The Effect of Physical Exercise on Cognitive Impairment in Neurodegenerative Disease: From Pathophysiology to Clinical and Rehabilitative Aspects
Abstract
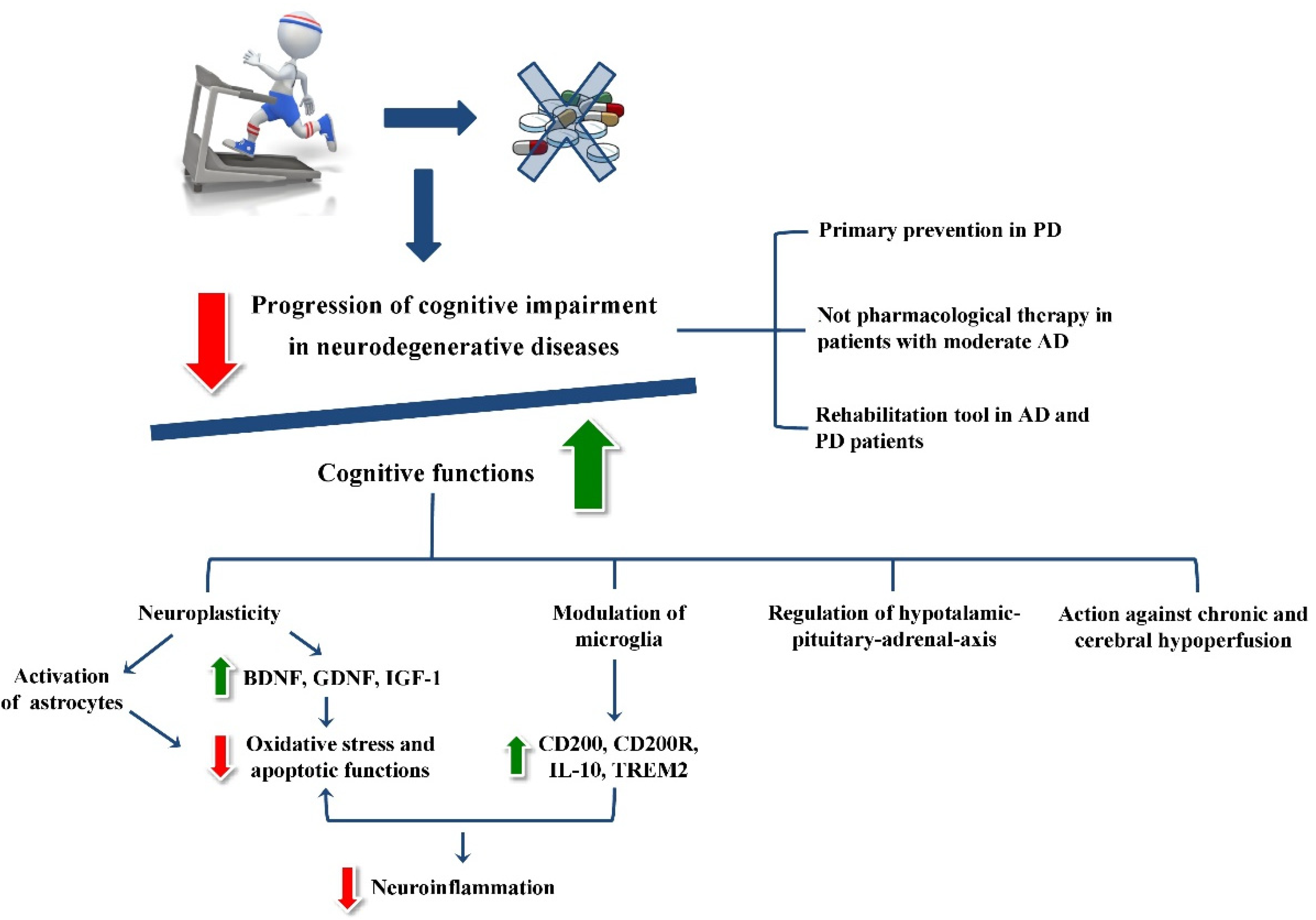
:1. Introduction
2. Physical Exercise-Related Neurobiological Processes in Neurodegenerative Diseases
2.1. Neurotrophin Modulation Induced by Physical Exercise
2.2. Impact of Physical Exercise on Astrocytic Functions
2.3. The Physical Exercise Modulation of Microglia
2.4. The Influence of Physical Exercise on Hormonal Activity
3. Physical Exercise-Related Clinical and Rehabilitative Effects in Neurodegenerative Diseases
3.1. The Role of Physical Exercise in Alzheimer’s Disease (AD)
3.2. The Role of Physical Exercise in Parkinson’s Disease (PD)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heemels, M.T. Neurodegenerative diseases. Nature 2016, 539, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42 (Suppl. 3), S125–S152. [Google Scholar] [CrossRef] [Green Version]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Jong-wook, L. Global health improvement and WHO: Shaping the future. Lancet 2003, 362, 2083–2088. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Dzhemileva, L.; Gloriozova, T.; D’yakonov, V. Natural and synthetic drugs used for the treatment of the dementia. Biochem. Biophys. Res. Commun. 2020, 524, 772–783. [Google Scholar] [CrossRef]
- Reddy, P.H. Lifestyle and Risk Factors of Dementia in Rural West Texas. J. Alzheimers Dis. 2019, 72, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Marques-Aleixo, I.; Beleza, J.; Sampaio, A.; Stevanović, J.; Coxito, P.; Gonçalves, I.; Ascensão, A.; Magalhães, J. Preventive and Therapeutic Potential of Physical Exercise in Neurodegenerative Diseases. Antioxid. Redox Signal. 2021, 34, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Raichlen, D.A.; Gordon, A.D. Relationship between exercise capacity and brain size in mammals. PLoS ONE 2011, 6, e20601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassilhas, R.C.; Tufik, S.; de Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell. Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Kirk-Sanchez, N.J.; McGough, E.L. Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 2014, 9, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef]
- Bherer, L. Cognitive plasticity in older adults: Effects of cognitive training and physical exercise. Ann. N. Y. Acad. Sci. 2015, 1337, 1–6. [Google Scholar] [CrossRef]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural. Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.; Zhao, X.; van Praag, H.; Wodtke, K.; Gage, F.H.; Christie, B.R. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 2004, 124, 71–79. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, T.; Chu, J.M.; Chen, Y.; Dunnett, S.; Ho, Y.S.; Wong, G.T.; Chang, R.C. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Investig. 2019, 99, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Leu, D.; Zou, Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch. Biochem. Biophys. 2015, 576, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef] [Green Version]
