Subzero Nonfreezing Hypothermia with Insect Antifreeze Protein Dramatically Improves Survival Rate of Mammalian Cells
Abstract
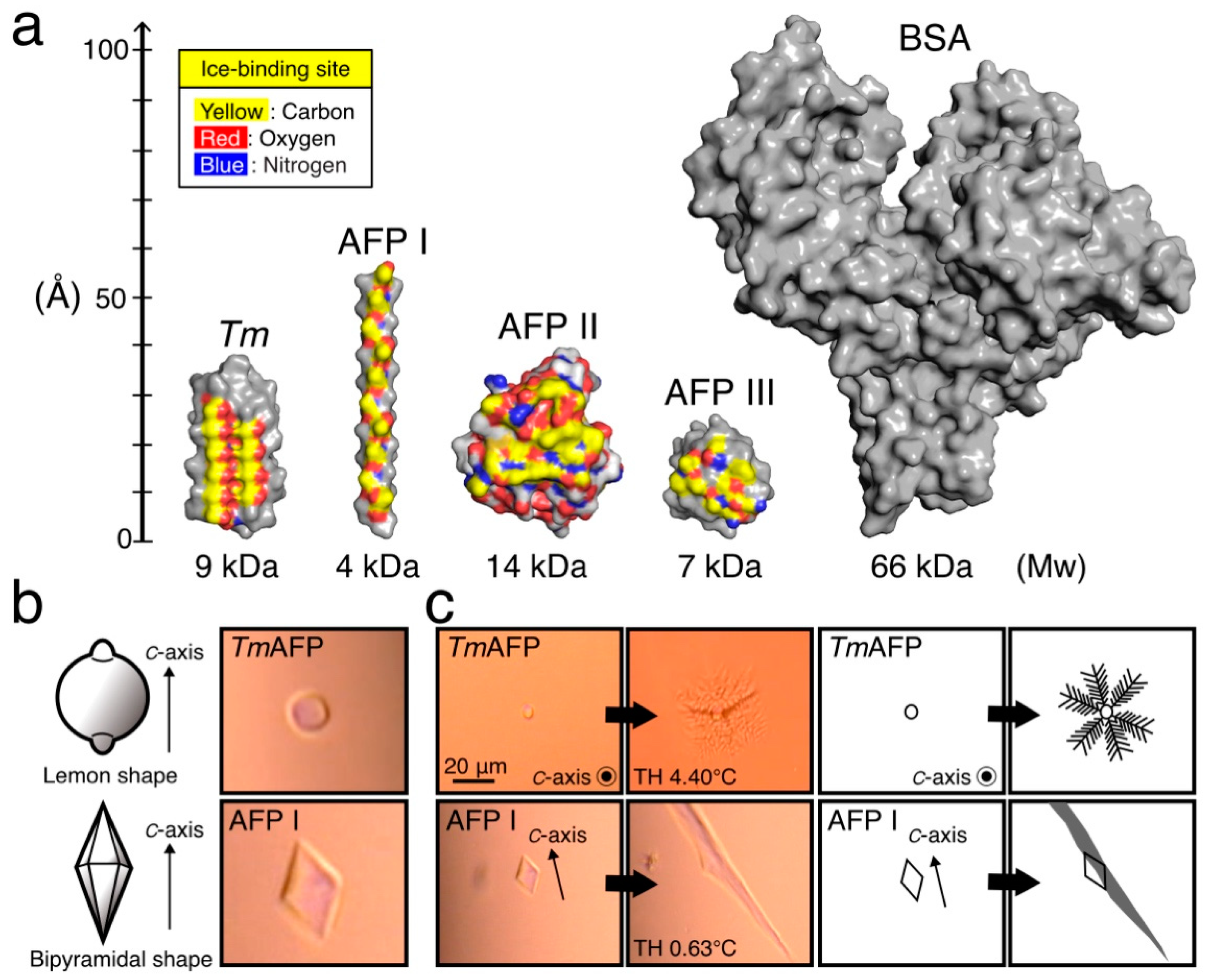
1. Introduction
2. Results and Discussion
2.1. Activity of AFPs in the Cell-Preservation Solution
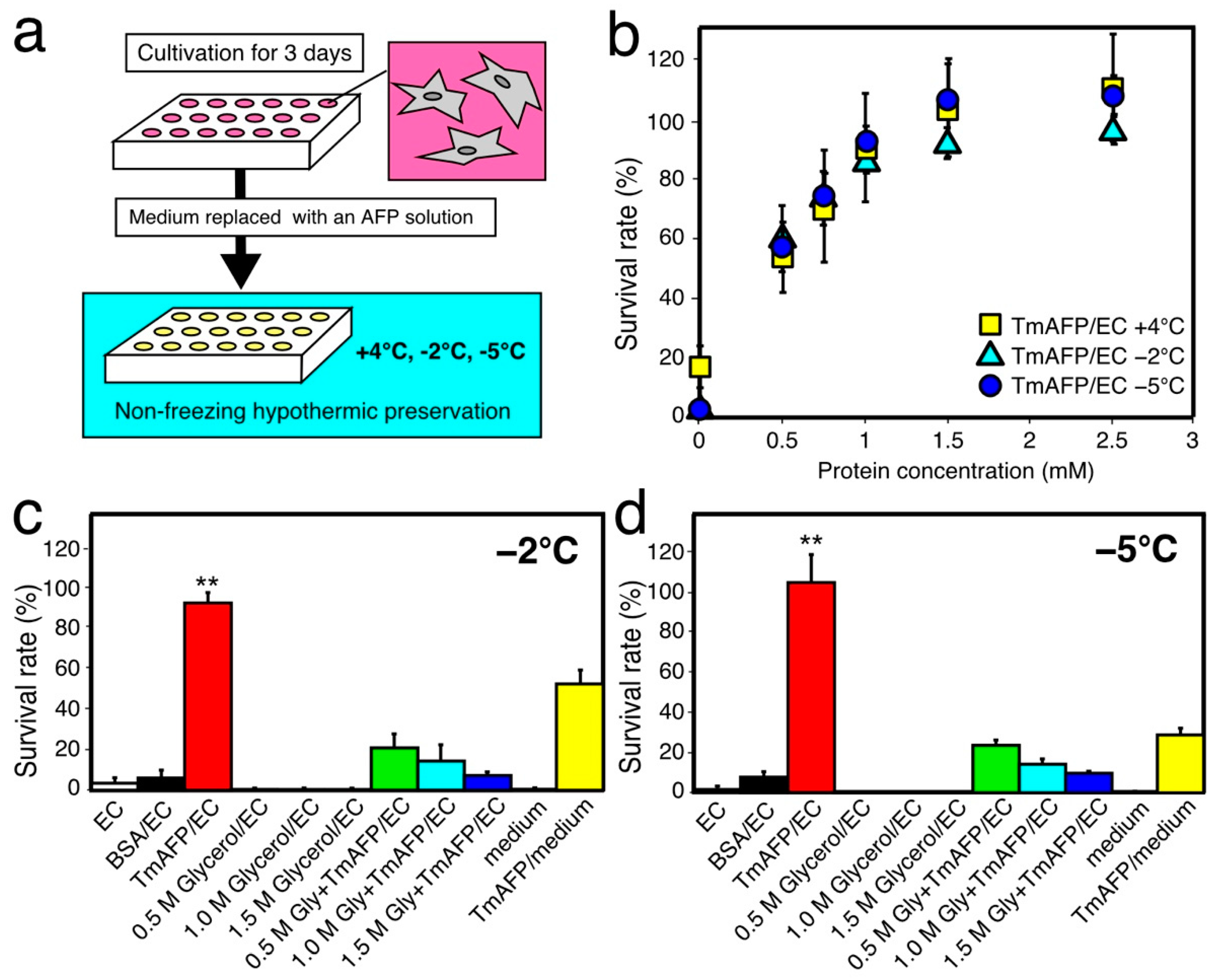
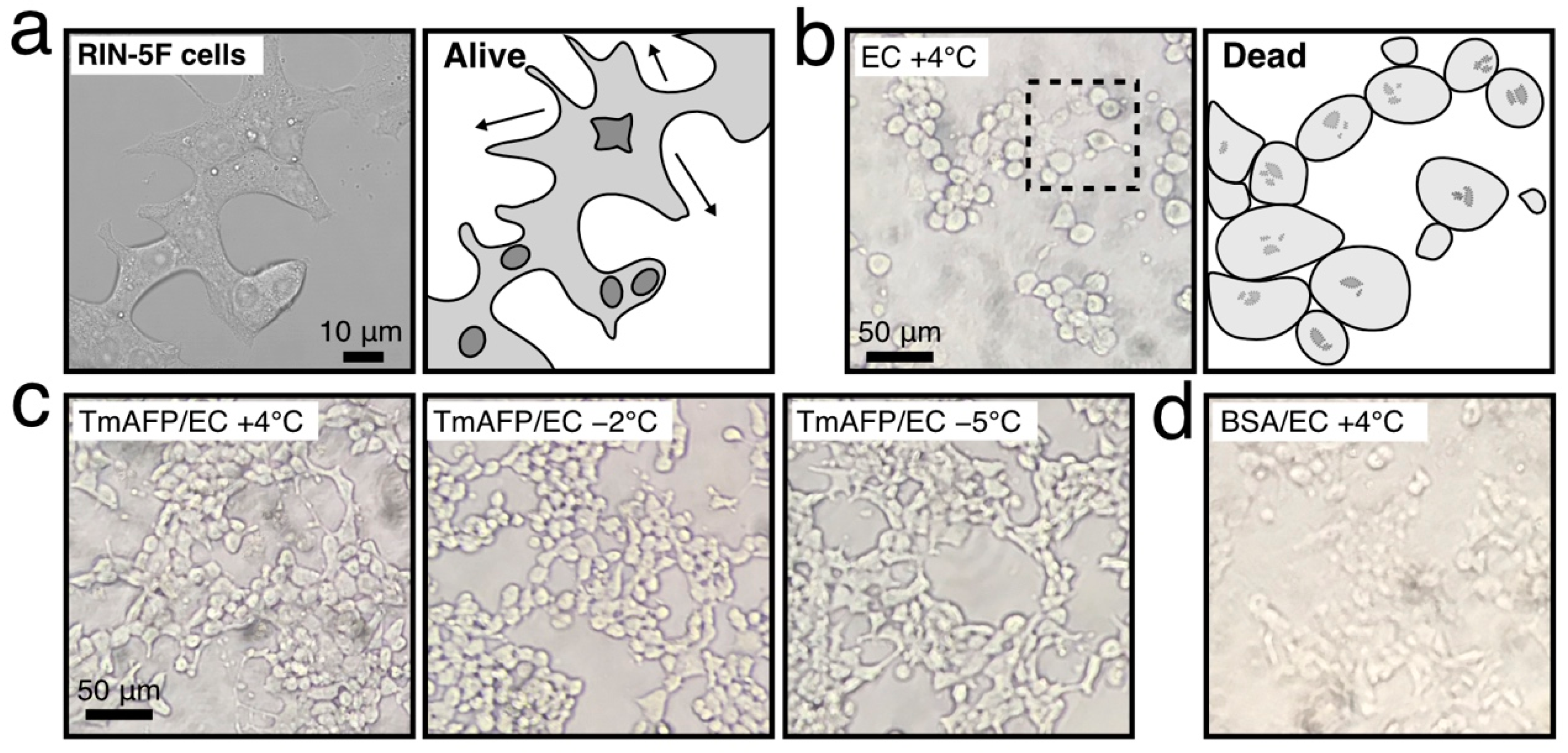
2.2. Preliminary Cell-Preservation Tests with TmAFP
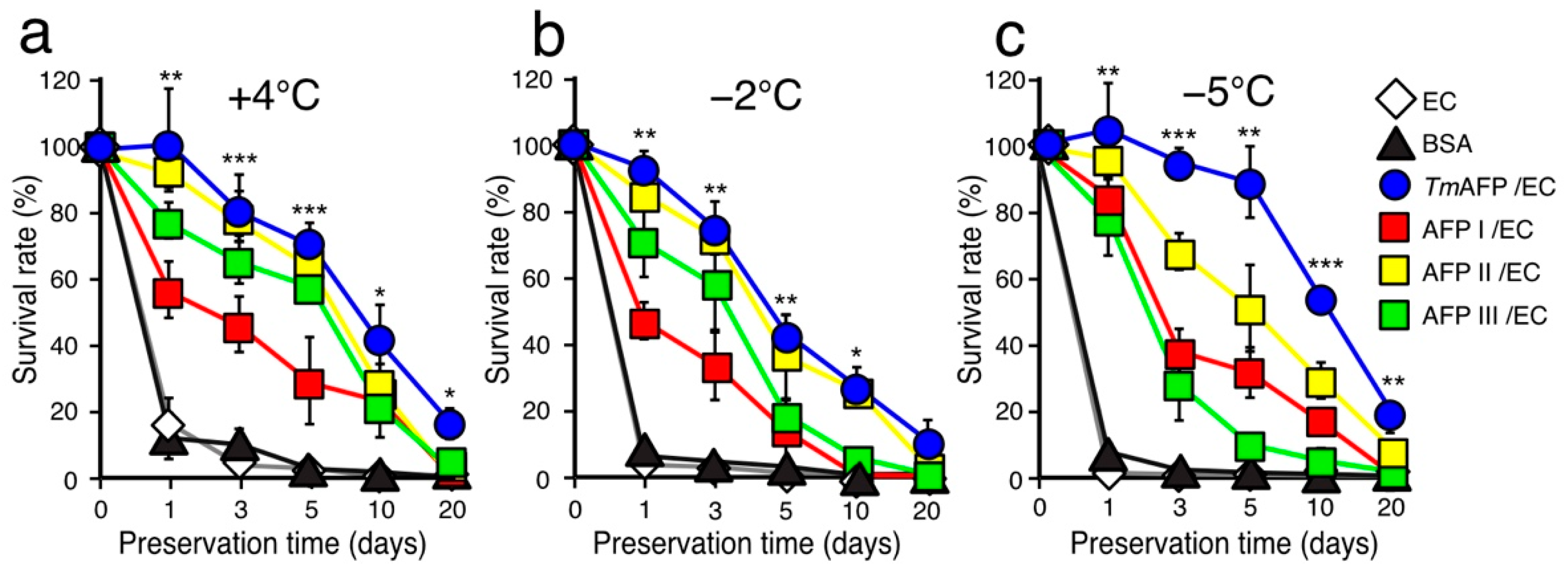
2.3. Comparison of Cell-Preservation Ability between the AFP Species
2.4. Further Cell-Viability Improvement by Employing UW Solution
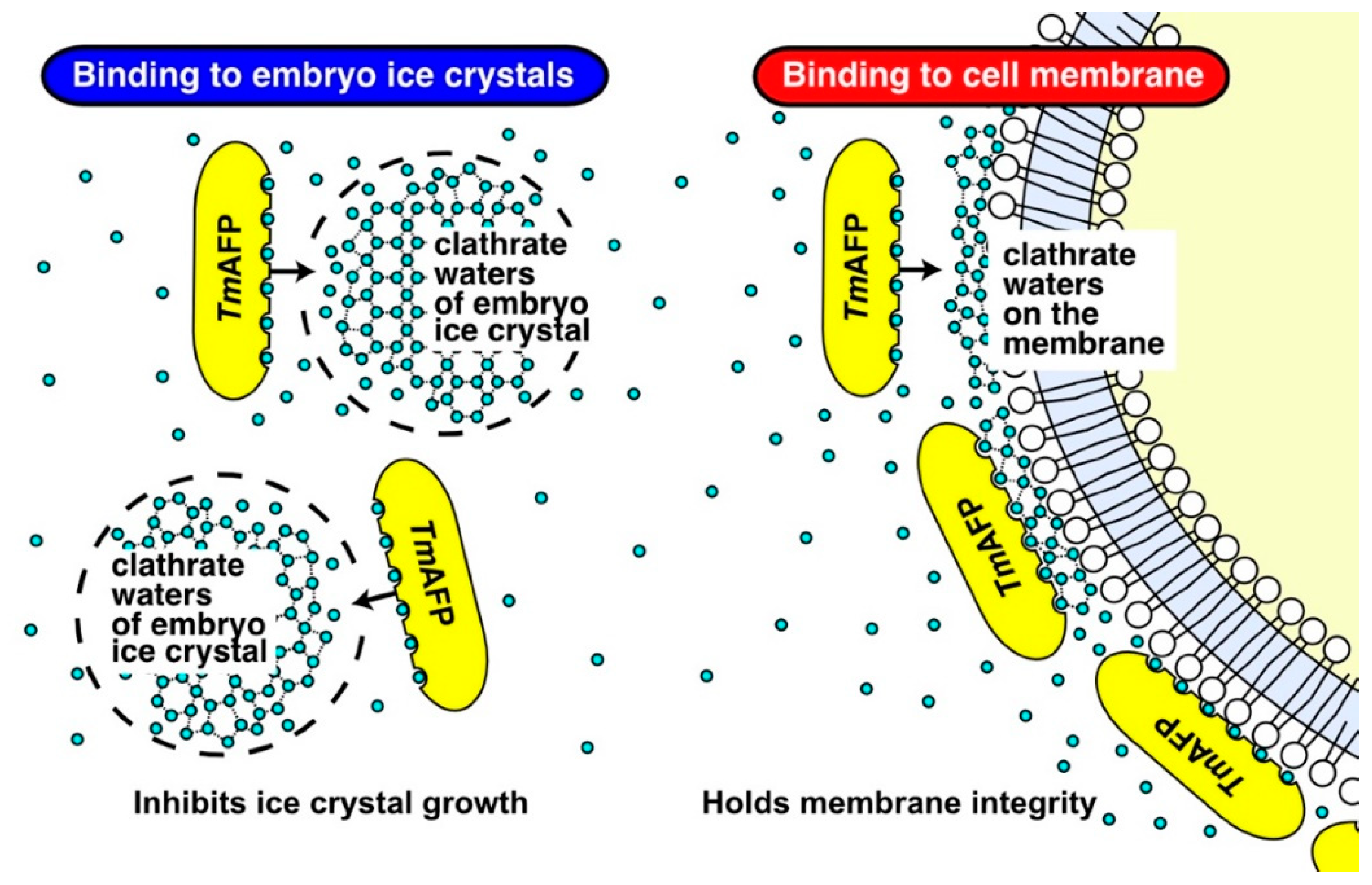
2.5. Mechanistic Consideration for the TmAFP Function
3. Materials and Methods
3.1. Cell-Preservation Solution
3.2. TH Measurement
3.3. Cell Cultivation
3.4. Cell Preservation Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFP | Antifreeze protein |
| TmAFP | Tenebrio molitor-derived AFP |
| BSA | Bovine Serum Albumin |
| RIN-5F | Rat Insulinoma Cell line code 5F |
| TH | Thermal hysteresis |
| IBS | Ice Binding Site |
| PBS | Phosphate Buffer Saline |
| EC | Euro Colins |
| UW | University of Washington |
References
- Rubinsky, B. Principles of low temperature cell preservation. Heart Fail. Rev. 2003, 8, 277–284. [Google Scholar] [CrossRef]
- Zachariassen, K.E.; Kristiansen, E. Ice nucleation and antinucleation in nature. Cryobiology 2000, 41, 257–279. [Google Scholar] [CrossRef]
- Yokoyama, H. Supercooled water and its structure, Bulletin of Yokohama City University. Nat. Sci. 2012, 62, 11–34. [Google Scholar]
