Derivation of Porcine Extra-Embryonic Endoderm Cell Lines Reveals Distinct Signaling Pathway and Multipotency States
Abstract
:1. Introduction
2. Results
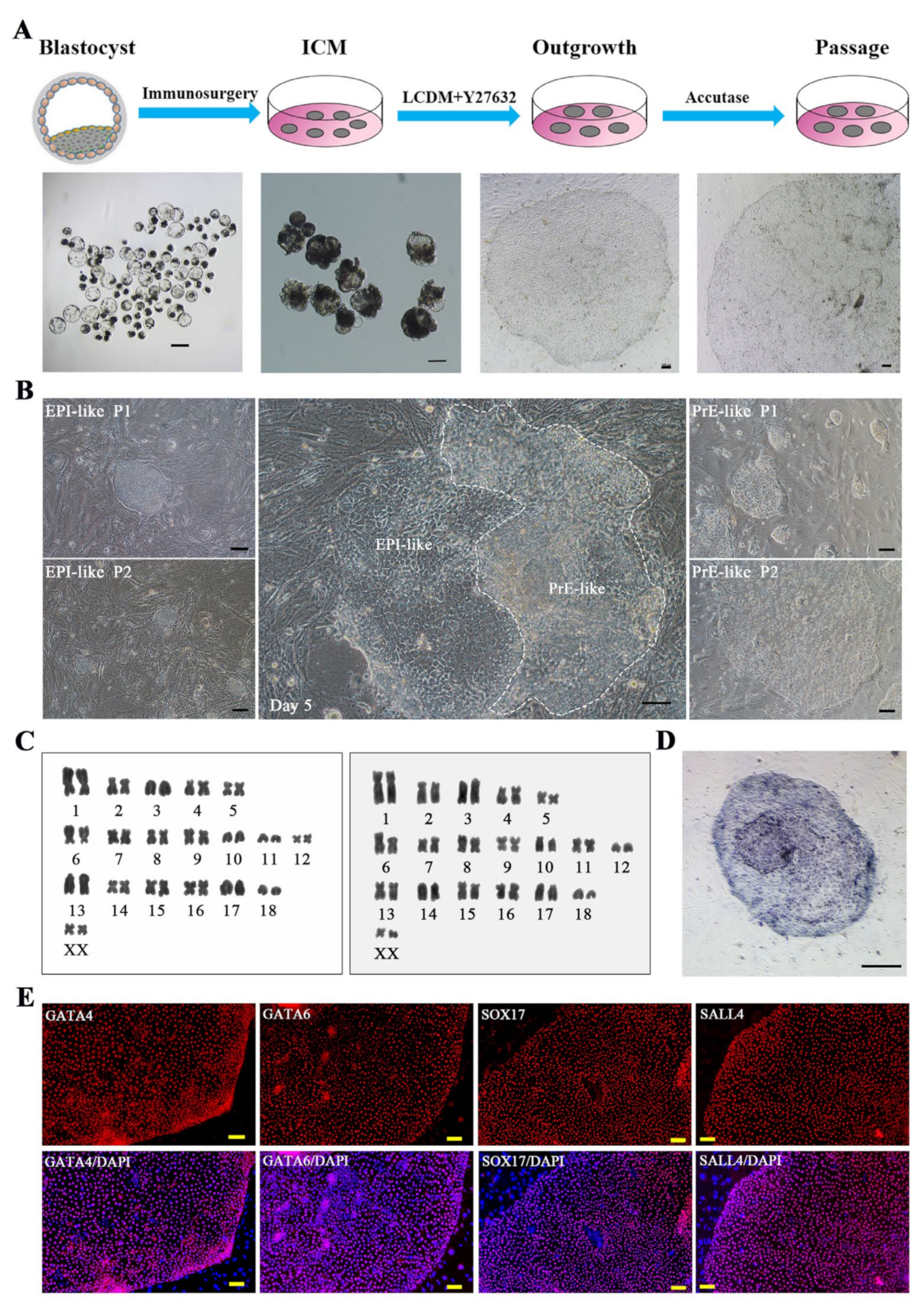
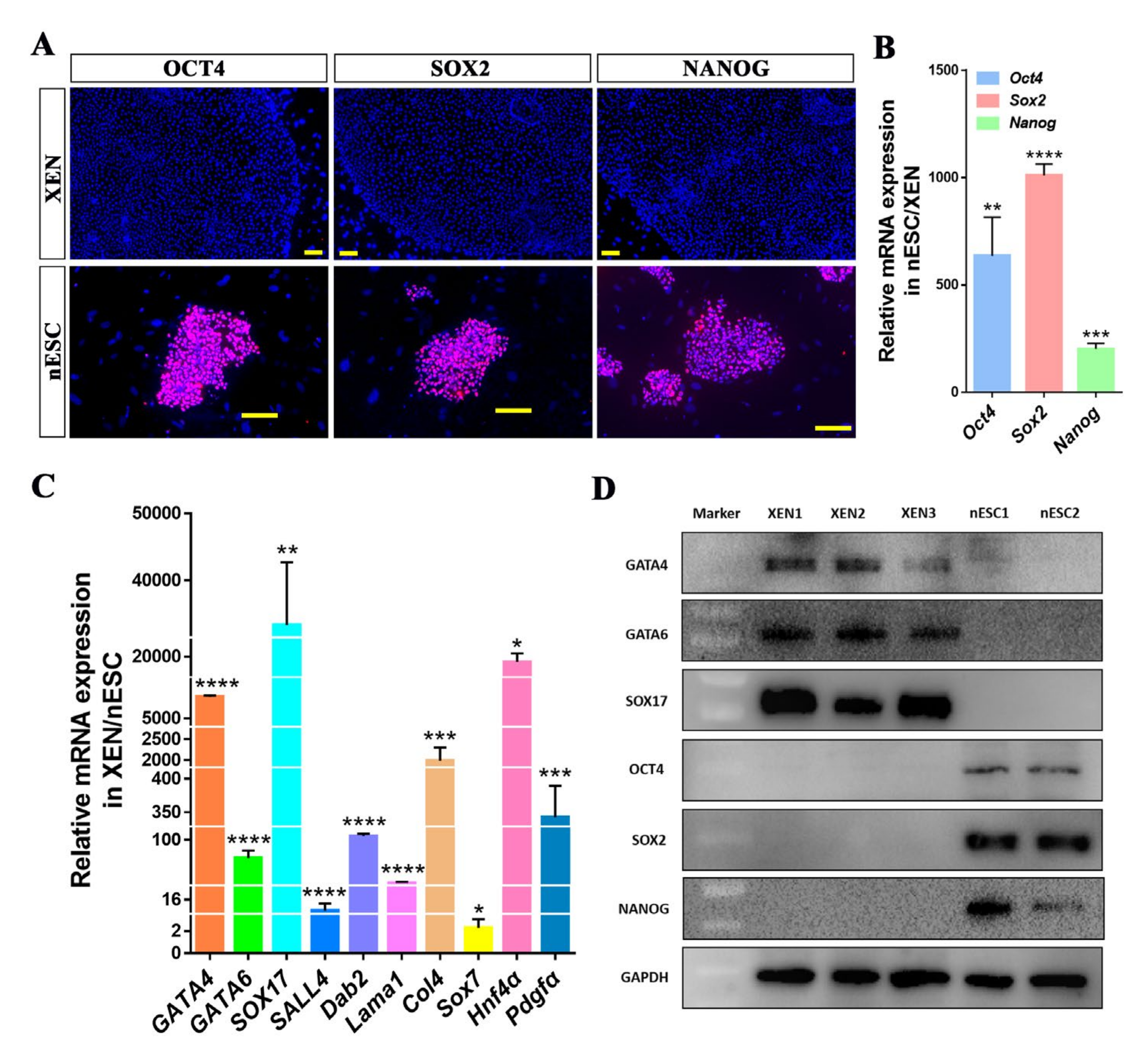
2.1. Derivation of Porcine XEN Cell Lines from Blastocysts
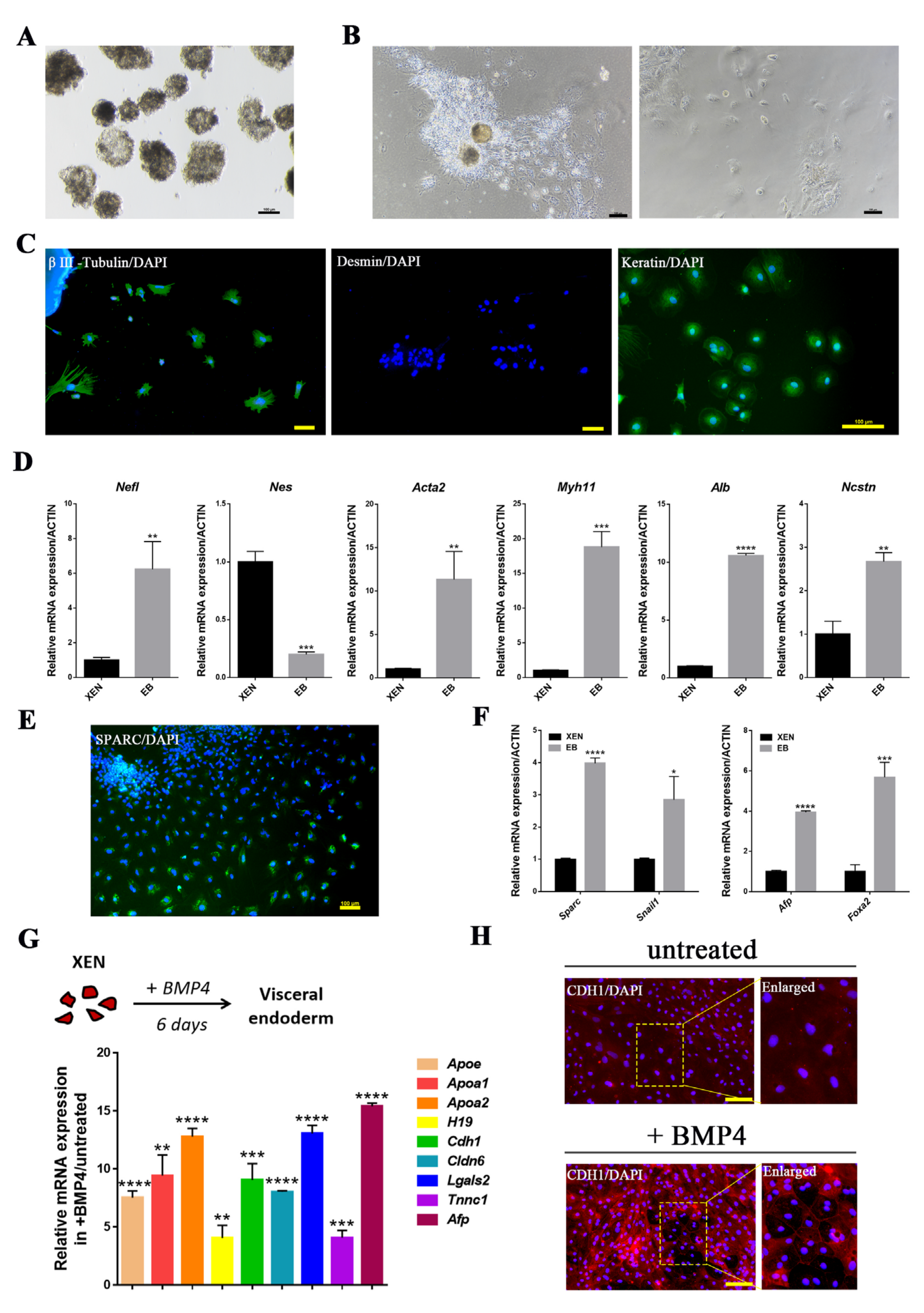
2.2. In Vitro Differentiation Potential of pXEN Cells
2.3. Maintenance of pXEN Cells Was Dependent on FGF/MEK and TGFβ Signaling
2.4. Conversion of Porcine Naïve-like ESCs to XEN Cells
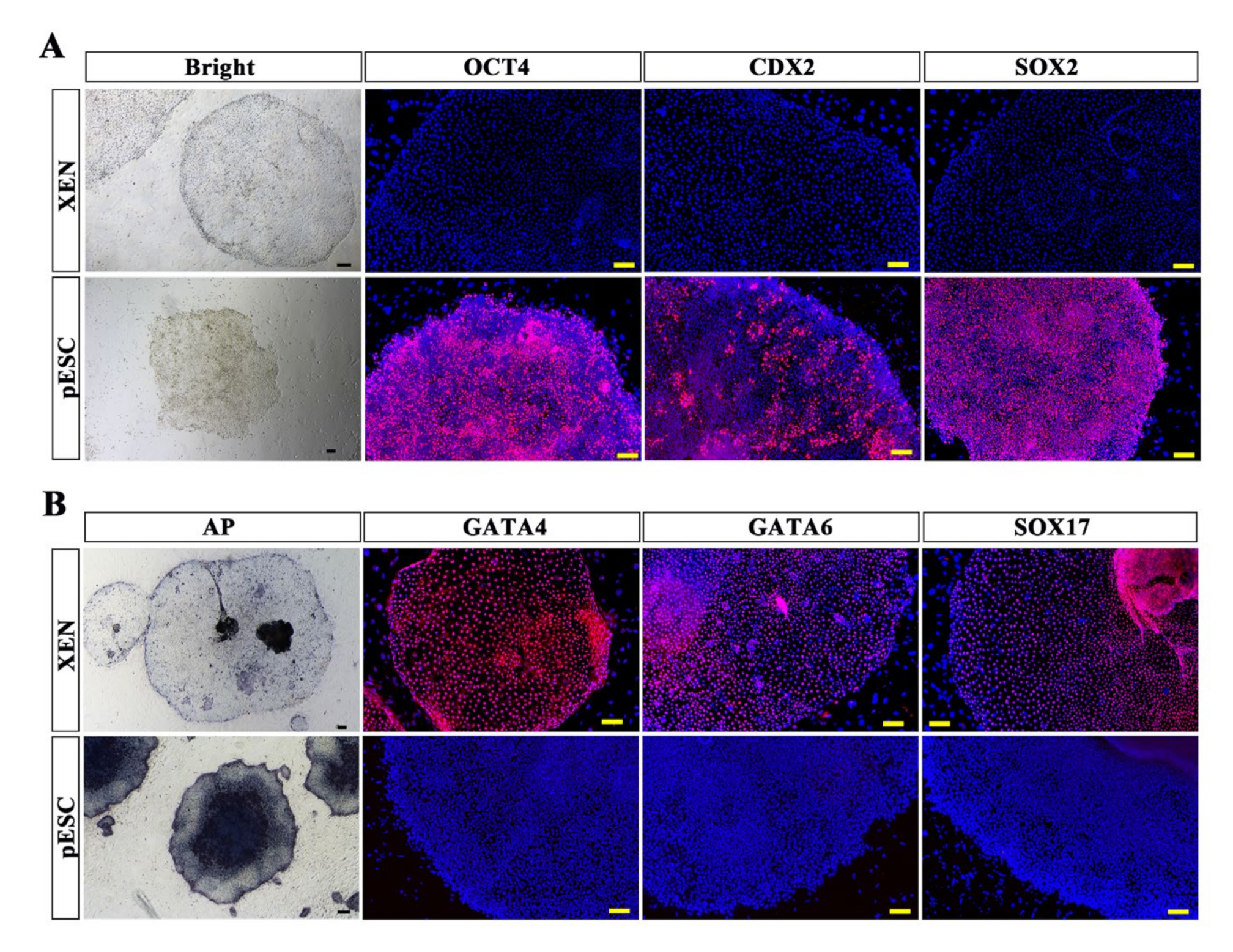
2.5. Comparison of pXEN Cells and Primed ESCs
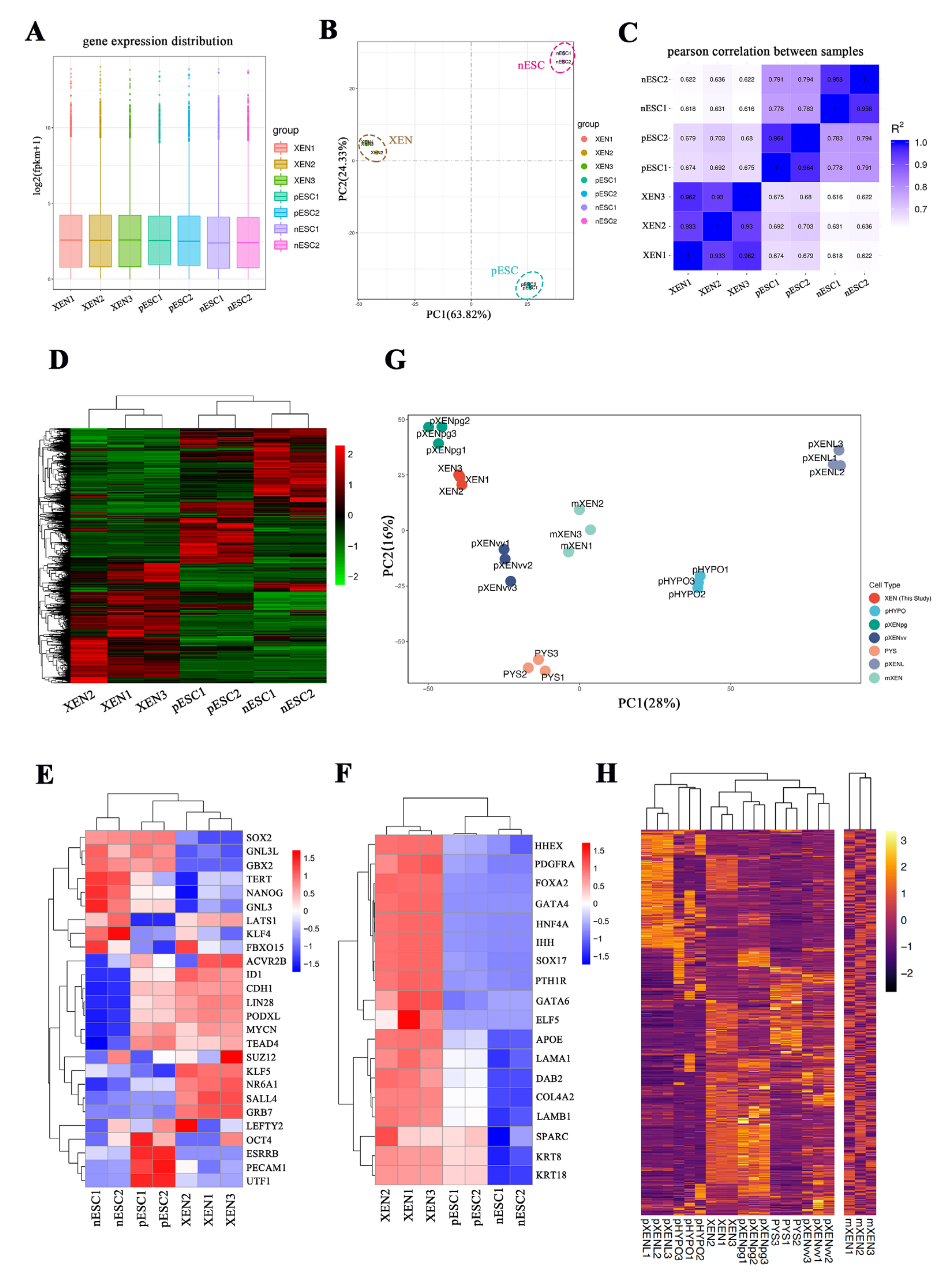
2.6. RNA-seq Analysis of Porcine XEN Cells, Naïve-like and Primed Embryonic Stem Cells
3. Discussion
4. Materials and Methods
4.1. Porcine XEN Cell Lines Derivation and Maintenance
4.2. Culture Conditions for Different States of Porcine Pluripotent Stem Cells
4.3. Alkaline Phosphatase Staining
4.4. Karyotyping
4.5. Immunofluorescence
4.6. Gene Expression Analysis
4.7. Western Blotting and the Quatification of Protein Levels
4.8. Embryoid Body (EB) Formation Assay
4.9. VE and PE Differentiation
4.10. Signaling Pathway Analysis
4.11. RNA-seq Analysis of Global Gene Expression
4.12. Transcriptomics Data Availability
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESCs | Embryonic stem cells |
| pXEN | Porcine extra-embryonic endoderm |
| VE | Visceral endoderm |
| PE | Parietal endoderm |
| ICM | Inner cell mass |
| TE | Trophectoderm |
| PrE | Primitive endoderm |
| EPI | Epiblast |
| ExEn | Extra-embryonic endoderm |
| nESCs | Naïve-like ESCs |
| pESCs | Primed ESCs |
| AP | Alkaline phosphatase |