- Leal, G.; Bramham, C.R.; Duarte, C.B. BDNF and Hippocampal Synaptic Plasticity. Vitam. Horm. 2017, 104, 153–195. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.-M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, M.A.; van Wegen, E.E.H.; Newman, M.A.; Heyn, P.C. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson’s disease: A systematic review and meta-analysis. Transl. Neurodegener. 2018, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Heyn, P.; Abreu, B.C.; Ottenbacher, K.J. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch. Phys. Med. Rehabil. 2004, 85, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Black, J.E.; Isaacs, K.R.; Anderson, B.J.; Alcantara, A.A.; Greenough, W.T. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. USA 1990, 87, 5568–5572. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P. Evolutionary aspects of human exercise--born to run purposefully. Ageing Res. Rev. 2012, 11, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.W.; Shih, Y.H.; Chen, S.J.; Lien, C.H.; Chang, C.Y.; Huang, T.Y.; Chen, S.H.; Jen, C.J.; Kuo, Y.M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol. Learn. Mem. 2015, 118, 189–197. [Google Scholar] [CrossRef]
- Fahimi, A.; Baktir, M.A.; Moghadam, S.; Mojabi, F.S.; Sumanth, K.; McNerney, M.W.; Ponnusamy, R.; Salehi, A. Physical exercise induces structural alterations in the hippocampal astrocytes: Exploring the role of BDNF-TrkB signaling. Brain Struct. Funct. 2017, 222, 1797–1808. [Google Scholar] [CrossRef]
- Zsuga, J.; Tajti, G.; Papp, C.; Juhasz, B.; Gesztelyi, R. FNDC5/irisin, a molecular target for boosting reward-related learning and motivation. Med. Hypotheses 2016, 90, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassilhas, R.C.; Viana, V.A.; Grassmann, V.; Santos, R.T.; Santos, R.F.; Tufik, S.; Mello, M.T. The impact of resistance exercise on the cognitive function of the elderly. Med. Sci. Sports Exerc. 2007, 39, 1401–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Linville, M.C.; Tucker, E.W.; Sonntag, W.E.; Brunso-Bechtold, J.K. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb. Cortex 2005, 15, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Trejo, J.L.; Piriz, J.; Llorens-Martin, M.V.; Fernandez, A.M.; Bolós, M.; LeRoith, D.; Nuñez, A.; Torres-Aleman, I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol. Psychiatry 2007, 12, 1118–1128. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Xu, B. BDNF (I)rising from exercise. Cell Metab. 2013, 18, 612–614. [Google Scholar] [CrossRef] [Green Version]
- Lourenco, M.V.; Frozza, R.L.; De Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- De Freitas, G.B.; Lourenco, M.V.; De Felice, F.G. Protective actions of exercise-related FNDC5/Irisin in memory and Alzheimer’s disease. J. Neurochem. 2020, 155, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006, 29, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Charles, A. Extracellular calcium sensing by glial cells: Low extracellular calcium induces intracellular calcium release and intercellular signaling. J. Neurochem. 1997, 69, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Vasile, F.; Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018, 136, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Salatino, J.W.; Ludwig, K.A.; Kozai, T.D.Y.; Purcell, E.K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 2017, 1, 862–877. [Google Scholar] [CrossRef]
