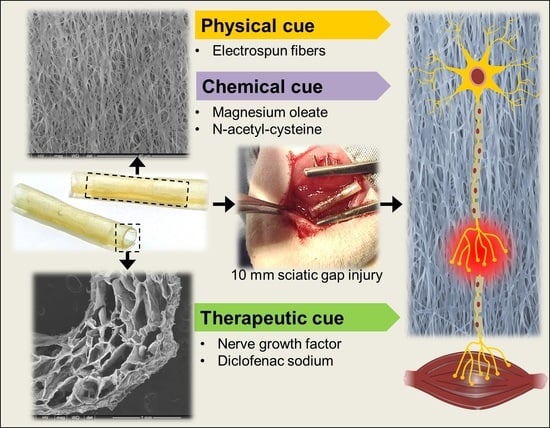
Gellan-Xanthan Hydrogel Conduits with Intraluminal Electrospun Nanofibers as Physical, Chemical and Therapeutic Cues for Peripheral Nerve Repair
Abstract
:1. Introduction
2. Results
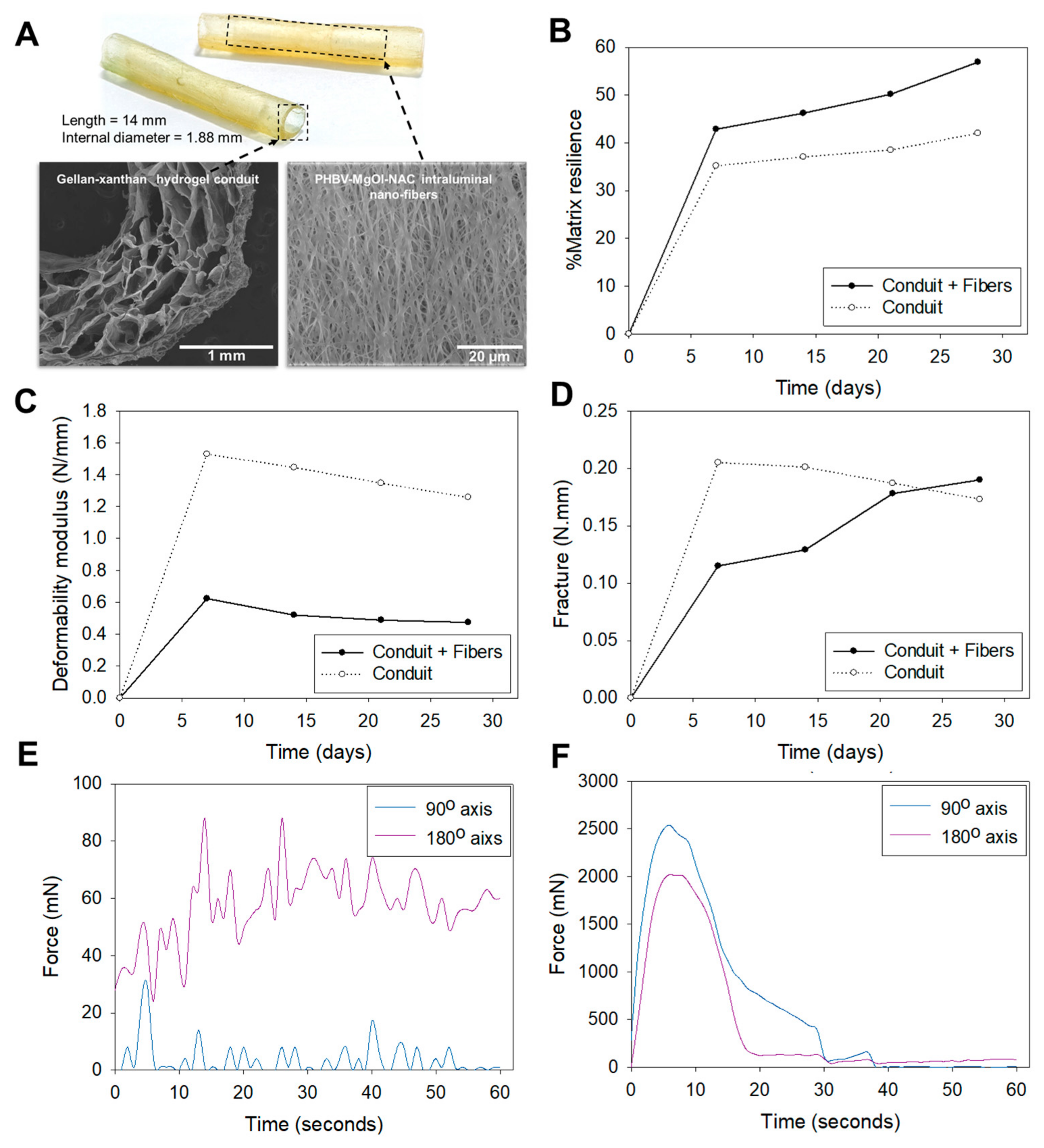
2.1. Mechanical Properties
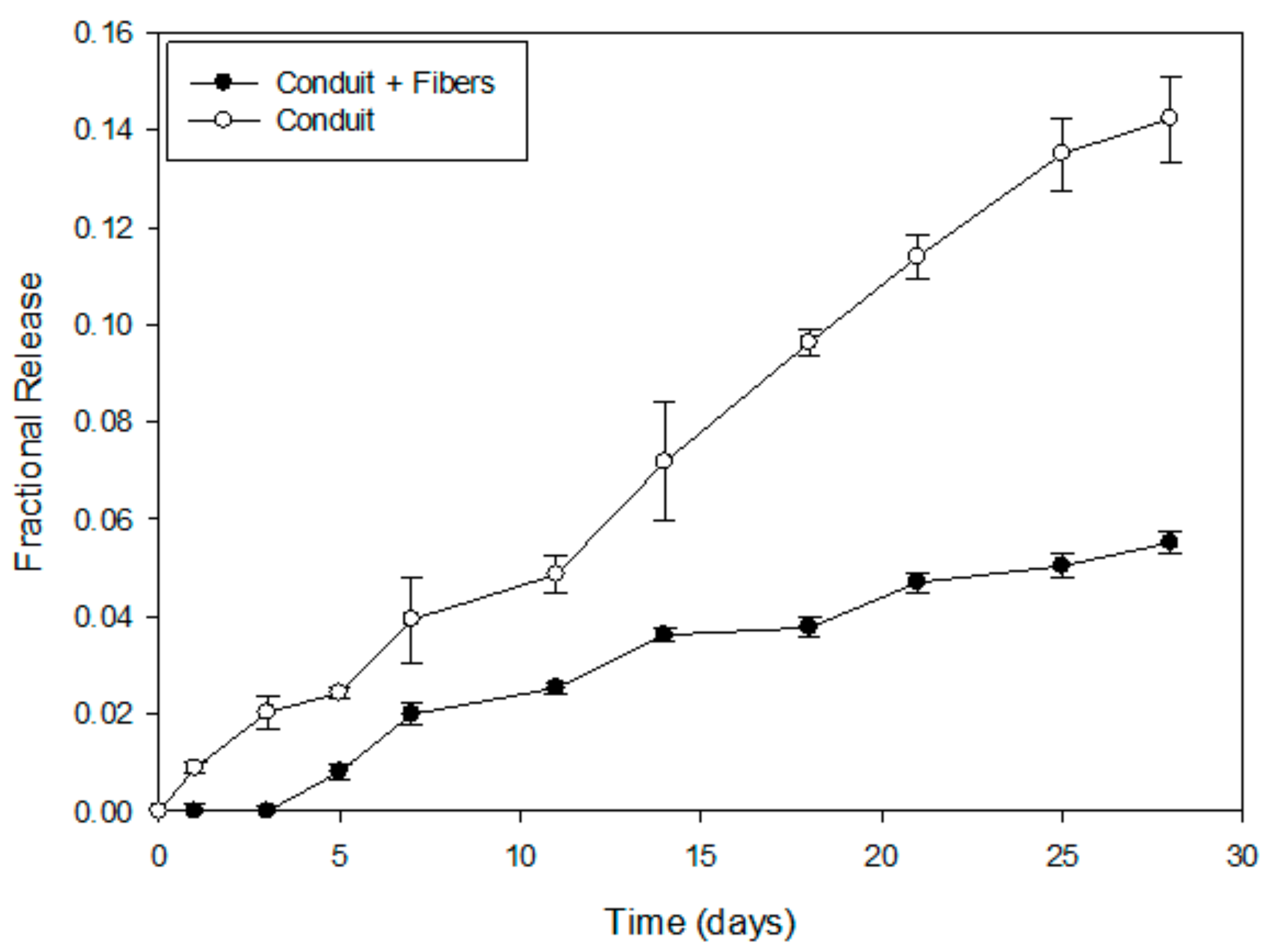
2.2. In Vitro NGF Release Profiles and Mathematical Modeling
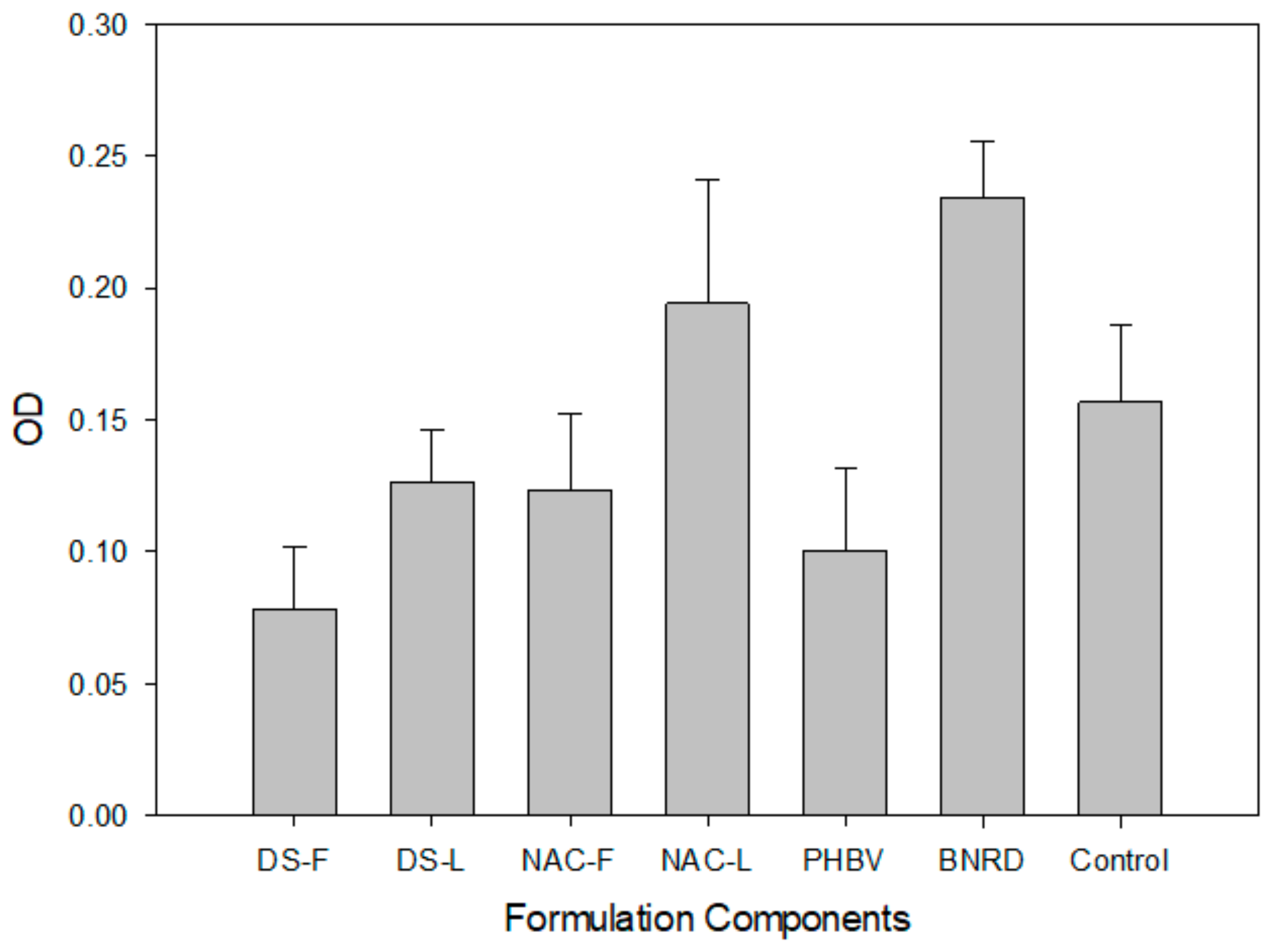
2.3. In Vitro PC12 Proliferation
2.4. SEM Imaging
2.5. Post-Implantation Evaluation
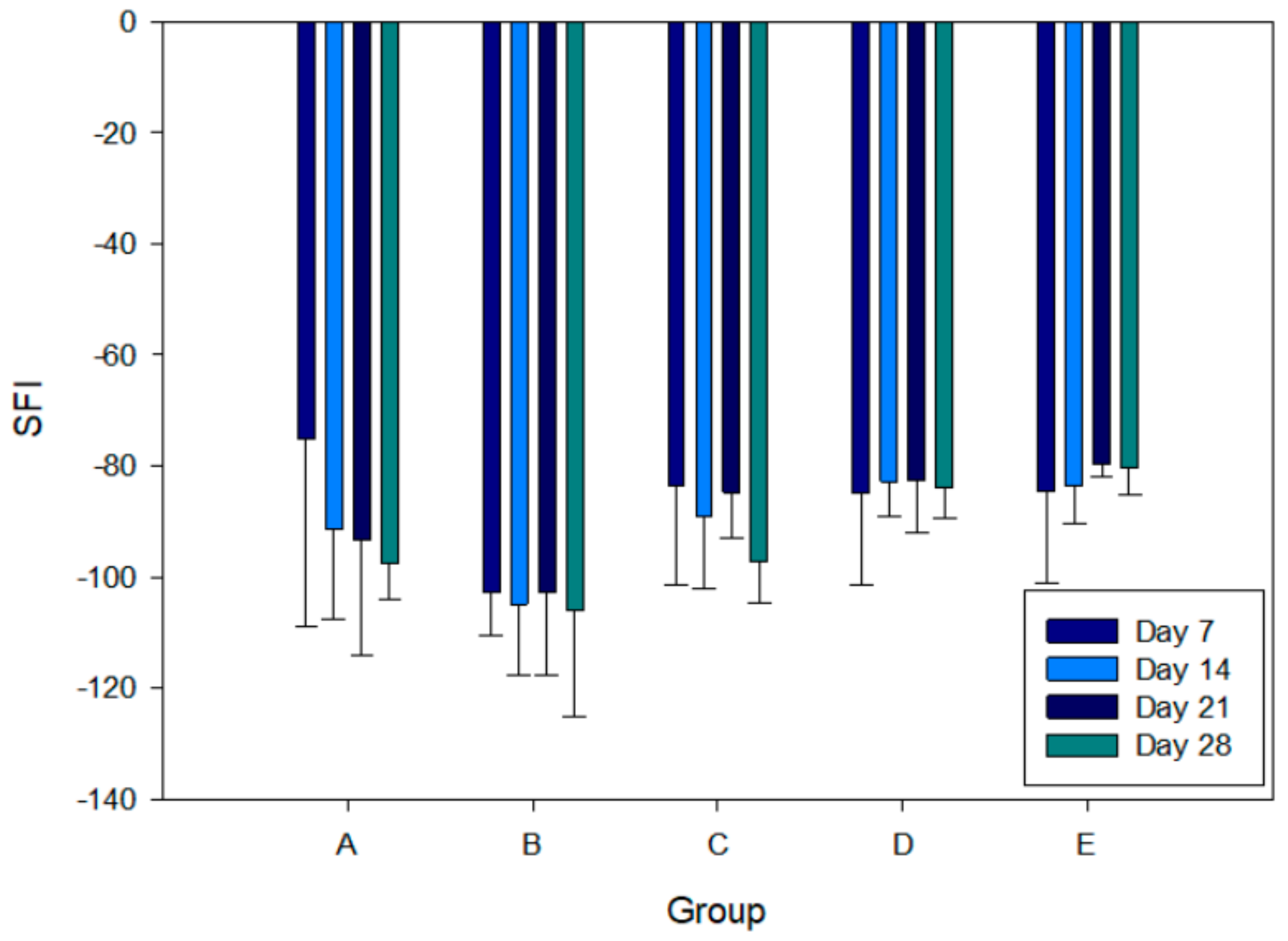
2.6. Sciatic Function Index
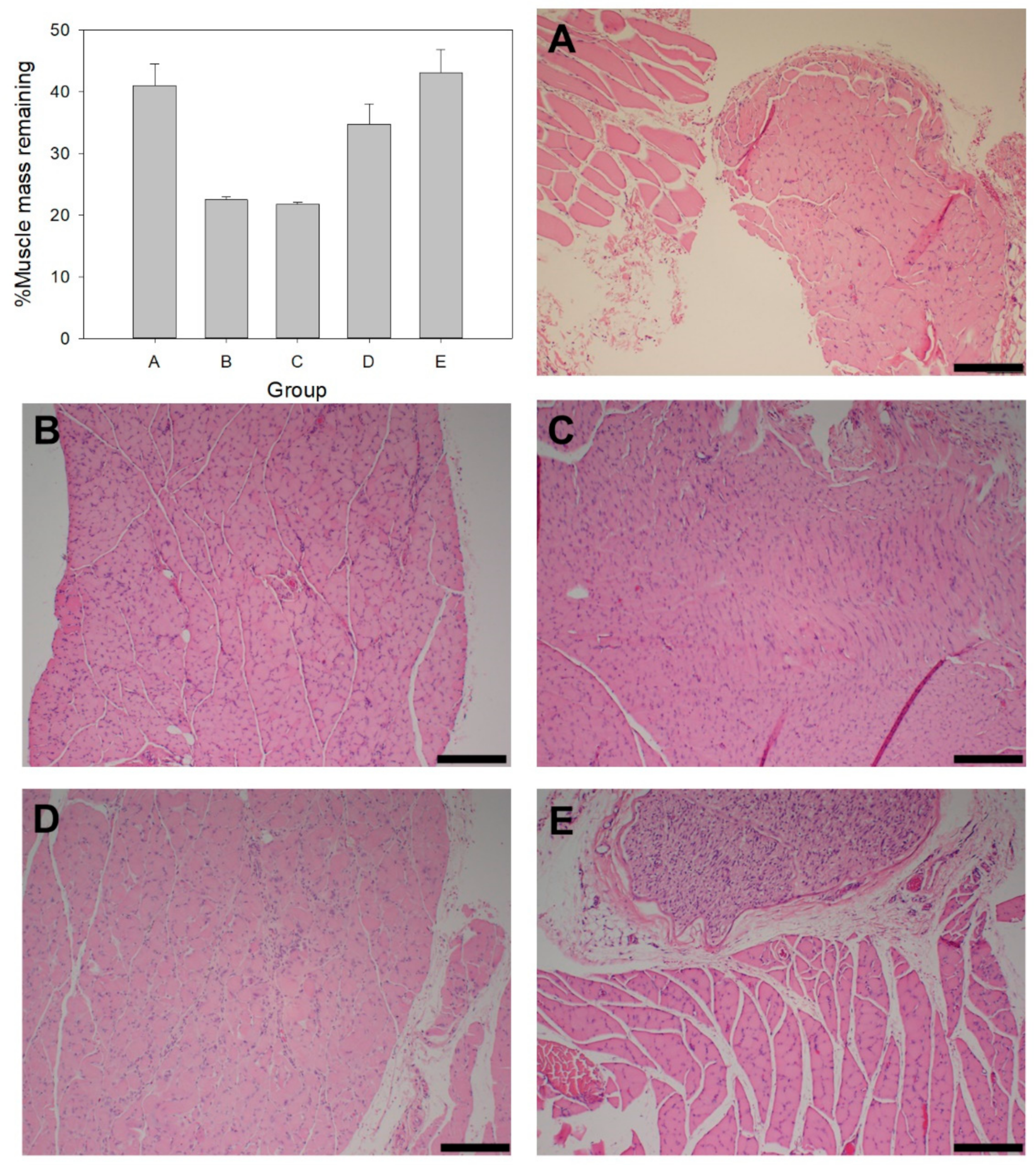
2.7. Muscle Mass and Histology
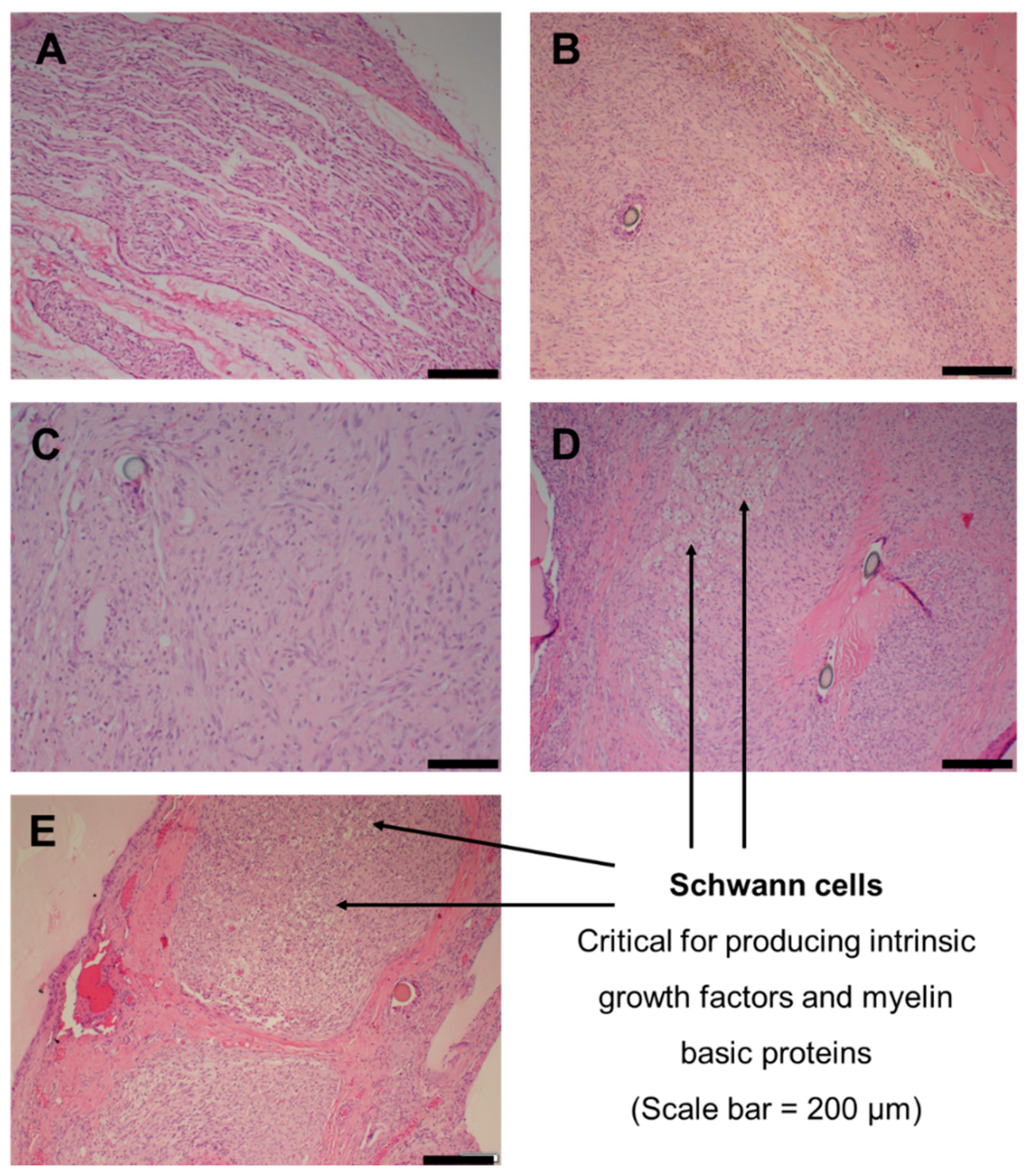
2.8. Nerve Histology
3. Discussion
3.1. Mechanical Properties
3.2. Drug Release Kinetics of NGF
3.3. In Vitro Cytocompatibility
3.4. Functional Recovery Evaluation
3.5. Gastrocnemius Muscle Mass and Histology
3.6. Sciatic Nerve Regeneration and Histology
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the Biosimulated Nerve Repair Conduit
4.3. Assessment of Mechanical Properties
4.4. In Vitro NGF Release Study
4.5. In Vitro Cell Proliferation Assessment
4.6. Visualization of Fiber and Cell Morphology
4.7. Animal Study
4.8. Surgical Procedures
4.9. Walking Track Analysis
4.10. High-Frequency Ultrasound Imaging
4.11. Gastrocnemius Muscle Mass Measurement
4.12. Muscle and Nerve Histological Assessment
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madduri, S.; di Summa, P.; Papaloïzos, M.; Kalbermatten, D.; Gander, B. Effect of controlled co-delivery of synergistic neurotrophic factors on early nerve regeneration in rats. Biomaterials 2010, 31, 8402–8409. [Google Scholar] [CrossRef]
