The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial
Abstract
:1. Vegetal Scaffolds Are New Players to the Broader Field of Tissue Engineering
2. Alternative Strategies to Current Chemical Decellularization Protocol
- -
- <50 ng dsDNA per mg extracellular matrix (ECM) dry weight;
- -
- <200 bp DNA fragment length;
- -
- Lack of visible nuclear material in tissue sections stained with DAPI or H&E.
3. Decellularized Vegetal Tissues Support Cell Culture
4. The Exploitation of the Inherent Vegetal Vein Network to Provide a Unique Vascularized Bioengineered Tissue Construct
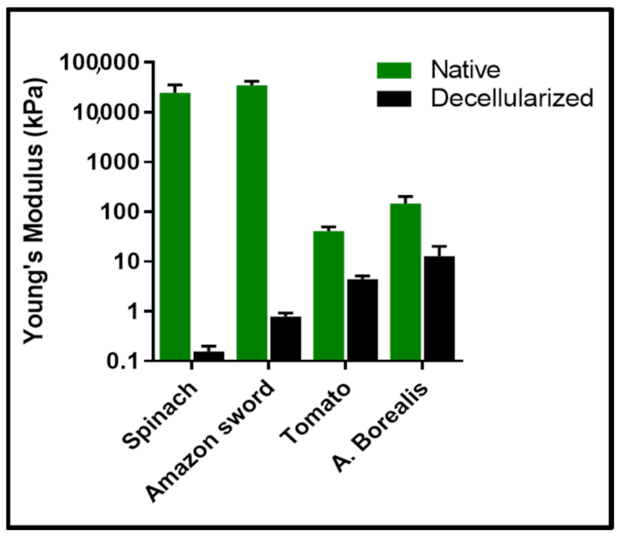
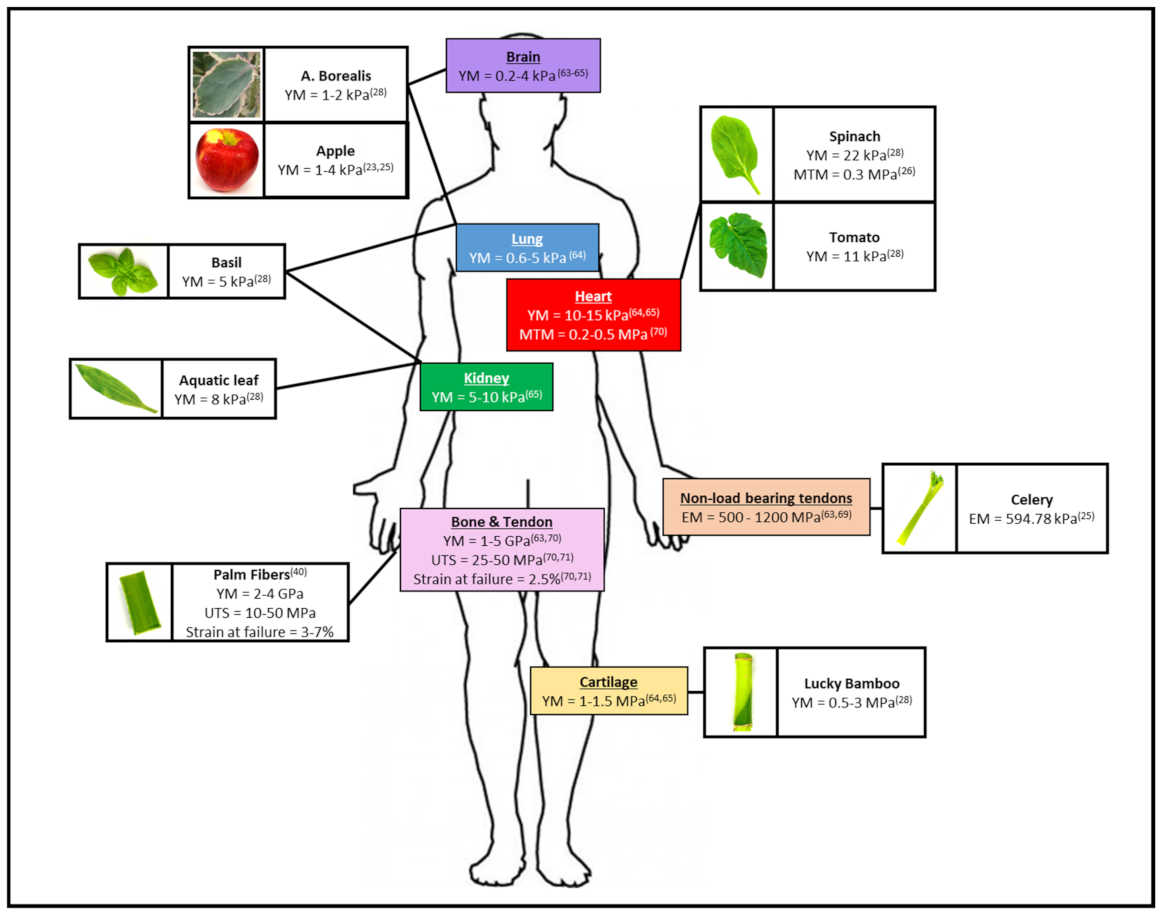
5. Decellularized Plant Tissues Exhibit a Wide Range of Mechanical Properties Which Can Be Matched to a Human Anatomical Site
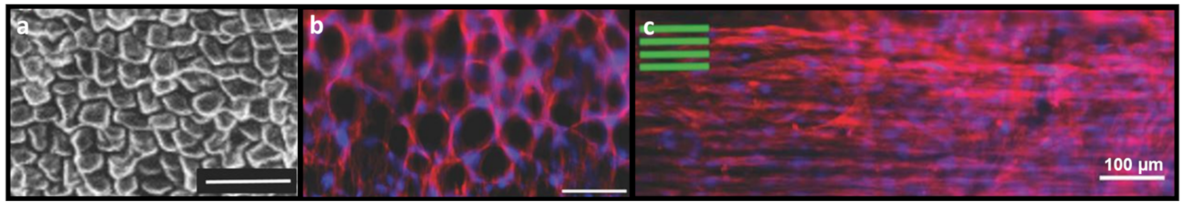
6. Natural Topographical Architecture Found in Plant Scaffolds Can Be Utilized to Direct Cell Behavior
7. Biocompatibility Demonstration and the First In Vivo Applications
8. Additional Considerations for Decellularized Plant-Based Biomaterials
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Available online: https://www.hindawi.com/journals/bmri/2017/9831534/ (accessed on 8 May 2019).