| EB | Embryoid body |
| KOSR | Knockout serum replacement |
References
- Gardner, R.L. Clonal analysis of early mammalian development. Philos. Trans. R. Soc. B Biol. Sci. 1985, 312, 163–178. [Google Scholar] [CrossRef]
- Rossant, J.; Tam, P.P.L. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 2009, 136, 701–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiz, N.; Plusa, B. Early cell fate decisions in the mouse embryo. Reproduction 2013, 145, R65–R80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [Green Version]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of Pluripotent Stem Cells in the Mammalian Embryo Depends on the POU Transcription Factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Avilion, A.A.; Nicolis, S.K.; Pevny, L.H.; Perez, L.; Vivian, N.; Lovell-Badge, R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003, 17, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional Expression Cloning of Nanog, a Pluripotency Sustaining Factor in Embryonic Stem Cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Kunath, T.; Hadjantonakis, A.-K.; Nagy, A.; Rossant, J. Promotion of Trophoblast Stem Cell Proliferation by FGF4. Science 1998, 282, 2072–2075. [Google Scholar] [CrossRef]
- Kunath, T.; Arnaud, D.; Uy, G.D.; Okamoto, I.; Chureau, C.; Yamanaka, Y.; Heard, E.; Gardner, R.L.; Avner, P.; Rossant, J. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development 2005, 132, 1649–1661. [Google Scholar] [CrossRef] [Green Version]
- Perleberg, C.; Kind, A.; Schnieke, A. Genetically engineered pigs as models for human disease. Dis. Model. Mech. 2018, 11, dmm030783. [Google Scholar] [CrossRef] [Green Version]
