Activated Carbon Fiber Cloth/Biomimetic Apatite: A Dual Drug Delivery System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Apatite Material Characterization
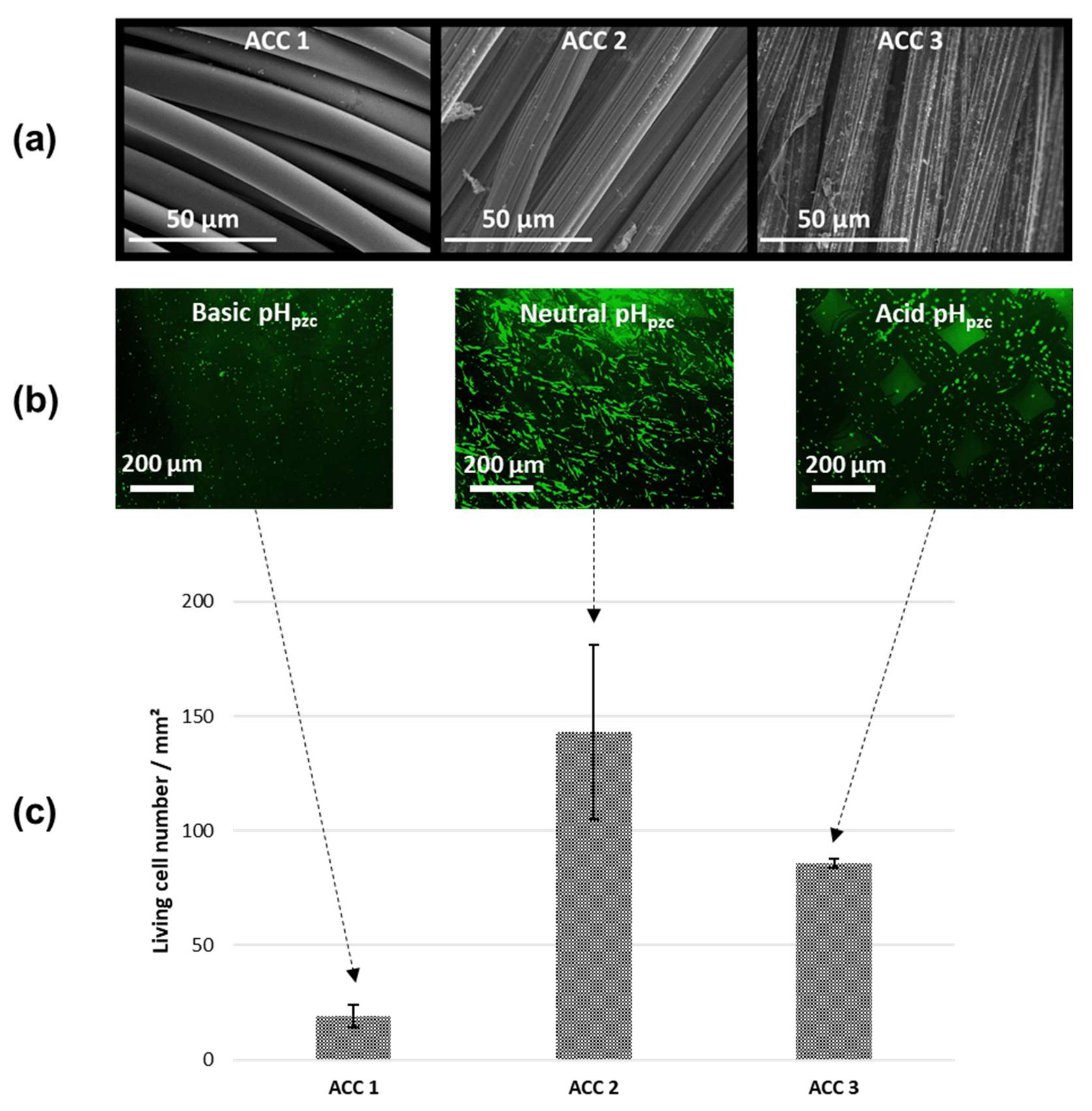
2.2. Characterization of Activated Carbon Fiber Cloth (ACC) Substrates
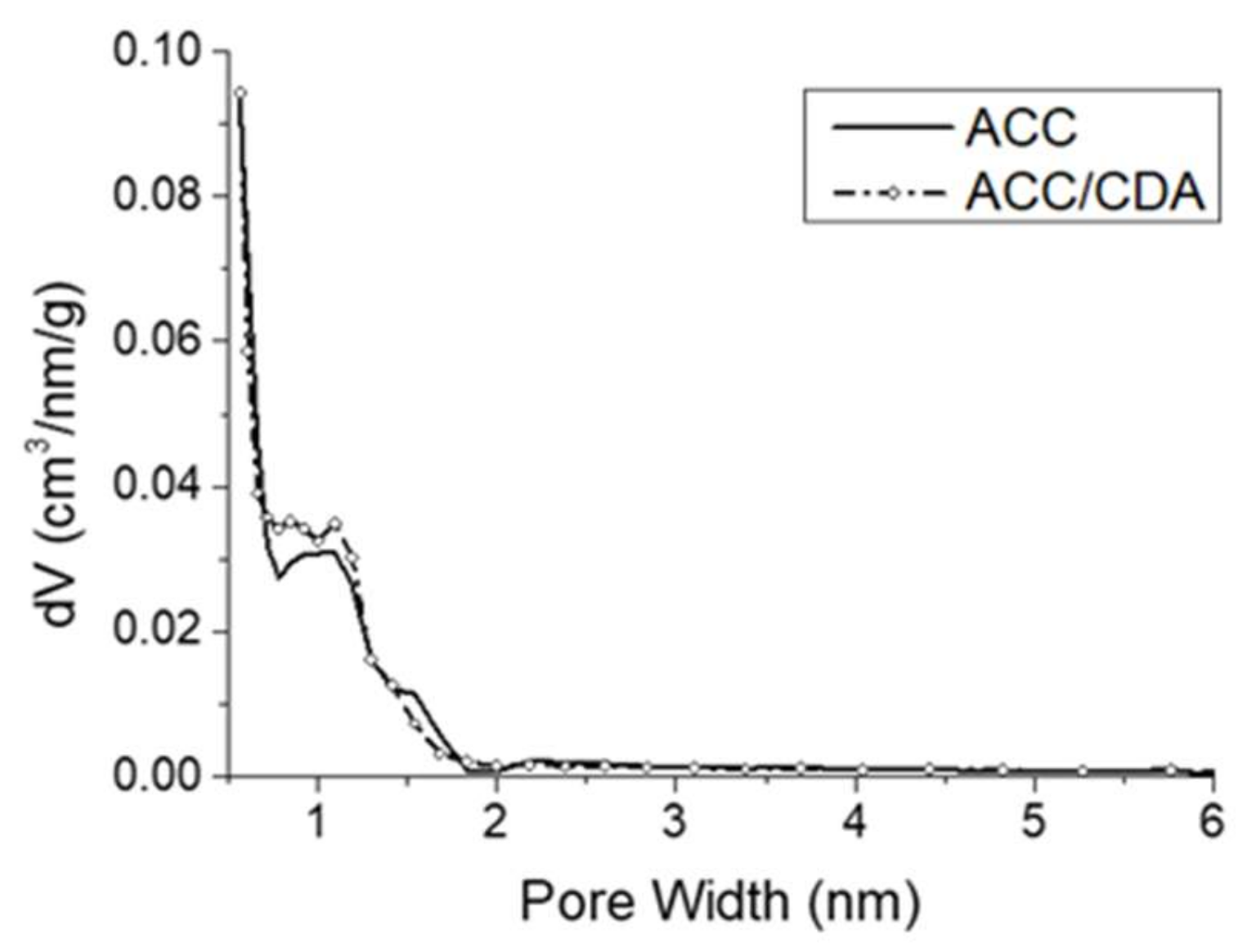
2.3. Characterization of ACC/CDA Porosity
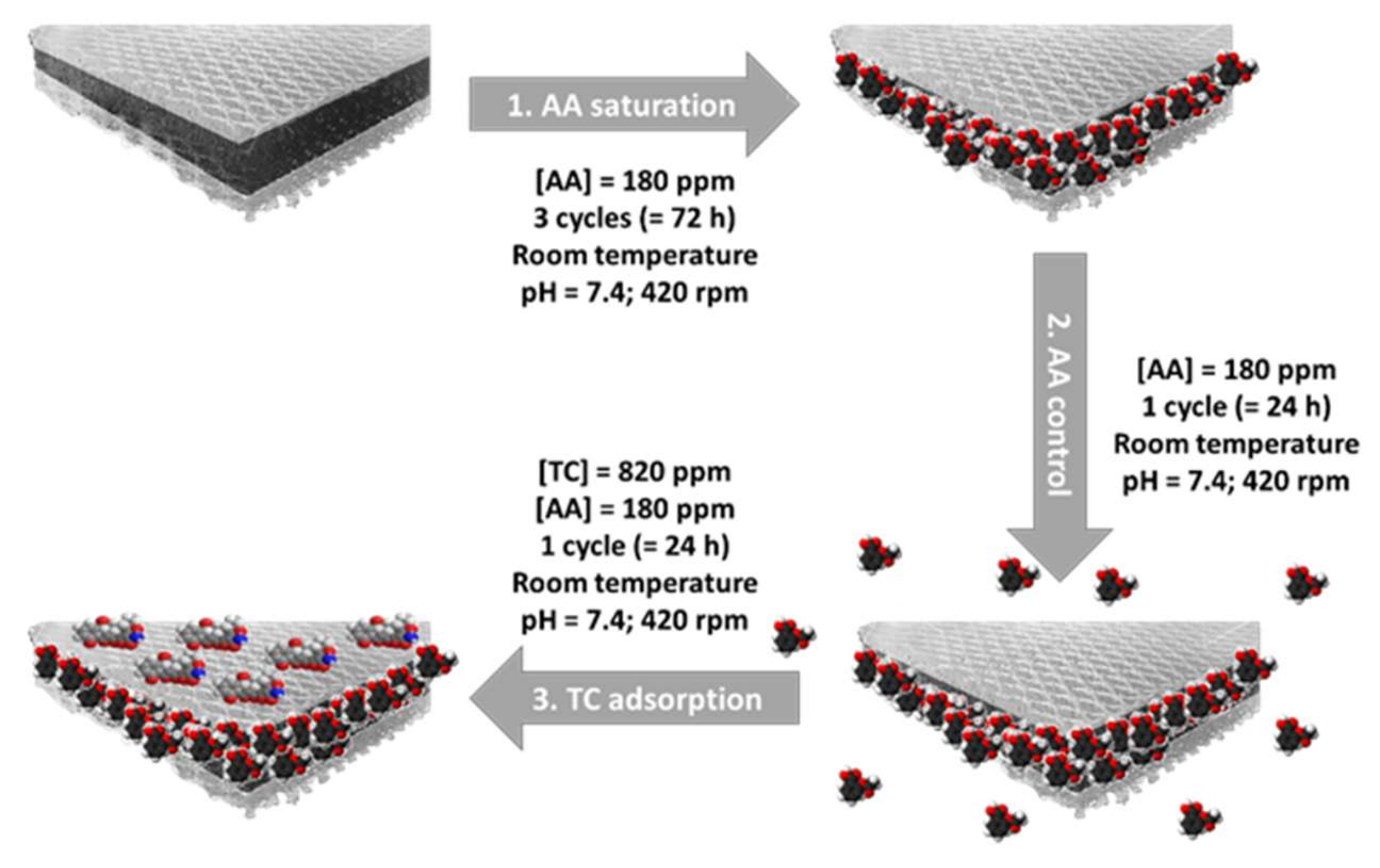
2.4. Tetracycline and Aspirin Adsorption Experiments
2.4.1. Adsorption Kinetics and Isotherms on Biomimetic Apatite Powder
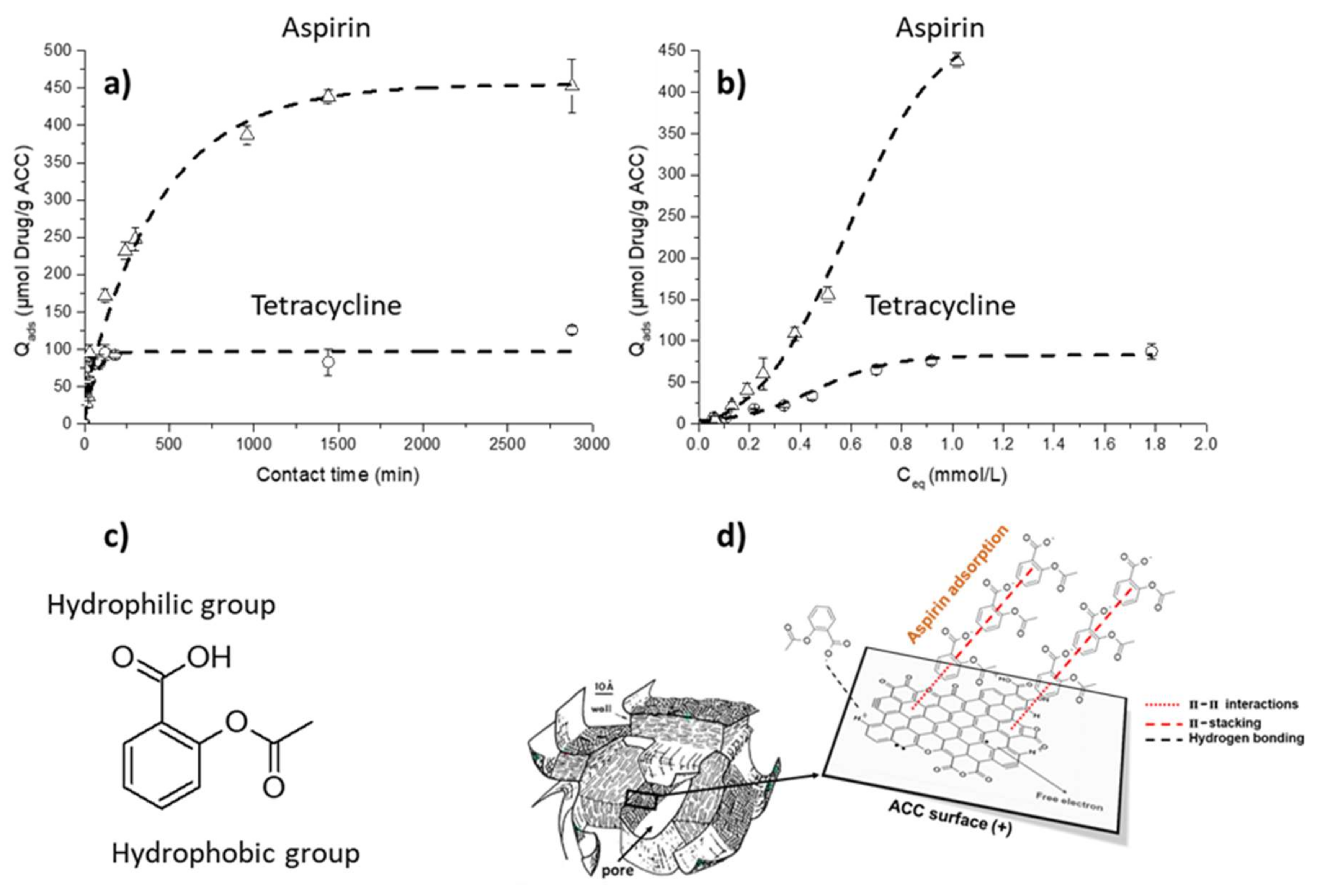
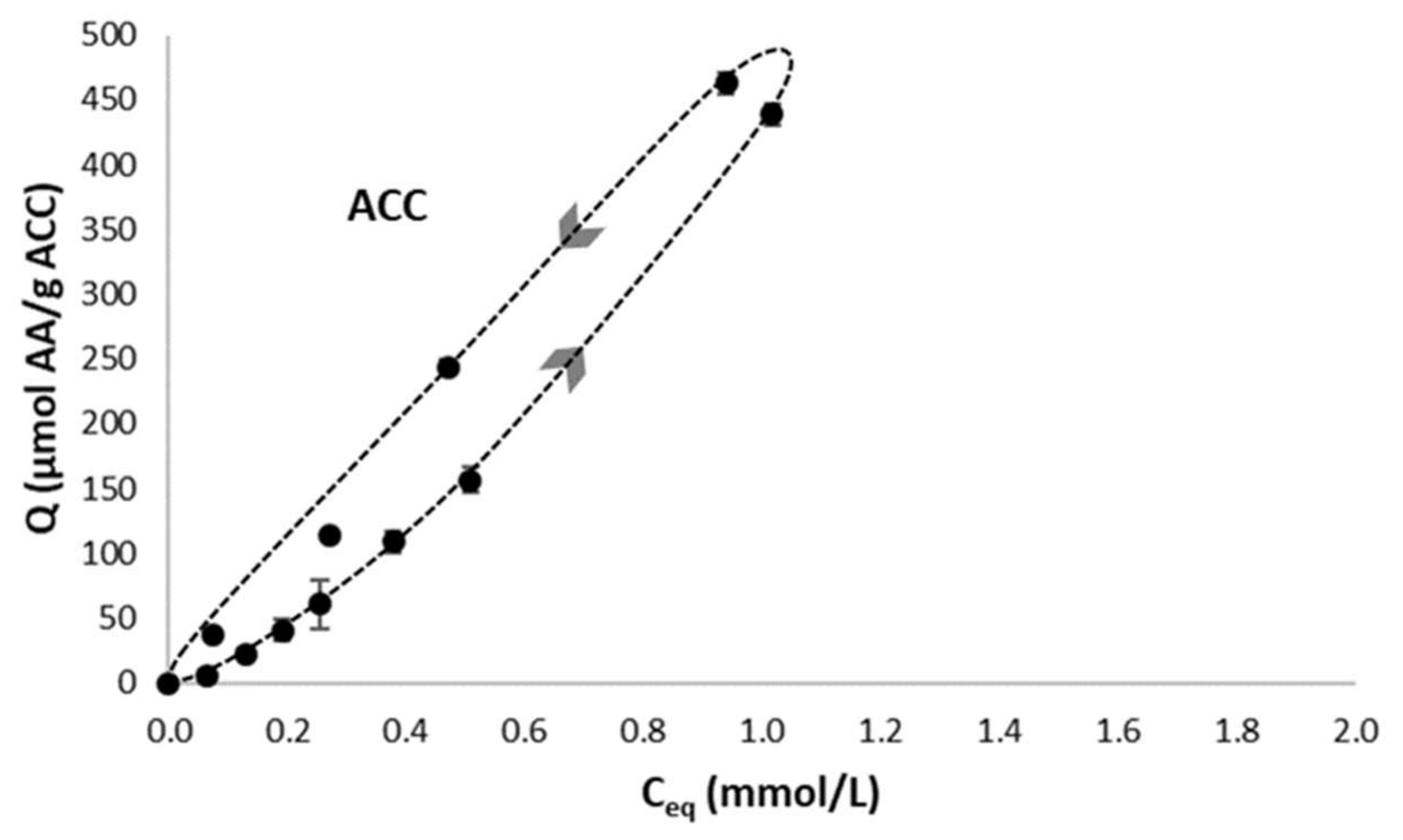
2.4.2. Adsorption Kinetics and Isotherms on ACC Material
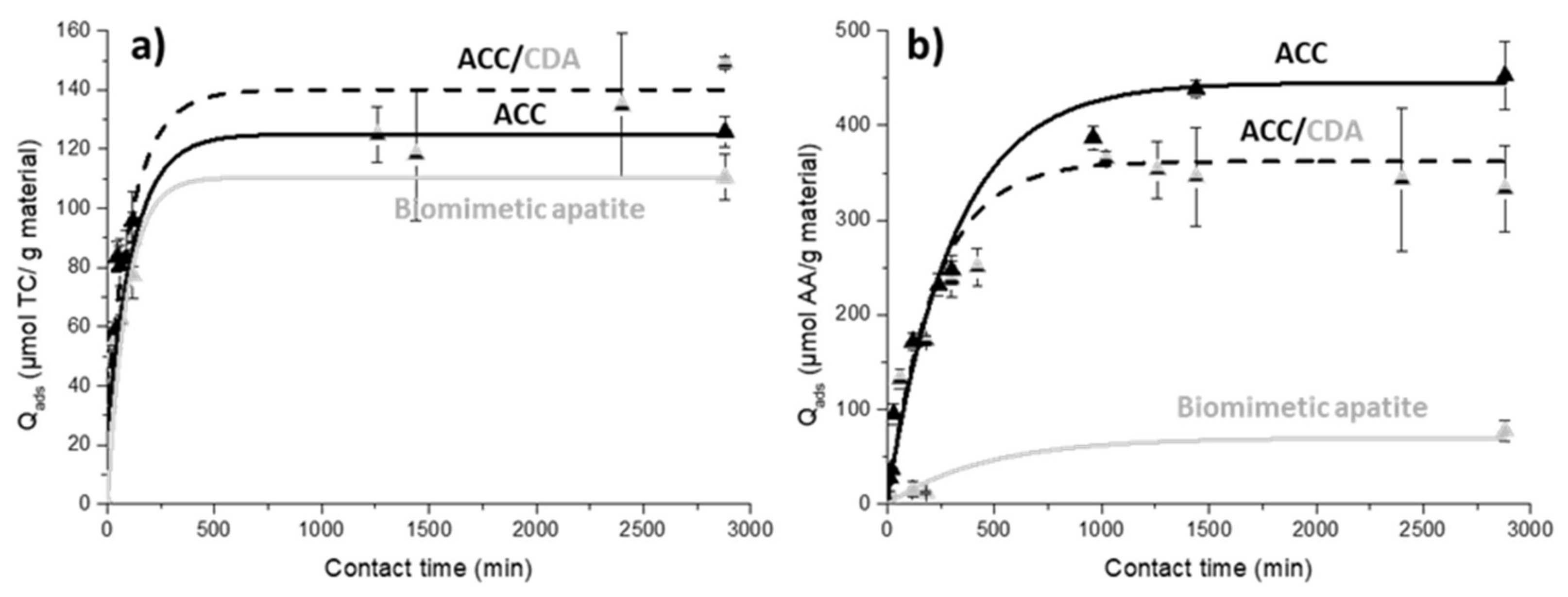
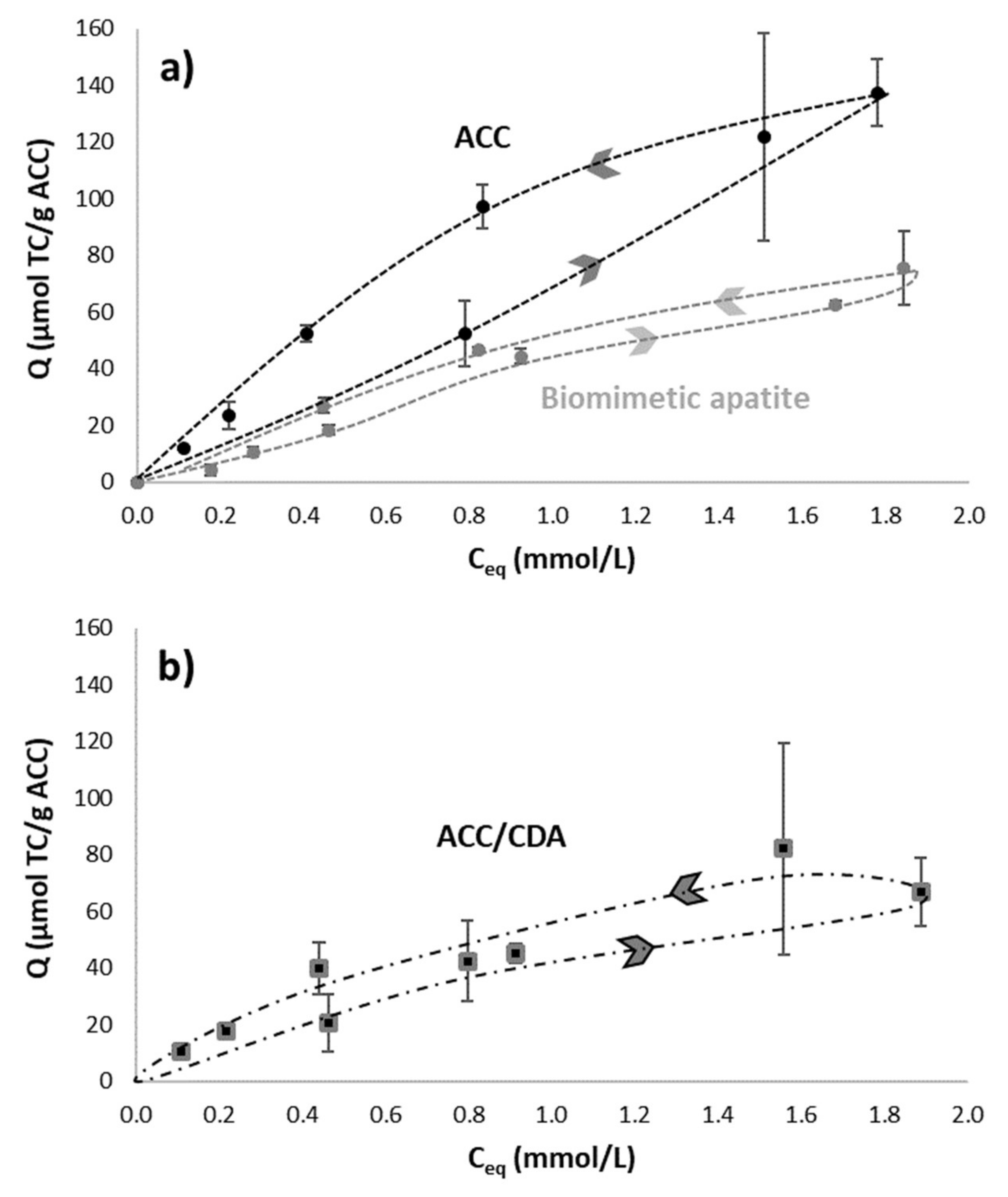
2.4.3. Adsorption Kinetics on ACC/CDA Material
2.4.4. Double Adsorption on ACC/CDA Material
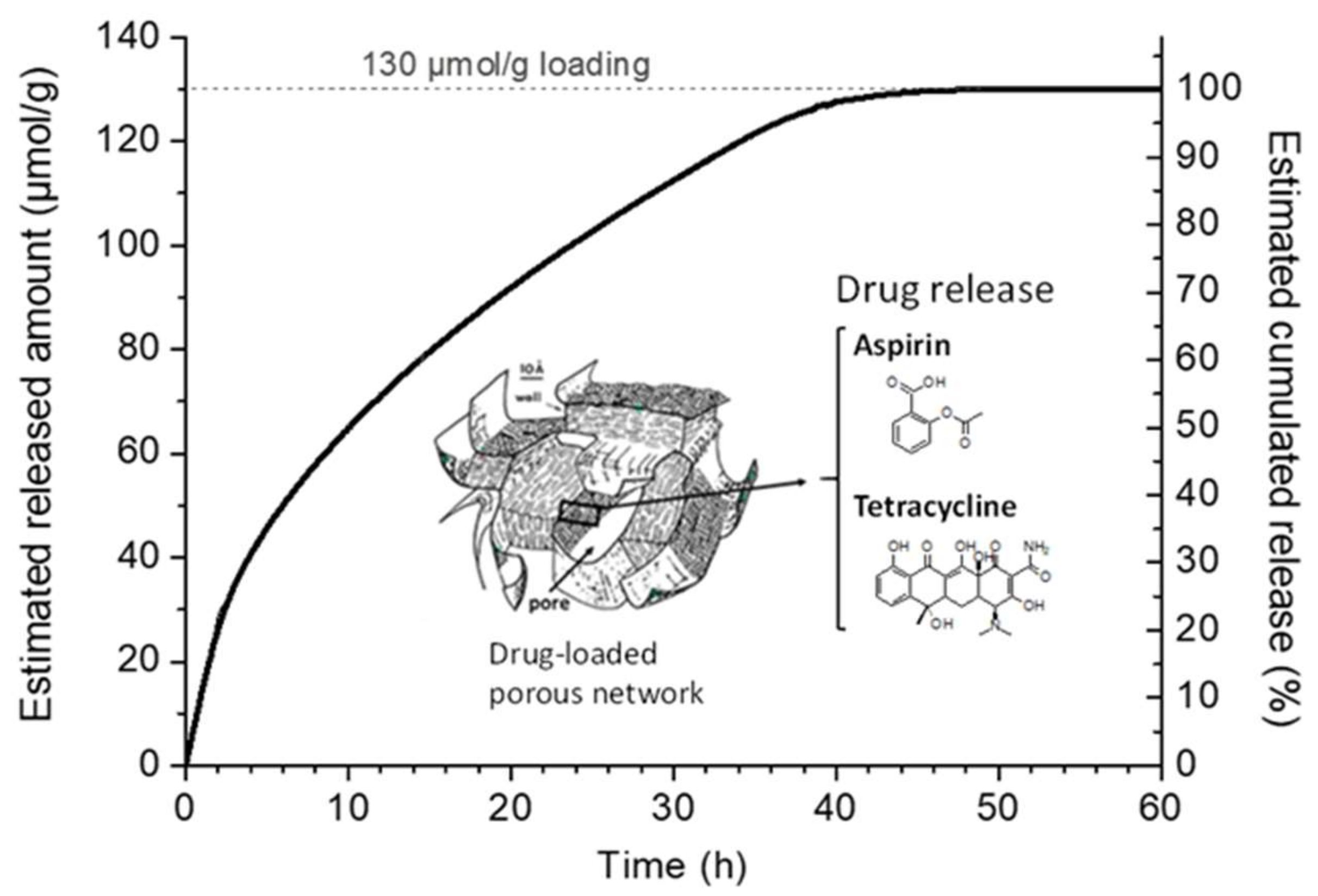
2.4.5. Desorption/Release Study
2.5. In Vitro Biocompatibility Assays on Human Osteoblasts
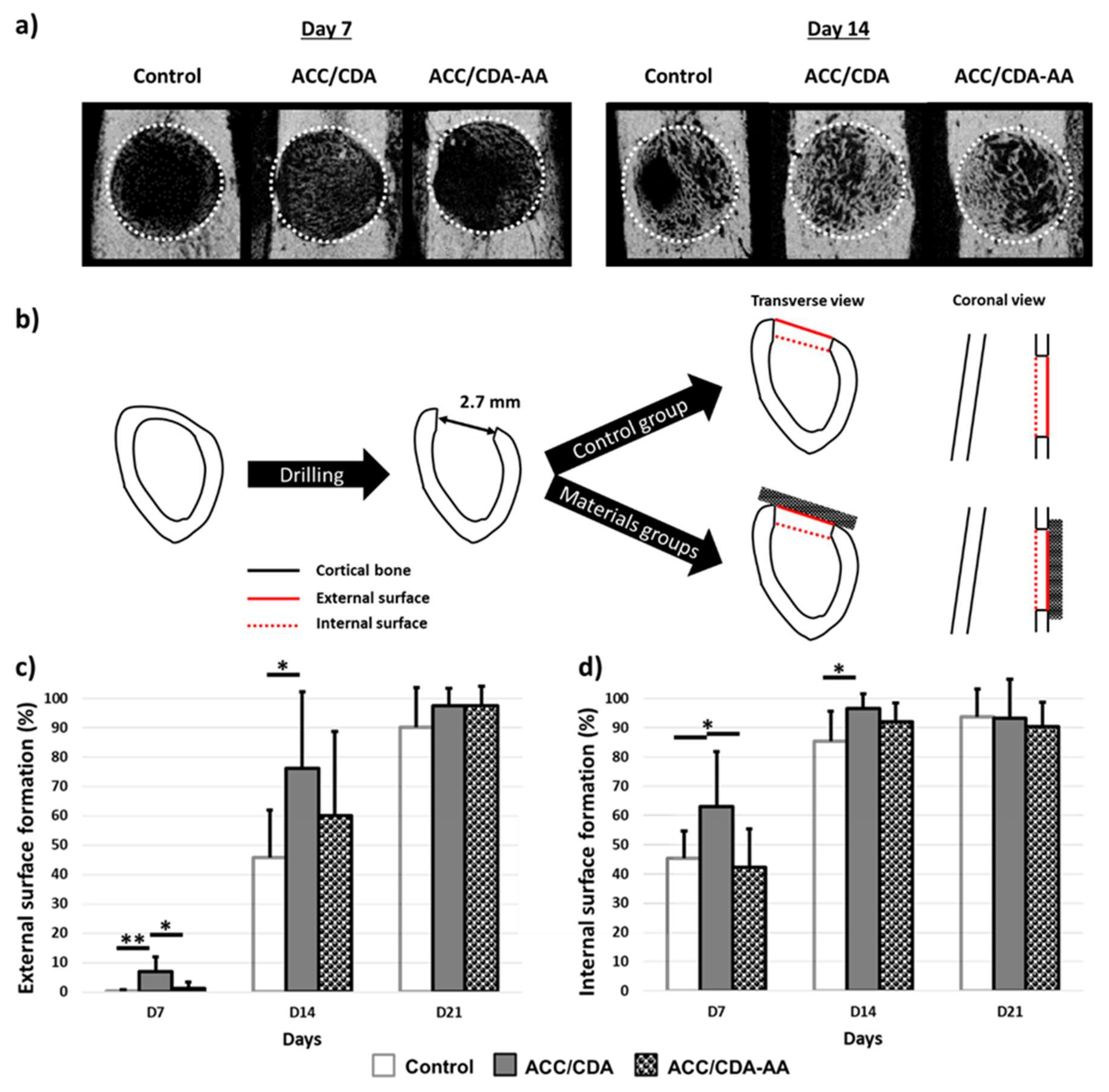
2.6. In Vivo Effect of ACC/CDA-AA Material on Cortical Bone Regeneration
3. Materials and Methods
3.1. Materials
3.1.1. Apatites
3.1.2. Activated Carbon Fiber Cloth (ACC) Substrates
3.1.3. Drugs
3.2. Drug Adsorption and Release Experiments
3.2.1. Drug Adsorption on Biomimetic Apatite Powder
3.2.2. Drug Adsorption on ACC and ACC/CDA Composite Materials
3.2.3. Double Drug Adsorption on ACC/CDA Composite Material
3.2.4. Drug Release
3.3. In Vitro Procedures
3.4. In Vivo Procedures
3.4.1. Surgical Procedure
3.4.2. AA Adsorption during Surgical Procedure
3.5. Micro-Computed Tomography Analysis
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarcho, M. Calcium Phosphate Ceramics as Hard Tissue Prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Canillas, M.; Pena, P.; de Aza, A.H.; Rodríguez, M.A. Calcium Phosphates for Biomedical Applications. Bol. Soc. Esp. Cerám. Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Harris, W.H. The Problem Is Osteolysis. Clin. Orthop. Relat. Res. 1995, 311, 46–53. [Google Scholar]
- Hing, K.A.; Best, S.M.; Bonfield, W. Characterization of Porous Hydroxyapatite. J. Mater. Sci. Mater. Med. 1999, 10, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, Present and for the Future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium Phosphates in Biomedical Applications: Materials for the Future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Verron, E.; Khairoun, I.; Guicheux, J.; Bouler, J.-M. Calcium Phosphate Biomaterials as Bone Drug Delivery Systems: A Review. Drug Discov. Today 2010, 15, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Von Euw, S.; Ajili, W.; Chan-Chang, T.