Functional Recovery Caused by Human Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles Administered 24 h after Stroke in Rats
Abstract
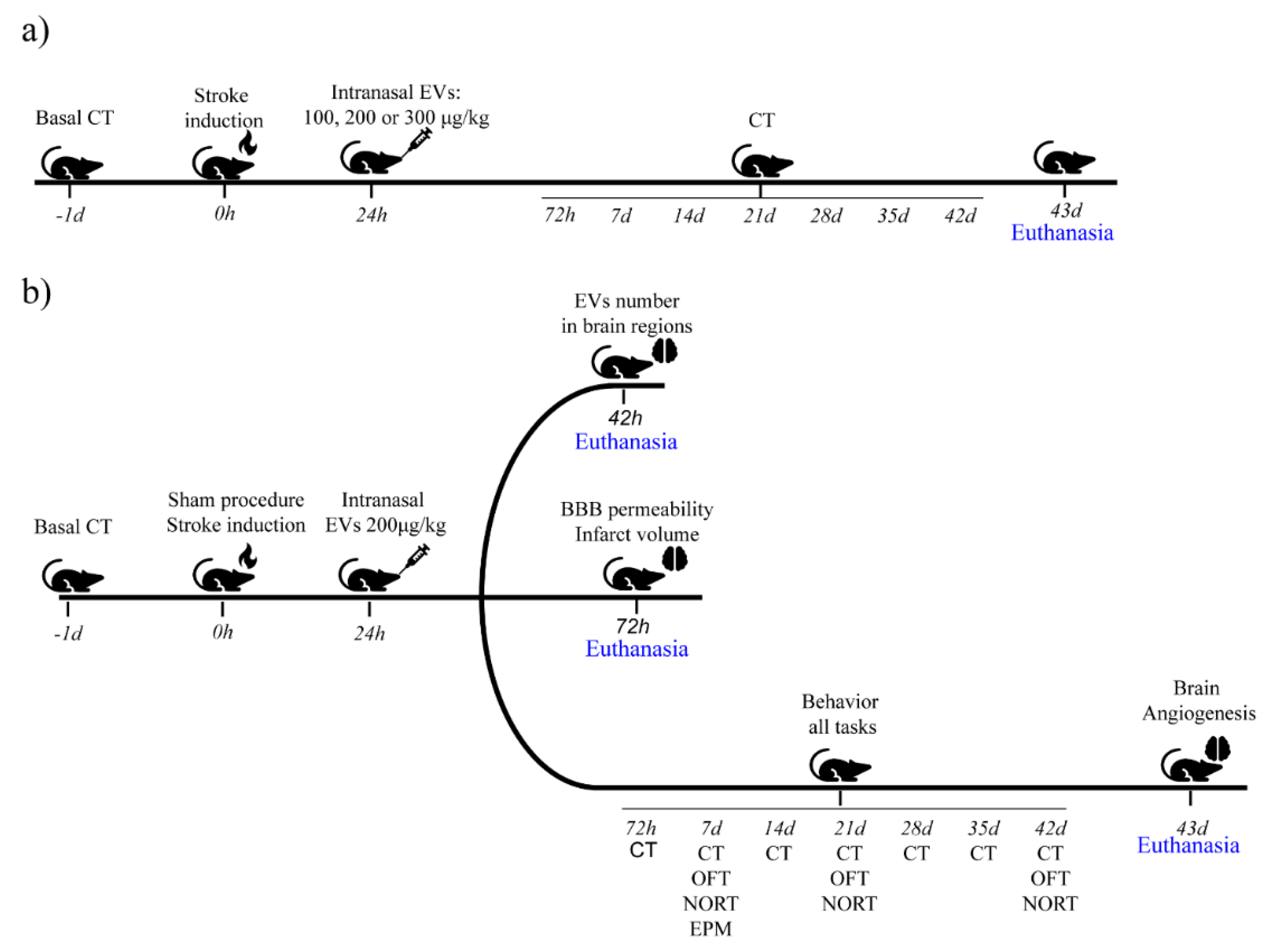
:1. Introduction
2. Results
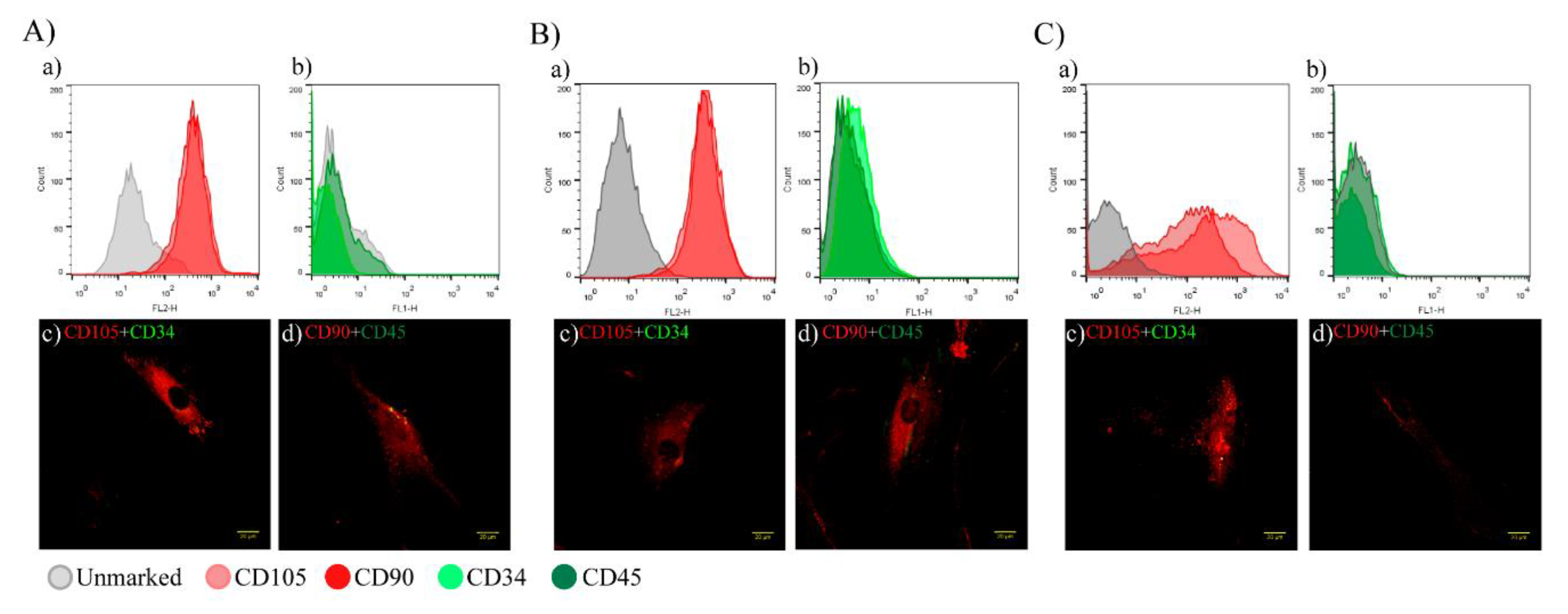
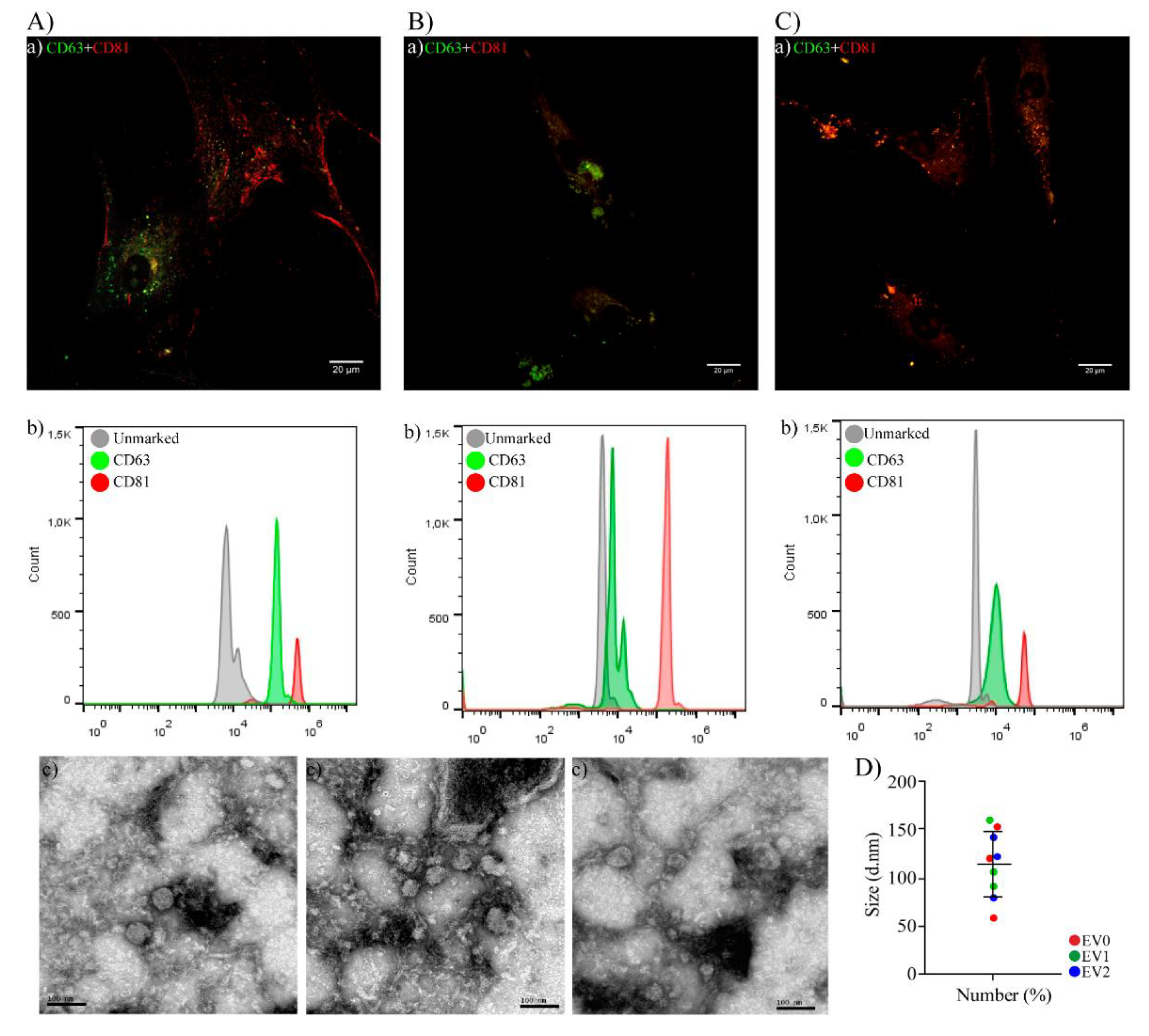
2.1. Characterization of hAT-MSCs and EVs
2.1.1. hAT-MSC Characterization