- Haraguchi, S.; Kikuchi, K.; Nakai, M.; Tokunaga, T. Establishment of Self-renewing Porcine Embryonic Stem Cell-like Cells by Signal Inhibition. J. Reprod. Dev. 2012, 58, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.-H.; Lee, D.-K.; Kim, S.W.; Woo, S.-H.; Kim, D.-Y.; Lee, C.-K. Chemically Defined Media Can Maintain Pig Pluripotency Network In Vitro. Stem Cell Rep. 2019, 13, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Telugu, B.P.V.L.; Ezashi, T.; Sinha, S.; Alexenko, A.P.; Spate, L.; Prather, R.; Roberts, R.M. Leukemia Inhibitory Factor (LIF)-dependent, Pluripotent Stem Cells Established from Inner Cell Mass of Porcine Embryos. J. Biol. Chem. 2011, 286, 28948–28953. [Google Scholar] [CrossRef] [Green Version]
- Hou, D.-R.; Jin, Y.; Nie, X.-W.; Zhang, M.-L.; Ta, N.; Zhao, L.-H.; Yang, N.; Chen, Y.; Wu, Z.-Q.; Jiang, H.-B.; et al. Derivation of Porcine Embryonic Stem-Like Cells from In Vitro-Produced Blastocyst-Stage Embryos. Sci. Rep. 2016, 6, 25838. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, C.; Jiang, H.; Liu, M.; Yang, N.; Zhao, L.; Hou, D.; Jin, Y.; Chen, Q.; Chen, Y.; et al. Derivation of novel naive-like porcine embryonic stem cells by a reprogramming factor-assisted strategy. FASEB J. 2019, 33, 9350–9361. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, B.; Xu, J.; Wang, J.; Wu, J.; Shi, C.; Xu, Y.; Dong, J.; Wang, C.; Lai, W.; et al. Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell 2017, 169, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Nowak-Imialek, M.; Chen, X.; Chen, D.; Herrmann, D.; Ruan, D.; Chen, A.C.H.; Eckersley-Maslin, M.A.; Ahmad, S.; Lee, Y.L.; et al. Establishment of porcine and human expanded potential stem cells. Nat. Cell. Biol. 2019, 21, 687–699. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.A.; Teo, R.T.Y.; Li, H.; Robson, P.; Glover, D.M.; Zernicka-Goetz, M. Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo. Proc. Natl. Acad. Sci. USA 2010, 107, 6364–6369. [Google Scholar] [CrossRef] [Green Version]
- Capo-Chichi, D.C.; Rula, M.E.; Smedberg, J.L.; Vanderveer, L.; Parmacek, M.; Morrisey, E.E.; Godwin, A.K.; Xu, X.-X. Perception of differentiation cues by GATA factors in primitive endoderm lineage determination of mouse embryonic stem cells. Dev. Biol. 2005, 286, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Wamaitha, S.E.; del Valle, I.; Cho, L.T.; Wei, Y.; Fogarty, N.M.; Blakeley, P.; Sherwood, R.I.; Ji, H.; Niakan, K.K. Gata6 potently initiates reprograming of pluripotent and differentiated cells to extraembryonic endoderm stem cells. Genes Dev. 2015, 29, 1239–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niakan, K.K.; Ji, H.; Maehr, R.; Vokes, S.A.; Rodolfa, K.T.; Sherwood, R.I.; Yamaki, M.; Dimos, J.T.; Chen, A.E.; Melton, D.A.; et al. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev. 2010, 24, 312–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niakan, K.; Schrode, N.; Cho, L.T.Y.; Hadjantonakis, A.-K. Derivation of extraembryonic endoderm stem (XEN) cells from mouse embryos and embryonic stem cells. Nat. Protoc. 2013, 8, 1028–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, L.T.Y.; Wamaitha, S.; Tsai, I.J.; Artus, J.; Sherwood, R.I.; Pedersen, R.A.; Hadjantonakis, A.