Structural Analysis of the Ancestral Haloalkane Dehalogenase AncLinB-DmbA
Abstract
:1. Introduction
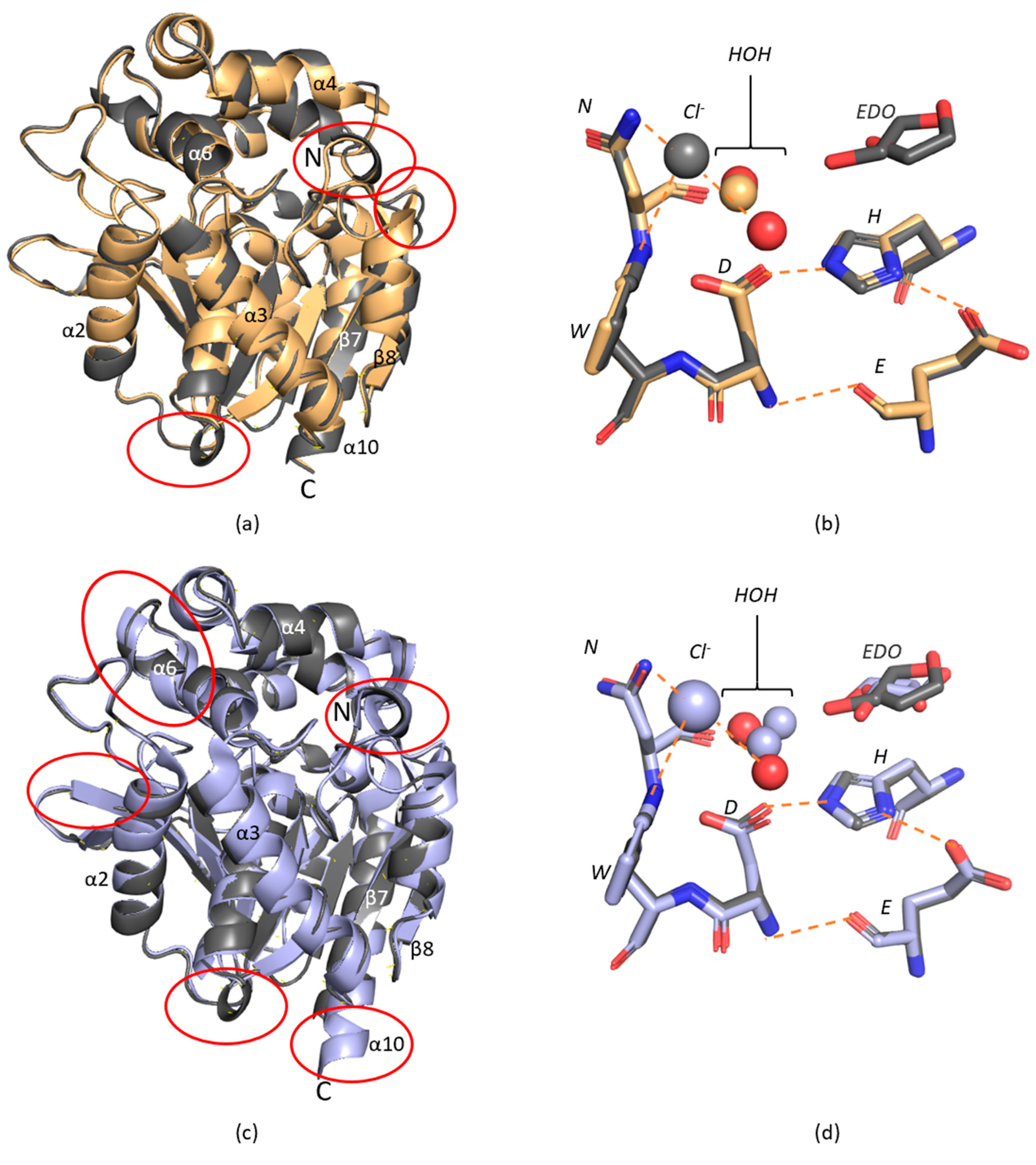
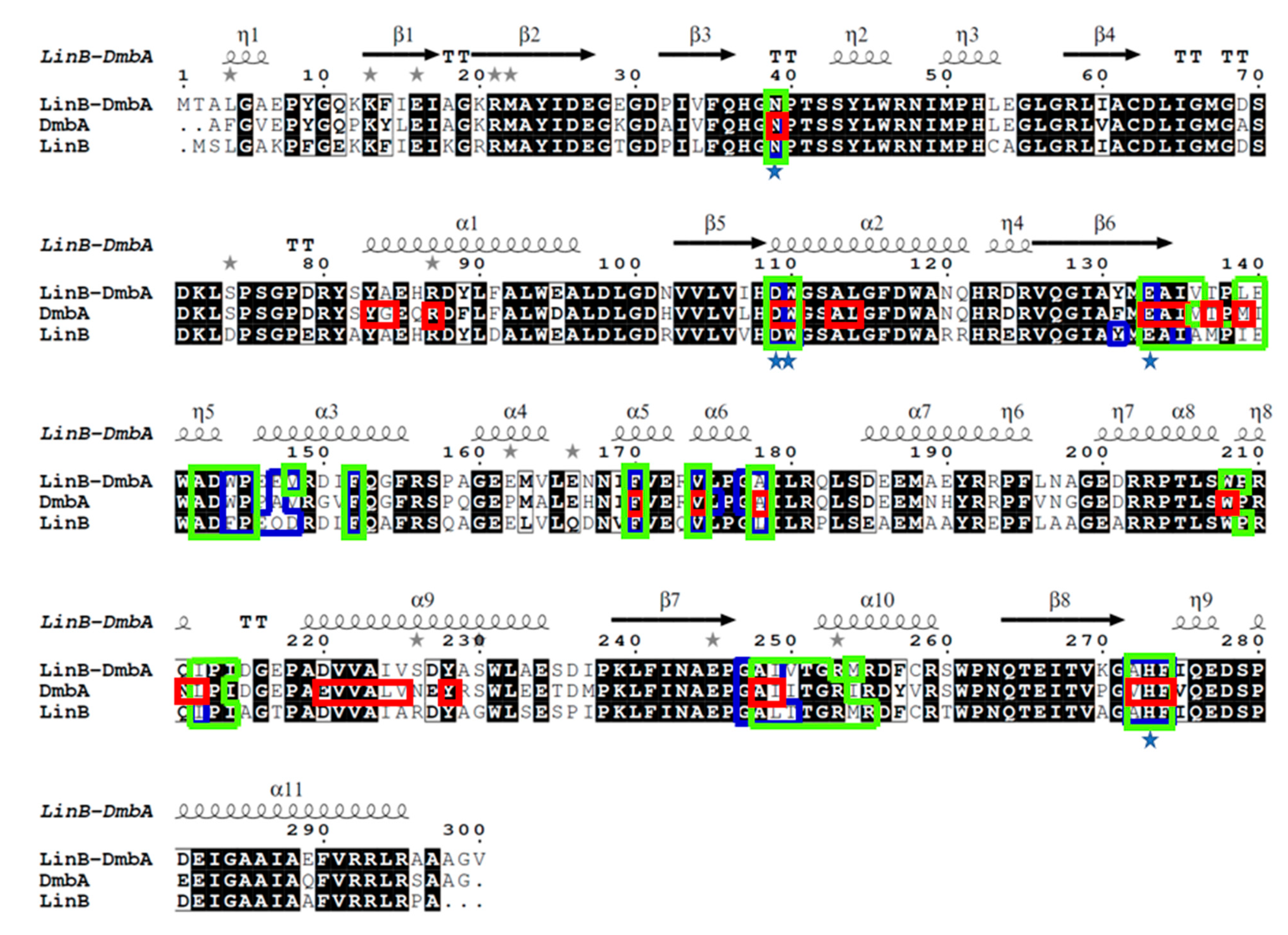
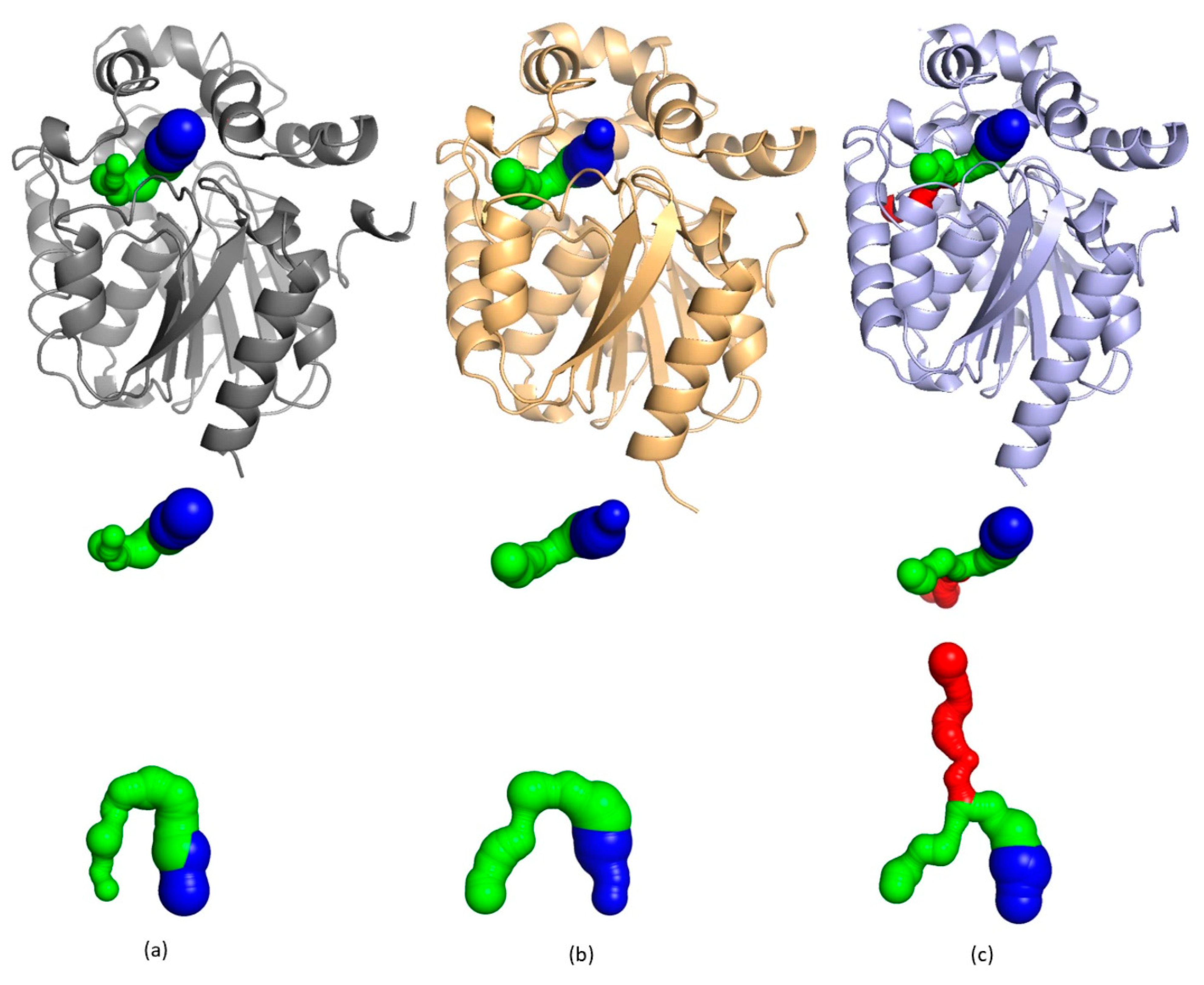
2. Results and Discussion
3. Materials and Methods
3.1. Ancestral Sequence Reconstruction and Gene Synthesis
3.2. Protein Expression and Purification
3.3. Crystallization
3.4. Data Collection
3.5. Structure Solution and Refinement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerba, C.P. Environmental Toxicology. In Environmental and Pollution Science, 3rd ed.; Brusseau, M.L., Pepper, I.L., Gerba, C.P., Eds.; Academic Press: New York, NY, USA, 2019; pp. 511–540. [Google Scholar]
- Ollis, D.L.; Cheah, E.; Cygler, M.; Dijkstra, B.; Frolow, F.; Franken, S.M.; Harel, M.; Remington, S.J.; Silman, I.; Schrag, J.; et al. The alpha/beta hydrolase fold. Protein Eng. 1992, 5, 197–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jong, R.M.; Tiesinga, J.J.; Rozeboom, H.J.; Kalk, K.H.; Tang, L.; Janssen, D.B.; Dijkstra, B.W. Structure and mechanism of a bacterial haloalcohol dehalogenase: A new variation of the short-chain dehydrogenase/reductase fold without an NAD(P)H binding site. EMBO J. 2003, 22, 4933–4944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verschueren, K.H.; Seljée, F.; Rozeboom, H.J.; Kalk, K.H.; Dijkstra, B.W. Crystallographic analysis of the catalytic mechanism of haloalkane dehalogenase. Nature 1993, 363, 693–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koudelakova, T.; Bidmanova, S.; Dvorak, P.; Pavelka, A.; Chaloupkova, R.; Prokop, Z.; Damborsky, J. Haloalkane dehalogenases: Biotechnological applications. Biotechnol. J. 2013, 8, 32–45. [Google Scholar] [CrossRef]
- Janssen, D.B.; Dinkla, I.J.; Poelarends, G.J.; Terpstra, P. Bacterial degradation of xenobiotic compounds: Evolution and distribution of novel enzyme activities. Environ. Microbiol. 2005, 7, 1868–1882. [Google Scholar] [CrossRef] [Green Version]