2.1.2. EVs Characterization
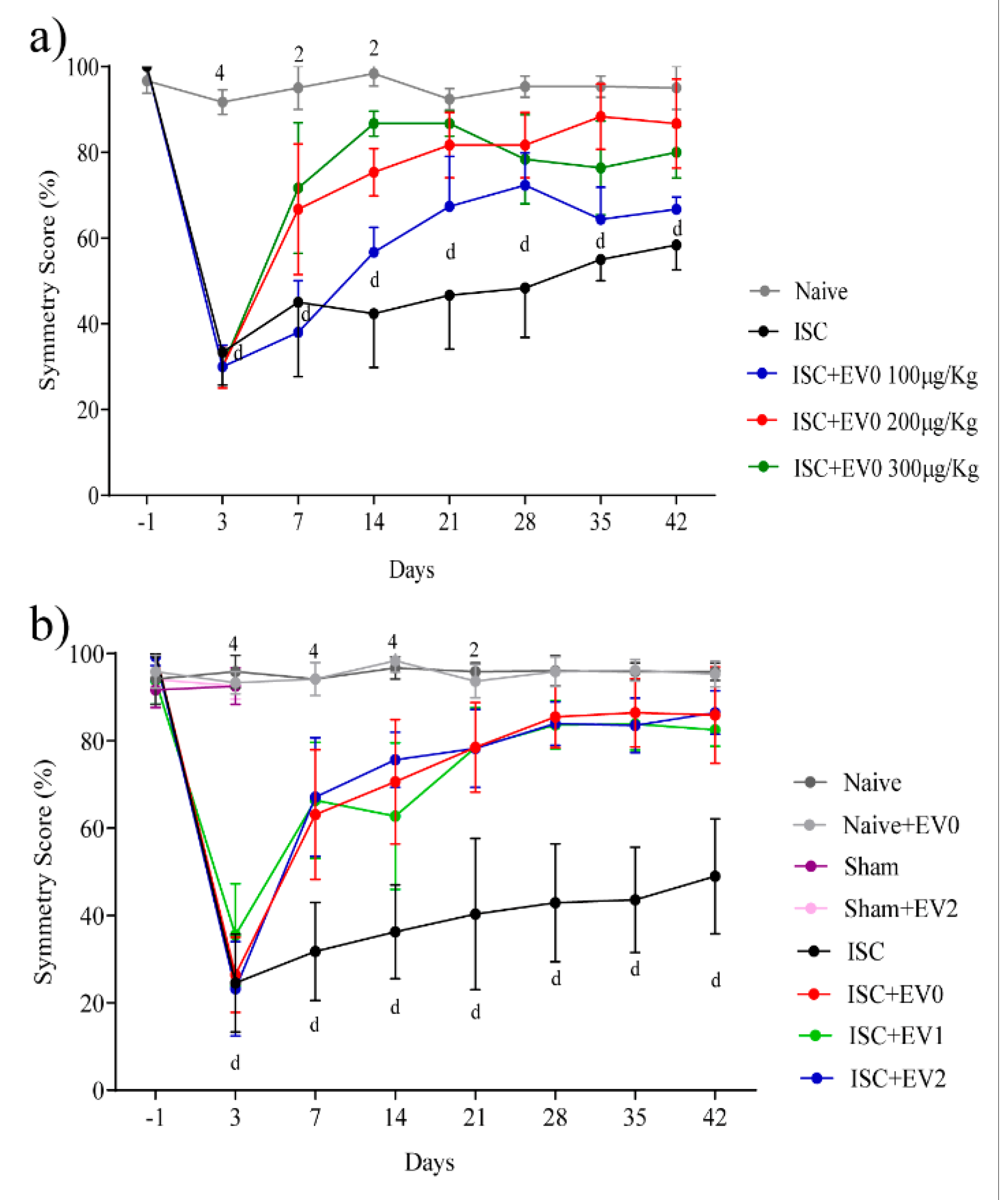
2.2. Front Paws Symmetry
Effect of Stroke on Paws Symmetry
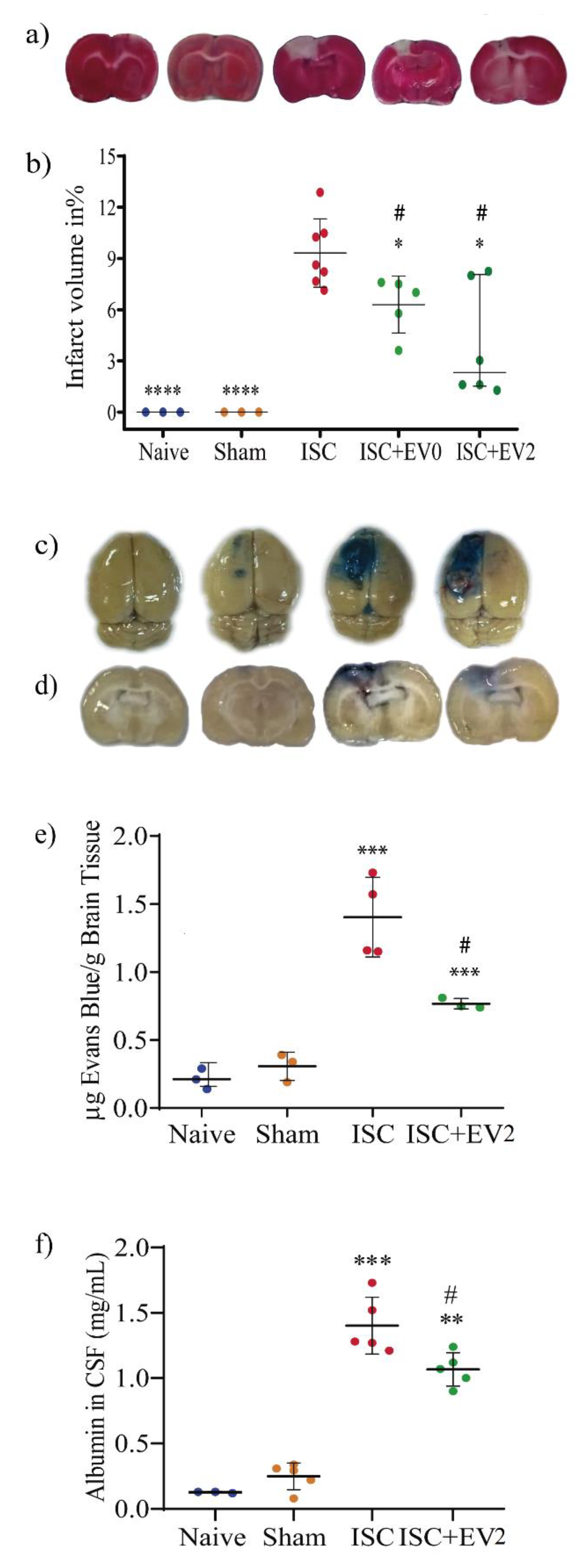
2.3. Infarct Volume and BBB Permeability
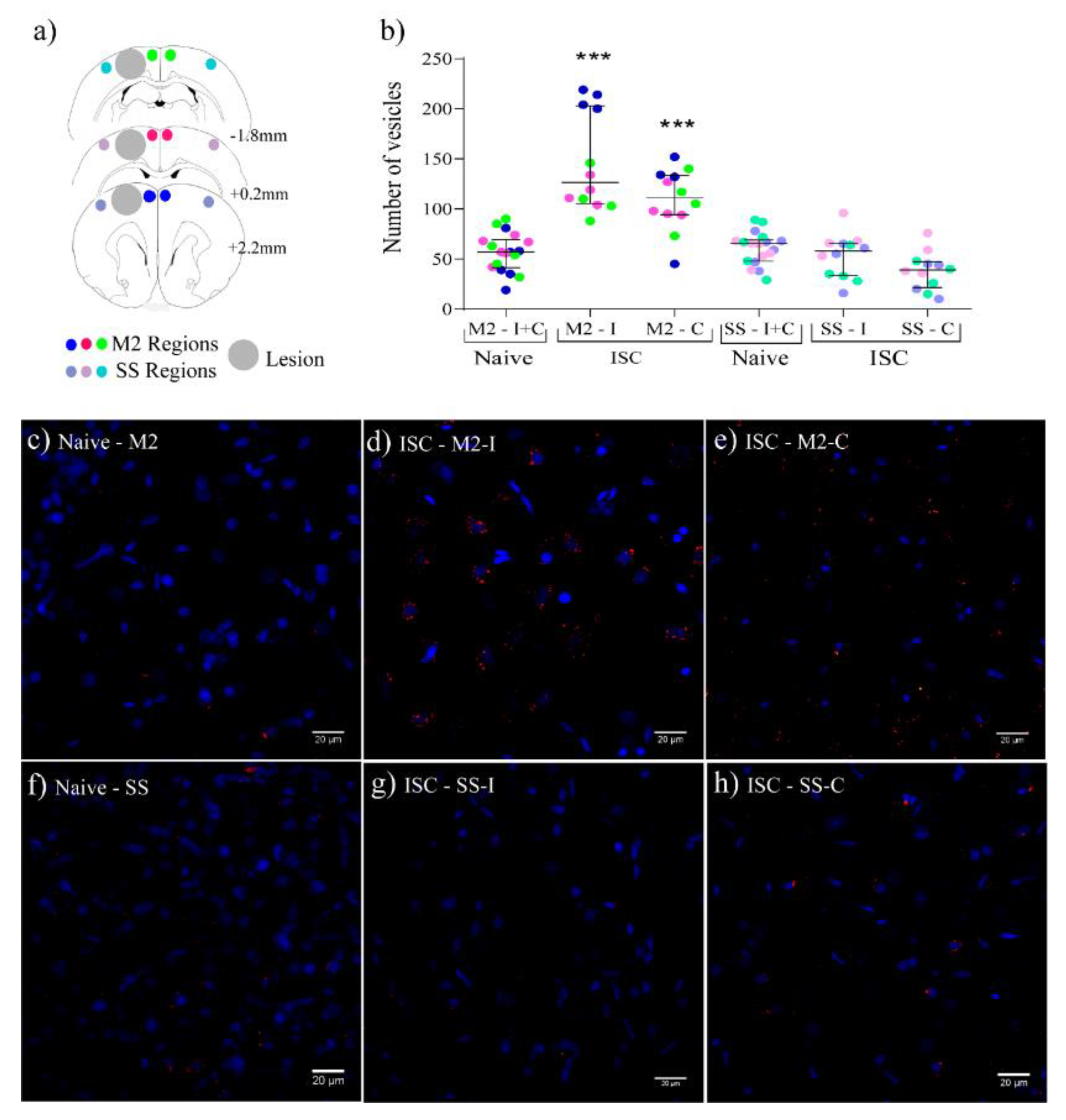
2.4. EVs Distribution in the Brain Parenchyma
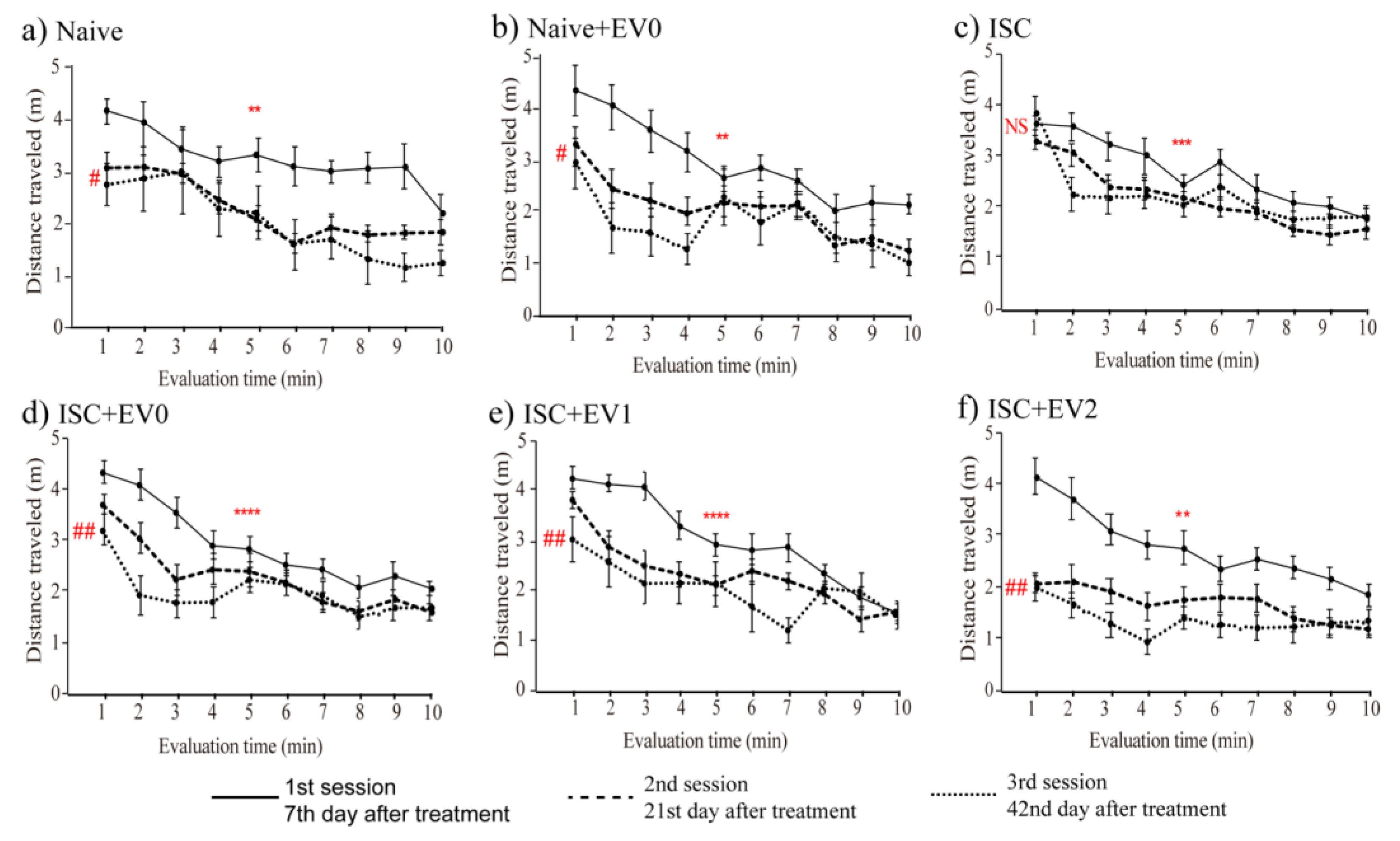
2.5. Open Field Task (OFT)