-K.; Niakan, K.K. Conversion from mouse embryonic to extra-embryonic endoderm stem cells reveals distinct differentiation capacities of pluripotent stem cell states. Development 2012, 139, 2866–2877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parenti, A.; Halbisen, M.A.; Wang, K.; Latham, K.; Ralston, A. OSKM Induce Extraembryonic Endoderm Stem Cells in Parallel to Induced Pluripotent Stem Cells. Stem Cell Rep. 2016, 6, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wu, S.; Yu, Y.; Zhang, H.; Wei, R.; Lv, J.; Cai, M.; Yang, X.; Zhang, Y.; Liu, Z. Derivation of porcine extraembryonic endoderm-like cells from blastocysts. Cell Prolif. 2020, 53, e12782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.-H.; Jeoung, Y.-H.; Uh, K.-J.; Park, K.-E.; Bridge, J.; Powell, A.; Li, J.; Pence, L.; Zhang, L.; Liu, T.; et al. Extraembryonic Endoderm (XEN) Cells Capable of Contributing to Embryonic Chimeras Established from Pig Embryos. Stem Cell Rep. 2021, 16, 212–223. [Google Scholar] [CrossRef]
- Shen, Q.; Yu, S.; Zhang, Y.; Zhou, Z.; Zhu, Z.; Pan, Q.; Lv, S.; Niu, H.; Li, N.; Peng, S.; et al. Characterization of porcine extraembryonic endoderm cells. Cell Prolif. 2019, 52, e12591. [Google Scholar] [CrossRef] [Green Version]
- Artus, J.; Douvaras, P.; Piliszek, A.; Isern, J.; Baron, M.H.; Hadjantonakis, A.-K. BMP4 signaling directs primitive endoderm-derived XEN cells to an extraembryonic visceral endoderm identity. Dev. Biol. 2012, 361, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.G.V.; Hamilton, W.B.; Roske, F.V.; Azad, A.; Knudsen, T.; Canham, M.; Forrester, L.M.; Brickman, J.M. Insulin fine-tunes self-renewal pathways governing naive pluripotency and extra-embryonic endoderm. Nature 2017, 19, 1164–1177. [Google Scholar] [CrossRef]