- Schmidt-Kastner, R.; Aguirre-Chen, C.; Saul, I.; Yick, L.; Hamasaki, D.; Busto, R.; Ginsberg, M.D. Astrocytes react to oligemia in the forebrain induced by chronic bilateral common carotid artery occlusion in rats. Brain Res. 2005, 1052, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Leardini-Tristão, M.; Borges, J.P.; Freitas, F.; Rangel, R.; Daliry, A.; Tibiriçá, E.; Estato, V. The impact of early aerobic exercise on brain microvascular alterations induced by cerebral hypoperfusion. Brain Res. 2017, 1657, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Chiba, K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals 2014, 7, 1028–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laakso, M.P.; Soininen, H.; Partanen, K.; Helkala, E.L.; Hartikainen, P.; Vainio, P.; Hallikainen, M.; Hänninen, T.; Riekkinen, P.J., Sr. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: Correlation with memory functions. J. Neural Transm. Park. Dis. Dement. Sect. 1995, 9, 73–86. [Google Scholar] [CrossRef]
- Shrikant, P.; Weber, E.; Jilling, T.; Benveniste, E.N. Intercellular adhesion molecule-1 gene expression by glial cells. Differential mechanisms of inhibition by IL-10 and IL-6. J. Immunol. 1995, 155, 1489–1501. [Google Scholar]
- Filipello, F.; Morini, R.; Corradini, I.; Zerbi, V.; Canzi, A.; Michalski, B.; Erreni, M.; Markicevic, M.; Starvaggi-Cucuzza, C.; Otero, K.; et al. The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 2018, 48, 979–991.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapolsky, R.M.; Plotsky, P.M. Hypercortisolism and its possible neural bases. Biol. Psychiatry 1990, 27, 937–952. [Google Scholar] [CrossRef]
- McEwen, B.S.; De Kloet, E.R.; Rostene, W. Adrenal steroid receptors and actions in the nervous system. Physiol. Rev. 1986, 66, 1121–1188. [Google Scholar] [CrossRef]
- Heuser, I.J.; Gotthardt, U.; Schweiger, U.; Schmider, J.; Lammers, C.H.; Dettling, M.; Holsboer, F. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: Importance of gender. Neurobiol. Aging 1994, 15, 227–231. [Google Scholar] [CrossRef]
- Holsboer, F.; Von Bardeleben, U.; Wiedemann, K.; Müller, O.A.; Stalla, G.K. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression. Implications for pathophysiology of DST nonsuppression. Biol. Psychiatry 1987, 22, 228–234. [Google Scholar] [CrossRef]
- Robinson, M.M.; Lowe, V.J.; Nair, K.S. Increased Brain Glucose Uptake after 12 Weeks of Aerobic High-Intensity Interval Training in Young and Older Adults. J. Clin. Endocrinol. Metab. 2018, 103, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfranco, F.; Strasburger, C.J. (Eds.) Sports Endocrinology; Karger Medical and Scientific Publishers: Basel, Switzerland, 2016; Volume 47, pp. 12–26. [Google Scholar] [CrossRef]
- Duclos, M.; Guinot, M.; Le Bouc, Y. Cortisol and GH: Odd and controversial ideas. Appl. Physiol. Nutr. Metab. 2007, 32, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.; Minkhar, M.; Sarrieau, A.; Bonnemaison, D.; Manier, G.; Mormede, P. Reversibility of endurance training-induced changes on glucocorticoid sensitivity of monocytes by an acute exercise. Clin. Endocrinol. 1999, 51, 749–756. [Google Scholar] [CrossRef]
