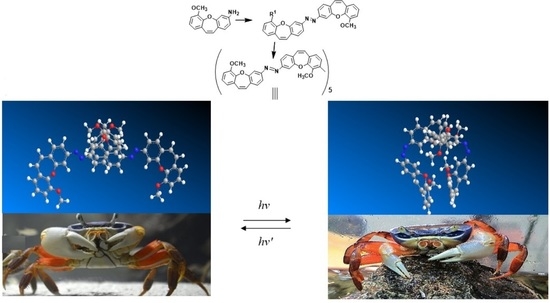
Synthesis and Investigations of Building Blocks with Dibenzo[b,f] Oxepine for Use in Photopharmacology
Abstract
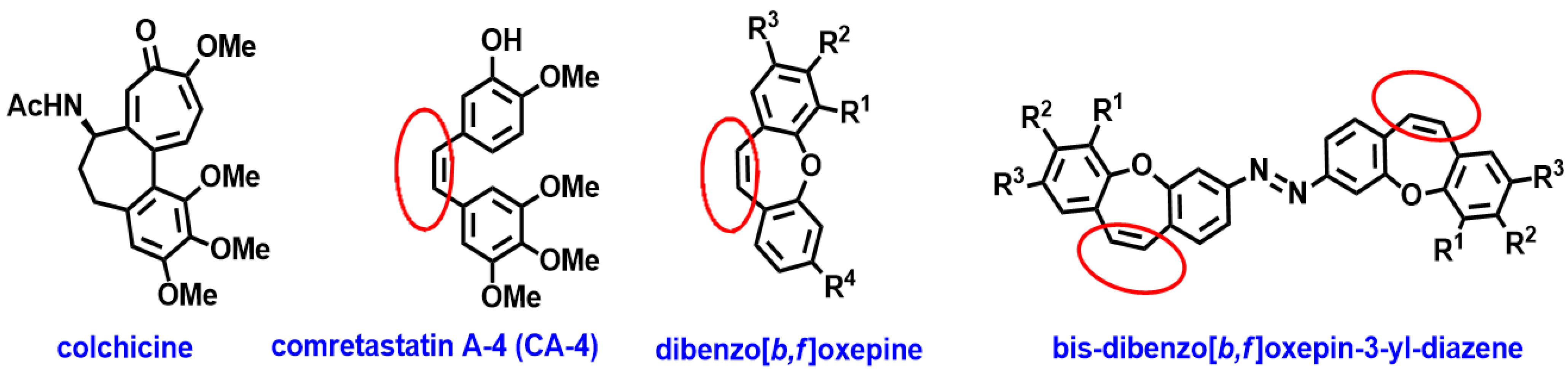
:1. Introduction
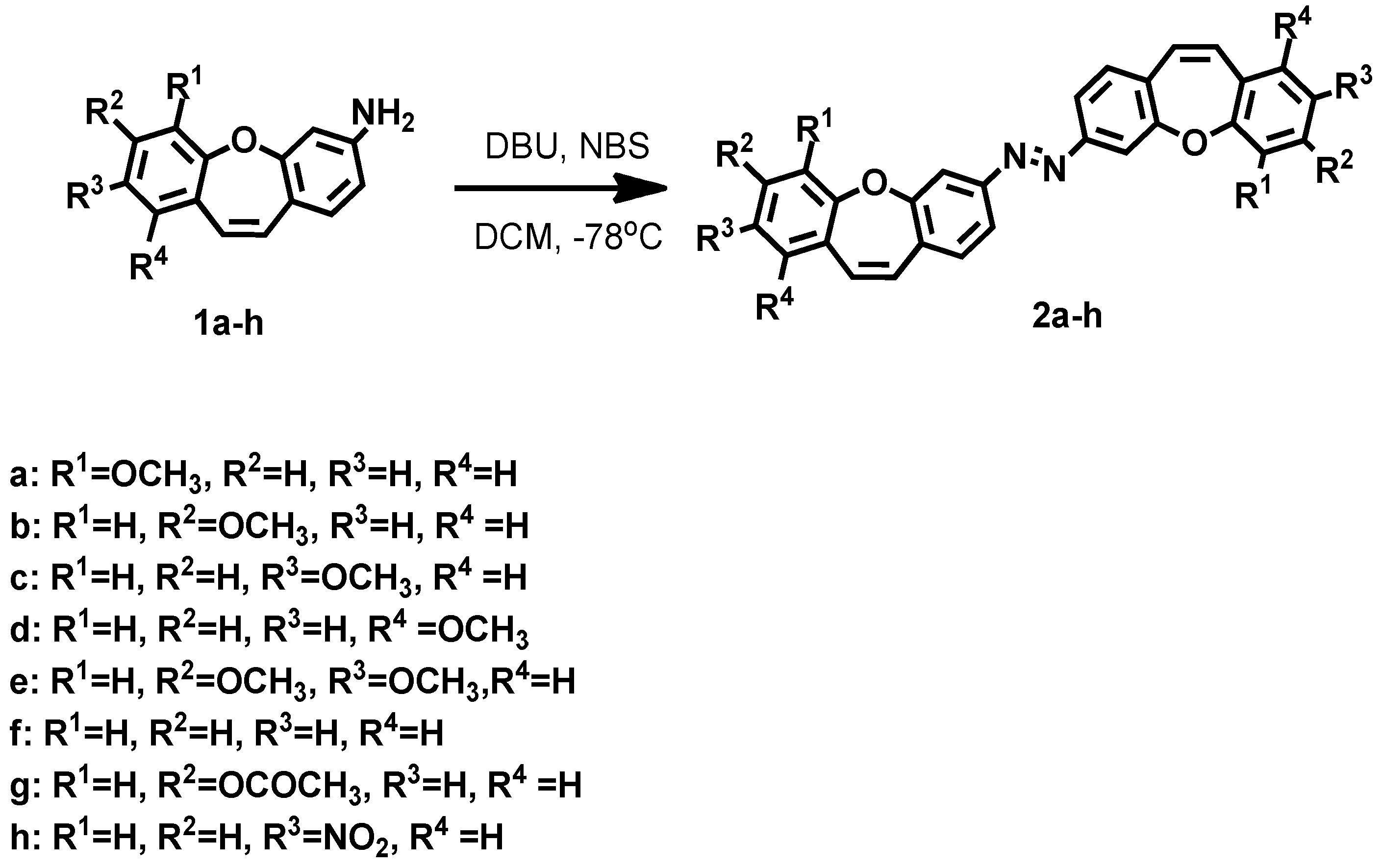
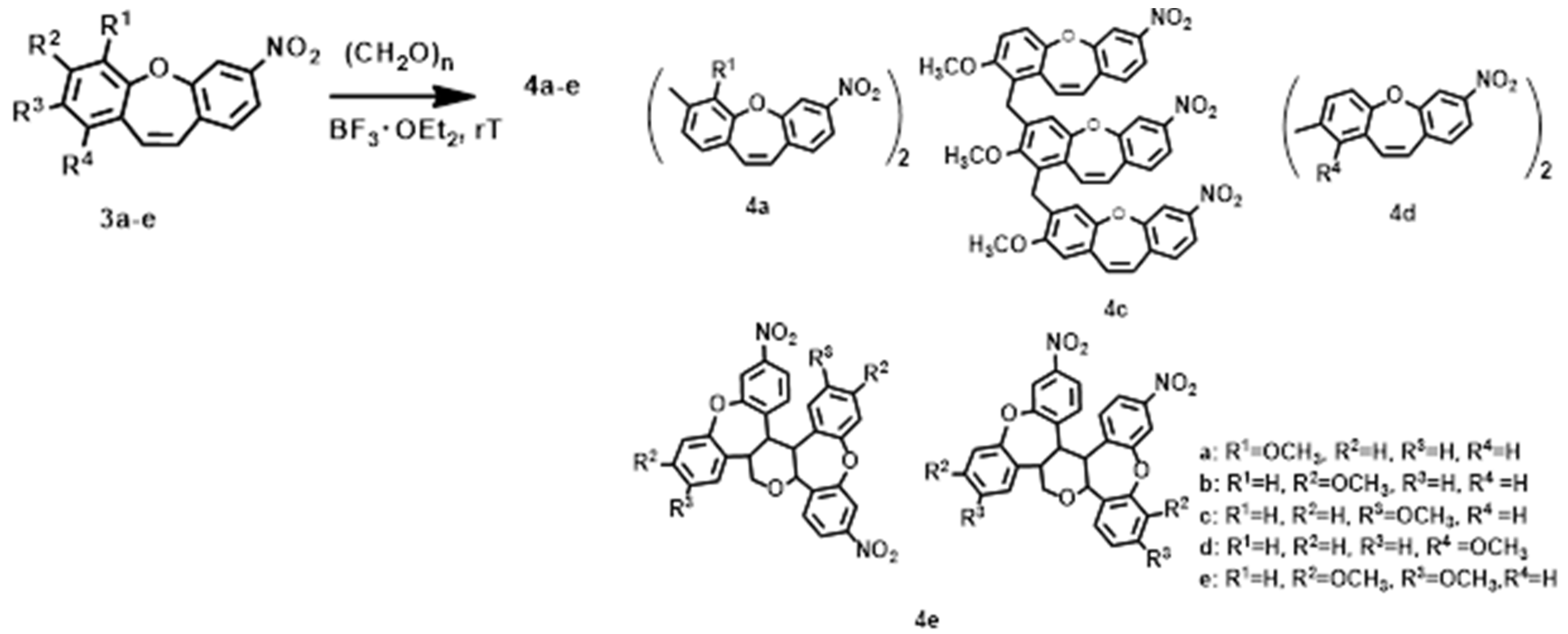
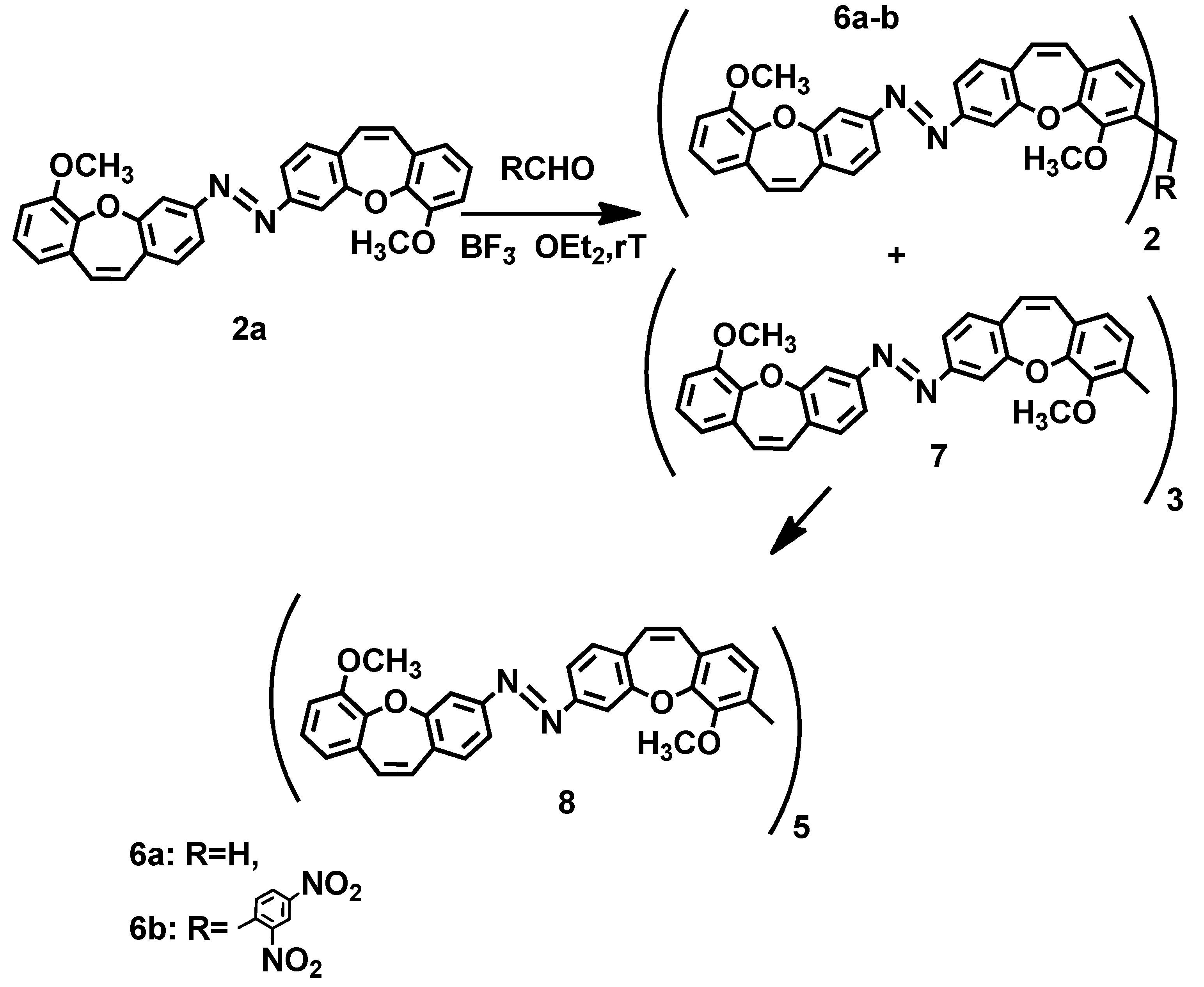
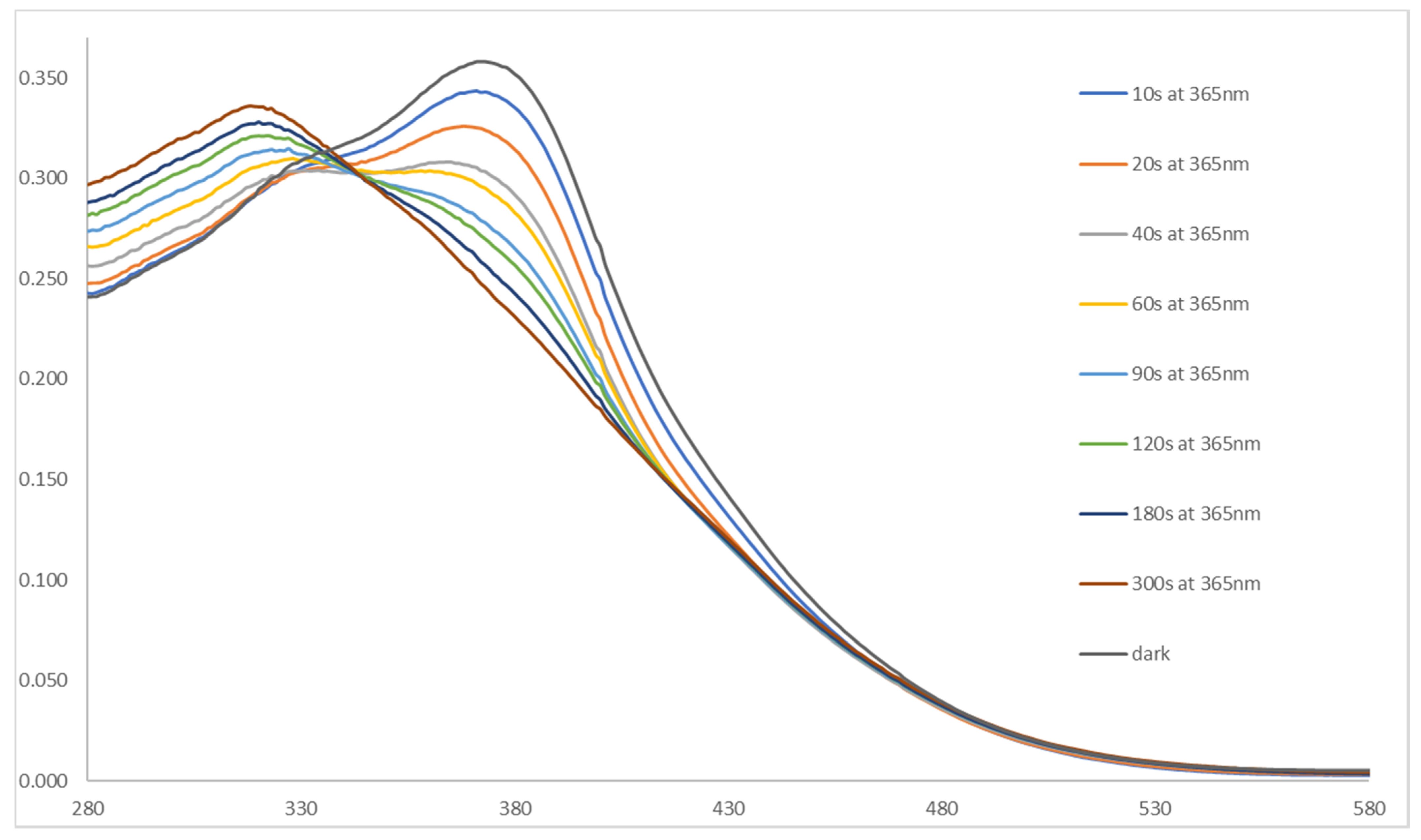
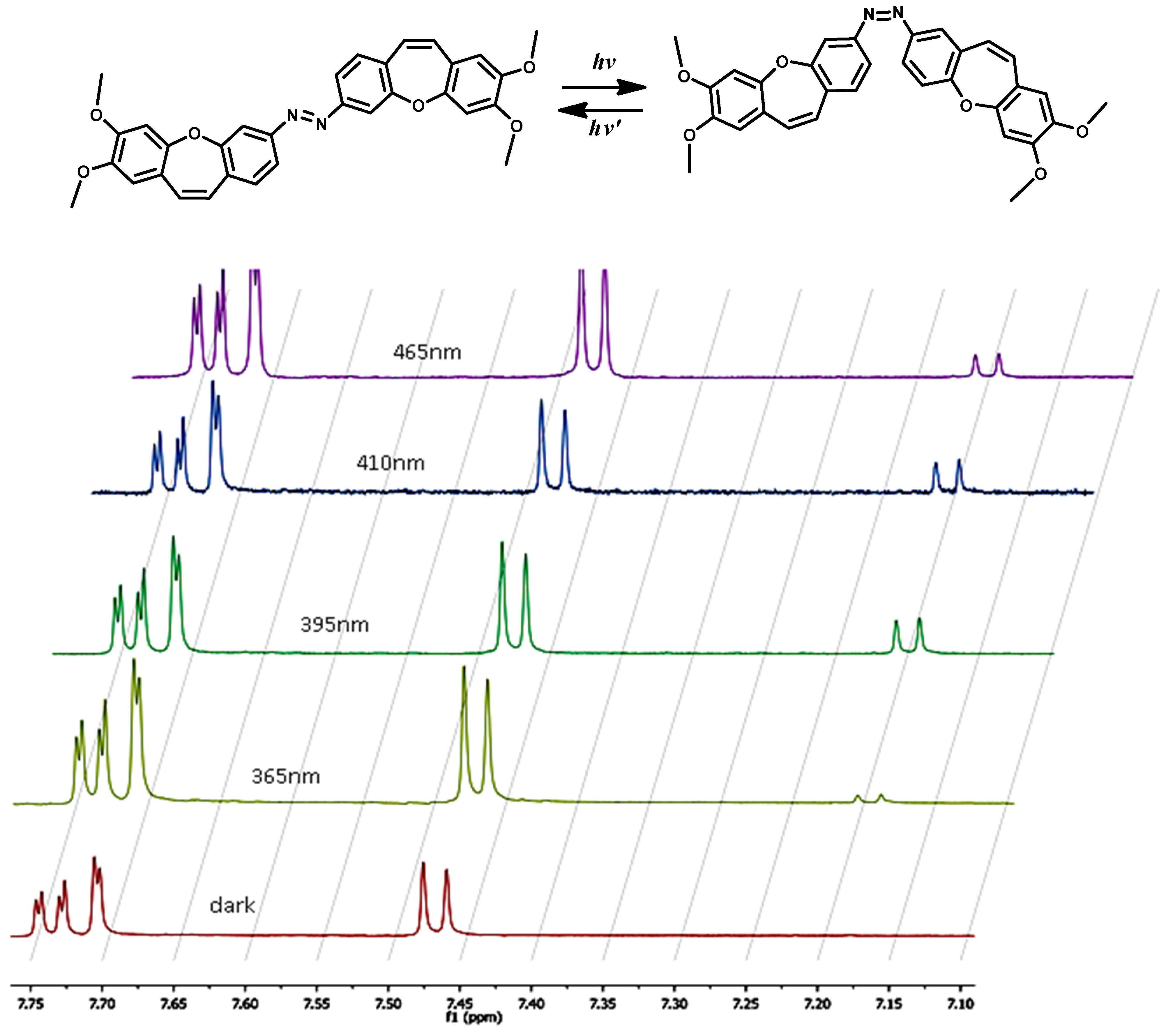
2. Results and Discussion
3. The Application in Photopharmacology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Borys, F.; Krawczyk, H.; Joachimiak, E.; Fabczak, H. Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells. I. Molecules 2020, 25, 3705. [Google Scholar] [CrossRef] [PubMed]
- Borys, F.; Tobiasz, P.; Poterała, M.; Krawczyk, H. Development of novel derivatives of stilbene and macrocyclic compounds as potent of anti-microtubule factors. Biomed. Pharmacother. 2021, 133, 110973. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.; Ocean, A.J. Peripheral neuropathy with microtubule-targeting agents: Occurrence and management approach. Clin. Breast Cancer 2011, 11, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrouenraets, M.B.; Visser, G.W.; Snow, G.B.; van Dongen, G.A. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003, 23, 505–522. [Google Scholar]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Acosta-Ruiz, A.; Broichhagen, J.; Levitz, J. Optical regulation of class C GPCRs by photoswitchable orthogonal remotely tethered ligands. Methods. Mol. Biol. 2019, 1947, 103–136. [Google Scholar]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2219. [Google Scholar] [CrossRef] [Green Version]
- Broichhagen, J.; Frank, J.A.; Trauner, D. A roadmap to success in photopharmacology. Acc. Chem. Res. 2015, 48, 1947–1960. [Google Scholar] [CrossRef]
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978−10999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleger, D.; Hecht, S. Visible-Light-Activated Molecular Switches. Angew. Chem. Int. Ed. 2015, 54, 11338–11349. [Google Scholar] [CrossRef]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Weissleder, R.; Ntziachristos, V. Shedding light onto live molecular targets. Nat. Med. 2003, 9, 123–128. [Google Scholar] [CrossRef]