- Chazaud, C.; Yamanaka, Y.; Pawson, T.; Rossant, J. Early Lineage Segregation between Epiblast and Primitive Endoderm in Mouse Blastocysts through the Grb2-MAPK Pathway. Dev. Cell 2006, 10, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhang, H.; Jiang, H.; Zhang, M.; Wang, J.; Zhao, L.; Wang, C.; Liu, M.; Li, R. Conversion between porcine naïve-like and primed ESCs and specific pluripotency marker identification. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 412–423. [Google Scholar] [CrossRef]
- Grabarek, J.B.; Żyżyńska, K.; Saiz, N.; Piliszek, A.; Frankenberg, S.; Nichols, J.; Hadjantonakis, A.K.; Plusa, B. Differential plasticity of epiblast and primitive endoderm precursors within the ICM of the early mouse embryo. Development 2011, 139, 129. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Choi, T.; Kim, M.; Jung, K.H.; Chai, Y.G.; Binas, B. Isolation of primitive mouse extraembryonic endoderm (pXEN) stem cell lines. Stem Cell Res. 2018, 30, 100–112. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Sang, F.; Zhu, Q.; Tang, W.W.C.; Withey, S.; Klisch, D.; Wood, L.; Loose, M.; Surani, M.A.; Alberio, R. Pluripotency and X chromosome dynamics revealed in pig pre-gastrulating embryos by single cell analysis. Nat. Commun. 2019, 10, 500. [Google Scholar] [CrossRef] [Green Version]
- Keefer, C.; Pant, D.; Blomberg, L.; Talbot, N. Challenges and prospects for the establishment of embryonic stem cell lines of domesticated ungulates. Anim. Reprod. Sci. 2007, 98, 147–168. [Google Scholar] [CrossRef]
