Stemness, Inflammation and Epithelial–Mesenchymal Transition in Colorectal Carcinoma: The Intricate Network
Abstract
:1. Introduction
2. Stem Cells and Their Markers in Colorectal Carcinoma
3. CRC Stemness and Epithelial–Mesenchymal Transition
4. Inflammation and Its Role in EMT and Cancer Stemness
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Benard, F.; Barkun, A.; Martel, M.; von Renteln, D. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J. Gastroenterol. 2018, 24, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Grady, W.M. Epigenetic events in the colorectum and in colon cancer. Biochem. Soc. Trans. 2005, 33, 684–688. [Google Scholar] [CrossRef]
- Smith, R.; Andrews, K.; Brooks, D.; Fedewa, S.; Manassaram-Baptiste, D.; Saslow, D.; Brawley, O.; Wender, R. Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2018, 68, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.; Robbins, E.C.; Pack, K.; Stenson, I.; Kirby, P.L.; Patel, B.; Rutter, M.; Veitch, A.M.; Saunders, B.P.; Duffy, S.W.; et al. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: A multicentre, retrospective, cohort study. Gut 2020, 69, 1645–1648. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 1–9. [Google Scholar] [CrossRef]
- Shinagawa, T.; Tanaka, T.; Nozawa, H.; Emoto, S.; Murono, K.; Kaneko, M.; Sasaki, K.; Otani, K.; Nishikawa, T.; Hata, K.; et al. Comparison of the guidelines for colorectal cancer in Japan, the USA and Europe. Ann. Gastroenterol. Surg. 2017, 2, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Liu, Y.; Zhang, C.; Li, H.; Lai, B.A.-O. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med. 2020, 9, 361–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biserova, K.; Jakovlevs, A.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells 2021, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Yang, X.; Wu, J.; Huang, C.; Miao, Y.; Fu, Z. HIST2H2BF Potentiates the Propagation of Cancer Stem Cells via Notch Signaling to Promote Malignancy and Liver Metastasis in Colorectal Carcinoma. Front. Oncol. 2021, 11, 677646. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Fabrizi, E.; Palio, E.; De Maria, R. Colon cancer stem cells. J. Mol. Med. 2009, 87, 1097. [Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abugomaa, A.; Elbadawy, M.; Yamawaki, H.; Usui, T.; Sasaki, K. Emerging Roles of Cancer Stem Cells in Bladder Cancer Progression, Tumorigenesis, and Resistance to Chemotherapy: A Potential Therapeutic Target for Bladder Cancer. Cells 2020, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Wijnands, A.M.; Mahmoud, R.; Lutgens, M.; Oldenburg, B. Surveillance and management of colorectal dysplasia and cancer in inflammatory bowel disease: Current practice and future perspectives. Eur. J. Intern. Med. 2021, 93, 35–41. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W.; et al. Effects of exosomes on pre-metastatic niche formation in tumors. Mol. Cancer 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papiewska-Pająk, I.; Przygodzka, P.; Krzyżanowski, D.; Soboska, K.; Szulc-Kiełbik, I.; Stasikowska-Kanicka, O.; Boncela, J.; Wągrowska-Danilewicz, M.; Kowalska, M. Snail Overexpression Alters the microRNA Content of Extracellular Vesicles Released from HT29 Colorectal Cancer Cells and Activates Pro-Inflammatory State In Vivo. Cancers 2021, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.I.; Aller, M.; Arias, J. Cancer cell: Using inflammation to invade the host. Mol. Cancer 