- Zhou, J.; Giannakakou, P. Anti-Cancer Agents. Curr. Med. Chem. 2005, 5, 65–71. [Google Scholar]
- Bohn, A.B.; Wittenborn, T.; Brems-Eskildsen, A.S.; Laurberg, T.; Bertelsen, L.B.; Nielsen, T.; Stødkilde-Jørgensen, H.; Møller, B.K.; Horsman, M.R. A combretastatin-mediated decrease in neutrophil concentration in peripheral blood and the impact on the anti-tumor activity of this drug in two different murine tumor models. PLoS ONE 2014, 9, e110091. [Google Scholar] [CrossRef]
- Horsman, R.M.; Bohn, A.B.; Busk, M. Vascular targeting therapy: Potential benefit depends on tumor and host related effects. Exp. Oncol. 2010, 32, 143–148. [Google Scholar] [PubMed]
- Iversen, A.B.; Busk, M.; Bertelsen, L.B.; Laustsen, C.; Munk, O.L.; Nielsen, T.; Wittenborn, T.R.; Bussink, J.; Lok, J.; Stødkilde-Jørgensen, H.; et al. The potential of hyperpolarized 13C magnetic resonance spectroscopy to monitor the effect of combretastatin based vascular disrupting agents. Acta Oncol. 2017, 56, 1626–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharyya, B.; Panda, D.; Gupta, S.; Banerjee, M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 2008, 28, 155–183. [Google Scholar] [CrossRef]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [Green Version]
- Pettit, G.R.; Rhodes, M.R.; Herald, D.L.; Hamel, E.; Schmidt, J.M.; Pettit, R.K. Antineoplastic agents. 445. Synthesis and evaluation of structural modifications of (Z)- and (E)-combretastatin A-41. J. Med. Chem. 2005, 48, 4087–4099. [Google Scholar] [CrossRef]
- Lawrence, N.J.; Rennison, D.; Woo, M.; McGown, A.T.; Hadfield, J.A. Antimitotic and cell growth inhibitory properties of combretastatin A-4-like ethers. Bioorg. Med. Chem. Lett. 2001, 11, 51–54. [Google Scholar] [CrossRef]
- Jakubowska, J.; Mikuła-Pietrasik, J.; Książek, K.; Krawczyk, H. Cytotoxicity studies of novel combretastatin and pterostilbene derivatives. BioMed Res. Int. 2014, 2014, 320895. [Google Scholar] [CrossRef]