- Fujikura, J.; Yamato, E.; Yonemura, S.; Hosoda, K.; Masui, S.; Nakao, K.; Miyazaki, J.-I.; Niwa, H. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002, 16, 784–789. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yu, L.; Guo, J.; Xiang, J.; Zheng, Z.; Gao, D.; Shi, B.; Hao, H.; Jiao, D.; Zhong, L.; et al. Generation of pig induced pluripotent stem cells using an extended pluripotent stem cell culture system. Stem Cell Res. Ther. 2019, 10, 193. [Google Scholar] [CrossRef]
- Ogino, H.; Nozaki, T.; Gunji, A.; Maeda, M.; Suzuki, H.; Ohta, T.; Murakami, Y.; Nakagama, H.; Sugimura, T.; Masutani, M. Loss of Parp-1 affects gene expression profile in a genome-wide manner in ES cells and liver cells. BMC Genom. 2007, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; Huss, M.; Tong, G.Q.; Wang, C.; Sun, L.L.; Clarke, N.D.; Robson, P. Resolution of Cell Fate Decisions Revealed by Single-Cell Gene Expression Analysis from Zygote to Blastocyst. Dev. Cell 2010, 18, 675–685. [Google Scholar] [CrossRef] [Green Version]
- Moscatelli, D.; Presta, M.; Joseph-Silverstein, J.; Rifkin, D.B. Both normal and tumor cells produce basic fibroblast growth factor. J. Cell. Physiol. 1986, 129, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Mesnard, D.; Guzman-Ayala, M.; Constam, D.B. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development 2006, 133, 2497–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhao, T.; Guan, J.; Zhang, X.; Fu, Y.; Ye, J.; Zhu, J.; Meng, G.; Ge, J.; Yang, S.; et al. A XEN-like State Bridges Somatic Cells to Pluripotency during Chemical Reprogramming. Cell 2015, 163, 1678–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, D.; Ma, Y.; Du, X.; Jing, J.; Wang, L.; Xie, B.; Sun, D.; Sun, S.; Jin, X.; et al. Direct Reprogramming of Fibroblasts via a Chemically Induced XEN-like State. Cell Stem Cell 2017, 21, 264–273. [Google Scholar] [CrossRef]