- Yokoyama, H.; Kannami, M.; Kanno, H. Existence of clathrate-like structures in supercooled water: X-ray diffraction evidence. Chem. Phys. Lett. 2008, 463, 99–102. [Google Scholar] [CrossRef]
- De Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Supercooling extends preservation time of human livers. Nat. Biotechnol. 2019, 37, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Brockbank, K.G.M.; Duman, J.D.; Chen, Z.; Greene, E.D.; Vu, H.M.; Campbell, L.H. Cell, tissue, and organ preservation with insect-derived antifreeze proteins. In Antifreeze Proteins; Ramløv, H., Eriis, D.S., Eds.; Springer Nature: Cham, Switzerland, 2020; Volume 2, pp. 261–285. [Google Scholar]
- Boutilier, R.G. Mechanisms of cell survival in hypoxia and hypothermia. J. Exp. Biol. 2001, 204, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Qin, M. Hypothermic preservation of Hepatocytes. Biotechnol. Prog. 2003, 19, 1118–1127. [Google Scholar] [CrossRef]
- Quinn, P.J. A lipid-phase separation model of low-temperature damage to biological membranes. Cryobiology 1985, 22, 128–146. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Z.; Cao, J.; Cui, H.; Ma, Z. Cold stress increase reactive oxygen species formation via TRPA1 activation in A549 cells. Cell Stress Chaperones 2016, 21, 367–372. [Google Scholar] [CrossRef]
- Nickel, A.; Kohlhaas, M.; Maack, C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell. Cardiol. 2014, 73, 26–33. [Google Scholar] [CrossRef]