2007, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigdar, S.; Li, Y.; Bhattacharya, S.; O’Connor, M.; Pu, C.; Lin, J.; Wang, T.; Xiang, D.; Kong, L.; Wei, M.Q.; et al. Inflammation and cancer stem cells. Cancer Lett. 2014, 345, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Oh, H.K.; Park, S.H.; Bong, J.G. Association between inflammation and cancer stem cell phenotype in breast cancer. Oncol. Lett. 2018, 15, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Fedyanin, M.; Anna, P.; Elizaveta, P.; Sergei, T. Role of Stem Cells in Colorectal Cancer Progression and Prognostic and Predictive Characteristics of Stem Cell Markers in Colorectal Cancer. Curr. Stem Cell Res. Ther. 2017, 12, 19–30. [Google Scholar] [CrossRef]
- Hirata, K.; Suzuki, H.; Imaeda, H.; Matsuzaki, J.; Tsugawa, H.; Nagano, O.; Asakura, K.; Saya, H.; Hibi, T. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br. J. Cancer 2013, 109, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manhas, J.; Bhattacharya, A.; Agrawal, S.K.; Gupta, B.; Das, P.; Deo, S.V.; Pal, S.; Sen, S. Characterization of cancer stem cells from different grades of human colorectal cancer. Tumour Biol. 2016, 37, 14069–14081. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Guo, J.; Manning, H.C.; Gore, J.C.; Guo, N. Evaluation of CD44 and CD133 as cancer stem cell markers for colorectal cancer. Oncol. Rep. 2012, 28, 1301–1308. [Google Scholar] [CrossRef] [Green Version]
- Muraro, M.G.; Mele, V.; Däster, S.; Han, J.; Heberer, M.; Cesare Spagnoli, G.; Iezzi, G. CD133+, CD166+CD44+, and CD24+CD44+ phenotypes fail to reliably identify cell populations with cancer stem cell functional features in established human colorectal cancer cell lines. Stem Cells Transl. Med. 2012, 1, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Fonseca, A.; Florek, M.; Freund, D.; Jászai, J.; Bornhäuser, M.; Fargeas, C.; Corbeil, D. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissues Organs 2008, 188, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.J.; Lin, W.H.; Chang, Y.W.; Wei, K.C.; Liang, C.L.; Chen, S.C.; Lee, J.L. Polarized cell migration induces cancer type-specific CD133/integrin/Src/Akt/GSK3β/β-catenin signaling required for maintenance of cancer stem cell properties. Oncotarget 2015, 6, 38029–38045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevchenko, V.; Arnotskaya, N.; Zaitsev, S.; Sharma, A.; Sharma, H.S.; Bryukhovetskiy, A.; Pak, O.; Khotimchenko, Y.; Bryukhovetskiy, I. Proteins of Wnt signaling pathway in cancer stem cells of human glioblastoma. Int. Rev. Neurobiol. 2020, 35, 185–200. [Google Scholar] [CrossRef]
- Wu, K.; Xu, T.; Song, X.; Shen, J.; Zheng, S.; Zhang, L.; Tao, G.; Jiang, B. LncRNA SLCO4A1-AS1 modulates colon cancer stem cell properties by binding to miR-150-3p and positively regulating SLCO4A1. Lab. Investig. 2021, 101, 908–920. [Google Scholar] [CrossRef]
- Czeczko, L.; Ribas, C.; Czeczko, N.; Skare, T.; Yamakawa, C.; Gionedis, G.; Vasconcelos, C.; Bremer, F.; Castoldi, D.; Gasser, M.; et al. Are stem cell marker expression and CD133 analysis relevant to differentiate colorectal cancer? Arq. Bras. Cir. Dig. 2021, 33, e1568. [Google Scholar] [CrossRef] [PubMed]
- Ilie, D.S.; Mitroi, G.; Păun, I.; Ţenea-Cojan, T.; Neamţu, C.; Totolici, B.D.; Sapalidis, K.; Mogoantă, S.; Murea, A. Pathological and immunohistochemical study of colon cancer. Evaluation of markers for colon cancer stem cells. Rom. J. Morphol. Embryol. 2021, 62, 117–124. [Google Scholar] [CrossRef]
