Discovery of Lactoferrin as a Stimulant for hADSC-Derived EV Secretion and Proof of Enhancement of Resulting EVs through Skin Model
Abstract
:1. Introduction
2. Results
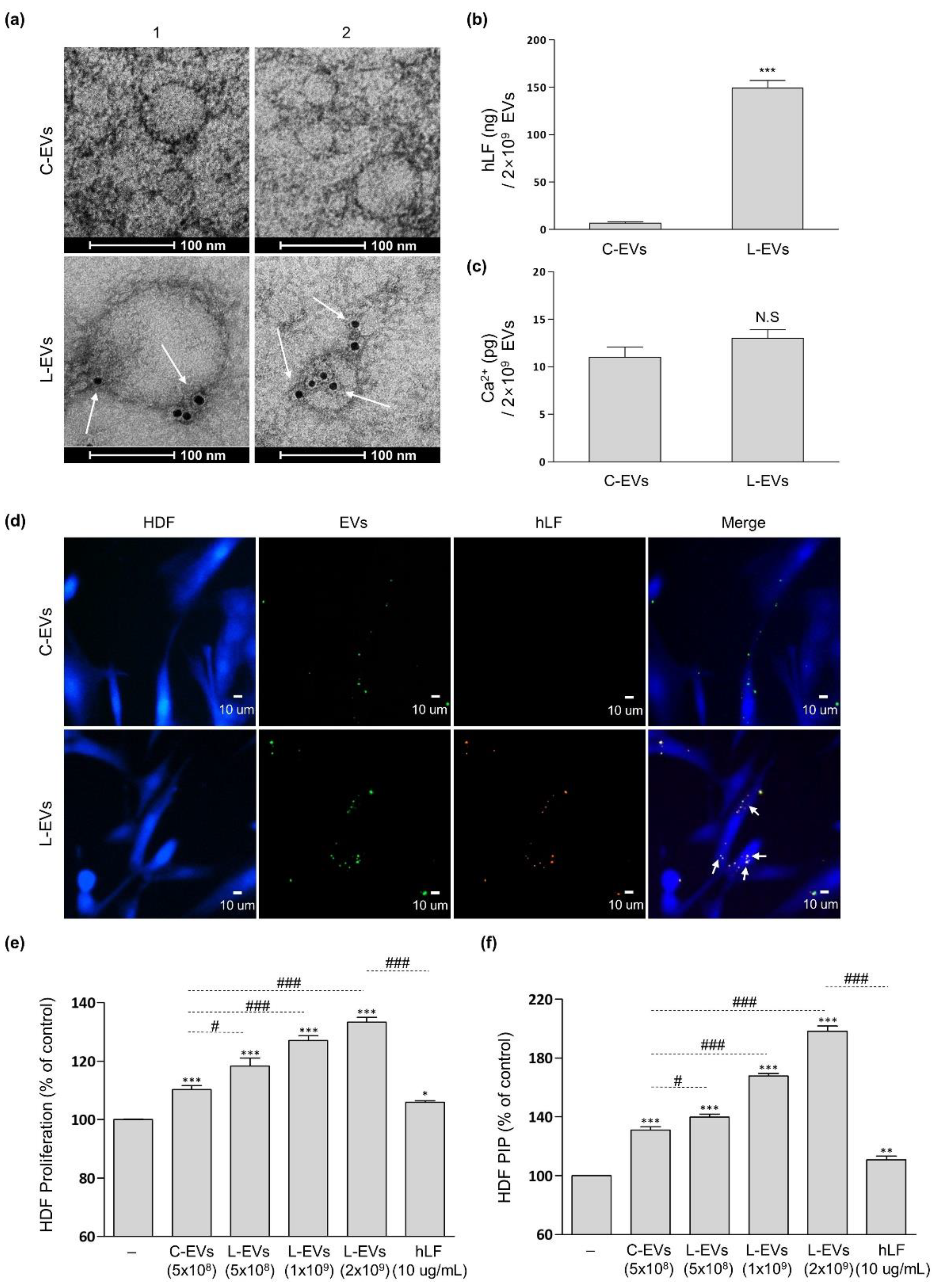
2.1. Intracellular Calcium Regulation by hLF and EVs Secretion
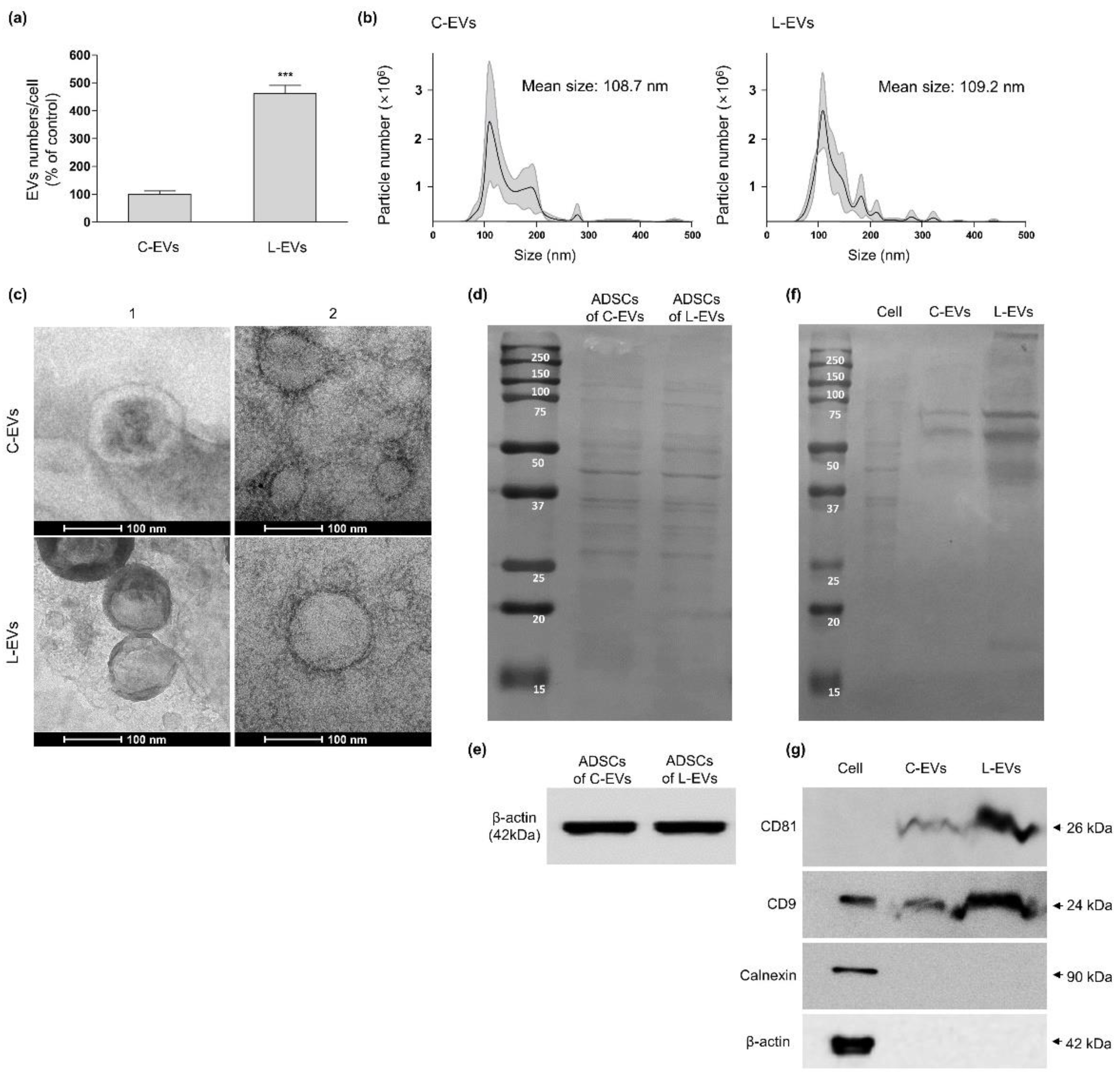
2.2. Productivity and Characteristic Change of EVs Generated from hLF-Stimulated hADSCs
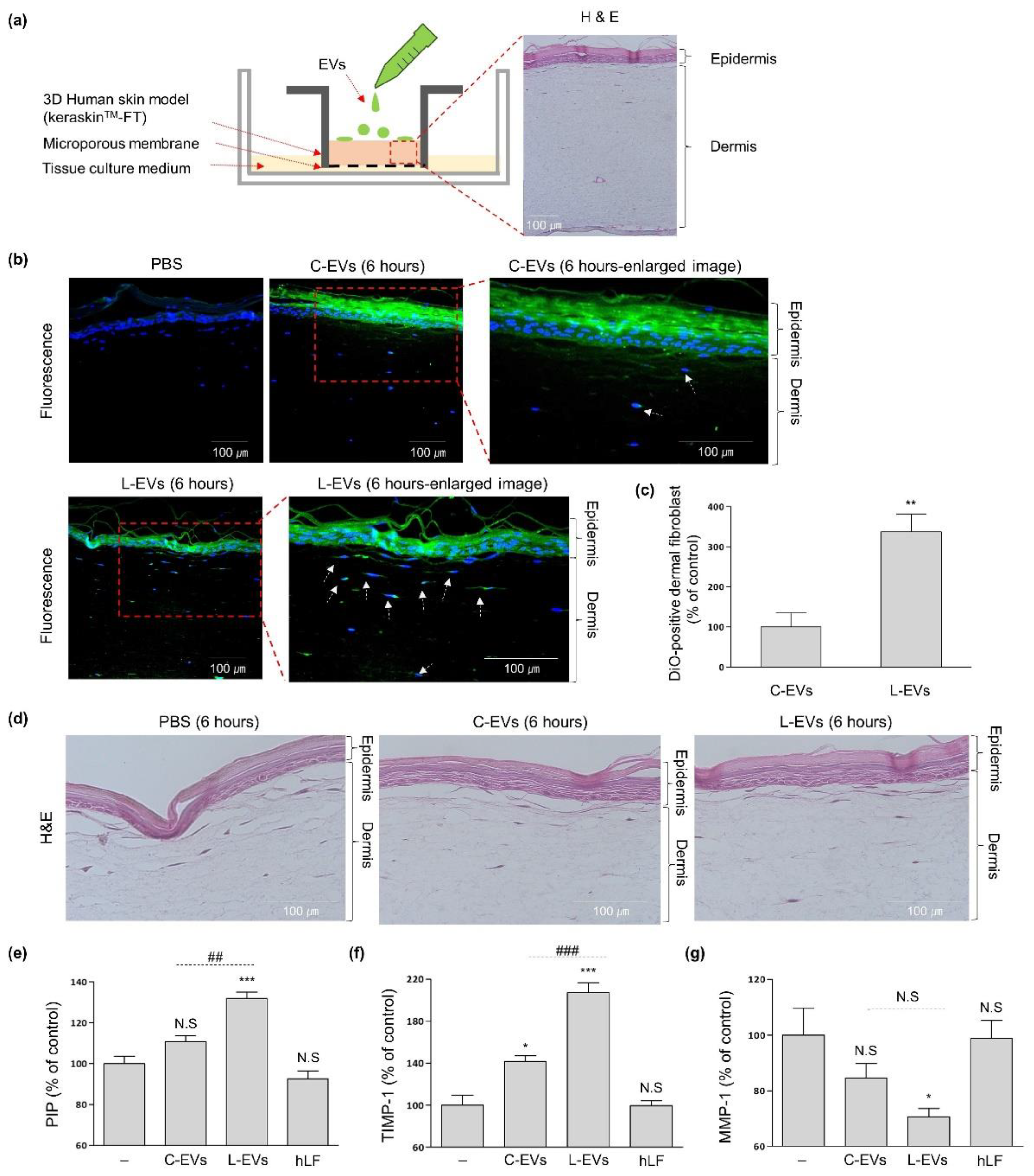
2.3. L-EVs Generated through hLF Are Effective for ECM Synthesis of Skin Tissue Model
3. Discussion
4. Materials and Methods
4.1. hADSC Isolation and Culture
4.2. Preparation of hADSC-Conditioned Media
4.3. EV Separation & Characterization
4.4. Effects of hADSCs-EVs on 2-Dimensional (2D) HDFs
4.5. Effects of hADSCs-EVs on 3-Dimensional (3D) Skin Model
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riazifar, M.; Pone, E.J.; Lotval, J.; Zhao, W. Stem Cell Extracellular Vesicles: Extended messages of regeneration. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 125–154. [Google Scholar] [CrossRef] [Green Version]
- Gomzikova, M.O.; Rizvanov, A.A. Current Trends in regenerative medicine: From cell to cell-free therapy. BioNanoScience 2017, 7, 240–245. [Google Scholar] [CrossRef]
- Galieva, L.R.; James, V.; Mukhamedshina, Y.O.; Rizvanov, A.A. Therapeutic potential of extracellular vesicles for the treatment of nerve disorders. Front. Neurosci. 2019, 13, 163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Sokic, S.; Schardt, J.S.; Raiker, R.S.; Lin, J.W.; Jay, S.M. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 2015, 21, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Bang, O.Y.; Kim, E.H. Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Stroke: Challenges and Progress. Front. Neurol. 2019, 10, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlongan, C.V.; Hadman, M.; Sanberg, C.D.; Sanberg, P.R. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke 2004, 35, 2385–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Yoo S mi Park, H.H.; Lim, H.J.; Kim, Y.L.; Lee, S.; Seo, K.W.; Kang, K.S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates regeneration of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef]
- Choi, E.W.; Seo, M.K.; Woo, E.Y.; Kim, S.H.; Park, E.J.; Kim, S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp. Dermatol. 2018, 27, 1170–1172. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ebisawa, K.; Kambe, M.; Kasai, T.; Suga, H.; Nakamura, K.; Narita, Y.; Ogata, A.; Kamei, Y. Effects of exosomes derived from the induced pluripotent stem cells on skin wound healing. Nagoya J. Med. Sci. 2018, 80, 141–153. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Yamashita, T.; Takahashi, Y.; Takakura, Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 2018, 41, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019, 9, 1015–1028. [Google Scholar] [CrossRef]
- Hedlund, M.; Nagaeva, O.; Kargl, D.; Baranov, V.; Mincheva-Nilsson, L. Thermal and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS ONE 2011, 6, e16899. [Google Scholar] [CrossRef]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysoczynski, M.; Ratajczak, M.Z. Lung cancer secreted microvesicles: Underappreciated modulators of microenvironment in expanding tumors. Int. J. Cancer 2009, 125, 1595–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Colone, M. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezgin, E.; Kaiser, H.J.; Baumgart, T.; Schwille, P.; Simons, K.; Levental, I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat. Protoc. 