- Belkas, J.S.; Shoichet, M.S.; Midha, R. Peripheral nerve regeneration through guidance tubes. Neurol. Res. 2013, 26, 151–160. [Google Scholar] [CrossRef]
- Mukhatyar, V.; Pai, B.; Clements, I.; Srinivasan, A.; Huber, R.; Mehta, A.; Mukhopadaya, S.; Rudra, S.; Patel, G.; Karumbaiah, L.; et al. Molecular sequelae of topographically guided peripheral nerve repair. Ann. Biomed. Eng. 2014, 42, 1436–1455. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Eisenberg, H.M.; Jia, X. Advances and future applications of augmented peripheral nerve regeneration. Int. J. Mol. Sci. 2016, 17, 1494. [Google Scholar] [CrossRef]
- Chang, W.; Shah, M.B.; Lee, P.; Yu, X. Tissue-engineered spiral nerve guidance conduit for peripheral nerve regeneration. Acta Biomater. 2018, 73, 302–311. [Google Scholar] [CrossRef]
- Talebi, A.; Labbaf, S.; Atari, M.; Parhizkar, M. Polymeric Nanocomposite Structures Based on Functionalized Graphene with Tunable Properties for Nervous Tissue Replacement. ACS Biomater. Sci. Eng. 2021, 7, 4591–4601. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern trends for peripheral nerve repair and regeneration: Beyond the hollow nerve guidance conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halim, A.; Qu, K.Y.; Zhang, X.F.; Huang, N.P. Recent Advances in the Application of Two-Dimensional Nanomaterials for Neural Tissue Engineering and Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 3503–3529. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Gordon, T.W. Peripheral nerve regeneration and muscle reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.G.; Kung, T.A. Regenerative Peripheral Nerve Interfaces for the Treatment and Prevention of Neuromas and Neuroma Pain. Hand Clin. 2021, 37, 361–371. [Google Scholar] [CrossRef]
- Suhar, R.A.; Marquardt, L.M.; Song, S.; Buabbas, H.; Doulames, V.M.; Johansson, P.K.; Klett, K.C.; Dewi, R.E.; Enejder, A.M.; Plant, G.W.; et al. Elastin-like Proteins to Support Peripheral Nerve Regeneration in Guidance Conduits. ACS Biomater. Sci. Eng. 2021, 7, 4209–4220. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Zhang, Z.; Luo, J.; Cai, Z.; Wang, Y.; Li, Y. Repairing transected peripheral nerve using a biomimetic nerve guidance conduit containing intraluminal sponge fillers. Adv. Healthc. Mater. 2019, 8, 1900913. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Wang, H.; Wang, Y.; Zhang, K.; Fan, C.; Wang, H.; Mo, X. Biomimetic and hierarchical nerve conduits from multifunctional nanofibers for guided peripheral nerve regeneration. Acta Biomater. 2020, 117, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [Green Version]
- Madduri, S.; Gander, B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J. Control. Release 2012, 161, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P.; Foy, C. Restoration of neurological function following peripheral nerve trauma. Int. J. Mol. Sci. 2020, 21, 1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Li, D.H.; Zhang, H.Y.; Wang, J.; Li, X.K.; Xiao, J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol. Sin. 2020, 41, 1289–1300. [Google Scholar] [CrossRef]