- Brandenberger, G.; Follenius, M.; Hietter, B. Feedback from meal-related peaks determines diurnal changes in cortisol response to exercise. J. Clin. Endocrinol. Metab. 1982, 54, 592–596. [Google Scholar] [CrossRef]
- Goudsmit, E.; Hofman, M.A.; Fliers, E.; Swaab, D.F. The supraoptic and paraventricular nuclei of the human hypothalamus in relation to sex, age and Alzheimer’s disease. Neurobiol. Aging 1990, 11, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Crewther, B.T.; Cook, C.; Cardinale, M.; Weatherby, R.P.; Lowe, T. Two emerging concepts for elite athletes: The short-term effects of testosterone and cortisol on the neuromuscular system and the dose-response training role of these endogenous hormones. Sports Med. 2011, 41, 103–123. [Google Scholar] [CrossRef]
- Klaperski, S.; Von Dawans, B.; Heinrichs, M.; Fuchs, R. Does the level of physical exercise affect physiological and psychological responses to psychosocial stress in women? Psychol. Sport Exerc. 2013, 14, 266–274. [Google Scholar] [CrossRef]
- Ebrecht, M.; Buske-Kirschbaum, A.; Hellhammer, D.; Kern, S.; Rohleder, N.; Walker, B.; Kirschbaum, C. Tissue specificity of glucocorticoid sensitivity in healthy adults. J. Clin. Endocrinol. Metab. 2000, 85, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Wenk, G.L. Neuropathologic changes in Alzheimer’s disease. J. Clin. Psychiatry 2003, 64, 7–10. [Google Scholar]
- Sutoo, D.; Akiyama, K. Regulation of brain function by exercise. Neurobiol. Dis. 2003, 13, 1–14. [Google Scholar] [CrossRef]
- Radak, Z.; Hart, N.; Sarga, L.; Koltai, E.; Atalay, M.; Ohno, H.; Boldogh, I. Exercise plays a preventive role against Alzheimer’s disease. J. Alzheimers Dis. 2010, 20, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Geda, Y.E.; Roberts, R.O.; Knopman, D.S.; Christianson, T.J.; Pankratz, V.S.; Ivnik, R.J.; Boeve, B.F.; Tangalos, E.G.; Petersen, R.C.; Rocca, W.A. Physical exercise, aging, and mild cognitive impairment: A population-based study. Arch. Neurol. 2010, 67, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, T. Physical exercise alleviates debilities of normal aging and Alzheimer’s disease. Acta Neurol. Scand. 2011, 123, 221–238. [Google Scholar] [CrossRef]
- Winchester, J.; Dick, M.B.; Gillen, D.; Reed, B.; Miller, B.; Tinklenberg, J.; Mungas, D.; Chui, H.; Galasko, D.; Hewett, L.; et al. Walking stabilizes cognitive functioning in Alzheimer’s disease (AD) across one year. Arch. Gerontol. Geriatr. 2013, 56, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794, Erratum in 2014, 13, 1070. [Google Scholar] [CrossRef] [Green Version]
- Larson, E.B.; Wang, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Crane, P.; Kukull, W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 2006, 144, 73–81. [Google Scholar] [CrossRef]