- Fung, H.K.; Gadd, M.S.; Drury, T.A.; Cheung, S.; Guss, J.M.; Coleman, N.V.; Matthews, J.M. Biochemical and biophysical characterisation of haloalkane dehalogenases DmrA and DmrB in Mycobacterium strain JS60 and their role in growth on haloalkanes. Mol. Microbiol. 2015, 97, 439–453. [Google Scholar] [CrossRef]
- Holmquist, M. Alpha/Beta-hydrolase fold enzymes: Structures, functions and mechanisms. Curr. Protein Pept. Sci. 2000, 1, 209–235. [Google Scholar] [CrossRef]
- Chaloupkova, R.; Prudnikova, T.; Rezacova, P.; Prokop, Z.; Koudelakova, T.; Daniel, L.; Brezovsky, J.; Ikeda-Ohtsubo, W.; Sato, Y.; Kuty, M.; et al. Structural and functional analysis of a novel haloalkane dehalogenase with two halide-binding sites. Acta Crystallogr. Sect. D Biol. Crystallogr. 2014, 70, 1884–1897. [Google Scholar] [CrossRef]
- Chovancova, E.; Kosinski, J.; Bujnicki, J.M.; Damborsky, J. Phylogenetic analysis of haloalkane dehalogenases. Proteins 2007, 67, 305–316. [Google Scholar] [CrossRef]
- Ang, T.F.; Maiangwa, J.; Salleh, A.B.; Normi, Y.M.; Leow, T.C. Dehalogenases: From Improved Performance to Potential Microbial Dehalogenation Applications. Molecules 2018, 23, 1100. [Google Scholar] [CrossRef] [Green Version]
- Marek, J.; Vevodova, J.; Smatanova, I.K.; Nagata, Y.; Svensson, L.A.; Newman, J.; Takagi, M.; Damborsky, J. Crystal structure of the haloalkane dehalogenase from Sphingomonas paucimobilis UT26. Biochemistry 2000, 39, 14082–14086. [Google Scholar] [CrossRef]
- Nagata, Y.; Miyauchi, K.; Takagi, M. Complete analysis of genes and enzymes for gamma-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26. J. Ind. Microbiol. Biotechnol. 1999, 23, 380–390. [Google Scholar] [CrossRef]
- Mazumdar, P.A.; Hulecki, J.C.; Cherney, M.M.; Garen, C.R.; James, M.N. X-ray crystal structure of Mycobacterium tuberculosis haloalkane dehalogenase Rv2579. Biochim. Biophys. Acta 2008, 1784, 351–362. [Google Scholar] [CrossRef]