- Wang, L.; Song, D.; Zhang, X.; Ding, Z.; Kong, X.; Lu, Q.; Kaplan, D.L. Silk–graphene hybrid hydrogels with multiple cues to induce nerve cell behavior. ACS Biomater. Sci. Eng. 2019, 5, 613–622. [Google Scholar] [CrossRef]
- Ramburrun, P.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Design and characterization of neurodurable gellan-xanthan pH-responsive hydrogels for controlled drug delivery. Expert Opin. Drug Deliv. 2017, 14, 291–306. [Google Scholar] [CrossRef]
- Ramburrun, P.; Kumar, P.; Choonara, Y.E.; Du Toit, L.C.; Pillay, V. Design and characterisation of PHBV-magnesium oleate directional nanofibers for neurosupport. Biomed. Mater. 2019, 14, 065015. [Google Scholar] [CrossRef]
- Schlaepfer, W.W. Calcium-induced degeneration of axoplasm in isolated segments of rat peripheral nerve. Brain Res. 1974, 69, 203–215. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Lehning, E.J. Mechanism of calcium entry during axon injury and degeneration. Toxicol. Appl. Pharmacol. 1997, 143, 233–244. [Google Scholar] [CrossRef]
- Coleman, M. Axon degeneration mechanisms: Commonality amid diversity. Nat. Rev. Neurosci. 2005, 6, 889–898. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zheng, Z.; Yan, J.; Zhang, L.; Li, Y.; Zhang, J.; Li, G.; Wang, X.; Kaplan, D. Porous nerve guidance conduits reinforced with braided composite structures of silk/magnesium filaments for peripheral nerve repair. Acta Biomater. 2021, in press. [Google Scholar] [CrossRef]
- Madduri, S.; Gander, B. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J. Peripher. Nerv. Syst. 2010, 15, 93–103. [Google Scholar] [CrossRef]