- Briede, I.; Strumfa, I.; Vanags, A.; Gardovskis, J. The Association Between Inflammation, Epithelial Mesenchymal Transition and Stemness in Colorectal Carcinoma. J. Inflamm. Res. 2020, 8, 15–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mare, M.; Colarossi, L.; Veschi, V.; Turdo, A.; Giuffrida, D.; Memeo, L.; Stassi, G.; Colarossi, C. Cancer Stem Cell Biomarkers Predictive of Radiotherapy Response in Rectal Cancer: A Systematic Review. Genes 2021, 12, 1502. [Google Scholar] [CrossRef]
- Simtniece, Z.; Vanags, A.; Strumfa, I.; Sperga, M.; Vasko, E.; Prieditis, P.; Trapencieris, P.; Gardovskis, J. Morphological and immunohistochemical profile of pancreatic neuroendocrine neoplasms. Pol. J. Pathol. 2015, 66, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Tang, Y.; Xie, L.; Huang, A.; Xue, C.; Gu, Z.; Wang, K.; Zong, S. The Prognostic and Clinical Value of CD44 in Colorectal Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 309. [Google Scholar] [CrossRef]
- Abugomaa, A.; Elbadawy, M. Patient-derived organoid analysis of drug resistance in precision medicine: Is there a value? Expert Rev. Precis. Med. Drug Dev. 2020, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Elbadawy, M.; Usui, T.; Mori, T.; Tsunedomi, R.; Hazama, S.; Nabeta, R.; Uchide, T.; Fukushima, R.; Yoshida, T.; Shibutani, M.; et al. Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture. Cancer Sci. 2019, 110, 2806–2821. [Google Scholar] [CrossRef] [PubMed]
- Suwannakul, N.; Ma, N.; Thanan, R.; Pinlaor, S.; Ungarreevittaya, P.; Midorikawa, K.; Hiraku, Y.; Oikawa, S.; Kawanishi, S.; Murata, M. Overexpression of CD44 Variant 9: A Novel Cancer Stem Cell Marker in Human Cholangiocarcinoma in Relation to Inflammation. Mediat. Inflamm. 2018, 2018, 4867234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shangguan, W.A.-O.X.; Fan, C.; Chen, X.; Lu, R.; Liu, Y.; Li, Y.; Shang, Y.; Yin, D.; Zhang, S.; Huang, Q.; et al. Endothelium originated from colorectal cancer stem cells constitute cancer blood vessels. Cancer Sci. 2017, 108, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Tian, X.; Zhang, Y.; Huang, X.; Li, Q.; Li, W.; Li, S. LINC00319 promotes cancer stem cell-like properties in laryngeal squamous cell carcinoma via E2F1-mediated upregulation of HMGB3. Exp. Mol. Med. 2021, 53, 1218–1228. [Google Scholar] [CrossRef]
- Segev, L.; Naboishchikov, I.; Kazanov, D.; Bernstein, E.; Shaked, M.; Arber, N.; Shapira, S. A Dinucleotide Deletion in the CD24 Gene Is a Potential Risk Factor for Colorectal Cancer. Am. Surg. 2020, 86, 480–485. [Google Scholar] [CrossRef]
- Kapeleris, J.; Zou, H.; Qi, Y.; Gu, Y.; Li, J.; Schoning, J.; Monteiro, M.; Gu, W. Cancer stemness contributes to cluster formation of colon cancer cells and high metastatic potentials. Clin. Exp. Pharmacol. Physiol. 2020, 47, 838–847. [Google Scholar] [CrossRef]
- Hagihara, T.; Kondo, J.; Endo, H.; Ohue, M.; Sakai, Y.; Inoue, M. Hydrodynamic stress stimulates growth of cell clusters via the ANXA1/PI3K/AKT axis in colorectal cancer. Sci. Rep. 2019, 9, 20027. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Park, S.; Jeong, H.O.; Park, S.; Kumar, S.; Jang, A.; Lee, S.; Kim, D.U.; Cho, Y. Circulating Tumor Cell Clusters Are Cloaked with Platelets and Correlate with Poor Prognosis in Unresectable Pancreatic Cancer. Cancers 2021, 13, 5272. [Google Scholar] [CrossRef]
- Weichert, W.; Knösel, T.; Bellach, J.; Dietel, M.; Kristiansen, G. ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J. Clin. Pathol. 2004, 57, 1160–1164. [Google Scholar] [CrossRef]