-H.-C.; Delices, A.; Laurent, G.; Babonneau, F.; Nassif, N.; Azaïs, T. Amorphous Surface Layer versus Transient Amorphous Precursor Phase in Bone—A Case Study Investigated by Solid-State NMR Spectroscopy. Acta Biomater. 2017, 59, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Cazalbou, S.; Eichert, D.; Ranz, X.; Drouet, C.; Combes, C.; Harmand, M.F.; Rey, C. Ion Exchanges in Apatites for Biomedical Applications. J. Mater. Sci. Mater. Med. 2005, 16, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Drouet, C.; Carayon, M.-T.; Combes, C.; Rey, C. Surface Enrichment of Biomimetic Apatites with Biologically-Active Ions Mg2+ and Sr2+: A Preamble to the Activation of Bone Repair Materials. Mater. Sci. Eng. C 2008, 28, 1544–1550. [Google Scholar] [CrossRef] [Green Version]
- Cazalbou, S.; Bertrand, G.; Drouet, C. Tetracycline-Loaded Biomimetic Apatite: An Adsorption Study. J. Phys. Chem. B 2015, 119, 3014–3024. [Google Scholar] [CrossRef] [Green Version]
- Sarda, S.; Errassifi, F.; Marsan, O.; Geffre, A.; Trumel, C.; Drouet, C. Adsorption of Tranexamic Acid on Hydroxyapatite: Toward the Development of Biomaterials with Local Hemostatic Activity. Mater. Sci. Eng. C 2016, 66, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combes, C.; Bareille, R.; Rey, C. Calcium Carbonate–Calcium Phosphate Mixed Cement Compositions for Bone Reconstruction. J. Biomed. Mater. Res. Part A 2006, 79A, 318–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elahpour, N.; Rabiee, S.M.; Ebrahimzadeh, M.H.; Moradi, A. In-Vitro Formation and Growth Kinetics of Apatite on a New Light-Cured Composite Calcium Phosphate Cement. Ceram. Int. 2018, 44, 15317–15322. [Google Scholar] [CrossRef]
- Autefage, H.; Briand-Mesange, F.; Cazalbou, S.; Drouet, C.; Fourmy, D.; Goncalves, S.; Salles, J.; Combes, C.; Swider, P.; Rey, C. Adsorption and Release of BMP-2 on Nanocrystalline Apatite-coated and Uncoated Hydroxyapatiteβ-tricalcium Phosphate Porous Ceramics. J. Biomed. Mater. Res. Part B 2009, 529–530, 475–479. [Google Scholar]
- Zhou, H.; Hou, S.; Zhang, M.; Yang, M.; Deng, L.; Xiong, X.; Ni, X. Deposition of Calcium Phosphate Coatings Using Condensed Phosphates (P 2 O 7 4− and P 3 O 10 5−) as Phosphate Source through Induction Heating. Mater. Sci. Eng. C 2016, 69, 337–342. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Girod-Fullana, S.; Charvillat, C.; Ternet-Fontebasso, H.; Dufour, P.; Dexpert-Ghys, J.; Santran, V.; Bordère, J.; Pipy, B.; Bernad, J.; et al. Biomimetic Nanocrystalline Apatites: Emerging Perspectives in Cancer Diagnosis and Treatment. Int. J. Pharm. 