- Nomi, M.; Atala, A.; Coppi, P.D.; Soker, S. Principals of Neovascularization for Tissue Engineering. Mol. Asp. Med. 2002, 23, 463–483. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 17. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, D.R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Groff, K.; Bachli, E.; Lansdowne, M.; Capaldo, T. Review of Evidence of Environmental Impacts of Animal Research and Testing. Environments 2014, 1, 14–30. [Google Scholar] [CrossRef] [Green Version]
- Taylor, K.; Alvarez, L.R. An Estimate of the Number of Animals Used for Scientific Purposes Worldwide in 2015. Altern. Lab. Anim. 2019, 47, 196–213. [Google Scholar] [CrossRef] [PubMed]
- JOINT MOTION FOR A RESOLUTION on Plans and Actions to Accelerate the Transition to Innovation without the Use of Animals in Research, Regulatory Testing and Education. Available online: https://www.europarl.europa.eu/doceo/document/RC-9-2021-0425_EN.html (accessed on 27 October 2021).
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of Tissues and Organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Takahashi, S.; Ito, H.; Inagaki, H.; Noishiki, Y. Tissue Biocompatibility of Cellulose and Its Derivatives. J. Biomed. Mater. Res. 1989, 23, 125–133. [Google Scholar] [CrossRef]
- Märtson, M.; Viljanto, J.; Hurme, T.; Laippala, P.; Saukko, P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20, 1989–1995. [Google Scholar] [CrossRef]
- Bo, S.; Min, Z.; Jing, S.; Zhibin, H.; Pedram, F.; Yonghao, N. Applications of Cellulose-Based Materials in Sustained Drug Delivery Systems. Curr. Med. Chem. 2019, 26, 2485–2501. [Google Scholar]
- Müller, F.A.; Müller, L.; Hofmann, I.; Greil, P.; Wenzel, M.M.; Staudenmaier, R. Cellulose-Based Scaffold Materials for Cartilage Tissue Engineering. Biomaterials 2006, 27, 3955–3963. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, Y.S. The Role of Bacterial Cellulose in Artificial Blood Vessels. Mol. Cell. Toxicol. 2017, 13, 257–261. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial Cellulose as a Material for Wound Treatment: Properties and Modifications. A Review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial Cellulose-Based Materials and Medical Devices: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef]
- Novotna, K.; Havelka, P.; Sopuch, T.; Kolarova, K.; Vosmanska, V.; Lisa, V.; Svorcik, V.; Bacakova, L. Cellulose-Based Materials as Scaffolds for Tissue Engineering. Cellulose 2013, 20, 2263–2278. [Google Scholar] [CrossRef] [Green Version]
- McCulloh, K.A.; Sperry, J.S.; Adler, F.R. Water Transport in Plants Obeys Murray’s Law. Nature 2003, 421, 939–942. [Google Scholar] [CrossRef]
- Sarker, M.D.; Naghieh, S.; Sharma, N.K.; Chen, X. 3D Biofabrication of Vascular Networks for Tissue Regeneration: A Report on Recent Advances. J. Pharm. Anal. 2018, 8, 277–296. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-Derived Nanostructures: Types and Applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Hickey, R.J.; Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Customizing the Shape and Microenvironment Biochemistry of Biocompatible Macroscopic Plant-Derived Cellulose Scaffolds. ACS Biomater. Sci. Eng. 2018, 4, 3726–3736. [Google Scholar] [CrossRef] [Green Version]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.-L.; Guzzardi, M.A.; Shulman, C.; Milwid, J.; Kobayashi, N.; Tilles, A.; Berthiaume, F.; et al. Organ Reengineering through Development of a Transplantable Recellularized Liver Graft Using Decellularized Liver Matrix. Nat. Med. 2010, 16, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-Decellularized Matrix: Using Nature’s Platform to Engineer a Bioartificial Heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Liao, J.; Joyce, E.M.; Sacks, M.S. Effects of Decellularization on the Mechanical and Structural Properties of the Porcine Aortic Valve Leaflet. Biomaterials 2008, 29, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Pomerantseva, I.; Bassett, E.K.; Bowley, C.M.; Zhao, X.; Bichara, D.A.; Kulig, K.M.; Vacanti, J.P.; Randolph, M.A.; Sundback, C.A. Engineering Ear Constructs with a Composite Scaffold to Maintain Dimensions. Tissue Eng. Part A 2011, 17, 1573–1581. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.; Chen, S.; Li, Z.; Sun, X.; Feng, C.; Wang, H.; Xu, Y. In vitro biodegradability of bacterial cellulose by cellulase in simulated body fluid and compatibility in vivo. Cellulose 2016, 23, 3187–3198. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Mohan, C.C.; Unnikrishnan, P.S.; Krishnan, A.G.; Nair, M.B. Decellularization and Oxidation Process of Bamboo Stem Enhance Biodegradation and Osteogenic Differentiation. Mater. Sci. Eng. C 2021, 119, 111500. [Google Scholar] [CrossRef]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple Derived Cellulose Scaffolds for 3D Mammalian Cell Culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef] [Green Version]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of Subcutaneously Implanted Plant-Derived Cellulose Biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef] [Green Version]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.K.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing Kingdoms: Using Decellularized Plants as Perfusable Tissue Engineering Scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.-S.; Matsumoto, S.; Le, H.D.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized Plants as Diverse Biomaterials for Human Cell Culture. Adv. Healthc. Mater. 2017, 6, 1601225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Zhang, Q.; Wang, S.; Zhang, J.; Fan, S.; Lin, X. Current Advances in the Development of Decellularized Plant Extracellular Matrix. Front. Bioeng. Biotechnol. 2021, 9, 650. [Google Scholar] [CrossRef]
- Petersen, T.H.; Calle, E.A.; Colehour, M.B.; Niklason, L.E. Matrix Composition and Mechanics of Decellularized Lung Scaffolds. CTO 2012, 195, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Sawada, K.; Terada, D.; Yamaoka, T.; Kitamura, S.; Fujisato, T. Cell Removal with Supercritical Carbon Dioxide for Acellular Artificial Tissue. J. Chem. Technol. Biotechnol. 2008, 83, 943–949. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Mirmalek-Sani, S.-H.; Deegan, D.B.; Baptista, P.M.; Aboushwareb, T.; Atala, A.; Yoo, J.J. Decellularization Methods of Porcine Kidneys for Whole Organ Engineering Using a High-Throughput System. Biomaterials 2012, 33, 7756–7764. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Tahtinen, M.; Shearier, E.; Qian, Z.; Zhao, F. Decellularization of Fibroblast Cell Sheets for Natural Extracellular Matrix Scaffold Preparation. Tissue Eng. Part C Methods 2015, 21, 77–87. [Google Scholar] [CrossRef]
- Meyer, S.R.; Chiu, B.; Churchill, T.A.; Zhu, L.; Lakey, J.R.T.; Ross, D.B. Comparison of Aortic Valve Allograft Decellularization Techniques in the Rat. J. Biomed. Mater. Res. Part A 2006, 79A, 254–262. [Google Scholar] [CrossRef]
- Petersen, T.H.; Calle, E.A.; Zhao, L.; Lee, E.J.; Gui, L.; Raredon, M.B.; Gavrilov, K.; Yi, T.; Zhuang, Z.W.; Breuer, C.; et al. Tissue-engineered lungs for in vivo implantation. Science 2010, 329, 538–541. [Google Scholar] [CrossRef] [Green Version]
- Gilpin, S.E.; Guyette, J.P.; Gonzalez, G.; Ren, X.; Asara, J.M.; Mathisen, D.J.; Vacanti, J.P.; Ott, H.C. Perfusion Decellularization of Human and Porcine Lungs: Bringing the Matrix to Clinical Scale. J. Heart Lung Transplant. 2014, 33, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Csiki, R.; Tron, A.; Saldamli, B.; Tübel, J.; Florian, K.; Siebenlist, S.; Balmayor, E.; Burgkart, R. Optimized Protocol for Whole Organ Decellularization. Eur. J. Med. Res. 2017, 22, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Bian, Y.; Su, W.; Shi, L.; Li, S.; Song, Y.; Zheng, G.; Xie, A.; Xue, J. Comparison of Various Reagents for Preparing a Decellularized Porcine Cartilage Scaffold. Am. J. Transl. Res. 2019, 11, 1417–1427. [Google Scholar]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Kawazoe, N. 3.1-Preparation of Polymer Scaffolds by Ice Particulate Method for Tissue Engineering. In Biomaterials Nanoarchitectonics; Ebara, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 77–95. ISBN 978-0-323-37127-8. [Google Scholar]