- Voets, I.K. From ice-binding proteins to bio-inspired antifreeze materials. Soft Matter 2017, 12, 4808–4823. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.A.; DeVries, A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L.; Wohlschlag, D.E. Freezing resistance in some Antarctic fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Mattioli, M.; Devries, A.L. The effect of antifreeze glycopeptides on membrane-potential changes at hypothermic temperatures. Biochem. Biophys. Res. Commun. 1990, 173, 1369–1374. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Fletcher, G.L. Hypothermic protection—A fundamental property of “antifreeze protein”. Biochem. Biophys. Res. Commun. 1991, 180, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, P.V. Ice Physics; Oxford University Press: London, UK, 1974; pp. 461–523. [Google Scholar]
- Mahatabuddin, S.; Tsuda, S. Applications of Antifreeze Proteins: Practical Use of the Quality Products from Japanese Fishes. Adv. Exp. Med. Biol. 2018, 1081, 321–337. [Google Scholar] [CrossRef]
- Scotter, A.J.; Marshall, C.B.; Graham, L.A.; Gilbert, J.A.; Garnham, C.P.; Davies, P.L. The basis for hyperactivity of antifreeze proteins. Cryobiology 2006, 53, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Takamichi, M.; Nishimiya, Y.; Miura, A.; Tsuda, S. Fully active QAE isoform confers thermal hysteresis activity on a defective SP isoform of type III antifreeze protein. FEBS J. 2009, 276, 1471–1479. [Google Scholar] [CrossRef]
- Celik, Y.; Graham, L.A.; Mok, Y.-F.; Bar, M.; Davies, P.L.; Braslavsky, I. Superheating of ice crystals in antifreeze protein solutions. Proc. Natl. Acad. Sci. USA 2010, 107, 5423–5428. [Google Scholar] [CrossRef]
- Davies, P.L. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem. Sci. 2017, 39, 548–555. [Google Scholar] [CrossRef]