2012, 423, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Tourbin, M.; Al-Kattan, A.; Drouet, C. Study on the Stability of Suspensions Based on Biomimetic Apatites Aimed at Biomedical Applications. Powder Technol. 2014, 255, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Adamiano, A.; Iafisco, M.; Sandri, M.; Basini, M.; Arosio, P.; Canu, T.; Sitia, G.; Esposito, A.; Iannotti, V.; Ausanio, G.; et al. On the Use of Superparamagnetic Hydroxyapatite Nanoparticles as an Agent for Magnetic and Nuclear in Vivo Imaging. Acta Biomater. 2018, 73, 458–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drouet, C.; Choimet, M.; Simon, M.; Devès, G.; Barberet, P.; Rassu, G.; Marsan, O.; Tourrette, A. Colloidal Apatite Particles: A Multifunctional Platform in (Nano)Medicine. J. Mater. Sci. 2020, 6, 1–9. [Google Scholar]
- Gautier, H.; Plumecocq, A.; Amador, G.; Weiss, P.; Merle, C.; Bouler, J.-M. In Vitro Characterization of Calcium Phosphate Biomaterial Loaded with Linezolid for Osseous Bone Defect Implantation. J. Biomater. Appl. 2012, 26, 811–828. [Google Scholar] [CrossRef]
- de Souza, C.A.S.; Colombo, A.P.V.; Souto, R.M.; Silva-Boghossian, C.M.; Granjeiro, J.M.; Alves, G.G.; Rossi, A.M.; Rocha-Leão, M.H.M. Adsorption of Chlorhexidine on Synthetic Hydroxyapatite and in Vitro Biological Activity. Colloids Surf. B Biointerfaces 2011, 87, 310–318. [Google Scholar] [CrossRef]
- Eichert, D.; Combes, C.; Drouet, C.; Rey, C. Formation and Evolution of Hydrated Surface Layers of Apatites. Key Eng. Mater. 2005, 284–286, 3–6. [Google Scholar] [CrossRef]
- Weber, C.G.; Mueller, M.; Vandecandelaere, N.; Trick, I.; Burger-Kentischer, A.; Maucher, T.; Drouet, C. Enzyme-Functionalized Biomimetic Apatites: Concept and Perspectives in View of Innovative Medical Approaches. J. Mater. Sci. Mater. Med. 2014, 25, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Bosco, R.; Iafisco, M.; van den Beucken, J.; Leeuwenburgh, S.C.G.; Jansen, J.A. Adsorption of Alendronate onto Biomimetic Apatite Nanocrystals to Develop Drug Carrier Coating for Bone Implants. Key Eng. Mater. 2012, 529–530, 475–479. [Google Scholar] [CrossRef]
- Errassifi, F.; Sarda, S.; Barroug, A.; Legrouri, A.; Sfihi, H.; Rey, C. Infrared, Raman and NMR Investigations of Risedronate Adsorption on Nanocrystalline Apatites. J. Colloid Interface Sci. 2014, 420, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.H.K.; Weir, M.D.; Simon, C.G. Injectable and Strong Nano-Apatite Scaffolds for Cell/Growth Factor Delivery and Bone Regeneration. Dent. Mater. 2008, 24, 1212–1222. [Google Scholar] [CrossRef] [Green Version]