- Conti, A.M.; Brimijoin, S.; Miller, L.J.; Windebank, A.J. Suppression of neurite outgrowth by high dose nerve growth factor is independent of functional p75NTR receptors. Neurobiol. Dis. 2004, 15, 106–114. [Google Scholar] [CrossRef]
- Kemp, S.W.P.; Webb, A.A.; Dhaliwal, S.; Syed, S.; Walsh, S.K.; Midha, R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp. Neurol. 2011, 229, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modelling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Mady, O. Mechanisms and percent of drug release of each new mathematic approach. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 56–69. [Google Scholar]
- Maity, S.; Sa, B. Ca-carboxymethyl xanthan gum mini-matrices: Swelling, erosion and their impact on drug release mechanism. Int. J. Biol. Macromol. 2014, 68, 78–85. [Google Scholar] [CrossRef]
- Hart, A.M.; Terenghi, G.; Wiberg, M. Neuronal death after peripheral nerve injury and experimental strategies for neuroprotection. Neurol. Res. 2008, 30, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Tabernero, A.; Medina, J.M. Role of oleic acid as a neurotrophic factor is supported in vivo by the expression of GAP-43 subsequent to the activation of SREBP-1 and the up-regulation of stearoyl-CoA desaturase during postnatal development of the brain. Brain Res. 2003, 977, 103–111. [Google Scholar] [CrossRef]
- Kojour, M.A.M.; Ebrahimi-Barough, S.; Kouchesfehani, H.M.; Jalali, H.; Ebrahim, M.H.K. Oleic acid promotes the expression of neural markers in differentiated human endometrial stem cells. J. Chem. Neuroanat. 