- Barrett, J. Thermal hysteresis proteins. Int. J. Biochem. Cell Biol. 2001, 33, 105–117. [Google Scholar] [CrossRef]
- Takamichi, M.; Nishimiya, Y.; Miura, A.; Tsuda, S. Effect of annealing time of an ice crystal on the activity of type III antifreeze protein. FEBS J. 2007, 274, 6469–6476. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Nishimiya, Y.; Kowata, K.; Mizutani, F.; Tsuda, S.; Komatsu, Y. Construction of time-lapse scanning electrochemical microscopy with temperature control and its application to evaluate the preservation effects of antifreeze proteins on living cells. Anal. Chem. 2008, 80, 9349–9354. [Google Scholar] [CrossRef] [PubMed]
- Kamijima, T.; Sakashita, M.; Miura, A.; Nishimiya, Y.; Tsuda, S. Antifreeze protein prolongs the lifetime of insulinoma cells during hypothermic preservation. PLoS ONE 2013, 8, e73643. [Google Scholar] [CrossRef]
- Hays, L.M.; Feeney, R.E.; Crowe, L.M.; Crowe, J.H.; Oliver, A.E. Antifreeze glycoproteins inhibit leakage from liposomes during thermotropic phase transitions. Proc. Natl. Acad. Sci. USA 1996, 93, 6835–6840. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Banoub, J.; Goddard, S.V.; Kao, M.H.; Fletcher, G.L. Antifreeze glycoproteins: Relationship between molecular weight, thermal hysteresis and the inhibition of leakage from liposomes during thermotropic phase transition. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 128, 265–273. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Hincha, D.K.; Estrada, S.D.; Wolkers, W.F.; Crowe, L.M.; Feeney, R.E.; Tablin, F.; Crowe, J.H. A mechanism for stabilization of membranes at low temperatures by an antifreeze protein. Biophys. J. 2002, 82, 874–881. [Google Scholar] [CrossRef]