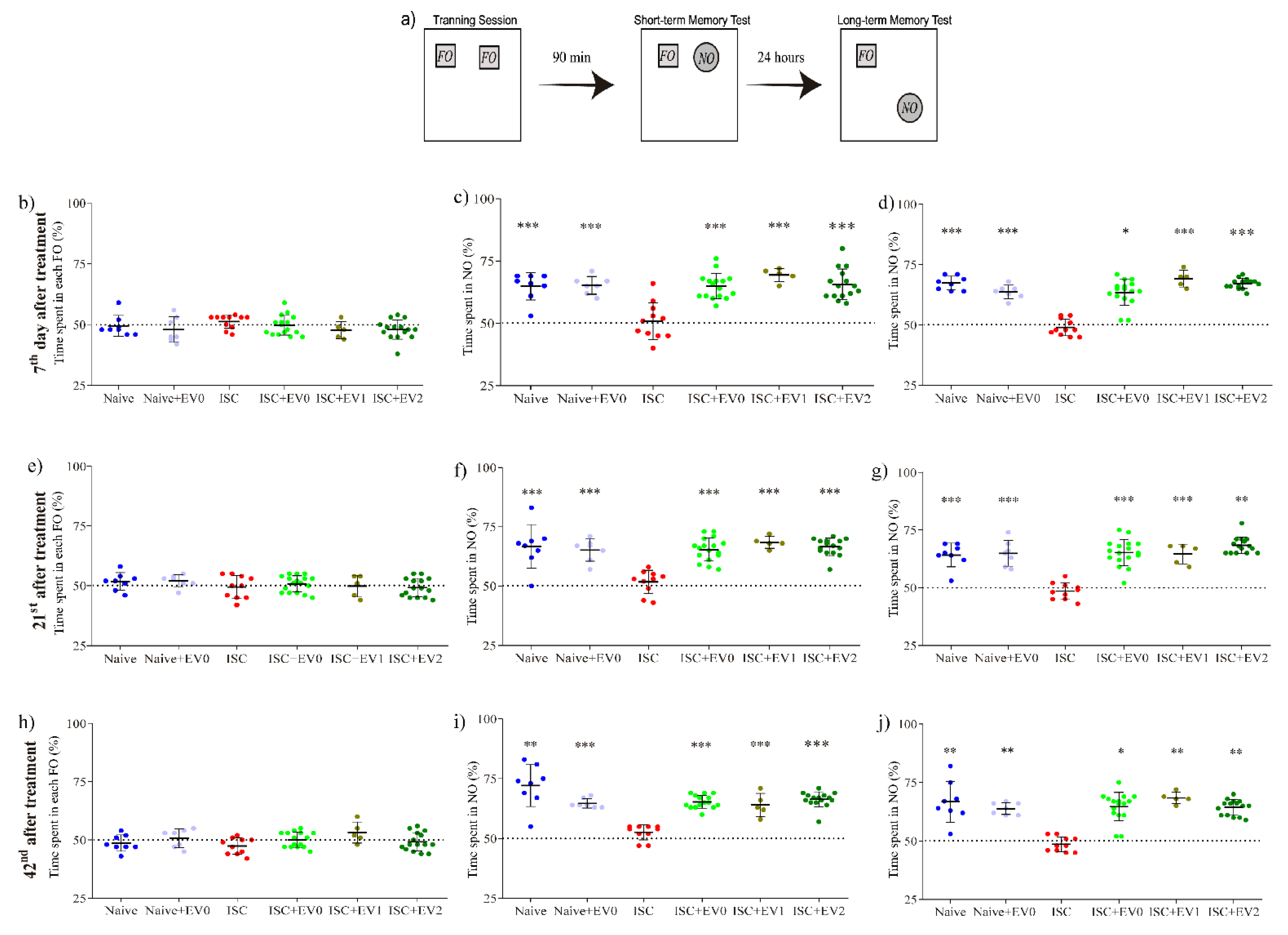
2.6. Novel Object Recognition Task (NORT)
2.7. Elevated Plus-Maze Task (EPMT)
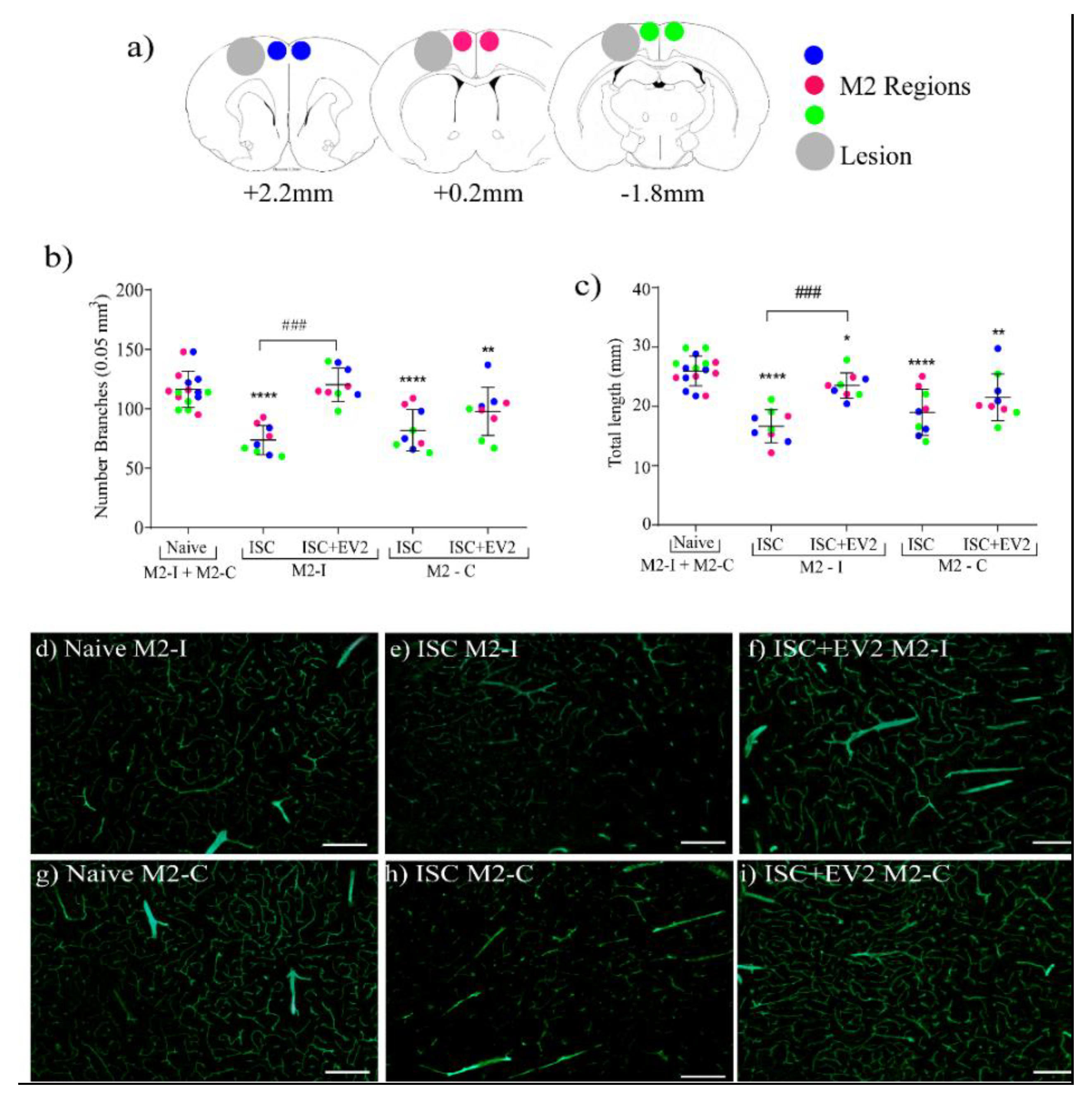
2.8. Brain Angiogenesis
3. Discussion
4. Materials and Methods
4.1. hAT-MSCs: Sources, Culture and Characterization
4.1.1. Commercial hAT-MSCs
4.1.2. Patient-Derived hAT-MSCs
4.1.3. Flow Cytometry
4.1.4. Confocal Microscopy
4.2. Extracellular Vesicles (EVs)
4.2.1. EV Isolation and Purification
4.2.2. EVs Characterization
4.2.3. EVs Measurement
4.2.4. EVs Labeling
4.3. In Vivo Experiments
4.3.1. Animals
4.3.2. Intranasal EV Treatment
4.4. Brain Analysis
4.4.1. Extracellular Fluorescent Vesicle (EV) Detection in the Rat Brain
4.4.2. Short-Term Evaluation of Infarct Volume
4.4.3. Brain Angiogenesis
4.4.4. BBB Permeability
4.5. Behavioral Tasks
4.5.1. Cylinder Task (CT)
4.5.2. Open Field Task (OFT)
4.5.3. Novel Object Recognition Task (NORT)
4.5.4. Elevated Plus-Maze Task (EPMT)
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. The Top 10 Causes of Death. 2020. Available online: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 4 July 2021).