2012, 7, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Charras, G.T.; Hu, C.K.; Coughlin, M.; Mitchison, T.J. Reassembly of contractile actin cortex in cell blebs. J. Cell Biol. 2006, 175, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Savina, A.; Furlán, M.; Vidal, M.; Colombo, M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 2003, 278, 20083–20090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassar-Gentina, V.; Rojas, E.; Luxoro, M. Rise in cytoplasmic Ca2+ induced by monensin in bovine medullary chromaffin cells. Cell Calcium 1994, 16, 475–480. [Google Scholar] [CrossRef]
- Sandova, M.E.; Aquino, G.; Chávez, J. Sodium-dependent, calmodulin-dependent transmitter release from synaptosomes. Neurosci. Lett. 1985, 56, 271–277. [Google Scholar] [CrossRef]
- Taylor, J.; Bebawy, M. Proteins regulating microvesicle biogenesis and multidrug resistance in cancer. Proteomics 2019, 19, 1800165. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, S.; Morihara, H.; Yokoe, S.; Nakagawa, T.; Moriwaki, K.; Tomoda, K.; Asahi, M. Overexpression of Na+/H+ exchanger 1 specifically induces cell death in human iPS cells via sustained activation of the Rho kinase ROCK. J. Biol. Chem. 2019, 294, 19577–19588. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Takase, M. Lactoferrin research, technology and applications. Int. Dairy J. 2006, 16, 1241–1251. [Google Scholar] [CrossRef]
- Takayama, Y.; Aoki, R. Roles of lactoferrin on skin wound healing. Biochem. Cell Biol. 2012, 90, 497–503. [Google Scholar] [CrossRef]
- Rousseau, E.; Michel, P.P.; Hirsch, E.C. The iron-binding protein lactoferrin protects vulnerable dopamine neurons from degeneration by preserving mitochondrial calcium homeostasis. Mol. Pharmacol. 2013, 84, 888–898. [Google Scholar] [CrossRef] [Green Version]