- Sgarminato, V.; Tonda-Turo, C.; Ciardelli, G. Reviewing Recently Developed Technologies to Direct Cell Activity through the Control of Pore Size: From the Macro- to the Nanoscale. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1176–1185. [Google Scholar] [CrossRef]
- Brennan, C.M.; Eichholz, K.F.; Hoey, D.A. The Effect of Pore Size within Fibrous Scaffolds Fabricated Using Melt Electrowriting on Human Bone Marrow Stem Cell Osteogenesis. Biomed. Mater. 2019, 14, 065016. [Google Scholar] [CrossRef]
- Woodfield, T.B.F.; Blitterswijk, C.A.V.; Wijn, J.D.; Sims, T.J.; Hollander, A.P.; Riesle, J. Polymer Scaffolds Fabricated with Pore-Size Gradients as a Model for Studying the Zonal Organization within Tissue-Engineered Cartilage Constructs. Tissue Eng. 2005, 11, 1297–1311. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the Effect of Mean Pore Size on Cell Activity in Collagen-Glycosaminoglycan Scaffolds. Cell Adhes. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jung, H.; Park, N.; Park, S.-H.; Ju, J.H. Induced Osteogenesis in Plants Decellularized Scaffolds. Sci. Rep. 2019, 9, 20194. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Toffoletto, N.; Farè, S.; Altomare, L. Plant Tissues as 3D Natural Scaffolds for Adipose, Bone and Tendon Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 723. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Efficient Mineralization and Osteogenic Gene Overexpression of Mesenchymal Stem Cells on Decellularized Spinach Leaf Scaffold. Gene 2020, 757, 144852. [Google Scholar] [CrossRef]
- Lacombe, J.; Harris, A.F.; Zenhausern, R.; Karsunsky, S.; Zenhausern, F. Plant-Based Scaffolds Modify Cellular Response to Drug and Radiation Exposure Compared to Standard Cell Culture Models. Front. Bioeng. Biotechnol. 2020, 8, 932. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Shiwarski, D.J.; Ball, R.L.; Whitehead, K.A.; Feinberg, A.W. Engineering Aligned Skeletal Muscle Tissue Using Decellularized Plant-Derived Scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 3046–3054. [Google Scholar] [CrossRef]
- Walawalkar, S.; Almelkar, S. Fabricating a Pre-Vascularized Large-Sized Metabolically-Supportive Scaffold Using Brassica Oleracea Leaf. J. Biomater. Appl. 2020, 36, 0885328220968388. [Google Scholar] [CrossRef]
- Harris, A.F.; Lacombe, J.; Liyanage, S.; Han, M.Y.; Wallace, E.; Karsunky, S.; Abidi, N.; Zenhausern, F. Supercritical Carbon Dioxide Decellularization of Plant Material to Generate 3D Biocompatible Scaffolds. Sci. Rep. 2021, 11, 3643. [Google Scholar] [CrossRef]
- Adamski, M.; Fontana, G.; Gershlak, J.R.; Gaudette, G.R.; Le, H.D.; Murphy, W.L. Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications. JoVE (J. Vis. Exp.) 2018, 135, e57586. [Google Scholar] [CrossRef]
- Jansen, K.; Evangelopoulou, M.; Casellas, C.P.; Abrishamcar, S.; Jansen, J.; Vermonden, T.; Masereeuw, R. Spinach and Chive for Kidney Tubule Engineering: The Limitations of Decellularized Plant Scaffolds and Vasculature. AAPS J. 2020, 23, 11. [Google Scholar] [CrossRef]
- Toker, M.; Rostami, S.; Kesici, M.; Gul, O.; Kocaturk, O.; Odabas, S.; Garipcan, B. Decellularization and Characterization of Leek: A Potential Cellulose-Based Biomaterial. Cellulose 2020, 27, 7331–7348. [Google Scholar] [CrossRef]
- Dikici, S.; Claeyssens, F.; MacNeil, S. Decellularised Baby Spinach Leaves and Their Potential Use in Tissue Engineering Applications: Studying and Promoting Neovascularisation. J. Biomater. Appl. 2019, 34, 546–559. [Google Scholar] [CrossRef]
- Robbins, E.R.; Pins, G.D.; Laflamme, M.A.; Gaudette, G.R. Creation of a contractile biomaterial from a decellularized spinach leaf without ECM protein coating: An in vitro study. J. Biomed. Mater. Res. Part A 2020, 108, 2123–2132. [Google Scholar] [CrossRef]
- James, B.D.; Ruddick, W.N.; Vasisth, S.E.; Dulany, K.; Sulekar, S.; Porras, A.; Marañon, A.; Nino, J.C.; Allen, J.B. Palm Readings: Manicaria Saccifera Palm Fibers Are Biocompatible Textiles with Low Immunogenicity. Mater. Sci. Eng. C 2020, 108, 110484. [Google Scholar] [CrossRef]