- Hirano, Y.; Nishimiya, Y.; Matsumoto, S.; Matsushita, M.; Todo, S.; Miura, A.; Komatsu, Y.; Tsuda, S. Hypothermic preservation effect on mammalian cells of type III antifreeze proteins from notched-fin eelpout. Cryobiology 2008, 57, 46–51. [Google Scholar] [CrossRef]
- Rubinsky, L.; Raichman, N.; Lavee, J.; Frenck, H.; Ben-Jacob, E.; Bickler, P.E. Antifreeze protein suppresses spontaneous neural activity and protects neurons from hypothermia/re-warming injury. Neurosci. Res. 2010, 67, 256–259. [Google Scholar] [CrossRef]
- Amir, G.; Horowitz, L.; Rubinsky, B.; Yousif, B.S.; Lavee, J.; Smolinsky, A.K. Subzero nonfreezing cryopreservation of rat hearts using antifreeze protein I and antifreeze protein III. Cryobiology 2004, 48, 273–282. [Google Scholar] [CrossRef]
- Ideta, A.; Aoyagi, Y.; Tsuchiya, K.; Nakamura, Y.; Hayama, K.; Shirasawa, A.; Sakaguchi, K.; Tominaga, N.; Nishimiya, Y.; Tsuda, S. Prolonging hypothermic storage (4 °C) of bovine embryos with fish antifreeze protein. J. Reprod. Dev. 2015, 61, 1–6. [Google Scholar] [CrossRef]
- Duman, J.G. Thermal-hysteresis-factors in overwintering insects. J. Insect Physiol. 1979, 25, 805–810. [Google Scholar] [CrossRef]