- Koudelakova, T.; Chovancova, E.; Brezovsky, J.; Monincova, M.; Fortova, A.; Jarkovsky, J.; Damborsky, J. Substrate specificity of haloalkane dehalogenases. Biochem. J. 2011, 435, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Nagata, Y.; Miyauchi, K.; Damborsky, J.; Manova, K.; Ansorgova, A.; Takagi, M. Purification and characterization of a haloalkane dehalogenase of a new substrate class from a gamma-hexachlorocyclohexane-degrading bacterium, Sphingomonas paucimobilis UT26. Appl. Environ. Microbiol. 1997, 63, 3707–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kmunicek, J.; Hynkova, K.; Jedlicka, T.; Nagata, Y.; Negri, A.; Gago, F.; Wade, R.C.; Damborsky, J. Quantitative analysis of substrate specificity of haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26. Biochemistry 2005, 44, 3390–3401. [Google Scholar] [CrossRef]
- Jesenska, A.; Pavlova, M.; Strouhal, M.; Chaloupkova, R.; Tesinska, I.; Monincova, M.; Prokop, Z.; Bartos, M.; Pavlik, I.; Rychlik, I.; et al. Cloning, biochemical properties, and distribution of mycobacterial haloalkane dehalogenases. Appl. Environ. Microbiol. 2005, 71, 6736–6745. [Google Scholar] [CrossRef] [Green Version]
- Degtjarik, O.; Chaloupkova, R.; Rezacova, P.; Kuty, M.; Damborsky, J.; Kuta Smatanova, I. Differences in crystallization of two LinB variants from Sphingobium japonicum UT26. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 284–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okai, M.; Ohtsuka, J.; Imai, L.F.; Mase, T.; Moriuchi, R.; Tsuda, M.; Nagata, K.; Nagata, Y.; Tanokura, M. Crystal structure and site-directed mutagenesis analyses of haloalkane dehalogenase LinB from Sphingobium sp. strain MI1205. J. Bacteriol. 2013, 195, 2642–2651. [Google Scholar] [CrossRef] [Green Version]
- Iermak, I.; Degtjarik, O.; Havlickova, P.; Kuty, M.; Chaloupkova, R.; Damborsky, J.; Prudnikova, T.; Kuta Smatanova, I. Description of Transport Tunnel in Haloalkane Dehalogenase Variant LinB D147C+L177C from Sphingobium japonicum. Catalysts 2021, 11, 5. [Google Scholar] [CrossRef]