- Grigorieva, D.V.; Gorudko, I.V.; Shamova, E.V.; Terekhova, M.S.; Maliushkova, E.V.; Semak, I.V.; Cherenkevich, S.N.; Sokolov, A.V.; Timoshenko, A.V. Effects of recombinant human lactoferrin on calcium signaling and functional responses of human neutrophils. Arch. Biochem. Biophys. 2019, 675, 108122. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Takayama, Y.; Mizumachi, K.; Suzuki, C. Lactoferrin promotes hyaluronan synthesis in human dermal fibroblasts. Biotechnol. Lett. 2011, 33, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wu, J.J.; Ma, Q.; Cui, T.; Andreopoulos, F.M.; Gil, J.; Li, J. Human lactoferrin stimulates skin keratinocyte function and wound re-epithelialization. Br. J. Dermatol. 2010, 163, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, H.; Sheokand, N.; Kumar, S.; Chauhan, A.S.; Kumar, M.; Jakhar, P.; Boradia, V.M.; Raje, C.I.; Raje, M. Exosomes: Tunable nano vehicles for macromolecular delivery of transferrin and lactoferrin to specific intracellular compartment. J. Biomed. Nanotechnol. 2016, 12, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.-K.; Lee, J.; Kwon, H.H.; Park, G.-H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Franquesa, M.; Hoogduijn, M.J.; Ripoll, E.; Luk, F.; Salih, M.; Betjes, M.G.H.; Merino, A.M. Update on controls for isolation and quantification methodology of extracellular vesicles derived from adipose tissue mesenchymal stem cells. Front. Immunol. 2014, 5, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Cho, W.L.; Choi, Y.J.; Kim, J.D.; Park, H.A.; Kim, S.Y.; Cho, Y.W. Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J. Extracell. Vesicles 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Varga, J.; Pasche, B. Antitransforming growth factor-beta therapy in fibrosis: Recent progress and implications for systemic sclerosis. Curr. Opin. Rheumatol. 