- Liou, Y.-C.; Tocilj, A.; Davies, P.L.; Jia, A. Mimicry of ice structure by surface hydroxyls and water of a β-helix antifreeze protein. Nature 2000, 406, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Mahatabuddin, S.; Hanada, Y.; Nishimiya, Y.; Miura, A.; Kondo, H.; Davies, P.L.; Tsuda, S. Concentration-dependent oligomerization of an alpha-helical antifreeze polypeptide makes it hyperactive. Sci. Rep. 2017, 7, 42501. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, Y.; Kondo, H.; Takamichi, M.; Sugimoto, H.; Suzuki, M.; Miura, A.; Tsuda, S. Crystal structure and mutational analysis of Ca2+-independent type II antifreeze protein from Longsnout poacher, Brachyopsis rostratus. J. Mol. Biol. 2008, 382, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Mahatabuddin, S.; Fukami, D.; Arai, T.; Nishimiya, Y.; Shimizu, R.; Shibazaki, C.; Kondo, H.; Adachi, M.; Tsuda, S. Polypentagonal ice-like water networks emerge solely in an activity-improved variant of ice-binding protein. Proc. Natl. Acad. Sci. USA 2018, 115, 5456–5464. [Google Scholar] [CrossRef]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumin. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Hew, C.L.; Yang, D.S.C. Protein interaction with ice. Eur. J. Biochem. 1992, 203, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Tomalty, H.E.; Graham, L.A.; Eves, R.; Gruneberg, A.K.; Davies, P.L. Laboratory-Scale Isolation of Insect Antifreeze Protein for Cryobiology. Biomolecules 2019, 9, 180. [Google Scholar] [CrossRef]
- Usta, O.B.; Kim, Y.; Ozer, S.; Bruinsma, B.G.; Lee, J.; Demir, E.; Berendsen, T.A.; Puts, C.F.; Izamis, M.-L.; Uygun, K.; et al. Supercooling as a viable non-freezing cell preservation method of Rat hepatocytes. PLoS ONE 2013, 8, e69334. [Google Scholar] [CrossRef]
