daniel Garcia de la Serrana, Francisco Gil, and Alfonsa García-Ayala. 2021. "The Effect of 17α-Ethynilestradiol and GPER1 Activation on Body and Muscle Growth, Muscle Composition and Growth-Related Gene Expression of Gilthead Seabream, Sparus aurata L" International Journal of Molecular Sciences 22, no. 23: 13118. https://doi.org/10.3390/ijms222313118
APA StyleAyala, M. D., Gómez, V., Cabas, I., García Hernández, M. P., Chaves-Pozo, E., Arizcun, M., Garcia de la Serrana, D., Gil, F., & García-Ayala, A. (2021). The Effect of 17α-Ethynilestradiol and GPER1 Activation on Body and Muscle Growth, Muscle Composition and Growth-Related Gene Expression of Gilthead Seabream, Sparus aurata L. International Journal of Molecular Sciences, 22(23), 13118. https://doi.org/10.3390/ijms222313118