- Janowska, K.; Matczak, R.; Zakrzewski, J.; Krawczyk, H. A novel regioselective method for aminostilbene preparation—The role of sodium azide. Tetrahedron Lett. 2012, 53, 6504–6507. [Google Scholar] [CrossRef]
- Garbicz, D.; Mielecki, D.; Wrzesiński, M.; Pilżys, T.; Marcinkowski, M.; Piwowarski, J.; Dębski, J.; Palak, E.; Szczeciński, P.; Krawczyk, H.; et al. Evaluation of anti-cancer activity of stilbene and methoxydibenzo[b,f]oxepin derivatives. Curr. Cancer Drug Tar. 2018, 18, 706–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobiasz, P.; Poterała, M.; Jaśkowska, E.; Krawczyk, H. Synthesis and investigation of new cyclic molecules using the stilbene scaffold. RSC Adv. 2018, 8, 30678–30682. [Google Scholar] [CrossRef] [Green Version]
- Krawczyk, H.; Wrzesiński, M.; Mielecki, D.; Szczeciński, P.; Grzesiuk, E. Synthesis of derivatives of methoxydibenzo[b,f]oxepine in the presence of sodium azide. Tetrahedron 2016, 72, 3877–3884. [Google Scholar] [CrossRef] [Green Version]
- Garbicz, D.; Tobiasz, P.; Borys, F.; Poterała, M.; Grzesiuk, E.; Krawczyk, H. The stilbene and dibenzo[b,f]oxepine derivatives as anticancer compounds. Biomed. Pharmacother. 2020, 123, 109781. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, H. The derivative of stilbene, nucleosides, and nucleosides modified by stilbene derivatives. Bioorg Chem. 2019, 90, 103073. [Google Scholar] [CrossRef] [PubMed]
- Tojo, E.; Dominguez, D.; Castedo, L. Alkaloids from Sarcocapnos enneaphylla. Phytochemistry 1991, 30, 1005–1010. [Google Scholar] [CrossRef]
- Qian, T.-X.; Li, L.-N. Isosalvianolic acid C, a depside possessing a dibenzooxepin skeleton. Phytochemistry 1992, 31, 1068–1070. [Google Scholar]
- Lu, Y.H.; Lin, C.N.; Ko, H.H.; Yang, S.Z.; Tsao, L.T.; Wang, J.P. Novel anti-inflammatory constituents of Artocarpus rigida. Helv. Chim. Acta 2003, 86, 2566–2572. [Google Scholar] [CrossRef]
- Kittakoop, P.; Nopichai, S.; Thongon, N.; Charoenchai, P.; Thebtaranonth, Y. Bauhinoxepins A and B: New antimycobacterial dibenzo[b,f]oxepins from Bauhinia saccocalyx. Helv. Chim. Acta 2004, 87, 175–179. [Google Scholar] [CrossRef]