- Puts, C.F.; Berendsen, T.A.; Bruinsma, B.G.; Ozer, S.; Luitje, M.; Usta, O.B.; Yarmush, M.L.; Uygun, K. Polyethylene glycol protects primary hepatocytes during supercooling preservation. Cryobiology 2015, 71, 125–129. [Google Scholar] [CrossRef]
- Graether, S.P.; Sykes, B.D. Cold survival in freeze-intolerant insects: The structure and function of β-helical antifreeze proteins. Eur. J. Biochem. 2004, 271, 3285–3296. [Google Scholar] [CrossRef] [PubMed]
- Rubinsky, B.; Mattioil, M.; Arav, A.; Barboni, B.; Fletcher, G.L. Inhibition of Ca2+ and K+ currents by “antifreeze” proteins. Am. J. Physiol. 1992, 262, R542–R545. [Google Scholar] [CrossRef] [PubMed]
- Murzyn, K.; Zhao, W.; Karttunen, M.; Kurdziel, M.; Róg, T. Dynamics of water at membrane surface: Effect of headgroup structure. Biointerphases 2006, 1, 98–105. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Wu, W.-G. Structure and dynamics of primary hydration shell of phosphatidylcholine bilayers at subzero temperatures. Biophys. J. 1996, 71, 3278–3287. [Google Scholar] [CrossRef][Green Version]
- Chakraborty, S.; Jana, B. Optimum number of anchored clathrate water and its instantaneous fluctuations dictate ice plane recognition specificities of insect antifreeze protein. J. Phys. Chem. B 2018, 122, 3056–3067. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Yamauchi, A.; Khan, N.M.; Arai, T.; Mahatabuddin, S.; Miura, A.; Kondo, H. Fish-derived antifreeze proteins and antifreeze glycoprotein exhibit a different ice-binding property with increasing concentration. Biomolecules 2020, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Yamauchi, A.; Miura, A.; Kondo, H.; Nishimiya, Y.; Sasaki, Y.C.; Tsuda, S. Discovery of hyperactive antifreeze protein from phylogenetically distant beetles questions its evolutionary origin. Int. J. Mol. Sci. 2021, 22, 3637. [Google Scholar] [CrossRef]