- Brezovsky, J.; Babkova, P.; Degtjarik, O.; Fortova, A.; Gora, A.; Iermak, I.; Rezacova, P.; Dvorak, P.; Smatanova, I.K.; Prokop, Z.; et al. Engineering a de Novo Transport Tunnel. ACS Catal. 2016, 6, 7597–7610. [Google Scholar] [CrossRef]
- Kokkonen, P.; Slanska, M.; Dockalova, V.; Pinto, G.P.; Sanchez-Carnerero, E.M.; Damborsky, J.; Klan, P.; Prokop, Z.; Bednar, D. The impact of tunnel mutations on enzymatic catalysis depends on the tunnel-substrate complementarity and the rate-limiting step. Comput. Struct. Biotechnol. J. 2020, 18, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, P.; Bednar, D.; Pinto, G.; Prokop, Z.; Damborsky, J. Engineering enzyme access tunnels. Biotechnol. Adv. 2019, 37, 107386. [Google Scholar] [CrossRef]
- Li, A.; Shao, Z. Biochemical characterization of a haloalkane dehalogenase DadB from Alcanivorax dieselolei B-5. PLoS ONE 2014, 9, e89144. [Google Scholar] [CrossRef]
- Harms, M.J.; Thornton, J.W. Analyzing protein structure and function using ancestral gene reconstruction. Curr. Opin. Struct. Biol. 2010, 20, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62, 72–82. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokop, Z.; Monincová, M.; Chaloupková, R.; Klvana, M.; Nagata, Y.; Janssen, D.B.; Damborský, J. Catalytic mechanism of the maloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26. J. Biol. Chem. 2003, 278, 45094–45100. [Google Scholar] [CrossRef] [Green Version]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [Green Version]
- Brezovsky, J.; Kozlikova, B.; Damborsky, J. Computational Analysis of Protein Tunnels and Channels. Methods Mol. Biol. 2018, 1685, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Stourac, J.; Vavra, O.; Kokkonen, P.; Filipovic, J.; Pinto, G.; Brezovsky, J.; Damborsky, J.; Bednar, D. Caver Web 1.0: Identification of tunnels and channels in proteins and analysis of ligand transport. Nucleic Acids Res. 2019, 47, W414–W422. [Google Scholar] [CrossRef] [Green Version]
- Damborsky, J.; Chaloupkova, R.; Pavlova, M.; Chovancova, E.; Brezovsky, J. Structure–Function Relationships and Engineering of Haloalkane Dehalogenases. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin, Germany, 2010; pp. 1081–1098. [Google Scholar]