- Pettit, G.R.; Numata, A.; Iwamoto, C.; Usami, Y.; Yamada, T.; Ohishi, H.; Cragg, G.M. Antineoplastic agents. 551. Isolation and structures of bauhiniastatins 1-4 from Bauhinia purpurea. J. Nat. Prod. 2006, 69, 323–327. [Google Scholar] [CrossRef]
- Ong, H.H.; Profitt, J.A.; Anderson, V.B.; Spaulding, T.C.; Wilker, J.C.; Geyer, H.M., III; Kruse, H. Tricyclics with analgesic and antidepressant activity.1. [[(Alkylamino)ethyl]thio]dibenz[b,f]oxepins and 10,11-dihydro derivatives. J. Med. Chem. 1980, 23, 494–501. [Google Scholar] [CrossRef]
- Fernandez, J.; Alonso, J.M.; Andres, J.I.; Cid, J.M.; Diaz, A.; Iturrino, L.; Gil, P.; Megens, A.; Sipido, V.K.; Trabanco, A.A. Discovery of new tetracyclic tetrahydrofuran derivatives as potential broad-spectrum psychotropic agents. J. Med. Chem. 2005, 48, 1709–1712. [Google Scholar] [CrossRef]
- Trabanco, A.A.; Alonso, J.M.; Cid, J.M.; Font, L.M.; Megens, A. Synthesis of 2-N,N-dimethylaminomethyl-2,3,3a,12b-tetrahydrodibenzo[b,f]furo[2,3-d]oxepine derivatives as potential anxiolytic agents. Part 2: Substitutions by methyl groups on the tetrahydrofuran ring. Farmaco 2005, 60, 241–248. [Google Scholar] [CrossRef]
- Ueda, I.; Sato, Y.; Maeno, S.; Umio, S. The synthesis of 10-(4-methylpiperazino)dibenzo [b,f]thiepin and related compounds. Neurotropic and psychotropic agents. Chem. Pharm. Bull. 1975, 23, 2223–2231. [Google Scholar] [CrossRef] [Green Version]
- Mu, L.-H.; Li, J.-B.; Yang, J.-Z.; Zhang, D.-M. New dibenz[b,f]oxepins from Cercis chinensis Bunge. J. Asian Nat. Prod. Res. 2007, 9, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Irie, A.; Nakamura, H.; Hino, K.; Uno, H.; Nishimura, H. Nonsteroidal antiinflammatory agents. 1. 10,11-Dihydro-11-oxodibenz[b,f]oxepinacetic acids and related compounds. J. Med. Chem. 1982, 25, 1065–1070. [Google Scholar] [CrossRef]
- RCSB Protein Data Bank—RCSB PDB. Available online: http://www.rcsb.org/pdb/home/home.do (accessed on 23 February 2004).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tron, G.C.; Pirali, T.; Sorba, G.; Pagliai, F.; Busacca, S.; Genazzani, A.A. Medicinal chemistry of combretastatin A4: Present and future directions. J. Med. Chem. 2006, 49, 3033–3044. [Google Scholar] [CrossRef]