- Phan, N.V.; Wright, T.; Rahman, M.M.; Xu, J.; Coburn, J.M. In vitro biocompatibility of decellularized cultured plant cell-derived matrices. ACS Biomater. Sci. Eng. 2020, 6, 822–832. [Google Scholar] [CrossRef]
- White, A.; Burns, D.; Christensen, T.W. Effective Terminal Sterilization Using Supercritical Carbon Dioxide. J. Biotechnol. 2006, 123, 504–515. [Google Scholar] [CrossRef]
- Dillow, A.K.; Dehghani, F.; Hrkach, J.S.; Foster, N.R.; Langer, R. Bacterial Inactivation by Using Near- and Supercritical Carbon Dioxide. Proc. Natl. Acad. Sci. USA 1999, 96, 10344–10348. [Google Scholar] [CrossRef] [Green Version]
- Banuti, D.; Raju, M.; Ma, P.C.; Ihme, M.; Hickey, J.-P. Seven Questions about Supercritical Fluids-Towards a New Fluid State Diagram. In Proceedings of the 55th AIAA Aerospace Sciences Meeting; American Institute of Aeronautics and Astronautics, Grapevine, TX, USA, 9 January 2017. [Google Scholar]
- Bullington, L.S.; Lekberg, Y.; Larkin, B.G. Insufficient Sampling Constrains Our Characterization of Plant Microbiomes. Sci. Rep. 2021, 11, 3645. [Google Scholar] [CrossRef]
- Wang, Y.; Dominko, T.; Weathers, P.J. Using Decellularized Grafted Leaves as Tissue Engineering Scaffolds for Mammalian Cells. In Vitro Cell. Dev. Biol.-Plant 2020. [Google Scholar] [CrossRef]
- Lucas, A.D.; Merritt, K.; Hitchins, V.M.; Woods, T.O.; McNamee, S.G.; Lyle, D.B.; Brown, S.A. Residual Ethylene Oxide in Medical Devices and Device Material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 66B, 548–552. [Google Scholar] [CrossRef]
- Ruoslahti, E. Rgd and Other Recognition Sequences for Integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Moshiul Alam, A.K.M.; Beg, M.D.H.; Reddy Prasad, D.M.; Khan, M.R.; Mina, M.F. Structures and Performances of Simultaneous Ultrasound and Alkali Treated Oil Palm Empty Fruit Bunch Fiber Reinforced Poly(Lactic Acid) Composites. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1921–1929. [Google Scholar] [CrossRef] [Green Version]
- Saska, S.; Novaes Teixeira, L.; de Oliveira, P.T.; Gaspar, A.M.M.; Ribeiro, S.J.L.; Messaddeq, Y.; Marchetto, R. Bacterial Cellulose-Collagen Nanocomposite for Bone Tissue Engineering. J. Mater. Chem. 2012, 22, 22102–22112. [Google Scholar] [CrossRef]
- Vacanti, J.P.; Langer, R. Tissue Engineering: The Design and Fabrication of Living Replacement Devices for Surgical Reconstruction and Transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Jain, R.K.; Au, P.; Tam, J.; Duda, D.G.; Fukumura, D. Engineering Vascularized Tissue. Nat. Biotechnol. 2005, 23, 821–823. [Google Scholar] [CrossRef]
- Gui, L.; Niklason, L.E. Vascular Tissue Engineering: Building Perfusable Vasculature for Implantation. Curr. Opin. Chem. Eng. 2014, 3, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Escobar, E.L.N.; da Silva, T.A.; Pirich, C.L.; Corazza, M.L.; Pereira Ramos, L. Supercritical Fluids: A Promising Technique for Biomass Pretreatment and Fractionation. Front. Bioeng. Biotechnol. 2020, 8, 252. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, P.G.; McNaughton, K.G. Stomatal Control of Transpiration: Scaling Up from Leaf to Region. In Advances in Ecological Research; MacFadyen, A., Ford, E.D., Eds.; Academic Press: Cambridge, MA, USA, 1986; Volume 15, pp. 1–49. [Google Scholar]
- Iskratsch, T.; Wolfenson, H.; Sheetz, M.P. Appreciating Force and Shape—The Rise of Mechanotransduction in Cell Biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833. [Google Scholar] [CrossRef]
- Romani, P.; Valcarcel-Jimenez, L.; Frezza, C.; Dupont, S. Crosstalk between Mechanotransduction and Metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 22–38. [Google Scholar] [CrossRef]
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in Mechanotransduction Pathways That Control Cell Morphology. Annu. Rev. Physiol. 2019, 81, 585–605. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and Extracellular Matrix Homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Broders-Bondon, F.; Ho-Bouldoires, T.H.N.; Fernandez-Sanchez, M.-E.; Farge, E. Mechanotransduction in Tumor Progression: The Dark Side of the Force. J. Cell. Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef] [Green Version]