- Damia, C.; Marchat, D.; Lemoine, C.; Douard, N.; Chaleix, V.; Sol, V.; Larochette, N.; Logeart-Avramoglou, D.; Brie, J.; Champion, E. Functionalization of Phosphocalcic Bioceramics for Bone Repair Applications. Mater. Sci. Eng. C 2019, 95, 343–354. [Google Scholar] [CrossRef]
- Iafisco, M.; Palazzo, B.; Martra, G.; Margiotta, N.; Piccinonna, S.; Natile, G.; Gandin, V.; Marzano, C.; Roveri, N. Nanocrystalline Carbonate-Apatites: Role of Ca/P Ratio on the Upload and Release of Anticancer Platinum Bisphosphonates. Nanoscale 2012, 4, 206–217. [Google Scholar] [CrossRef]
- Martinez, T.; Sarda, S.; Dupret-Bories, A.; Charvillat, C.; Projetti, F.; Drouet, C. Toward a Doxorubicin-Loaded Bioinspired Bone Cement for the Localized Treatment of Osteosarcoma. Future Oncol. 2021, 17, 3511–3528. [Google Scholar] [CrossRef]
- Šupová, M. Substituted Hydroxyapatites for Biomedical Applications: A Review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Sampath Kumar, T.S.; Madhumathi, K.; Rubaiya, Y.; Doble, M. Dual Mode Antibacterial Activity of Ion Substituted Calcium Phosphate Nanocarriers for Bone Infections. Front. Bioeng. Biotechnol. 2015, 3, 59. [Google Scholar] [CrossRef] [Green Version]
- Lowry, N.; Han, Y.; Meenan, B.J.; Boyd, A.R. Strontium and Zinc Co-Substituted Nanophase Hydroxyapatite. Ceram. Int. 2017, 43, 12070–12078. [Google Scholar] [CrossRef]
- Ullah, I.; Li, W.; Lei, S.; Zhang, Y.; Zhang, W.; Farooq, U.; Ullah, S.; Ullah, M.W.; Zhang, X. Simultaneous Co-Substitution of Sr2+/Fe3+ in Hydroxyapatite Nanoparticles for Potential Biomedical Applications. Ceram. Int. 2018, 44, 21338–21348. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, G.; Lin, Y.; Zhang, D.; Liao, H.; Li, Z.; Tian, J.; Wu, Q. A Novel Flexible Electrode with Coaxial Sandwich Structure Based Polyaniline-Coated MoS 2 Nanoflakes on Activated Carbon Cloth. Electrochim. Acta 2018, 264, 91–100. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, W.; Gao, F.; Qiu, C.; Wang, Q.; Gao, F.; Zhao, C. Thermally Activated Multilayered Carbon Cloth as Flexible Supercapacitor Electrode Material with Significantly Enhanced Areal Energy Density. ChemElectroChem 2019, 6, 1768–1775. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Ahmed Shifa, T.; Wang, D.; Wu, X.; Cui, Y. Hierarchical MnO2/Activated Carbon Cloth Electrode Prepared by Synchronized Electrochemical Activation and Oxidation for Flexible Asymmetric Supercapacitors. Chem. Eng. J. 2019, 372, 1047–1055. [Google Scholar] [CrossRef]