- Liang, J.; Edelsbrunner, H.; Woodward, C. Anatomy of protein pockets and cavities: Measurement of binding site geometry and implications for ligand design. Protein Sci. 1998, 7, 1884–1897. [Google Scholar] [CrossRef] [Green Version]
- Kunka, A.; Damborsky, J.; Prokop, Z. Haloalkane Dehalogenases From Marine Organisms. Methods Enzymol. 2018, 605, 203–251. [Google Scholar] [CrossRef]
- Buryska, T.; Babkova, P.; Vavra, O.; Damborsky, J.; Prokop, Z. A Haloalkane Dehalogenase from a Marine Microbial Consortium Possessing Exceptionally Broad Substrate Specificity. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Babkova, P.; Sebestova, E.; Brezovsky, J.; Chaloupkova, R.; Damborsky, J. Ancestral Haloalkane Dehalogenases Show Robustness and Unique Substrate Specificity. ChemBioChem 2017, 18, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Chaloupkova, R.; Liskova, V.; Toul, M.; Markova, K.; Sebestova, E.; Hernychova, L.; Marek, M.; Pinto, G.P.; Pluskal, D.; Waterman, J.; et al. Light-Emitting Dehalogenases: Reconstruction of Multifunctional Biocatalysts. ACS Catal. 2019, 9, 4810–4823. [Google Scholar] [CrossRef]
- Babkova, P.; Dunajova, Z.; Chaloupkova, R.; Damborsky, J.; Bednar, D.; Marek, M. Structures of hyperstable ancestral haloalkane dehalogenases show restricted conformational dynamics. Comput. Struct. Biotechnol. J. 2020, 18, 1497–1508. [Google Scholar] [CrossRef]
- McPherson, A.; Gavira, J.A. Introduction to protein crystallization. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014, 70, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Mueller, U.; Förster, R.; Hellmig, M.; Huschmann, F.U.; Kastner, A.; Malecki, P.; Pühringer, S.; Röwer, M.; Sparta, K.; Steffien, M.; et al. The macromolecular crystallography beamlines at BESSY II of the Helmholtz-Zentrum Berlin: Current status and perspectives. Eur. Phys. J. Plus 2015, 130, 141. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vagin, A.; Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Skubak, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef] [Green Version]