- Han, S.; Yang, W.; Zong, S.; Li, H.; Liu, S.; Li, W.; Shi, Q.; Hou, F. Clinicopathological, prognostic and predictive value of CD166 expression in colorectal cancer: A meta-analysis. Oncotarget 2017, 8, 64373–64384. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Kim, J.; Kang, J.; Park, Y.; Park, S.; Cheon, J.; Kim, W.; Kim, H.; Park, J.; Kim, T. mTOR Signaling Combined with Cancer Stem Cell Markers as a Survival Predictor in Stage II Colorectal Cancer. Yonsei Med. J. 2020, 61, 572–578. [Google Scholar] [CrossRef]
- Geng, S.; Guo, Y.; Wang, Q.; Li, L.; Wang, J. Cancer stem-like cells enriched with CD29 and CD44 markers exhibit molecular characteristics with epithelial-mesenchymal transition in squamous cell carcinoma. Arch. Dermatol. Res. 2013, 305, 35–47. [Google Scholar] [CrossRef]
- Holah, N.S.; Aiad, H.A.; Asaad, N.Y.; Elkhouly, E.A.; Lasheen, A.G. Evaluation of the Role of ALDH1 as Cancer Stem Cell Marker in Colorectal Carcinoma: An Immunohistochemical Study. Clin. Diagn. Res. 2017, 11, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Liu, Y.; Dong, Y.; Wang, Y.; Kassab, M.A.; Fan, W.; Yu, X.; Wu, C. LGR5 regulates gastric adenocarcinoma cell proliferation and invasion via activating Wnt signaling pathway. Oncogenesis 2018, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Usui, T.; Sakurai, M.; Umata, K.; Elbadawy, M.; Ohama, T.; Yamawaki, H.; Hazama, S.; Takenouchi, H.; Nakajima, M.; Tsunedomi, R.; et al. Hedgehog Signals Mediate Anti-Cancer Drug Resistance in Three-Dimensional Primary Colorectal Cancer Organoid Culture. Int. J. Mol. Sci. 2018, 19, 1098. [Google Scholar] [CrossRef] [Green Version]
- Sahin, I.A.-O.X.; Gündoğdu, B.; Ceylan, A.C.; Erdem, H.B.; Tatar, A. High Expression of Stem Cell-Related Genes in Polyps with Villous Features and High-Grade Dysplasia Support Malignant Phenotype and Colorectal Carcinogenesis. Asian Pac. J. Cancer Prev. 2021, 22, 2429–2435. [Google Scholar] [CrossRef]
- Trautmann, F.; Cojoc, M.; Kurth, I.; Melin, N.; Bouchez, L.; Dubrovska, A.; Peitzsch, C. CXCR4 as biomarker for radioresistant cancer stem cells. Int J. Radiat. Biol. 2014, 90, 687–699. [Google Scholar] [CrossRef]
- Wu, W.; Cao, J.; Ji, Z.; Wang, J.; Jiang, T.; Ding, H. Co-expression of Lgr5 and CXCR4 characterizes cancer stem-like cells of colorectal cancer. Oncotarget 2016, 7, 81144–81155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, C.; Dupré-Crochet, S.; Norman, M.; Kajita, M.; Zimmermann, C.; Pelling, A.E.; Piddini, E.; Baena-López, L.A.; Vincent, J.-P.; Itoh, Y.; et al. Characterization of the interface between normal and transformed epithelial cells. Nat. Cell Biol. 2009, 11, 460–467. [Google Scholar] [CrossRef]
- Grigore, A.D.; Jolly, M.K.; Jia, D.; Farach-Carson, M.C.; Levine, H. Tumor Budding: The Name is EMT. Partial EMT. J. Clin. Med. 2016, 5, 51. [Google Scholar] [CrossRef]
- Sato, K.; Uehara, T.; Nakajima, T.; Iwaya, M.; Miyagawa, Y.; Watanabe, T.; Ota, H. Inverse correlation between PD-L1 expression and LGR5 expression in tumor budding of stage II/III colorectal cancer. Ann. Diagn. Pathol. 2021, 52, 151739. [Google Scholar] [CrossRef]
- Luo, W.-R.; Gao, F.; Li, S.-Y.; Yao, K.-T. Tumour budding and the expression of cancer stem cell marker aldehyde dehydrogenase 1 in nasopharyngeal carcinoma. Histopathology 2012, 61, 1072–1081. [Google Scholar] [CrossRef]
- Attramadal, C.G.; Kumar, S.; Boysen, M.E.; Dhakal, H.P.; Nesland, J.M.; Bryne, M. Tumor Budding, EMT and Cancer Stem Cells in T1-2/N0 Oral Squamous Cell Carcinomas. Anticancer. Res. 2015, 35, 6111–6120. [Google Scholar] [PubMed]