- Cox, T.R.; Erler, J.T. Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. Dis. Models Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Skardal, A.; Mack, D.; Atala, A.; Soker, S. Substrate Elasticity Controls Cell Proliferation, Surface Marker Expression and Motile Phenotype in Amniotic Fluid-Derived Stem Cells. J. Mech. Behav. Biomed. Mater. 2013, 17, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.-J. Tissue Stiffness Dictates Development, Homeostasis, and Disease Progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, K.; Gu, X.; Leong, K.W. Biophysical Regulation of Cell Behavior—Cross Talk between Substrate Stiffness and Nanotopography. Engineering 2017, 3, 36–54. [Google Scholar] [CrossRef]
- Fekete, N.; Béland, A.V.; Campbell, K.; Clark, S.L.; Hoesli, C.A. Bags versus Flasks: A Comparison of Cell Culture Systems for the Production of Dendritic Cell–Based Immunotherapies. Transfusion 2018, 58, 1800–1813. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, K.; Lin-Gibson, S.; Wallace, W.E.; Parekh, S.H.; Lee, Y.J.; Cicerone, M.T.; Young, M.F.; Simon, C.G. The Effect of 3D Hydrogel Scaffold Modulus on Osteoblast Differentiation and Mineralization Revealed by Combinatorial Screening. Biomaterials 2010, 31, 5051–5062. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.; Mbimba, T.; Younesi, M.; Akkus, O. Effects of Substrate Stiffness on the Tenoinduction of Human Mesenchymal Stem Cells. Acta Biomater. 2017, 58, 244–253. [Google Scholar] [CrossRef]
- Bioengineering Human Myocardium on Native Extracellular Matrix|Circulation Research. Available online: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.115.306874?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 18 May 2021).
- Maganaris, C.N.; Paul, J.P. In vivo human tendon mechanical properties. J. Physiol. 1999, 521, 307–313. [Google Scholar] [CrossRef]
- Read, J.; Sanson, G.D. Characterizing Sclerophylly: The Mechanical Properties of a Diverse Range of Leaf Types. New Phytol. 2003, 160, 81–99. [Google Scholar] [CrossRef]
- Zajac, A.L.; Discher, D.E. Cell Differentiation through Tissue Elasticity-Coupled, Myosin-Driven Remodeling. Curr. Opin. Cell Biol. 2008, 20, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Hickey, R.J.; Latour, M.L.; Harden, J.L.; Pelling, A.E. Engineered tissue interfaces for in vitro and in vivo regeneration. bioRxiv 2020. [Google Scholar] [CrossRef]
- Xu, C.Y.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned Biodegradable Nanofibrous Structure: A Potential Scaffold for Blood Vessel Engineering. Biomaterials 2004, 25, 877–886. [Google Scholar] [CrossRef]
- Curtis, A.; Wilkinson, C. New Depths in Cell Behaviour: Reactions of Cells to Nanotopography. Biochem. Soc. Symp. 1999, 65, 15–26. [Google Scholar]
- Duffy, R.M.; Sun, Y.; Feinberg, A.W. Understanding the Role of ECM Protein Composition and Geometric Micropatterning for Engineering Human Skeletal Muscle. Ann. Biomed. Eng. 2016, 44, 2076–2089. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D Bioprinting System to Produce Human-Scale Tissue Constructs with Structural Integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-Coated Electrospun PLGA Nanofibers for Neural Tissue Applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Zhang, N.; Huang, Y.; Wen, X. Formation of Highly Aligned Grooves on Inner Surface of Semipermeable Hollow Fiber Membrane for Directional Axonal Outgrowth. J. Manuf. Sci. Eng. 2008, 130. [Google Scholar] [CrossRef]
- Phamduy, T.B.; Sweat, R.S.; Azimi, M.S.; Burow, M.E.; Murfee, W.L.; Chrisey, D.B. Printing Cancer Cells into Intact Microvascular Networks: A Model for Investigating Cancer Cell Dynamics during Angiogenesis. Integr. Biol. 2015, 7, 1068–1078. [Google Scholar] [CrossRef] [Green Version]