- Lai, L.; Prather, R.S. Production of Cloned Pigs by Using Somatic Cells as Donors. Cloning Stem Cells 2003, 5, 233–241. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.-L.; Jin, Y.; Zhao, L.-H.; Zhang, J.; Zhou, M.; Li, M.-S.; Yin, Z.-B.; Wang, Z.-X.; Zhao, L.-X.; Li, X.-H.; et al. Derivation of Porcine Extra-Embryonic Endoderm Cell Lines Reveals Distinct Signaling Pathway and Multipotency States. Int. J. Mol. Sci. 2021, 22, 12918. https://doi.org/10.3390/ijms222312918
Zhang M-L, Jin Y, Zhao L-H, Zhang J, Zhou M, Li M-S, Yin Z-B, Wang Z-X, Zhao L-X, Li X-H, et al. Derivation of Porcine Extra-Embryonic Endoderm Cell Lines Reveals Distinct Signaling Pathway and Multipotency States. International Journal of Molecular Sciences. 2021; 22(23):12918. https://doi.org/10.3390/ijms222312918
Chicago/Turabian StyleZhang, Man-Ling, Yong Jin, Li-Hua Zhao, Jia Zhang, Meng Zhou, Mei-Shuang Li, Zhi-Bao Yin, Zi-Xin Wang, Li-Xia Zhao, Xi-He Li, and et al. 2021. "Derivation of Porcine Extra-Embryonic Endoderm Cell Lines Reveals Distinct Signaling Pathway and Multipotency States" International Journal of Molecular Sciences 22, no. 23: 12918. https://doi.org/10.3390/ijms222312918
APA StyleZhang, M.-L., Jin, Y., Zhao, L.-H., Zhang, J., Zhou, M., Li, M.-S., Yin, Z.-B., Wang, Z.-X., Zhao, L.-X., Li, X.-H., & Li, R.-F. (2021). Derivation of Porcine Extra-Embryonic Endoderm Cell Lines Reveals Distinct Signaling Pathway and Multipotency States. International Journal of Molecular Sciences, 22(23), 12918. https://doi.org/10.3390/ijms222312918