- Voutsadakis, I.A. Prognostic role of tumor budding in breast cancer. World J. Exp. Med. 2018, 8, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Stemmler, M.A.-O.; Eccles, R.L.; Brabletz, S.; Brabletz, T. Non-redundant functions of EMT transcription factors. Nat. Cell Biol. 2019, 21, 102–112. [Google Scholar] [CrossRef]
- Fan, F.; Samuel, S.; Evans, K.; Lu, J.; Xia, L.; Zhou, Y.; Sceusi, E.; Tozzi, F.; Ye, X.; Mani, S.; et al. Overexpression of snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Cancer Med. 2012, 1, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Kroepil, F.; Fluegen, G.; Vallböhmer, D.; Baldus, S.; Dizdar, L.; Raffel, A.; Hafner, D.; Stoecklein, N.; Knoefel, W.T. Snail1 expression in colorectal cancer and its correlation with clinical and pathological parameters. BMC Cancer 2013, 13. [Google Scholar] [CrossRef] [Green Version]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. JNK Pathway Mediates Low Oxygen Level Induced Epithelial–Mesenchymal Transition and Stemness Maintenance in Colorectal Cancer Cells. Cancers 2020, 12, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stemmer, V.; de Craene, B.; Berx, G.; Behrens, J. Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene 2008, 27, 5075–5080. [Google Scholar] [CrossRef] [Green Version]
- Christie, M.; Jorissen, R.; Mouradov, D.; Sakthianandeswaren, A.; Li, S.; Day, F.; Tsui, C.; Lipton, L.; Desai, J.; Jones, I.; et al. Different APC genotypes in proximal and distal sporadic colorectal cancers suggest distinct WNT/β-catenin signalling thresholds for tumourigenesis. Oncogene 2013, 32, 4675–4682. [Google Scholar] [CrossRef] [PubMed]
- Baeg, G.H.; Matsumine, A.; Kuroda, T.; Bhattacharjee, R.; Miyashiro, I.; Toyoshima, K.; Akiyama, T. The tumour suppressor gene product APC blocks cell cycle progression from G0/G1 to S phase. EMBO J. 1995, 14, 5618–5625. [Google Scholar] [CrossRef]
- Pronobis, M.I.; Rusan, N.M.; Peifer, M. A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient βcatenin destruction. Elife 2015, 4, e08022. [Google Scholar] [CrossRef] [PubMed]
- Lecarpentier, Y.; Schussler, O.; Hébert, J.L.; Vallée, A. Multiple Targets of the Canonical WNT/β-Catenin Signaling in Cancers. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Katoh, M. Multi-layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β-catenin signaling activation (Review). Int. J. Mol. Med. 2018, 42, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Isobe, T.; Ariyama, H.; Nakano, M.; Kikushige, Y.; Takaishi, S.; Kusaba, H.; Takenaka, K.; Ueki, T.; Nakamura, M.; et al. Ecadherin regulates proliferation of colorectal cancer stem cells through NANOG. Oncol. Rep. 2018, 40, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Nevins, J.R. E2F: A link between the Rb tumor suppressor protein and viral oncoproteins. Science 1992, 258, 424–429. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Zhou, B.; Li, C.; Zhang, F.; Wang, X.; Zhang, G.; Bu, X.; Cai, S.; Du, J. Epithelial-mesenchymal transition (EMT) induced by TNF-alpha requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Liu, B.; Chen, F.; Li, J.; Liu, Y.; Zhu, J.; Shen, C. Ponicidin inhibits pro-inflammatory cytokine TNF-alpha-induced epithelial-mesenchymal transition and metastasis of colorectal cancer cells via suppressing the AKT/GSK-3beta/Snail pathway. Inflammopharmacology 2019, 27, 627–638. [Google Scholar] [CrossRef]