- The PyMOL Molecular Graphics System; Version 2.4; Schrödinger, LLC: New York, NY, USA, 2020.


| Data Collection | |
|---|---|
| Space Group | P4322 |
| a, b, c (Å) | 68.89, 68.89, 156.24 |
| α, β, γ (°) | 90.0, 90.0, 90.0 |
| Resolution range (Å) | 50–1.50 (1.59–1.50) |
| Total no. of reflections | 641,156 (102,219) |
| No. of unique reflections | 61,367 (9534) |
| Completeness (%) | 99.6 (97.8) |
| 〈I/σ(I)〉 | 13.68 (1.65) |
| Rmeas * | 102.9 (9.6) |
| CC1/2 | 0.99 (0.77) |
| Overall B factor from Wilson plot (Å2) | 27.062 |
| Refinement | |
| No. of reflections used for refinement | 58,297 |
| Rwork ‡/Rfree § (%) | 15.94/17.10 |
| No. of non-H atoms | 2810 |
| No. of protein atoms | 2475 |
| No. of chloride ions | 5 |
| No of ligands | 1 |
| No. of water molecules | 319 |
| Average B factor (Å2) | 22.357 |
| Ramachandran plot | |
| Most favored (%) | 95.89 |
| Allowed (%) | 3.79 |
| Outliers (%) | 0 |
| R.m.s. deviations | |
| Bonds (Å) | 0.019 |
| Angles (°) | 1.958 |
| PDB ID | 7PW1 |
| AncLinB-DmbA | LinB | DmbA | |
|---|---|---|---|
| PDB code | 7PW1 | 1CV2 | 2QVB |
| Resolution (Å) | 1.5 | 1.58 | 1.19 |
| Sequence identity to AncLinB-DmbA (%) | - | 81.7 | 83.2 |
| R.m.s.d. for AncLinB-DmbA | - | 0.778 | 0.995 |
| Active site cavity volume (Å3) | 460 | 406 | 375 |
| p1 main tunnel characteristics | |||
| Bottleneck radius (Å) | 2.0 | 1.4 | 2.1 |
| Number of residues | 17 | 22 | 18 |
| Length (Å) | 3.9 | 6.9 | 3.9 |
| Entrance radius (Å) | 2.5 | 1.4 | 2.2 |
| Distance to surface * (Å) | 3.9 | 6.7 | 3.5 |
| Curvature ‡ | 1 | 1 | 1.1 |
| p2a slot tunnel characteristics | |||
| Bottleneck radius (Å) | 1 | 1 | 0.9 |
| Number of residues | 32 | 34 | 30 |
| Length (Å) | 22.4 | 17 | 19.9 |
| Entrance radius (Å) | 1.0 | 1.7 | 1.6 |
| Distance to surface * (Å) | 8.1 | 11.5 | 11.3 |
| Curvature ‡ | 2.8 | 1.5 | 1.8 |
| p2b slot tunnel characteristics | |||
| Bottleneck radius (Å) | - | - | 0.9 |