- Luo, Y.; Qiu, K.M.; Lu, X.; Liu, K.; Fu, J.; Zhu, H.L. Synthesis, biological evaluation, and molecular modeling of cinnamic acyl sulfonamide derivatives as novel antitubulin agents. Bioorg. Med. Chem. 2011, 19, 4730–4738. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, Y.Y.; Wang, X.I.; Keenan, S.M.; Arora, S.; Welsh, W.J. Highly potent triazole-based tubulin polymerization inhibitors. J. Med. Chem. 2007, 50, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Massarotti, A.; Coluccia, A.; Silvestri, R.; Sorba, G.; Brancale, A. The Tubulin Colchicine Domain: A Molecular Modeling Perspective. Chem. Med. Chem. 2012, 7, 33–42. [Google Scholar] [CrossRef]
- Welleman, I.M.; Hoorens, M.W.H.; Feringa, B.L.; Boersma, H.H.; Szymanski, W. Photoresponsive molecular tools for emerging applications of light in medicine. Chem. Sci. 2020, 11, 11672–11691. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Bai, S.; Liu, B.; Wang, C.L.J. Oxidative Dehydrogenation of Hydrazobenzenes toward Azo Compounds Catalyzed by tert-Butyl Nitrite in EtOH. ACS Omega 2020, 5, 28856–28862. [Google Scholar] [CrossRef]
- Sahoo, M.K.; Saravanakumar, K.; Jaiswal, G.; Balaraman, E. Photocatalysis Enabling Acceptorless Dehydrogenation of Diaryl Hydrazines at Room Temperature. ACS Catal. 2018, 8, 7727–7733. [Google Scholar] [CrossRef]
- Jo, G.; Kim, M.H.; Kim, J. A practical route to azo compounds by metal-free aerobic oxidation of arylhydrazides using an NOX system. Org. Chem. Front. 2020, 7, 834–839. [Google Scholar] [CrossRef]
- Du, K.S.; Huang, J.M. Electrochemical dehydrogenation of hydrazines to azo compounds. Green Chem. 2019, 21, 1680–1685. [Google Scholar] [CrossRef]
- Lv, H.; Laishram, R.D.; Li, J.; Zhou, Y.; Xu, D.; More, S.; Dai, Y.; Fan, B. Photocatalyzed oxidative dehydrogenation of hydrazobenzenes to azobenzenes. J. Green Chem. 2019, 21, 4055–4406. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jana, S.; Kibriya, G.; Dey, A.; Hajra, A. Tert-Butyl nitrite mediated azo coupling between anilines and imidazoheterocycles. RSC Adv. 2016, 6, 34146–34152. [Google Scholar] [CrossRef]