2008, 20, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Wallach, T.; Srivastava, V.; Reyes, E.; Klein, O.; Gartner, Z. Lactoferrin Reverses Methotrexate Driven Epithelial Barrier Defect by Inhibiting TGF-β Mediated Epithelial to Mesenchymal Transition. bioRxiv 2019. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, L.; Li, F.R.; Li, X.; Wang, Z.; Zou, X.; Chen, M. Topical application of exosomes derived from human umbilical cord mesenchymal stem cells in combination with sponge spicules for treatment of photoaging. Int. J. Nanomed. 2020, 15, 2859–2872. [Google Scholar] [CrossRef] [Green Version]
- Mitragotri, S. Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J. Control. Release 2003, 86, 69–92. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; You, G.E.; Woo, M.; Chang, N.H.; Lee, J. Discovery of Lactoferrin as a Stimulant for hADSC-Derived EV Secretion and Proof of Enhancement of Resulting EVs through Skin Model. Int. J. Mol. Sci. 2021, 22, 10993. https://doi.org/10.3390/ijms222010993
Kim J, You GE, Woo M, Chang NH, Lee J. Discovery of Lactoferrin as a Stimulant for hADSC-Derived EV Secretion and Proof of Enhancement of Resulting EVs through Skin Model. International Journal of Molecular Sciences. 2021; 22(20):10993. https://doi.org/10.3390/ijms222010993
Chicago/Turabian StyleKim, Junho, Ga Eun You, Minkyu Woo, Nicole Hyesoo Chang, and Jungsun Lee. 2021. "Discovery of Lactoferrin as a Stimulant for hADSC-Derived EV Secretion and Proof of Enhancement of Resulting EVs through Skin Model" International Journal of Molecular Sciences 22, no. 20: 10993. https://doi.org/10.3390/ijms222010993
APA StyleKim, J., You, G. E., Woo, M., Chang, N. H., & Lee, J. (2021). Discovery of Lactoferrin as a Stimulant for hADSC-Derived EV Secretion and Proof of Enhancement of Resulting EVs through Skin Model. International Journal of Molecular Sciences, 22(20), 10993. https://doi.org/10.3390/ijms222010993