2017, 79, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, Y.S.; Lee, D.H.; Lee, S.H.; Park, H.J.; Lee, D.; Kim, H. Neuroprotective effects of oleic acid in rodent models of cerebral ischaemia. Sci. Rep. 2019, 9, 10732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, M.; Noble, M. N-acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc. Natl. Acad. Sci. USA 1994, 91, 7496–7500. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, G.; Yan, C.Y.; Greene, L.A. N-acetylcysteine (D-and L-stereoisomers) prevents apoptotic death of neuronal cells. J. Neurosci. 1995, 15, 2857–2866. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.R.; Yang, Y. Neuron differentiation and neuritogenesis stimulated by N-acetylcysteine (NAC). Acta Pharmacol. Sin. 2009, 30, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Hirsaee, M.A.; Amini, K. Improvement of functional recovery of transected peripheral nerve by means of artery grafts filled with diclofenac. Int. J. Surg. 2013, 11, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Yurt, K.K.; Kaplan, S. As a painkiller: A review of pre-and postnatal non-steroidal anti-inflammatory drug exposure effects on the nervous systems. Inflammopharmacology 2018, 26, 15–28. [Google Scholar] [CrossRef]
- Tedesco, F.S.; Dellavalle, A.; Diaz-Manera, J.; Messina, G.; Cossu, G. Repairing skeletal muscle: Regenerative potential of skeletal muscle stem cells. J. Clin. Investig. 2010, 120, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malecova, B.; Puri, P.L. “Mix of Mics”-Phenotypic and Biological Heterogeneity of “Multipotent” Muscle Interstitial Cells (MICs). J. Stem Cell Res. Ther. 2012, (Suppl. 11), 004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceafalan, L.C.; Popescu, B.O.; Hinescu, M.E. Cellular players in skeletal muscle regeneration. BioMed Res. Int. 2014, 2014, 957014. [Google Scholar] [CrossRef]