- Ayranci, E.; Hoda, N. Adsorption Kinetics and Isotherms of Pesticides onto Activated Carbon-Cloth. Chemosphere 2005, 60, 1600–1607. [Google Scholar] [CrossRef]
- Masson, S.; Gineys, M.; Delpeux-Ouldriane, S.; Reinert, L.; Guittonneau, S.; Béguin, F.; Duclaux, L. Single, Binary, and Mixture Adsorption of Nine Organic Contaminants onto a Microporous and a Microporous/Mesoporous Activated Carbon Cloth. Microporous Mesoporous Mater. 2016, 234, 24–34. [Google Scholar] [CrossRef]
- Nieto-Delgado, C.; Partida-Gutierrez, D.; Rangel-Mendez, J.R. Preparation of Activated Carbon Cloths from Renewable Natural Fabrics and Their Performance during the Adsorption of Model Organic and Inorganic Pollutants in Water. J. Clean. Prod. 2019, 213, 650–658. [Google Scholar] [CrossRef]
- González-García, P. Activated Carbon from Lignocellulosics Precursors: A Review of the Synthesis Methods, Characterization Techniques and Applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Ilondu, N.A.; Afonne, O.J.; Ofoefule, S.I.; Orish, C.N. Acceleration of Body Clearance of DiethylCarbamazine By Oral Activated Charcoal. Pharmacol. Res. 2000, 42, 167–170. [Google Scholar] [CrossRef]
- Spiller, H.A.; Sawyer, T.S. Impact of Activated Charcoal After Acute Acetaminophen Overdoses Treated with N-Acetylcysteine. J. Emerg. Med. 2007, 33, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Peng, J.; Zhao, J.; Liang, S.; Shao, Y.; Wu, Q. Laser Light Triggered-Activated Carbon Nanosystem for Cancer Therapy. Biomaterials 2013, 34, 1820–1832. [Google Scholar] [CrossRef]
- Picard, Q.; Olivier, F.; Delpeux, S.; Chancolon, J.; Warmont, F.; Bonnamy, S. Development and Characterization of Biomimetic Carbonated Calcium-Deficient Hydroxyapatite Deposited on Carbon Fiber Scaffold. J. Carbon Res. 2018, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Olivier, F.; Picard, Q.; Delpeux-Ouldriane, S.; Chancolon, J.; Warmont, F.; Sarou-Kanian, V.; Fayon, F.; Bonnamy, S. Influence of Electrochemical Parameters on the Characteristics of Sono-Electrodeposited Calcium Phosphate-Coated Carbon Fiber Cloth. Surf. Coat. Technol. 2020, 389, 125507. [Google Scholar] [CrossRef]
- Olivier, F.; Rochet, N.; Delpeux-Ouldriane, S.; Chancolon, J.; Sarou-Kanian, V.; Fayon, F.; Bonnamy, S. Strontium Incorporation into Biomimetic Carbonated Calcium-Deficient Hydroxyapatite Coated Carbon Cloth: Biocompatibility with Human Primary Osteoblasts. Mater. Sci. Eng. C 2020, 116, 111192. [Google Scholar] [CrossRef] [PubMed]