- John, A.A.; Lin, Q. Synthesis of Azobenzenes Using N-Chlorosuccinimide and 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU). J. Org. Chem. 2017, 82, 9873–9876. [Google Scholar] [CrossRef] [PubMed]
- Agnetta, L.; Bermudez, M.; Riefolo, F.; Matera, C.; Claro, E.; Messerer, R.; Littmann, T.; Wolber, G.; Holzgrabe, U.; Decker, M. Fluorination of Photoswitchable Muscarinic Agonists Tunes Receptor Pharmacology and Photochromic Properties. J. Med. Chem. 2019, 62, 3009–3020. [Google Scholar] [CrossRef]
- Zhao, R.; Tan, C.; Xie, Y.; Gao, C.; Liu, H.; Jiang, Y. One step synthesis of azo compounds from nitroaromatics and anilines. Tetrahedron Lett. 2011, 52, 3805–3809. [Google Scholar] [CrossRef]
- Craik, D.J. Substituent Effects on Nuclear Shielding. Ann. Rep. NMR Spectrosc. 1984, 15, 1–104. [Google Scholar]
- Kara, Y.S. Substituent effect study on experimental 13C NMR chemical shifts of (3-(substituted phenyl)-cis-4,5-dihydroisoxazole-4,5-diyl)bis(methylene)diacetate derivatives. Spectrochim Acta A Mol. Biomol. Spectrosc. 2015, 151, 723–730. [Google Scholar] [CrossRef]
- Holik, M. Second-order regression of 13C substituent chemical shifts with Taft’s sigma constants. Magn. Reson. Chem. 1992, 30, 189–199. [Google Scholar] [CrossRef]
- Ewing, D.F. 13C substituent effects in monosubstituted benzenes. Magn. Reson. Chem. 1979, 12, 499–524. [Google Scholar] [CrossRef]
- Kappes, U.P.; Luo, D.; Potter, M.; Schulmeister, K.; Rünger, T.M. Short- and Long-Wave UV Light (UVB and UVA) Induce Similar Mutations in Human Skin Cells. J. Investig. Dermatol. 2006, 126, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juvekar, V.; Lee, C.S.L.D.J.; Park, S.J.; Song, G.O.; Kang, H.; Kim, H.M. An azo dye for photodynamic therapy that is activated selectively by two-photon excitation. Chem. Sci. 2021, 12, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, S.; Zahavy, E.; Wrasidlo, W.; Hayashi, T.; Norton, J.; Su, Y.; Adams, S.; Esener, S. Localized in vivo activation of a photoactivatable doxorubicinprodrug in deep tumor tissue. Photochem. Photobiol. 2013, 89, 698–708. [Google Scholar] [CrossRef] [Green Version]