- Chung, T.W.; Yang, M.C.; Tseng, C.C.; Sheu, S.H.; Wang, S.S.; Huang, Y.Y.; Chen, S.D. Promoting regeneration of peripheral nerves in-vivo using new PCL-NGF/Tirofiban nerve conduits. Biomaterials 2011, 32, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, K.; Chakrabarti, K.; De, S.K. Effect of oleic acid ligand on photophysical, photoconductive and magnetic properties of monodisperse SnO2 quantum dots. Dalton Trans. 2013, 42, 3434–3446. [Google Scholar] [CrossRef]
- Bain, J.R.; Mackinnon, S.E.; Hunter, D.A. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast. Reconstr. Surg. 1989, 83, 129–138. [Google Scholar] [CrossRef]
- Brown, C.J.; Evans, P.J.; Mackinnon, S.E.; Bain, J.R.; Makino, A.P.; Hunter, D.A.; Hare, G. Inter-and intraobserver reliability of walking-track analysis used to assess sciatic nerve function in rats. Microsurgery 1991, 12, 76–79. [Google Scholar] [CrossRef] [PubMed]


| Mathematical Model | Parameter | Conduit | Conduit + Fibers |
|---|---|---|---|
| Zero-Order | R2 | 0.907 | 0.960 |
| First-Order | R2 | 0.904 | 0.962 |
| Higuchi | R2 | 0.884 | 0.975 |
| Hopfenberg | R2 | 0.905 | 0.961 |
| Hixson–Crowell | R2 | 0.905 | 0.961 |
| Korsmeyer–Peppas | R2 n | 0.958 0.850 | 0.821 0.622 |
| Peppas–Sahlin | R2 K1 K2 R/F Ratio | 0.907 262.240 518.640 1.978 | 0.828 185.630 5034.400 27.121 |
| Best-fit model | Zero-Order | Higuchi |