- Schneider, O.D.; Loher, S.; Brunner, T.J.; Schmidlin, P.; Stark, W.J. Flexible, Silver Containing Nanocomposites for the Repair of Bone Defects: Antimicrobial Effect against E. Coli Infection and Comparison to Tetracycline Containing Scaffolds. J. Mater. Chem. 2008, 18, 2679. [Google Scholar] [CrossRef]
- Meng, D.; Francis, L.; Thompson, I.D.; Mierke, C.; Huebner, H.; Amtmann, A.; Roy, I.; Boccaccini, A.R. Tetracycline-Encapsulated P(3HB) Microsphere-Coated 45S5 Bioglass®-Based Scaffolds for Bone Tissue Engineering. J. Mater. Sci. Mater. Med. 2013, 24, 2809–2817. [Google Scholar] [CrossRef]
- Martin, V.; Bettencourt, A. Bone Regeneration: Biomaterials as Local Delivery Systems with Improved Osteoinductive Properties. Mater. Sci. Eng. C 2018, 82, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal Stem Cell–Based Tissue Regeneration Is Governed by Recipient T Lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef]
- Fang, X.; Lei, L.; Jiang, T.; Chen, Y.; Kang, Y. Injectable Thermosensitive Alginate-β-Tricalcium Phosphate-Aspirin Hydrogels for Bone Augmentation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, S.; Liu, Z.; Geng, H.; Lu, X.; Zhang, X.; Li, H.; Gao, C.; Zhang, X.; Gao, P. Guided Bone Regeneration with Asymmetric Collagen-Chitosan Membranes Containing Aspirin-Loaded Chitosan Nanoparticles. Int. J. Nanomed. 2017, 12, 8855–8866. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Mei, S.; Guo, L.; Su, Y.; Wang, H.; Liu, Y.; Zhao, Z.; Wang, S.; Liu, Y. Platelet-Rich Fibrin/Aspirin Complex Promotes Alveolar Bone Regeneration in Periodontal Defect in Rats. J. Periodontal Res. 2018, 53, 47–56. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Z.; Yuan, P.; Jin, R.; Wang, X.; Jiang, T.; Chen, X. Injectable Colloidal Hydrogel with Mesoporous Silica Nanoparticles for Sustained Co-Release of MicroRNA-222 and Aspirin to Achieve Innervated Bone Regeneration in Rat Mandibular Defects. J. Mater. Chem. B 2019, 7, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, N.; Zhang, T.; Sun, Q.; Han, B.; Yu, T. A Tetra-PEG Hydrogel Based Aspirin Sustained Release System Exerts Beneficial Effects on Periodontal Ligament Stem Cells Mediated Bone Regeneration. Front. Chem. 2019, 7, 682. [Google Scholar] [CrossRef] [Green Version]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic Apatite-Based Biomaterials: On the Critical Impact of Synthesis and Post-Synthesis Parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, T.I.; Frank-Kamenetskaya, O.V.; Kol’tsov, A.B.; Ugolkov, V.L. Crystal Structure of Calcium-Deficient Carbonated Hydroxyapatite. Thermal Decomposition. J. Solid State Chem. 2001, 160, 340–349. [Google Scholar] [CrossRef]