- Polosukhina, A.; Litt, J.; Tochitsky, I.; Nemargut, J.; Sychev, Y.; de Kouchkovsky, I.; Huang, T.; Borges, K.; Trauner, D.; van Gelder, R.N. Photochemical restoration of visual responses in blind mice. Neuron 2012, 75, 271–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
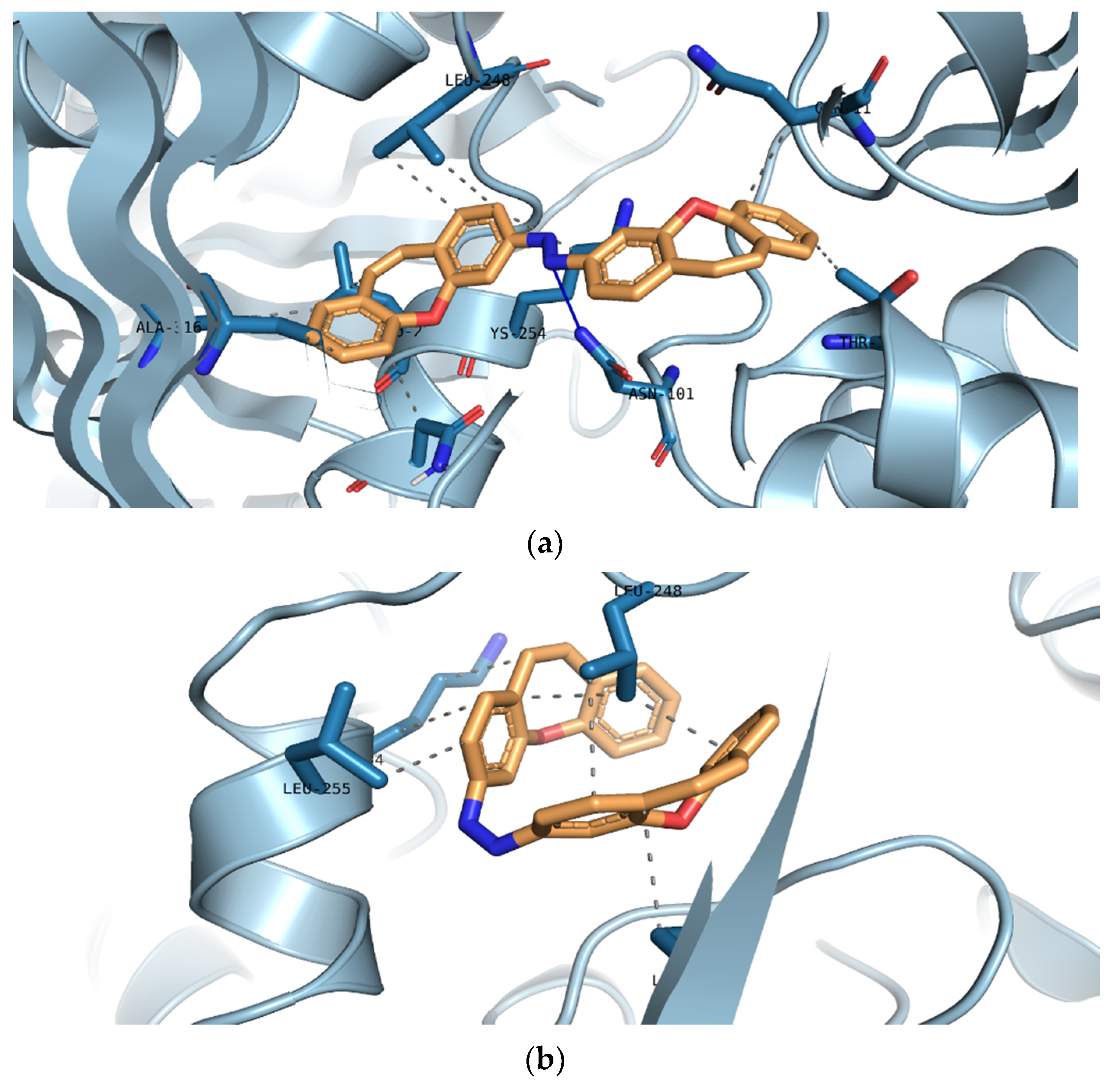

| Compound | The Affinity of Isomer E (kJ/mol) | The Affinity of Isomer Z (kJ/mol) | Δ Affinity (kJ/mol) |
|---|---|---|---|
| 2a | –13.9 | –12.3 | 1.6 |
| 2b | –13.5 | –14.2 | –0.7 |
| 2c | –14.2 | –13.5 | 0.7 |
| 2e | –12.8 | –12.4 | 0.4 |
| 2f | –13.4 | –12.4 | 1 |
| Method | Conditions | Yield |
|---|---|---|
| 1 [57] | NBS, DBU, CH2Cl2, Ar, −78 °C | 45% |
| 2 [56] | t-BuONO (1.2 eq.), EtOH, rt, 48 h | 0% |
| 3 [57] | KMnO4, CuSO4·5H2O, CH2Cl2,24 h, rt | 20% |
| 4 [57] | KMnO4, CuSO4·5H2O, CH2Cl2, grinding | 10% |
| 5 [58] | (1) oxone, H2O, CH2Cl2(2) AcOH, TFA, toluene | 5% |
| 6 [57] | NCS, DBU, CH2Cl2, Ar, −78 °C | 45% |
| 7 [57] | NIS, DBU, CH2Cl2, Ar, −78 °C | 37% |
| 8 [57] | NCS, DBN, CH2Cl2, Ar, −78 °C | 10% |
| 9 [57] | NCS, KOtBu, CH2Cl2, Ar, −78 °C | 20% |
| 10 [59] | KOH, DMF, N2, 150 °C | mixture |
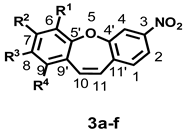
| R | C1 | C2 | C3 | C4 | C4′ | C5′ | C6 | C7 | C8 | C9 | C9′ | C10 | C11 | C11′ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1–R4 = H, * | 131.12 | 121.26 | 149.05 | 117.46 | 156.93 | 156.86 | 122.35 | 132.06 | 126.73 | 130.80 | 130.43 | 134.36 | 129.10 | 138.01 |
| 3(a); R1 = OCH3, R2–R4 = H | –0.05 | 0.02 | −0.19 | −0.27 | −0.2 | −12.4 | 29.91 | −17.08 | −4.98 | −3.99 | 1.11 | 0.03 | 0.09 | 0.3 |
| 3(b); R2 = OCH3, R1, R3,R4 = H | –0.27 | 0.07 | −0.29 | 0.23 | −0.66 | 1.09 | –14.34 | 30.97 | −13.93 | 0.82 | −7.34 | −0.08 | −2.49 | 0.54 |
| 3(c); R3 = OCH3, R1, R2,R4 = H | 0.08 | −0.13 | 0.01 | –0.18 | 0.35 | −6.34 | 0.75 | −14.87 | 30.84 | −15.84 | 0.69 | −0.06 | 0.32 | −0.03 |