- Kuriakose, D.; Xiao, Z. Pathophysiology and treatment of stroke: Present status and future perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019, 5, 1–22. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of motor function after stroke: A multiple systematic review focused on techniques to stimulate upper extremity recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferro, J.M.; Caeiro, L.; Figueira, M.L. Neuropsychiatric sequelae of stroke. Nat. Rev. Neurol. 2016, 12, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.; Inness, E.L.; Mcilroy, W.E. Stroke. Handb. Clin. Neurol. 2018, 159, 205–228. [Google Scholar] [CrossRef]
- Surawan, J.; Sirithanawutichai, T.; Areemit, S.; Tiamkao, S.; Saensak, S. Prevalence and factors associated with memory disturbance and dementia after acute ischemic stroke. Neurol. Int. 2018, 10, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushie, M.J.; Crawford, A.M.; Sylvain, N.J.; Hou, H.; Hackett, M.J.; George, G.N.; Kelly, M.E. Revealing the Penumbra through Imaging Elemental Markers of Cellular Metabolism in an Ischemic Stroke Model. ACS Chem. Neurosci. 2018, 9, 886–893. [Google Scholar] [CrossRef]
- Williamson, M.R.; Franzen, R.L.; Fuertes, C.J.A.; Dunn, A.K.; Drew, M.R.; Jones, T.A. A window of vascular plasticity coupled to behavioral recovery after stroke. J. Neurosci. 2020, 40, 7651–7667. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.H. A new penumbra: Transitioning from injury into repair after stroke. Nat. Med. 2008, 14, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Uzdensky, A.B. Apoptosis regulation in the penumbra after ischemic stroke: Expression of pro- and antiapoptotic proteins. Apoptosis 2019, 24, 687–702. [Google Scholar] [CrossRef]
- Businger, J.; Fort, A.C.; Vlisides, P.E.; Cobas, M.; Akca, O. Management of Acute Ischemic Stroke-Specific Focus on Anesthetic Management for Mechanical Thrombectomy. Anesth. Analg. 2020, 131, 1124–1134. [Google Scholar] [CrossRef]
- Munich, S.A.; Vakharia, K.; Levy, E.I. Overview of Mechanical Thrombectomy Techniques. Clin. Neurosurg. 2019, 85, S60–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Ali, M.; Tabassum, H.; Parveen, S.; Parvez, S. Pramipexole prevents ischemic cell death via mitochondrial pathways in ischemic stroke. DMM Dis. Model. Mech. 2019, 12, dmm033860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnowski, K.; Opielka, M.; Nazar, W.; Kowianski, P.; Smolenski, R.T. Neuroprotective effects of guanosine in ischemic stroke—small steps towards effective therapy. Int. J. Mol. Sci. 2021, 22, 6898. [Google Scholar] [CrossRef]
- Hansel, G.; Ramos, D.B.; Delgado, C.A.; Souza, D.G.; Almeida, R.F.; Portela, L.V.; Quincozes-Santos, A.; Souza, D.O. The potential therapeutic effect of guanosine after cortical focal ischemia in rats. PLoS ONE 2014, 9, e90693. [Google Scholar] [CrossRef] [Green Version]
- Hansel, G.; Tonon, A.C.; Guella, F.L.; Pettenuzzo, L.F.; Duarte, T.; Duarte, M.M.M.F.; Oses, J.P.; Achaval, M.; Souza, D.O. Guanosine Protects Against Cortical Focal Ischemia. Involvement of Inflammatory Response. Mol. Neurobiol. 2015, 52, 1791–1803. [Google Scholar] [CrossRef] [PubMed]
- Nonose, Y.; Gewehr, P.E.; Almeida, R.F.; da Silva, J.S.; Bellaver, B.; Martins, L.A.M.; Zimmer, E.R.; Greggio, S.; Venturin, G.T.; Da Costa, J.C.; et al. Cortical Bilateral Adaptations in Rats Submitted to Focal Cerebral Ischemia: Emphasis on Glial Metabolism. Mol. Neurobiol. 2018, 55, 2025–2041. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.C.; Loureiro, S.O.; Pettenuzzo, L.F.; Almeida, R.F.; Ynumaru, E.Y.; Guazzelli, P.A.; Meyer, F.S.; Pasquetti, M.V.; Ganzella, M.; Calcagnotto, M.E.; et al. Effects of intranasal guanosine administration on brain function in a rat model of ischemic stroke. Purinergic Signal. 2021, 17, 255–271. [Google Scholar] [CrossRef]
- Teixeira, L.V.; Almeida, R.F.; Rohden, F.; Martins, L.A.M.; Spritzer, P.M.; de Souza, D.O.G. Neuroprotective Effects of Guanosine Administration on In Vivo Cortical Focal Ischemia in Female and Male Wistar Rats. Neurochem. Res. 2018, 43, 1476–1489. [Google Scholar] [CrossRef]
- Sanchez-Bezanilla, S.; David Åberg, N.; Crock, P.; Walker, F.R.; Nilsson, M.; Isgaard, J.; Ong, L.K. Growth hormone promotes motor function after experimental stroke and enhances recovery-promoting mechanisms within the peri-infarct area. Int. J. Mol. Sci. 2020, 21, 606. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.M.; Farahmandnia, M.; Razi, Z.; Delavarifar, S.; Shakibajahromi, B. 12 Hours After Cerebral Ischemia Is the Optimal Time for Bone Marrow Mesenchymal Stem Cell Transplantation. Neural Regen. Res. 2015, 10, 904–908. [Google Scholar] [CrossRef]
- Xu, Y.; Du, S.W.; Yu, X.G.; Han, X.; Hou, J.C.; Guo, H. Human bone marrow mesenchymal stem cell transplantation attenuates axonal injury in stroke rats. Neural Regen. Res. 2014, 9, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V. Concise Review: Stem Cell Therapy for Stroke Patients: Are We There Yet? Stem Cells Transl. Med. 2019, 8, 983–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabrowska, S.; Andrzejewska, A.; Strzemecki, D.; Muraca, M.; Janowski, M.; Lukomska, B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J. Neuroinflammation 2019, 16, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero-Ortega, L.; Laso-García, F.; De Frutos, M.C.G.; Diekhorst, L.; Martínez-Arroyo, A.; Alonso-López, E.; García-Bermejo, M.L.; Rodríguez-Serrano, M.; Arrúe-Gonzalo, M.; Díez-Tejedor, E.; et al. Low dose of extracellular vesicles identified that promote recovery after ischemic stroke. Stem Cell Res. Ther. 2020, 11, 70. [Google Scholar] [CrossRef] [Green Version]