- Rolland, Y.; Andrieu, S.; Cantet, C.; Morley, J.E.; Thomas, D.; Nourhashemi, F.; Vellas, B. Wandering behavior and Alzheimer disease. The REAL.FR prospective study. Alzheimer Dis. Assoc. Disord. 2007, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, M.; Scarsini, R.; Schena, F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am. J. Alzheimers Dis. Other Demen. 2011, 26, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.X.; Liang, J.H.; Xu, Y.; Wang, Y.Q. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, Y.; Li, J.; Zhou, C.; Li, F.; Yang, X. Physical activity can improve cognition in patients with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Interv. Aging 2018, 13, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.I.; Raji, C.A.; Lopez, O.L.; Becker, J.T.; Rosano, C.; Newman, A.B.; Gach, H.M.; Thompson, P.M.; Ho, A.J.; Kuller, L.H. Physical activity predicts gray matter volume in late adulthood: The Cardiovascular Health Study. Neurology 2010, 75, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Svenningsson, P.; Westman, E.; Ballard, C.; Aarsland, D. Cognitive impairment in patients with Parkinson’s disease: Diagnosis, biomarkers, and treatment. Lancet Neurol. 2012, 11, 697–707. [Google Scholar] [CrossRef]
- Xu, Q.; Park, Y.; Huang, X.; Hollenbeck, A.; Blair, A.; Schatzkin, A.; Chen, H. Physical activities and future risk of Parkinson disease. Neurology 2010, 75, 341–348. [Google Scholar] [CrossRef]
- Thacker, E.L.; Chen, H.; Patel, A.V.; McCullough, M.L.; Calle, E.E.; Thun, M.J.; Schwarzschild, M.A.; Ascherio, A. Recreational physical activity and risk of Parkinson’s disease. Mov. Disord. 2008, 23, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mentis, A.A.; Dardiotis, E.; Efthymiou, V.; Chrousos, G.P. Non-genetic risk and protective factors and biomarkers for neurological disorders: A meta-umbrella systematic review of umbrella reviews. BMC Med. 2021, 19, 6. [Google Scholar] [CrossRef]
- Feng, Y.S.; Yang, S.D.; Tan, Z.X.; Wang, M.M.; Xing, Y.; Dong, F.; Zhang, F. The benefits and mechanisms of exercise training for Parkinson’s disease. Life Sci. 2020, 245, 117345. [Google Scholar] [CrossRef]
- Hashimoto, H.; Takabatake, S.; Miyaguchi, H.; Nakanishi, H.; Naitou, Y. Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: A quasi-randomized pilot trial. Complement. Ther. Med. 2015, 23, 210–219. [Google Scholar] [CrossRef]
- De Natale, E.R.; Paulus, K.S.; Aiello, E.; Sanna, B.; Manca, A.; Sotgiu, G.; Leali, P.T.; Deriu, F. Dance therapy improves motor and cognitive functions in patients with Parkinson’s disease. NeuroRehabilitation 2017, 40, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Nocera, J.R.; Amano, S.; Vallabhajosula, S.; Hass, C.J. Tai Chi Exercise to Improve Non-Motor Symptoms of Parkinson’s Disease. J. Yoga Phys. Ther. 2013, 3, 10.4172/2157–7595.1000137. [Google Scholar] [CrossRef] [Green Version]
- Kwok, J.Y.Y.; Kwan, J.C.Y.; Auyeung, M.; Mok, V.C.T.; Chan, H.Y.L. The effects of yoga versus stretching and resistance training exercises on psychological distress for people with mild-to-moderate Parkinson’s disease: Study prxotocol for a randomized controlled trial. Trials 2017, 18, 509. [Google Scholar] [CrossRef] [Green Version]
- Petzinger, G.M.; Fisher, B.E.; McEwen, S.; Beeler, J.A.; Walsh, J.P.; Jakowec, M.W. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol. 2013, 12, 716–726. [Google Scholar] [CrossRef] [Green Version]