| 3(d); R4 = OCH3, R1–R3 = H | −0.25 | 0.12 | −0.05 | 0.03 | 0.04 | 1.72 | −7.89 | 0.51 | −17.37 | 27.18 | −11.26 | −5.41 | −0.73 | 0.58 |
| 3(e); R2,R3 = OCH3, R1, R4 = H | −0.37 | −0.13 | −0.25 | 0.04 | −0.33 | −6.38 | −15.64 | 19.98 | 20.33 | −18.02 | −8.43 | 0.12 | −1.99 | 0.55 |
| 3(f) *; R1, R3 = OCH3, R2, R4 = H | −0.01 | −0.14 | −0.21 | −0.49 | 0.13 | −18.31 | 30.42 | −29.70 | 31.00 | −25.95 | 1.12 | 0.15 | 0.36 | 0.26 |
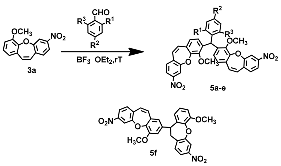
| Compound 3 or 5 | R | Yield (%) of 5 |
|---|---|---|
| a | R1=NO2, R2=H, R3=H | 26% |
| b | R1=H, R2=NO2, R3=H | 36% |
| c | R1=NO2, R2=NO2, R3=H | 66% |
| d | R1=Cl, R2=H, R3=H | 9% |
| e | R1=Br, R2=Cl, R3=H | 14% |
| f | R1=NO2, R2=H, R3=NO2 | 43% |
| Compound | Percent of (Z) Isomer at PSS at | ||||
|---|---|---|---|---|---|
| 365 [nm] | 395 [nm] | 410 [nm] | 465 [nm] | ||
| 2a |  | 44 | 51 | 46 | 48 |
| 2b |  | 28 | 47 | 50 | 48 |
| 2e | 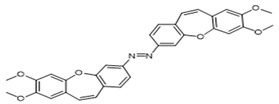 | 15 | 40 | 45 | 42 |
| 2g | 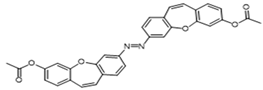 | 38 | 49 | 51 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobiasz, P.; Borys, F.; Borecka, M.; Krawczyk, H. Synthesis and Investigations of Building Blocks with Dibenzo[b,f] Oxepine for Use in Photopharmacology. Int. J. Mol. Sci. 2021, 22, 11033. https://doi.org/10.3390/ijms222011033
Tobiasz P, Borys F, Borecka M, Krawczyk H. Synthesis and Investigations of Building Blocks with Dibenzo[b,f] Oxepine for Use in Photopharmacology. International Journal of Molecular Sciences. 2021; 22(20):11033. https://doi.org/10.3390/ijms222011033
Chicago/Turabian StyleTobiasz, Piotr, Filip Borys, Marta Borecka, and Hanna Krawczyk. 2021. "Synthesis and Investigations of Building Blocks with Dibenzo[b,f] Oxepine for Use in Photopharmacology" International Journal of Molecular Sciences 22, no. 20: 11033. https://doi.org/10.3390/ijms222011033
APA StyleTobiasz, P., Borys, F., Borecka, M., & Krawczyk, H. (2021). Synthesis and Investigations of Building Blocks with Dibenzo[b,f] Oxepine for Use in Photopharmacology. International Journal of Molecular Sciences, 22(20), 11033. https://doi.org/10.3390/ijms222011033