- Smith, A.L.; Robin, T.P.; Ford, H.L. Molecular Pathways: Targeting the TGF-β Pathway for Cancer Therapy. Clin. Cancer Res. 2012, 18, 4514. [Google Scholar] [CrossRef] [Green Version]
- Cabarcas, S.M.; Mathews, L.; Farrar, W.L. The cancer stem cell niche--there goes the neighborhood? Int. J. Cancer 2011, 129, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.; Shakibaei, M. Resveratrol chemosensitizes TNF-beta-induced survival of 5-FU-treated colorectal cancer cells. Nutrients 2018, 10, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, T.; Goto, A.; Sugiyama, M.; Matsuoka, H.; Abe, N.; Sakamoto, A.; Atomi, Y. Possible contribution of CD44 variant 6 and nuclear β-catenin expression to the formation of budding tumor cells in patients with T1 colorectal carcinoma. Cancer 2001, 92, 2539–2546. [Google Scholar] [CrossRef]
- Yamada, N.; Sugai, T.; Eizuka, M.; Tsuchida, K.; Sugimoto, R.; Mue, Y.; Suzuki, M.; Osakabe, M.; Uesugi, N.; Ishida, K.; et al. Tumor budding at the invasive front of colorectal cancer may not be associated with the epithelial-mesenchymal transition. Hum. Pathol. 2017, 60, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Kim, S.; Lee, Y.; Hong, H.; Kim, T.; Kim, S.; Lee, W.; Cho, Y. Twist1-induced epithelial-mesenchymal transition according to microsatellite instability status in colon cancer cells. Oncotarget 2016, 7, 57066–57076. [Google Scholar] [CrossRef] [Green Version]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal cancer in Crohn’s disease: A Scandinavian population-based cohort study. Lancet Gastroenterol. Hepatol. 2020, 5, 475–484. [Google Scholar] [CrossRef]
- Witschen, P.M.; Chaffee, T.S.; Brady, N.A.-O.; Huggins, D.N.; Knutson, T.A.-O.; LaRue, R.S.; Munro, S.A.; Tiegs, L.; McCarthy, J.A.-O.; Nelson, A.A.-O.; et al. Tumor Cell Associated Hyaluronan-CD44 Signaling Promotes Pro-Tumor Inflammation in Breast Cancer. Cancers 2020, 12, 1325. [Google Scholar] [CrossRef]
- Chefetz, I.; Alvero, A.; Holmberg, J.; Lebowitz, N.; Craveiro, V.; Yang-Hartwich, Y.; Yin, G.; Squillace, L.; Gurrea Soteras, M.; Aldo, P.; et al. TLR2 enhances ovarian cancer stem cell self-renewal and promotes tumor repair and recurrence. Cell Cycle 2013, 12, 511–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Max, N.; Harbaum, L.; Pollheimer, M.J.; Lindtner, R.A.; Kornprat, P.; Langner, C. Tumour budding with and without admixed inflammation: Two different sides of the same coin? Br. J. Cancer 2016, 114, 368–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wyk, H.C.; Roseweir, A.; Alexander, P.; Park, J.H.; Horgan, P.G.; McMillan, D.C.; Edwards, J. The Relationship Between Tumor Budding, Tumor Microenvironment, and Survival in Patients with Primary Operable Colorectal Cancer. Ann. Surg. Oncol. 2019, 26, 4397–4404. [Google Scholar] [CrossRef]
- Sadek, S.A.; DM, A.R.; Fatima, S. The role of tumor budding in colorectal adenocarcinoma: Possible involvement of the intestinal cancer stem cell marker Lgr5. Indian J. Pathol. Microbiol. 2020, 63, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Uhlitz, F.; Bischoff, P.; Sieber, A.; Obermayer, B.; Blanc, E.; Lüthen, M.; Sawitzki, B.; Kamphues, C.; Beule, D.; Sers, C.; et al. A census of cell types and paracrine interactions in colorectal cancer. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Rao, H.; Chen, J.; Li, M.; Xiao, Y.; Fu, J.; Zeng, Y.; Cai, M.; Xie, D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Legrand, F.; Driss, V.; Delbeke, M.; Loiseau, S.; Hermann, E.; Dombrowicz, D.; Capron, M. Human eosinophils exert TNF-α and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J. Immunol. 2010, 185, 7443–7451. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, W.J.; Hogendoorn, P.; Jansen, F.; Zwinderman, A.; Trimbos, J.; Fleuren, G.J. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum. Pathol. 1996, 27, 904–911. [Google Scholar] [CrossRef]