| Group | Treatment | Bioactive Dose |
|---|---|---|
| A | Reversed autograft | - |
| B | Bioactive-free conduit | - |
| C | Bioactive conduit | DS = 4 mg NGF = 100 ng |
| D | Bioactive conduit + bioactive-free intraluminal fibers | DS = 4 mg NGF = 100 ng |
| E | Bioactive conduit + bioactive intraluminal fibers | DS = 4 mg NGF = 100 ng NAC = 0.7 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramburrun, P.; Kumar, P.; Ndobe, E.; Choonara, Y.E. Gellan-Xanthan Hydrogel Conduits with Intraluminal Electrospun Nanofibers as Physical, Chemical and Therapeutic Cues for Peripheral Nerve Repair. Int. J. Mol. Sci. 2021, 22, 11555. https://doi.org/10.3390/ijms222111555
Ramburrun P, Kumar P, Ndobe E, Choonara YE. Gellan-Xanthan Hydrogel Conduits with Intraluminal Electrospun Nanofibers as Physical, Chemical and Therapeutic Cues for Peripheral Nerve Repair. International Journal of Molecular Sciences. 2021; 22(21):11555. https://doi.org/10.3390/ijms222111555
Chicago/Turabian StyleRamburrun, Poornima, Pradeep Kumar, Elias Ndobe, and Yahya E. Choonara. 2021. "Gellan-Xanthan Hydrogel Conduits with Intraluminal Electrospun Nanofibers as Physical, Chemical and Therapeutic Cues for Peripheral Nerve Repair" International Journal of Molecular Sciences 22, no. 21: 11555. https://doi.org/10.3390/ijms222111555
APA StyleRamburrun, P., Kumar, P., Ndobe, E., & Choonara, Y. E. (2021). Gellan-Xanthan Hydrogel Conduits with Intraluminal Electrospun Nanofibers as Physical, Chemical and Therapeutic Cues for Peripheral Nerve Repair. International Journal of Molecular Sciences, 22(21), 11555. https://doi.org/10.3390/ijms222111555