- Pathipati, P.; Lecuyer, M.; Faustino, J.; Strivelli, J.; Phinney, D.G.; Vexler, Z.S. Mesenchymal Stem Cell (MSC)–Derived Extracellular Vesicles Protect from Neonatal Stroke by Interacting with Microglial Cells. Neurotherapeutics 2021, 18, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zagrean, A.M.; Hermann, D.M.; Opris, I.; Zagrean, L.; Popa-Wagner, A. Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. therapeutic implications. Front. Neurosci. 2018, 12, 811. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Duban, L.; Segura, E.; Væron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Saleki, K.; Javanmehr, N.; Khodaparast, J.; Saadat, P.; Nouri, H.R. Mesenchymal stem cell-derived extracellular vesicle-based therapies protect against coupled degeneration of the central nervous and vascular systems in stroke. Ageing Res. Rev. 2020, 62, 101106. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, W.; Lian, L.; Xu, Y.; Bai, X.; Xu, S.; Zhang, J. Stroke Treatment: Is Exosome Therapy Superior to Stem Cell Therapy? Biochimie 2020, 179, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, M.H.; Kink, J.A.; Hematti, P.; Capitini, C.M. Mesenchymal Stromal Cells and Exosomes: Progress and Challenges. Front. Cell Dev. Biol. 2020, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Prunevieille, A.; Babiker-Mohamed, M.H.; Aslami, C.; Gonzalez-Nolasco, B.; Mooney, N.; Benichou, G. T cell antigenicity and immunogenicity of allogeneic exosomes. Am. J. Transplant. 2021, 21, 2583–2589. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, L.L.; Li, Y.F.; Wu, K.M.; Duan, R.R.; Yao, Y.B.; Jing, L.J.; Gong, Z.; Teng, J.F.; Jia, Y.J. Exosomes derived from bone marrow mesenchymal stem cells protect the injured spinal cord by inhibiting pericyte pyroptosis. Neural Regen. Res. 2022, 17, 194–202. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of extracellular vesicles across the blood-brain barrier: Brain pharmacokinetics and effects of inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, T.J.; Kang, L.; Kim, Y.J.; Kang, M.K.; Kim, J.; Ryu, J.H.; Hyeon, T.; Yoon, B.W.; Ko, S.B.; et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials 2020, 243, 119942. [Google Scholar] [CrossRef]
- Xu, R.; Bai, Y.; Min, S.; Xu, X.; Tang, T.; Ju, S. In vivo monitoring and assessment of exogenous mesenchymal stem cell-derived exosomes in mice with ischemic stroke by molecular imaging. Int. J. Nanomed. 2020, 15, 9011–9023. [Google Scholar] [CrossRef]
- Kodali, M.; Castro, O.W.; Kim, D.K.; Thomas, A.; Shuai, B.; Attaluri, S.; Upadhya, R.; Gitai, D.; Madhu, L.N.; Prockop, D.J.; et al. Intranasally administered human msc-derived extracellular vesicles pervasively incorporate into neurons and microglia in both intact and status epilepticus injured forebrain. Int. J. Mol. Sci. 2020, 21, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longa, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, L.D.; Sode, D.; Patel, R.; Lui, A.; Hudson, C.; Patel, N.A.; Bickford, P.C. Intranasal delivery of exosomes from human adipose derived stem cells at forty-eight hours post injury reduces motor and cognitive impairments following traumatic brain injury. Neurochem. Int. 2021, 150, 105173. [Google Scholar] [CrossRef]
- Uder, C.; Brückner, S.; Winkler, S.; Tautenhahn, H.M.; Christ, B. Mammalian MSC from selected species: Features and applications. Cytom. Part A 2018, 93, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.L.; Crawford, J.R.; Dib, N.; Verkh, L.; Tankovich, N.; Cramer, S.C. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke 2019, 50, 2835–2841. [Google Scholar] [CrossRef]
- Jaillard, A.; Hommel, M.; Moisan, A.; Zeffiro, T.A.; Favre-Wiki, I.M.; Barbieux-Guillot, M.; Vadot, W.; Marcel, S.; Lamalle, L.; Grand, S.; et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: A Randomized Clinical Trial. Transl. Stroke Res. 2020, 11, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Perets, N.; Betzer, O.; Shapira, R.; Brenstein, S.; Angel, A.; Sadan, T.; Ashery, U.; Popovtzer, R.; Offen, D. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019, 19, 3422–3431. [Google Scholar] [CrossRef]
- de Vasconcelos dos Santos, A.; da Costa Reis, J.; Diaz Paredes, B.; Moraes, L.; Jasmin; Giraldi-Guimarães, A.; Mendez-Otero, R. Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats. Brain Res. 2010, 1306, 149–158. [Google Scholar] [CrossRef]
- Marcondes, L.A.; Nachtigall, E.G.; Zanluchi, A.; de Carvalho Myskiw, J.; Izquierdo, I.; Furini, C.R.G. Involvement of medial prefrontal cortex NMDA and AMPA/kainate glutamate receptors in social recognition memory consolidation. Neurobiol. Learn. Mem. 2020, 168, 107153. [Google Scholar] [CrossRef]
- Brenna, S.; Krisp, C.; Altmeppen, H.C.; Magnus, T.; Puig, B. Brain-derived extracellular vesicles in health and disease: A methodological perspective. Int. J. Mol. Sci. 2021, 22, 1365. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Z.; He, T.; Qu, M.; Jiang, L.; Li, W.; Shi, X.; Pan, J.; Zhang, L.; Wang, Y.; et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 2019, 9, 2910–2923. [Google Scholar] [CrossRef]
- Kanazawa, M.; Takahashi, T.; Ishikawa, M.; Onodera, O.; Shimohata, T.; del Zoppo, G.J. Angiogenesis in the ischemic core: A potential treatment target? J. Cereb. Blood Flow Metab. 2019, 39, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Forró, T.; Bajkó, Z.; Bălașa, A.; Bălașa, R. Dysfunction of the neurovascular unit in ischemic stroke: Highlights on micrornas and exosomes as potential biomarkers and therapy. Int. J. Mol. Sci. 2021, 22, 5621. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control Release 2017, 264, 112–126. [Google Scholar] [CrossRef]
- Jiang, X.; Andjelkovic, A.V.; Zhu, L.; Yang, T.; Bennett, M.V.L.; Chen, J.; Keep, R.F.; Shi, Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018, 163–164, 144–171. [Google Scholar] [CrossRef] [PubMed]
- Patabendige, A.; Singh, A.; Jenkins, S.; Sen, J.; Chen, R. Astrocyte activation in neurovascular damage and repair following ischaemic stroke. Int. J. Mol. Sci. 2021, 22, 4280. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Ohta, Y.; Takenaga, M.; Hamaguchi, A.; Ootaki, M.; Takeba, Y.; Kobayashi, T.; Watanabe, M.; Iiri, T.; Matsumoto, N. Isolation of adipose-derived stem/stromal cells from cryopreserved fat tissue and transplantation into rats with spinal cord injury. Int. J. Mol. Sci. 2018, 19, 1963. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.; Yang, S.; Siaw-Debrah, F.; Hu, J.; Wu, K.; He, Z.; Yang, J.; Pan, S.; Lin, X.; Ye, H.; et al. Exosomes derived from bone mesenchymal stem cells ameliorate early inflammatory responses following traumatic brain injury. Front. Neurosci. 2019, 13, 14. [Google Scholar] [CrossRef] [Green Version]