- Crizzle, A.M.; Newhouse, I.J. Is physical exercise beneficial for persons with Parkinson’s disease? Clin. J. Sport Med. 2006, 16, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Gison, A.; Rizza, F.; Bonassi, S.; Donati, V.; Giaquinto, S. Effects of dispositional optimism on quality of life, emotional distress and disability in Parkinson’s disease outpatients under rehabilitation. Funct. Neurol. 2015, 30, 105–111. [Google Scholar] [CrossRef] [Green Version]
- San Martín Valenzuela, C.; Moscardó, L.D.; López-Pascual, J.; Serra-Añó, P.; Tomás, J.M. Effects of Dual-Task Group Training on Gait, Cognitive Executive Function, and Quality of Life in People with Parkinson Disease: Results of Randomized Controlled DUALGAIT Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1849–1856.e1. [Google Scholar] [CrossRef]
- Keshner, E.A.; Fung, J. The quest to apply VR technology to rehabilitation: Tribulations and treasures. J. Vestib. Res. 2017, 27, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Van Diest, M.; Lamoth, C.J.; Stegenga, J.; Verkerke, G.J.; Postema, K. Exergaming for balance training of elderly: State of the art and future developments. J. Neuroeng. Rehabil. 2013, 10, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirelman, A.; Maidan, I.; Deutsch, J.E. Virtual reality and motor imagery: Promising tools for assessment and therapy in Parkinson’s disease. Mov. Disord. 2013, 28, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fu, Z.; Le, W. Exercise and Parkinson’s disease. Int. Rev. Neurobiol. 2019, 147, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Foreyt, J.P. The role of lifestyle modification in dysmetabolic syndrome management. Nestle Nutr. Workshop Ser. Clin. Perform. Programme 2006, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farì, G.; Lunetti, P.; Pignatelli, G.; Raele, M.V.; Cera, A.; Mintrone, G.; Ranieri, M.; Megna, M.; Capobianco, L. The Effect of Physical Exercise on Cognitive Impairment in Neurodegenerative Disease: From Pathophysiology to Clinical and Rehabilitative Aspects. Int. J. Mol. Sci. 2021, 22, 11632. https://doi.org/10.3390/ijms222111632
Farì G, Lunetti P, Pignatelli G, Raele MV, Cera A, Mintrone G, Ranieri M, Megna M, Capobianco L. The Effect of Physical Exercise on Cognitive Impairment in Neurodegenerative Disease: From Pathophysiology to Clinical and Rehabilitative Aspects. International Journal of Molecular Sciences. 2021; 22(21):11632. https://doi.org/10.3390/ijms222111632
Chicago/Turabian StyleFarì, Giacomo, Paola Lunetti, Giovanni Pignatelli, Maria Vittoria Raele, Alessandra Cera, Giulia Mintrone, Maurizio Ranieri, Marisa Megna, and Loredana Capobianco. 2021. "The Effect of Physical Exercise on Cognitive Impairment in Neurodegenerative Disease: From Pathophysiology to Clinical and Rehabilitative Aspects" International Journal of Molecular Sciences 22, no. 21: 11632. https://doi.org/10.3390/ijms222111632
APA StyleFarì, G., Lunetti, P., Pignatelli, G., Raele, M. V., Cera, A., Mintrone, G., Ranieri, M., Megna, M., & Capobianco, L. (2021). The Effect of Physical Exercise on Cognitive Impairment in Neurodegenerative Disease: From Pathophysiology to Clinical and Rehabilitative Aspects. International Journal of Molecular Sciences, 22(21), 11632. https://doi.org/10.3390/ijms222111632