- Dashti, A.; Ready, D.; Salih, V.; Knowles, J.C.; Barralet, J.E.; Wilson, M.; Donos, N.; Nazhat, S.N. In Vitro Antibacterial Efficacy of Tetracycline Hydrochloride Adsorbed onto Bio-Oss® Bone Graft. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93B, 394–400. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich Equation Used for the Analysis of Adsorption Kinetics in Dye-Chitosan Systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Adsorption of DNA on Biomimetic Apatites: Toward the Understanding of the Role of Bone and Tooth Mineral on the Preservation of Ancient DNA. Appl. Surf. Sci. 2014, 292, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Delpeux-Ouldriane, S.; Gineys, M.; Cohaut, N.; Béguin, F. The Role Played by Local PH and Pore Size Distribution in the Electrochemical Regeneration of Carbon Fabrics Loaded with Bentazon. Carbon 2015, 94, 816–825. [Google Scholar] [CrossRef]
- Boudrahem, N.; Delpeux-Ouldriane, S.; Khenniche, L.; Boudrahem, F.; Aissani-Benissad, F.; Gineys, M. Single and Mixture Adsorption of Clofibric Acid, Tetracycline and Paracetamol onto Activated Carbon Developed from Cotton Cloth Residue. Process. Saf. Environ. Prot. 2017, 111, 544–559. [Google Scholar] [CrossRef]
- Oberlin, A.; Villey, M.; Combaz, A. Influence of Elemental Composition on Carbonization: Pyrolysis of kerosene shale and kuckersite. Carbon 1980, 18, 347–353. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi Equation: Derivation, Applications, Use and Misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, T.; Boukhechba, F.; Clavé, A.; Bouvet-Gerbettaz, S.; Trojani, C.; Michiels, J.-F.; Laugier, J.-P.; Bouler, J.-M.; Carle, G.F.; Scimeca, J.-C.; et al. Biphasic Calcium Phosphate Microparticles for Bone Formation: Benefits of Combination with Blood Clot. Tissue Eng. Part A 2010, 16, 3495–3505. [Google Scholar] [CrossRef]
- Balaguer, T.; Fellah, B.H.; Boukhechba, F.; Traverson, M.; Mouska, X.; Ambrosetti, D.; Dadone, B.; Michiels, J.-F.; Amri, E.-Z.; Trojani, C.; et al. Combination of Blood and Biphasic Calcium Phosphate Microparticles for the Reconstruction of Large Bone Defects in Dog: A Pilot Study: BCP/Blood Clot Composite for Critical Bone Defect Reconstruction in Dog. J. Biomed. Mater. Res. Part A 2018, 106, 1842–1850. [Google Scholar] [CrossRef]

| CO3 %wt. | Morphology (SEM) | Structure (XRD) | Lattice Parameters | |||
|---|---|---|---|---|---|---|
| a (Å) | b (Å) | |||||