- Nalamolu, K.R.; Venkatesh, I.; Mohandass, A.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravallia, K.K. Exosomes treatment mitigates ischemic brain damage but does not improve post-stroke neurological outcome. Cell. Physiol. Biochem. 2019, 52, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomi, G.; Joerger-Messerli, M.; Haesler, V.; Muri, L.; Surbek, D.; Schoeberlein, A. Intranasally Administered Exosomes from Umbilical Cord Stem Cells Have Preventive Neuroprotective Effects and Contribute to Functional Recovery after Perinatal Brain Injury. Cells 2019, 8, 855. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholl, J.N.; De Fraga Dias, A.; Pizzato, P.R.; Lopes, D.V.; Moritz, C.E.J.; Jandrey, E.H.F.; Souto, G.D.; Colombo, M.; Rohden, F.; Sévigny, J.; et al. Characterization and antiproliferative activity of glioma-derived extracellular vesicles. Nanomedicine 2020, 15, 1001–1018. [Google Scholar] [CrossRef]
- da Silva, H.; Nucci, M.P.; Mamani, J.B.; Mendez-Otero, R.; Nucci, L.P.; Tannus, A.; Gamarra, L.F. Evaluation of temperature induction in focal ischemic thermocoagulation model. PLoS ONE 2018, 13, e0200135. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Aakriti; Gupta, V. A review on animal models of stroke: An update. Brain Res. Bull. 2016, 122, 35–44. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Ramos, D.B.; Muller, G.C.; Rocha, G.B.M.; Dellavia, G.H.; Almeida, R.F.; Pettenuzzo, L.F.; Loureiro, S.O.; Hansel, G.; Horn, Â.C.M.; Souza, D.O.; et al. Intranasal guanosine administration presents a wide therapeutic time window to reduce brain damage induced by permanent ischemia in rats. Purinergic Signal. 2016, 12, 149–159. [Google Scholar] [CrossRef]
- Carpentier, G.; Martinelli, M.; Courty, J.; Cascone, I. Angiogenesis Analyzer for ImageJ. In Proceedings of the 4th ImageJ User and Developer Conference Proceedings, Mondorf-Les-Bains, Luxembourg, 24–26 October 2012; pp. 198–201. [Google Scholar]
- Tsou, P.S.; Campbell, P.; Asif Amin, M.; Coit, P.; Miller, S.; Fox, D.A.; Khanna, D.; Sawalha, A.H. Inhibition of EZH2 prevents fibrosis and restores normal angiogenesis in scleroderma. Proc. Natl. Acad. Sci. USA 2019, 116, 3695–3702. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Willumeit-Römer, R.; Luthringer-Feyerabend, B.J.C. Effect of magnesium-degradation products and hypoxia on the angiogenesis of human umbilical vein endothelial cells. Acta Biomater. 2019, 98, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Lai, T.W. Optimization of Evans blue quantitation in limited rat tissue samples. Sci. Rep. 2014, 4, 6588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dal-Pizzol, F.; Rojas, H.A.; Dos Santos, E.M.; Vuolo, F.; Constantino, L.; Feier, G.; Pasquali, M.; Comim, C.M.; Petronilho, F.; Gelain, D.P.; et al. Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood-brain barrier dysfunction in an animal model of severe sepsis. Mol. Neurobiol. 2013, 48, 62–70. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Bioanalytical Method Validation Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK, 2012; pp. 1–23. [Google Scholar]
- FDA. Guidance for Industry: Bioanalytical Method Validation; FDA: Rockville, MD, USA, 2018; pp. 1–44.
- de Almeida, R.F.; Ganzella, M.; Machado, D.G.; Loureiro, S.O.; Leffa, D.; Quincozes-Santos, A.; Pettenuzzo, L.F.; Duarte, M.M.M.F.; Duarte, T.; Souza, D.O. Olfactory bulbectomy in mice triggers transient and long-lasting behavioral impairments and biochemical hippocampal disturbances. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 76, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar]
- Figueiredo, C.P.; Clarke, J.R.; Ledo, J.H.; Ribeiro, F.C.; Costa, C.V.; Melo, H.M.; Mota-Sales, A.P.; Saraiva, L.M.; Klein, W.L.; Sebollela, A.; et al. Memantine rescues transient cognitive impairment caused by high-molecular-weight Aβ oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J. Neurosci. 2013, 33, 9626–9634. [Google Scholar] [CrossRef] [Green Version]
- Almeida, R.F.; Cereser, V.H.; Faraco, R.B.; Böhmer, A.E.; Souza, D.O.; Ganzella, M. Systemic administration of GMP induces anxiolytic-like behavior in rats. Pharmacol. Biochem. Behav. 2010, 96, 306–311. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohden, F.; Teixeira, L.V.; Bernardi, L.P.; Ferreira, P.C.L.; Colombo, M.; Teixeira, G.R.; de Oliveira, F.d.S.; Cirne Lima, E.O.; Guma, F.C.R.; Souza, D.O. Functional Recovery Caused by Human Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles Administered 24 h after Stroke in Rats. Int. J. Mol. Sci. 2021, 22, 12860. https://doi.org/10.3390/ijms222312860
Rohden F, Teixeira LV, Bernardi LP, Ferreira PCL, Colombo M, Teixeira GR, de Oliveira FdS, Cirne Lima EO, Guma FCR, Souza DO. Functional Recovery Caused by Human Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles Administered 24 h after Stroke in Rats. International Journal of Molecular Sciences. 2021; 22(23):12860. https://doi.org/10.3390/ijms222312860
Chicago/Turabian StyleRohden, Francieli, Luciele Varaschini Teixeira, Luis Pedro Bernardi, Pamela Cristina Lukasewicz Ferreira, Mariana Colombo, Geciele Rodrigues Teixeira, Fernanda dos Santos de Oliveira, Elizabeth Obino Cirne Lima, Fátima Costa Rodrigues Guma, and Diogo Onofre Souza. 2021. "Functional Recovery Caused by Human Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles Administered 24 h after Stroke in Rats" International Journal of Molecular Sciences 22, no. 23: 12860. https://doi.org/10.3390/ijms222312860
APA StyleRohden, F., Teixeira, L. V., Bernardi, L. P., Ferreira, P. C. L., Colombo, M., Teixeira, G. R., de Oliveira, F. d. S., Cirne Lima, E. O., Guma, F. C. R., & Souza, D. O. (2021). Functional Recovery Caused by Human Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles Administered 24 h after Stroke in Rats. International Journal of Molecular Sciences, 22(23), 12860. https://doi.org/10.3390/ijms222312860