- Betts, J.G.; Young, K.A.; Wise, J.A.; Johnson, E.; Poe, B.; Kruse, D.H.; Korol, O.; Johnson, J.E.; Womble, M.; DeSaix, P. Structural Organization of the Human Body. In Anatomy and Physiology; OpenStax: Houston, TX, USA, 2013. [Google Scholar]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In Vivo Biocompatibility of Bacterial Cellulose. J. Biomed. Mater. Res. Part A 2006, 76A, 431–438. [Google Scholar] [CrossRef]
- Bai, H.; Xie, B.; Wang, Z.; Li, M.; Sun, P.; Wei, S.; Wang, W.; Wu, H.; Bai, L.; Li, J. Application of the Tissue-Engineered Plant Scaffold as a Vascular Patch. ACS Omega 2021, 6, 11595–11601. [Google Scholar] [CrossRef]
- Tommila, M.; Jokilammi, A.; Penttinen, R.; Ekholm, E. Cellulose—A Biomaterial with Cell-Guiding Property. In Cellulose-Medical, Pharmaceutical and Electronic Applications; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-Based Scaffolds in Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, E.; Lieth, H.J.; Jernstedt, J.A.; Labavitch, J.M.; Suslow, T.V.; Cantwell, M.I. Texture, Composition and Anatomy of Spinach Leaves in Relation to Nitrogen Fertilization. J. Sci. Food Agric. 2013, 93, 227–237. [Google Scholar] [CrossRef]
- Koyama, K.; Masuda, T. The Arrangement of Lateral Veins along the Midvein of Leaves Is Not Related to Leaf Phyllotaxis. Sci. Rep. 2018, 8, 16417. [Google Scholar] [CrossRef]
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision Genome Editing in Plants: State-of-the-Art in CRISPR/Cas9-Based Genome Engineering. BMC Plant Biol. 2020, 20, 234. [Google Scholar] [CrossRef]
- Young, J.F.; Skrivergaard, S. Cultured Meat on a Plant-Based Frame. Nat. Food 2020, 1, 195. [Google Scholar] [CrossRef]
- Jones, J.D.; Rebello, A.S.; Gaudette, G.R. Decellularized Spinach: An Edible Scaffold for Laboratory-Grown Meat. Food Biosci. 2021, 41, 100986. [Google Scholar] [CrossRef]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the Manufacture of Cultured Meat. Crit. Rev. Biotechnol. 2021, 1–13. [Google Scholar] [CrossRef]
- Campuzano, S.; Pelling, A.E. Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-Animal Sources. Front. Sustain. Food Syst. 2019, 3, 38. [Google Scholar] [CrossRef] [Green Version]
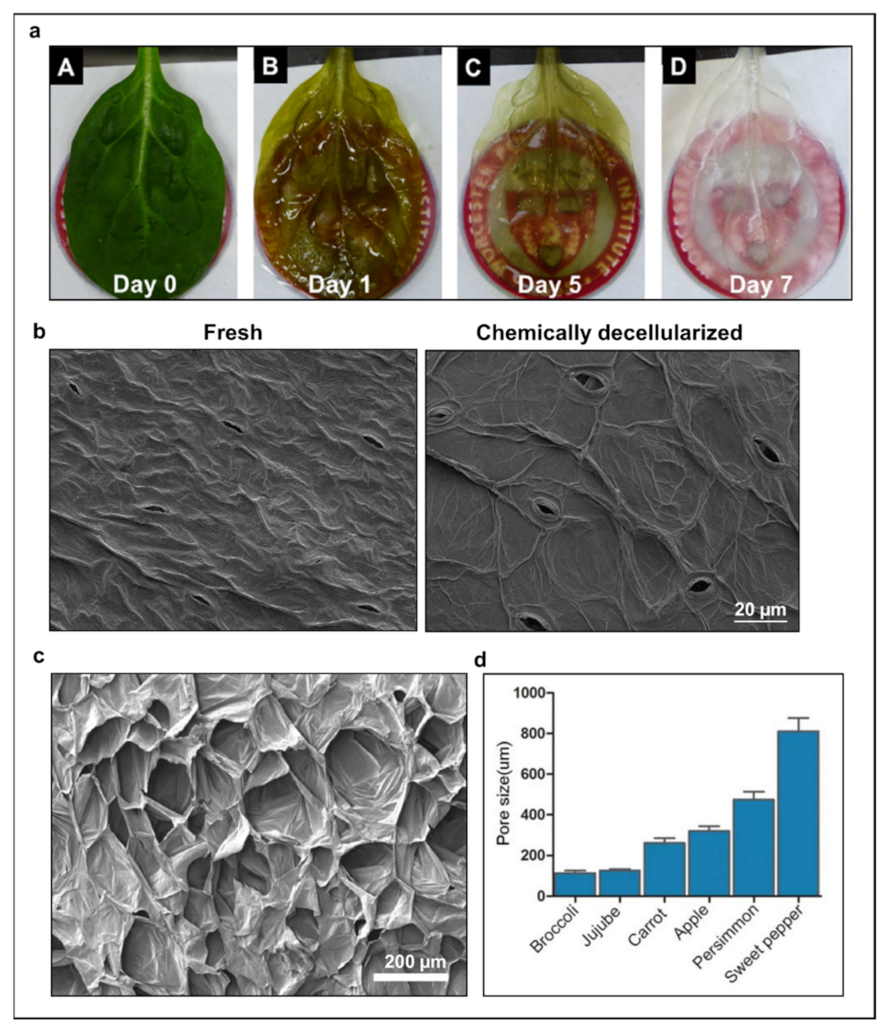
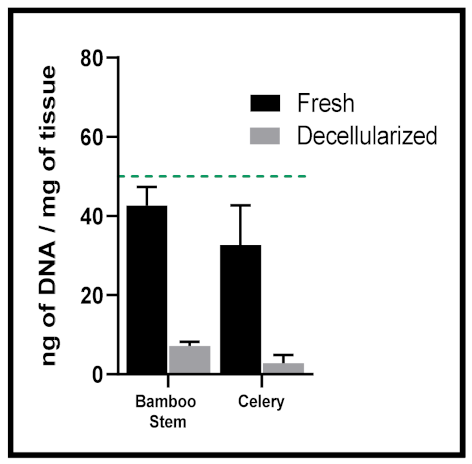
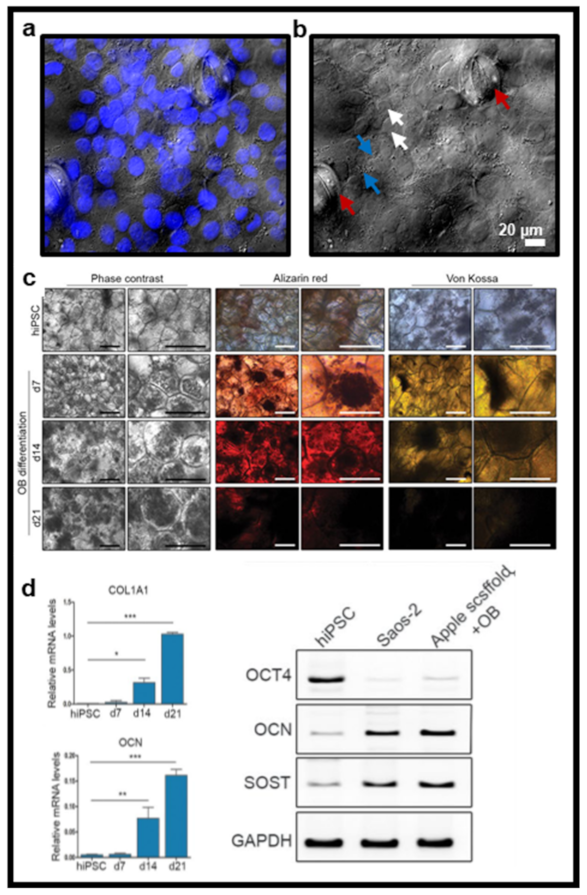
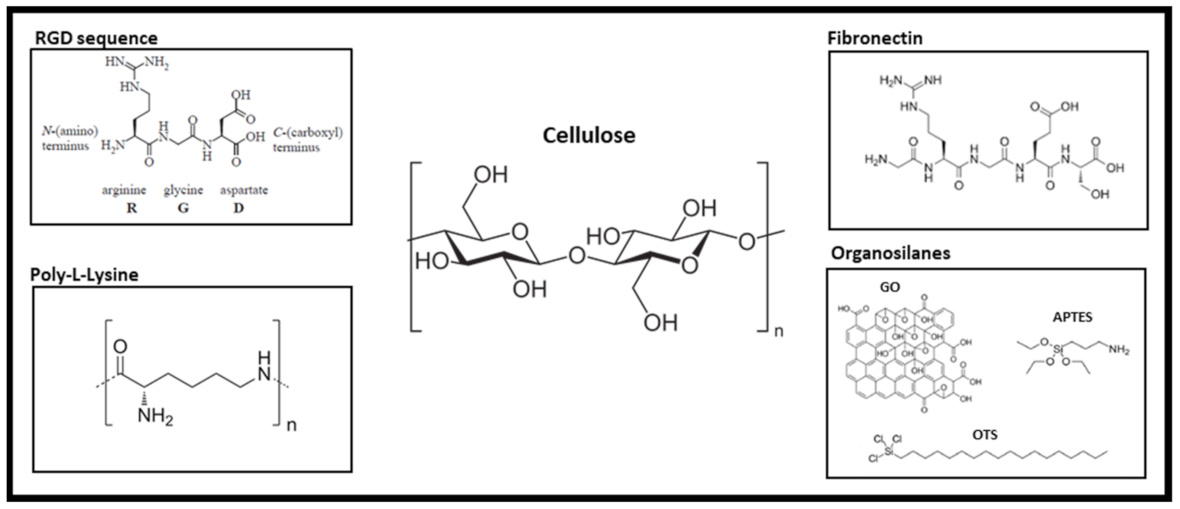


| Decellularization Treatment | Compounds | Time | Decellularized Plants | Advantages | Limitations |
|---|---|---|---|---|---|
| Chemical | SDS (0.1 to 10%, depending on plant material); Triton X-100; bleach (10%) Hexane pre- treatment can be performed when wax cuticle present | 12 h to 3 weeks, depending on the plant material | Amazon sword [55], Anthurium [35], Anthurium (queen) [35], Apple interior [24,32,33,53,56], Asparagus [56], Bamboo [31,35], Basil [55], Broccoli stem [52,56], Cabbage [57], Calathea zebrina [35], Carrot [52,56], Celery [53,56,58], Cucumber [56], Ficus hispida [59], Garcinia [59], Green onion [56,60], Impatiens capensis [35], Jujube [52], Leek [61], Lucky bamboo [55], Orchid pseudobulb [35], Pachira aquatica [59], Parsley stem [34,58], Peanut hairy root [34], Persimmon [52], Potato [56], Solenostemon “wasabi” [35], Spinach [34,54,55,58,60,62,63], Sweet yellow bell pepper [52], Sweet wormwood [34], summer lilac [35], tomato [55], Ubuçu Palm fibers [64], Vanilla [35] | Gold standard, well characterized; demonstrated ability to decellularize a multitude of plant materials with different structural and chemical compositions | Use of harsh chemicals; potential toxic residue thus, requires intense washing steps; time consuming; chemicals are environmentally toxic [46] |
| Detergent-Free [59] | Heated bleach and NaHCO3 solutions or bleach with surfactant | Minutes to hours, depending on the plant material | Bamboo stem, Ficus hispida, Garcinia, Pachira aquatica | Oxidation may enhance cellulose breakdown | Strong chemicals; able to degrade scaffold when heated [59] |
| Freeze/Enzymatic [65] | Lyophilization, DNAse I | 24 h | Transgenic plant cultured cell lines: Hairy root, Tobacco bright yellow (BY-2), Monocot rice cells (Oryza sativa L.) | Retains native proteins | Additional clearing with surfactant might be needed to remove debris [1] |