- Von Wasielewski, R.; Seth, S.; Franklin, J.; Fischer, R.; Hübner, K.; Hansmann, M.; Diehl, V.; Georgii, A. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin’s disease, allowing for known prognostic factors. Blood 2000, 95, 1207–1213. [Google Scholar] [CrossRef]
- Harbaum, L.; Pollheimer, M.; Kornprat, P.; Lindtner, R.; Bokemeyer, C.; Langner, C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod. Pathol. 2015, 28, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prizment, A.; Vierkant, R.; Smyrk, T.; Tillmans, L.; Lee, J.; Sriramarao, P.; Nelson, H.; Lynch, C.; Thibodeau, S.; Church, T.; et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa women’s health study. Mod. Pathol. 2016, 29, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Rao, G.; Wang, H.; Li, B.; Huang, L.; Xue, D.; Wang, X.; Jin, H.; Wang, J.; Zhu, Y.; Lu, Y.; et al. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin. Cancer Res. 2013, 19, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Li, S.; Zhang, J.; Wang, L.; Wu, X.; Wang, J.; Huang, Q.; Lai, M. Cancer Stemness, Immune Cells, and Epithelial–Mesenchymal Transition Cooperatively Predict Prognosis in Colorectal Carcinoma. Clin. Colorectal Cancer 2018, 17, e579–e592. [Google Scholar] [CrossRef]
- González, I.A.-O.; Bauer, P.S.; Liu, J.; Chatterjee, D.A.-O. Intraepithelial tumour infiltrating lymphocytes are associated with absence of tumour budding and immature/myxoid desmoplastic reaction, and with better recurrence-free survival in stages I-III colorectal cancer. Histopathology 2021, 78, 252–264. [Google Scholar] [CrossRef]
- Yu, X.; Wang, D.; Wang, X.; Sun, S.; Zhang, Y.; Wang, S.; Miao, R.; Xu, X.; Qu, X. CXCL12/CXCR4 promotes inflammation-driven colorectal cancer progression through activation of RhoA signaling by sponging miR-133a-3p. J. Exp. Clin. Cancer Res. 2019, 38, 32. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.-O.; Kaf, R.M.; Ahmed, M.M.; Elwan, A.; Ashour, H.R.; Ibrahim, A. The Prognostic Value of Cancer Stem Cell Markers (Notch1, ALDH1, and CD44) in Primary Colorectal Carcinoma. J. Gastrointest. Cancer 2019, 50, 824–837. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Liu, D.; Yin, L.; Kharbanda, S.; Kufe, D. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005, 65, 10413–10422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israël, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Xiong, J.; Takeuchi, M.; Kurama, T.; Goeddel, D.V. TRAF6 is a signal transducer for interleukin-1. Nature 1996, 383, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, N.; Jin, C.; Long, M.D.; Rajabi, H.; Yasumizu, Y.; Fushimi, A.; Yamashita, N.; Hagiwara, M.; Zheng, R.; et al. MUC1-C drives stemness in progression of colitis to colorectal cancer. JCI Insight 2020, 5, e137112. [Google Scholar] [CrossRef] [PubMed]
- Huynh, P.T.; Beswick, E.J.; Coronado, Y.A.; Johnson, P.; O’Connell, M.R.; Watts, T.; Singh, P.; Qiu, S.; Morris, K.; Powell, D.W.; et al. CD90(+) stromal cells are the major source of IL-6, which supports cancer stem-like cells and inflammation in colorectal cancer. Int. J. Cancer 2016, 138, 1971–1981. [Google Scholar] [CrossRef] [Green Version]