- Skelin, M.; Rupnik, M.; Cencic, A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX-Altern. Anim. Exp. 2010, 27, 105–113. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, A.; Miura, A.; Kondo, H.; Arai, T.; Sasaki, Y.C.; Tsuda, S. Subzero Nonfreezing Hypothermia with Insect Antifreeze Protein Dramatically Improves Survival Rate of Mammalian Cells. Int. J. Mol. Sci. 2021, 22, 12680. https://doi.org/10.3390/ijms222312680
Yamauchi A, Miura A, Kondo H, Arai T, Sasaki YC, Tsuda S. Subzero Nonfreezing Hypothermia with Insect Antifreeze Protein Dramatically Improves Survival Rate of Mammalian Cells. International Journal of Molecular Sciences. 2021; 22(23):12680. https://doi.org/10.3390/ijms222312680
Chicago/Turabian StyleYamauchi, Akari, Ai Miura, Hidemasa Kondo, Tatsuya Arai, Yuji C. Sasaki, and Sakae Tsuda. 2021. "Subzero Nonfreezing Hypothermia with Insect Antifreeze Protein Dramatically Improves Survival Rate of Mammalian Cells" International Journal of Molecular Sciences 22, no. 23: 12680. https://doi.org/10.3390/ijms222312680
APA StyleYamauchi, A., Miura, A., Kondo, H., Arai, T., Sasaki, Y. C., & Tsuda, S. (2021). Subzero Nonfreezing Hypothermia with Insect Antifreeze Protein Dramatically Improves Survival Rate of Mammalian Cells. International Journal of Molecular Sciences, 22(23), 12680. https://doi.org/10.3390/ijms222312680