| “Reference” nanocrystalline apatite | 1.46 ± 0.02 | 0 | Plate-like | Hexagonal, (P63/m) | 9.437 ± 0.004 | 6.871 ± 0.002 |
| Carbonated CDA | 1.4 ± 0.1 | 2–6 | Plate-like | Hexagonal, (P63/m) | 9.366 | 6.820 |
| Precursor | Sizing | Architecture | Fiber Diameter (µm) | SBET (m2/g) | VTOTAL (cm3/g) | Acidic Functional Groups (mmol/g) | pHpzc | |
|---|---|---|---|---|---|---|---|---|
| ACC 1 | Phenolic resin | – | Woven | 10 | 1693 | 0.68 | 0.48 | 9.4 |
| ACC 2 | Viscose | ZnAl2O4 | Knitted | 8–12 | 898 | 0.51 | 2.93 | 7.8 |
| ACC 3 | Viscose | ZnAl2O4 | Knitted | 12–15 | 1400 | 1.27 | 1.31 | 5.8 |
| SBET (m2/g) | Vmicro 1 N2, DFT (cm3/g) | Vmeso 1 N2, DFT (cm3/g) | Vmicro 2 N2, DR (cm3/g) | |
|---|---|---|---|---|
| ACC | 898 | 0.41 | 0.07 | 0.41 |
| ACC/CDA | 980 | 0.45 | 0.10 | 0.46 |
| Chemical Formula | M (g/mol) | Volume (Å3) | pKa (25 °C) | Log P | Log D (pH = 7.4) | Polar Surface Area (Å2) | |
|---|---|---|---|---|---|---|---|
| Tetracycline (TC) | 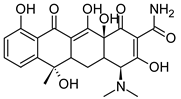 | 444.43 | 449 | 3.3 | −1.47 ± 0.81 | 0.48 | 182 |
| Aspirin (AA) |  | 180.16 | 232 | 3.5 | 1.19 ± 0.23 | 2.93 | 64 |
| AA Adsorption | TC Adsorption | |
|---|---|---|
| ACC | Apatite | |
| Qads (mmol/g) | 2.07 ± 0.06 | 0.06 ± 0.01 |
| ACC/CDA | ||
| Qads (mmol/g) | 2.10 ± 0.10 | 0.07 ± 0.01 |
| Drug Loading | Live/Dead Assay | ||||
|---|---|---|---|---|---|
| Qads (µmol/g) | Qads (µg) | Live Cells/mm2 | Dead Cells/mm2 | “r” (L/D) | |
| ACC/CDA | - | - | 18 ± 4 | 14 ± 2 | 1.30 |
| ACC/CDA-TC | 133 ± 36 | 500 ± 130 | 18 ± 9 | 6 ± 2 | 3.00 |
| ACC/CDA-AA | 134 ± 23 | 200 ± 30 | 51 ± 13 | 14 ± 2 | 3.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivier, F.; Bonnamy, S.; Rochet, N.; Drouet, C. Activated Carbon Fiber Cloth/Biomimetic Apatite: A Dual Drug Delivery System. Int. J. Mol. Sci. 2021, 22, 12247. https://doi.org/10.3390/ijms222212247
Olivier F, Bonnamy S, Rochet N, Drouet C. Activated Carbon Fiber Cloth/Biomimetic Apatite: A Dual Drug Delivery System. International Journal of Molecular Sciences. 2021; 22(22):12247. https://doi.org/10.3390/ijms222212247
Chicago/Turabian StyleOlivier, Florian, Sylvie Bonnamy, Nathalie Rochet, and Christophe Drouet. 2021. "Activated Carbon Fiber Cloth/Biomimetic Apatite: A Dual Drug Delivery System" International Journal of Molecular Sciences 22, no. 22: 12247. https://doi.org/10.3390/ijms222212247
APA StyleOlivier, F., Bonnamy, S., Rochet, N., & Drouet, C. (2021). Activated Carbon Fiber Cloth/Biomimetic Apatite: A Dual Drug Delivery System. International Journal of Molecular Sciences, 22(22), 12247. https://doi.org/10.3390/ijms222212247