| Number of residues | - | - | 36 |
| Length (Å) | - | - | 24.3 |
| Entrance radius (Å) | - | - | 1.6 |
| Distance to surface * (Å) | - | - | 19.4 |
| Curvature ‡ | - | - | 1.3 |
| Source Organism | Artificial Gene |
|---|---|
| DNA source | - |
| Restriction sites | NdeI/BamHI |
| Vector | pET21b |
| Expression host | E. coli |
| Complete amino acid sequence of the construct produced | MTALGAEPYGQKKFIEIAGKRMAYIDEGEGDPIVFQHGNPTSSYLWRNIMPHLEGLGRLIACDLIGMGDSDKLSPSGPDRYSYAEHRDYLFALWEALDLGDNVVLVIHDWGSALGFDWANQHRDRVQGIAYMEAIVTPLEWADWPEEVRDIFQGFRSPAGEEMVLENNIFVERVLPGAILRQLSDEEMAEYRRPFLNAGEDRRPTLSWPRQIPIDGEPADVVAIVSDYASWLAESDIPKLFINAEPGAIVTGRMRDFCRSWPNQTEITVKGAHFIQEDSPDEIGAAIAEFVRRLRAAAGV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, A.; Grinkevich, P.; Chaloupkova, R.; Havlickova, P.; Kascakova, B.; Kuty, M.; Damborsky, J.; Kuta Smatanova, I.; Prudnikova, T. Structural Analysis of the Ancestral Haloalkane Dehalogenase AncLinB-DmbA. Int. J. Mol. Sci. 2021, 22, 11992. https://doi.org/10.3390/ijms222111992
Mazur A, Grinkevich P, Chaloupkova R, Havlickova P, Kascakova B, Kuty M, Damborsky J, Kuta Smatanova I, Prudnikova T. Structural Analysis of the Ancestral Haloalkane Dehalogenase AncLinB-DmbA. International Journal of Molecular Sciences. 2021; 22(21):11992. https://doi.org/10.3390/ijms222111992
Chicago/Turabian StyleMazur, Andrii, Pavel Grinkevich, Radka Chaloupkova, Petra Havlickova, Barbora Kascakova, Michal Kuty, Jiri Damborsky, Ivana Kuta Smatanova, and Tatyana Prudnikova. 2021. "Structural Analysis of the Ancestral Haloalkane Dehalogenase AncLinB-DmbA" International Journal of Molecular Sciences 22, no. 21: 11992. https://doi.org/10.3390/ijms222111992
APA StyleMazur, A., Grinkevich, P., Chaloupkova, R., Havlickova, P., Kascakova, B., Kuty, M., Damborsky, J., Kuta Smatanova, I., & Prudnikova, T. (2021). Structural Analysis of the Ancestral Haloalkane Dehalogenase AncLinB-DmbA. International Journal of Molecular Sciences, 22(21), 11992. https://doi.org/10.3390/ijms222111992