| Supercritical Fluid (scCO2) [58] | scCO2 (2500 psi at 33 °C); PAA as cosolvent (2%); bleach if scaffold clearing required; Hexane pre- treatment can be performed when wax cuticle present | 3 h (+6 h if clearing required) | Celery, Parsley stem, Spinach leaf, Sweet mint leaf | Fast; use of soft approach with minimal amount of chemicals; sterilization step included | Needs to be characterized on a larger diversity of plants; specialized equipment required [58] |
| Plant | Modification | Mechanical Properties | Technique |
|---|---|---|---|
| Apple hypanthium [32,53] | None | YM = 1.10 ± 0.10 kPa | Nano-indentation |
| Collagen I | YM = 2.20 ± 0.20 kPa | ||
| Glutaraldehyde | YM = 4.10 ± 0.30 kPa | ||
| Poly-L-lysine (PLL) | YM = 4.33 ± 1.98 kPa EM = 4.17 ± 0.17 kPa Residual Strain = 6.42 ± 0.08% MS = 1.17 ± 0.28 kPa | Measurement of bulk dynamic tensile properties | |
| Amazon sword [55] | None | YM = 8.60 ± 0.70 kPa | Nano-indentation |
| Aurora Borealis leaf [55] | None | YM = 1.70 ± 0.30 kPa | Nano-indentation |
| Bamboo stem [31] | None | Compression = 1.52 ± 0.35 MPa | Measurement of bulk dynamic compression properties |
| Oxidation (0.01% NaIO4) | Compression = 1.36 ± 0.47 MPa | ||
| Oxidation (0.1% NaIO4) | Compression = 1.08 ± 0.20 MPa | ||
| Oxidation (0.5% NaIO4) | Compression = 0.60 ± 0.05 MPa | ||
| Basil plant leaf [55] | None | YM = 5.40 ± 2.60 kPa | Nano-indentation |
| Carrot taproot [53] | None | EM = 43.43 ± 5.22 kPa MS = 44.31 ± 8.59 kPa | Measurement of bulk dynamic tensile properties |
| Celery stalk [53] | None | EM = 594.78 ± 94.24 kPa MS = 175.93 ± 40.96 kPa | Measurement of bulk dynamic tensile properties |
| Ficus hispida leaf [59] | None | MTM = 2.00 MPa Strain at Failure = 0.30% UTS = 0.50 MPa | Measurement of bulk dynamic tensile properties |
| Leek [61] | None | EM = 4.42 ± 0.50 kPa Tensile strength = 1.89 ± 0.25 MPa | Measurement of bulk dynamic tensile properties |
| APTES | EM = 1.31 ± 0.15 kPa TS = 2.45 ± 0.27 MPa | ||
| OTS | EM = 0.54 ± 0.14 kPa TS = 1.08 ± 0.28 MPa | ||
| GO | EM = 1.50 ± 0.07 kPa Tensile strength = 1.93 ± 0.10 MPa | ||
| Lucky bamboo stem [55] | None | YM = 1.77 ± 1.20 MPa | Nano-indentation |
| Pachira aquatica [59] | None | MTM = 2.00 MPa Strain at Failure = 0.30% UTS = 0.50 MPa | Measurement of bulk dynamic tensile properties |
| Spinach leaf [34,54,55,58] | None | MTM = 0.30 MPa UTS = ~0.05 MPa Strain at Failure = ~7.00% | Measurement of bulk dynamic tensile properties |
| None | Tensile testing = 1.40 MPa Strain at Failure = 4.57% | Measurement of bulk dynamic tensile properties | |
| None Collagen + Fibronectin | YM = 21.27 ± 0.6 kPa YM = 37.64 ± 2.3 kPa | Nano-indentation | |
| None (scCO2 treated) | YM = 18.09 ± 7.14 kPa | Nano-indentation | |
| Tomato plant leaf [55] | None | YM = 10.70 ± 4.40 kPa | Nano-indentation |
| Ubuçu Palm fibers [64] | None | YM = 3.10 ± 1.04 GPa UTS = 33.96 ± 30.45 MPa Strain at Failure = 5.71 ± 2.4% | Measurement of bulk dynamic tensile properties |
| Alkali treatment | YM = 8.22 ± 4.86 GPa UTS = 72.38 ± 45.19 MPa Strain at Failure = 2.80 ± 1.52% | ||
| Alkali treatment + autoclaved | YM = 3.10 ± 1.04 GPa UTS = 33.96 ± 30.45 MPa Strain at Failure = 5.71 ± 2.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, A.F.; Lacombe, J.; Zenhausern, F. The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial. Int. J. Mol. Sci. 2021, 22, 12347. https://doi.org/10.3390/ijms222212347
Harris AF, Lacombe J, Zenhausern F. The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial. International Journal of Molecular Sciences. 2021; 22(22):12347. https://doi.org/10.3390/ijms222212347
Chicago/Turabian StyleHarris, Ashlee F., Jerome Lacombe, and Frederic Zenhausern. 2021. "The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial" International Journal of Molecular Sciences 22, no. 22: 12347. https://doi.org/10.3390/ijms222212347
APA StyleHarris, A. F., Lacombe, J., & Zenhausern, F. (2021). The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial. International Journal of Molecular Sciences, 22(22), 12347. https://doi.org/10.3390/ijms222212347