- McAndrews, K.M.; Vázquez-Arreguín, K.; Kwak, C.; Sugimoto, H.; Zheng, X.; Li, B.; Kirtley, M.L.; LeBleu, V.S.; Kalluri, R. αSMA+ fibroblasts suppress Lgr5+ cancer stem cells and restrain colorectal cancer progression. Oncogene 2021, 40, 4440–4452. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Hibiya, S.; Katsukura, N.; Kitagawa, S.; Sato, A.; Okamoto, R.; Watanabe, M.; Tsuchiya, K. Influence of chronic inflammation on the malignant phenotypes and the plasticity of colorectal cancer cells. Biochem. Biophys. Rep. 2021, 26, 101031. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Kwon, J.; Kim, J.; Oh, S.; Jin Lee, K.; Park, J.; Pil Hong, S.; Cheon, J.; Kim, T.; Kim, W. Nonsteroidal anti-inflammatory drugs suppress cancer stem cells via inhibiting PTGS2 (cyclooxygenase 2) and NOTCH/HES1 and activating PPARG in colorectal cancer. Int. J. Cancer 2014, 134, 519–529. [Google Scholar] [CrossRef]
- Khoo, B.L.; Grenci, G.; Lim, J.S.Y.; Lim, Y.P.; Fong, J.; Yeap, W.H.; Bin Lim, S.; Chua, S.L.; Wong, S.C.; Yap, Y.-S.; et al. Low-dose anti-inflammatory combinatorial therapy reduced cancer stem cell formation in patient-derived preclinical models for tumour relapse prevention. Br. J. Cancer 2019, 120, 407–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salnikov, A.V.; Bretz, N.P.; Perne, C.; Hazin, J.; Keller, S.; Fogel, M.; Herr, I.; Schlange, T.; Moldenhauer, G.; Altevogt, P. Antibody targeting of CD24 efficiently retards growth and influences cytokine milieu in experimental carcinomas. Br. J. Cancer 2013, 108, 1449–1459. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.Y.; Yang, C.Y.; Yeh, P.H.; Hsu, C.J.; Chang, L.W.; Chan, W.J.; Lin, C.P.; Lyu, Y.Y.; Wu, W.C.; Lee, C.W.; et al. Highly Correlated Recurrence Prognosis in Patients with Metastatic Colorectal Cancer by Synergistic Consideration of Circulating Tumor Cells/Microemboli and Tumor Markers CEA/CA19-9. Cells 2021, 10, 1149. [Google Scholar] [CrossRef]
- Mo, S.; Ku, H.J.; Choi, S.H.; Jeong, H.J.; Park, D.G.; Oh, M.H.; Ahn, J.C. 470 nm LED Irradiation Inhibits the Invasiveness of CD133-positive Human Colorectal Cancer Stem Cells by Suppressing the Cyclooxygenase-2/prostaglandin E2 Pathway. Anticancer Res. 2021, 41, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
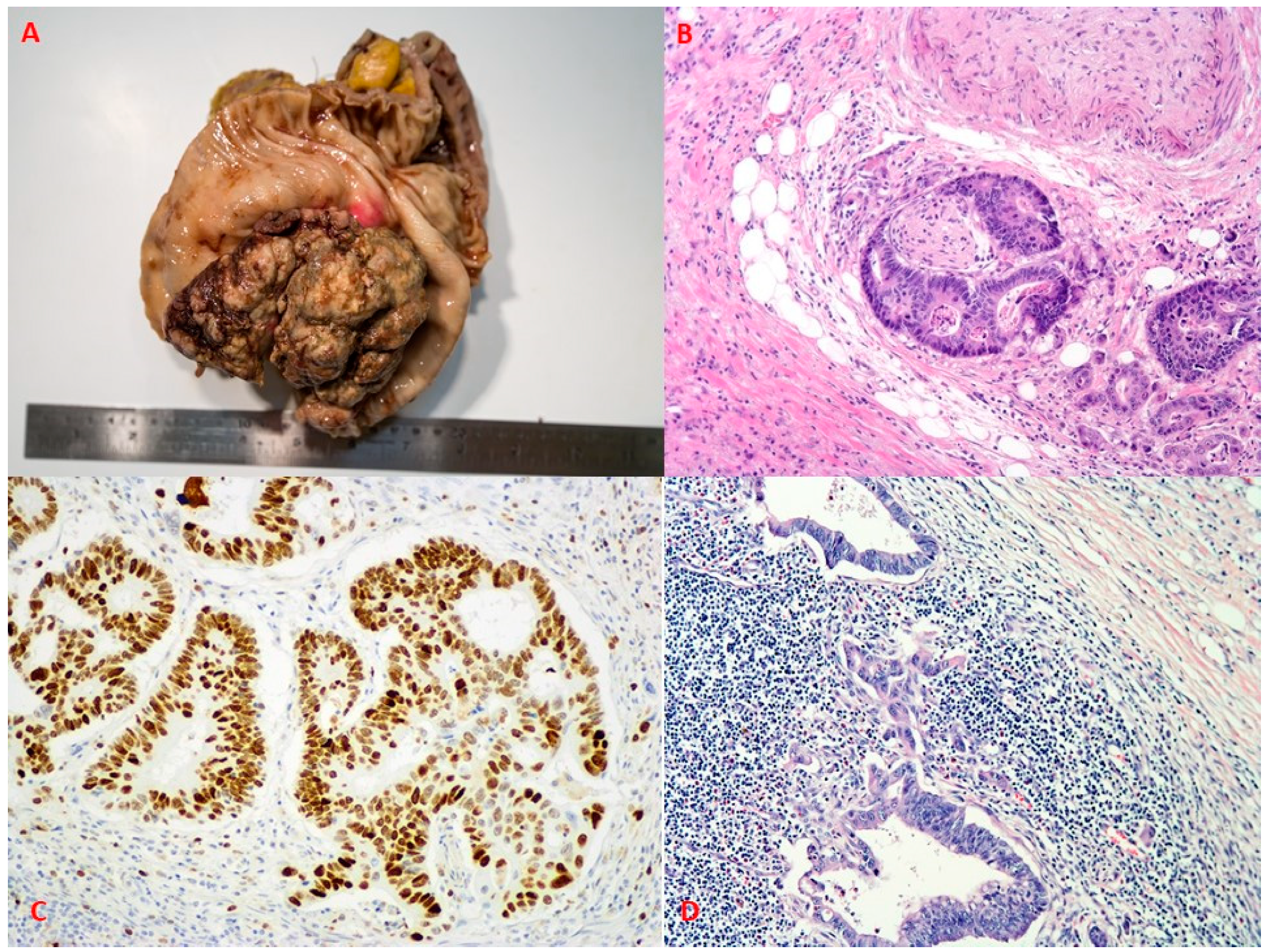

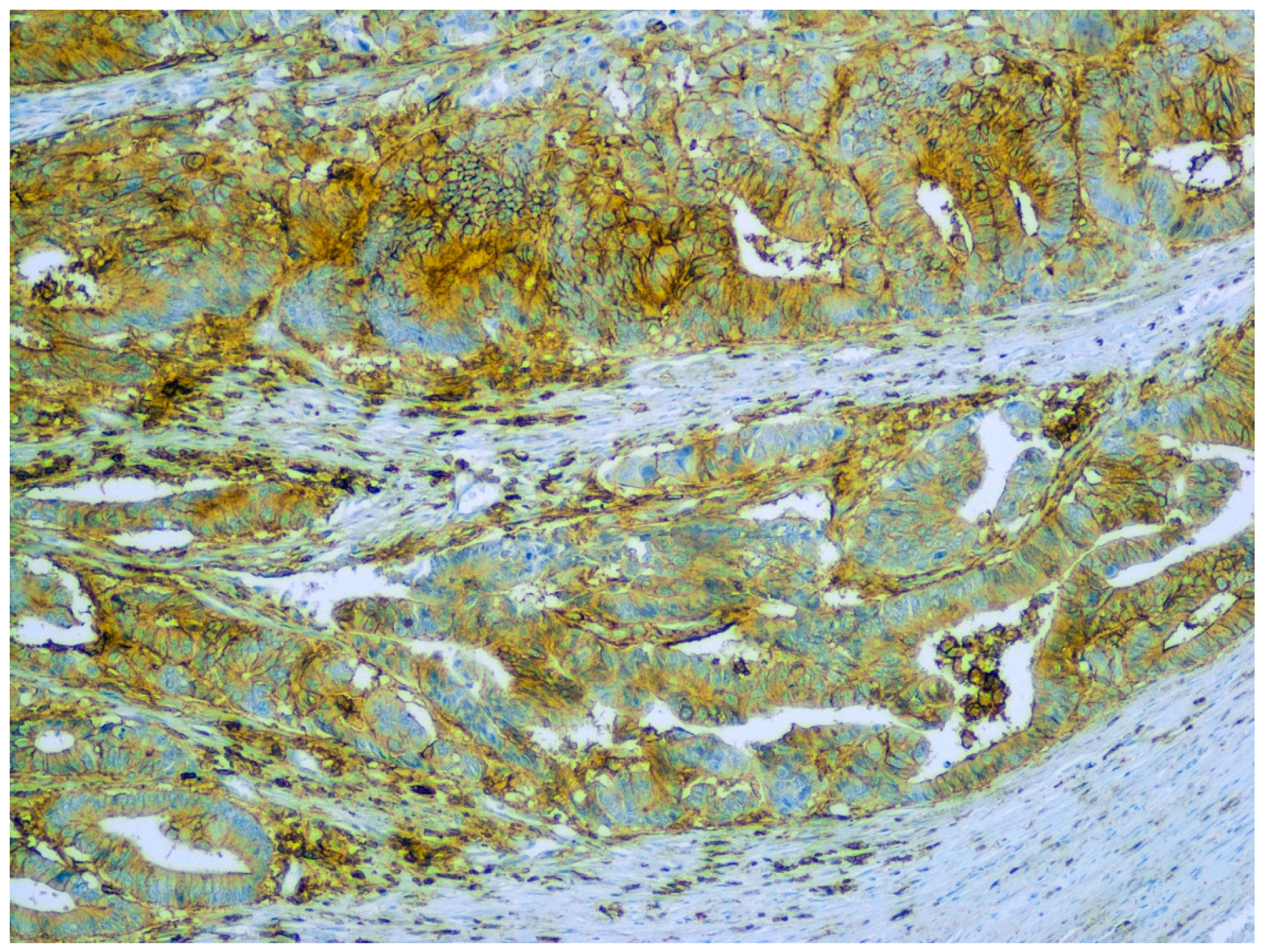
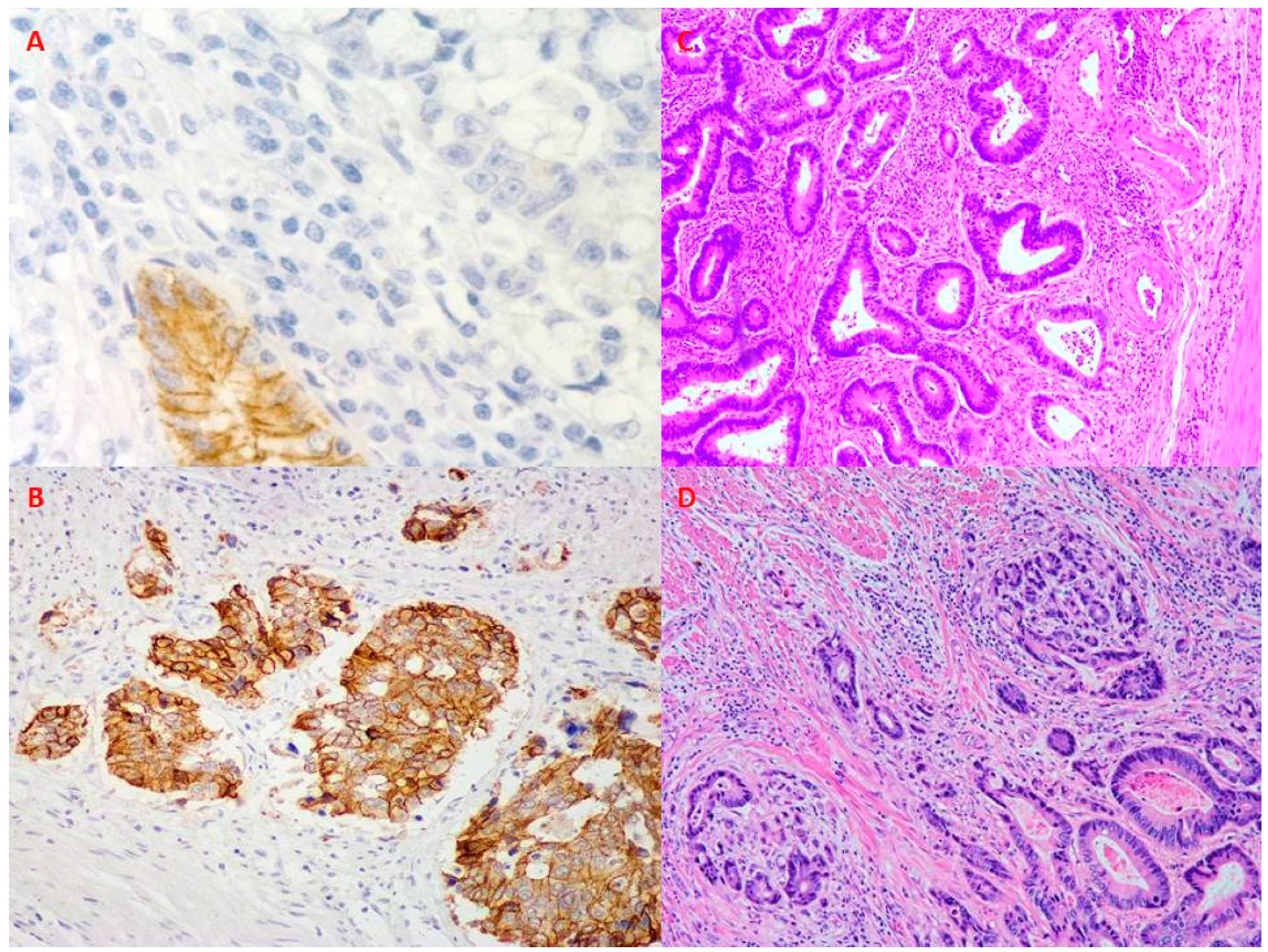
High risk
|
Intermediate risk
|
Low risk
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briede, I.; Balodis, D.; Gardovskis, J.; Strumfa, I. Stemness, Inflammation and Epithelial–Mesenchymal Transition in Colorectal Carcinoma: The Intricate Network. Int. J. Mol. Sci. 2021, 22, 12891. https://doi.org/10.3390/ijms222312891
Briede I, Balodis D, Gardovskis J, Strumfa I. Stemness, Inflammation and Epithelial–Mesenchymal Transition in Colorectal Carcinoma: The Intricate Network. International Journal of Molecular Sciences. 2021; 22(23):12891. https://doi.org/10.3390/ijms222312891
Chicago/Turabian StyleBriede, Inese, Dainis Balodis, Janis Gardovskis, and Ilze Strumfa. 2021. "Stemness, Inflammation and Epithelial–Mesenchymal Transition in Colorectal Carcinoma: The Intricate Network" International Journal of Molecular Sciences 22, no. 23: 12891. https://doi.org/10.3390/ijms222312891
APA StyleBriede, I., Balodis, D., Gardovskis, J., & Strumfa, I. (2021). Stemness, Inflammation and Epithelial–Mesenchymal Transition in Colorectal Carcinoma: The Intricate Network. International Journal of Molecular Sciences, 22(23), 12891. https://doi.org/10.3390/ijms222312891







