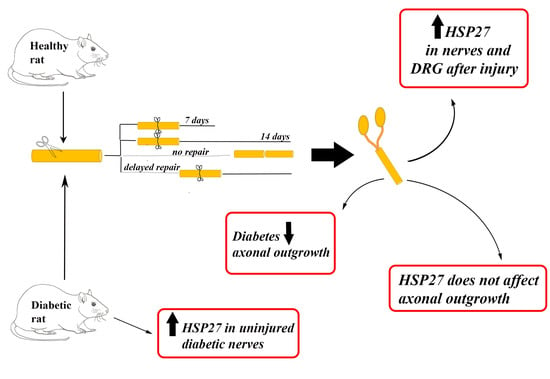
Injury-Induced HSP27 Expression in Peripheral Nervous Tissue Is Not Associated with Any Alteration in Axonal Outgrowth after Immediate or Delayed Nerve Repair
Abstract
1. Introduction
2. Results
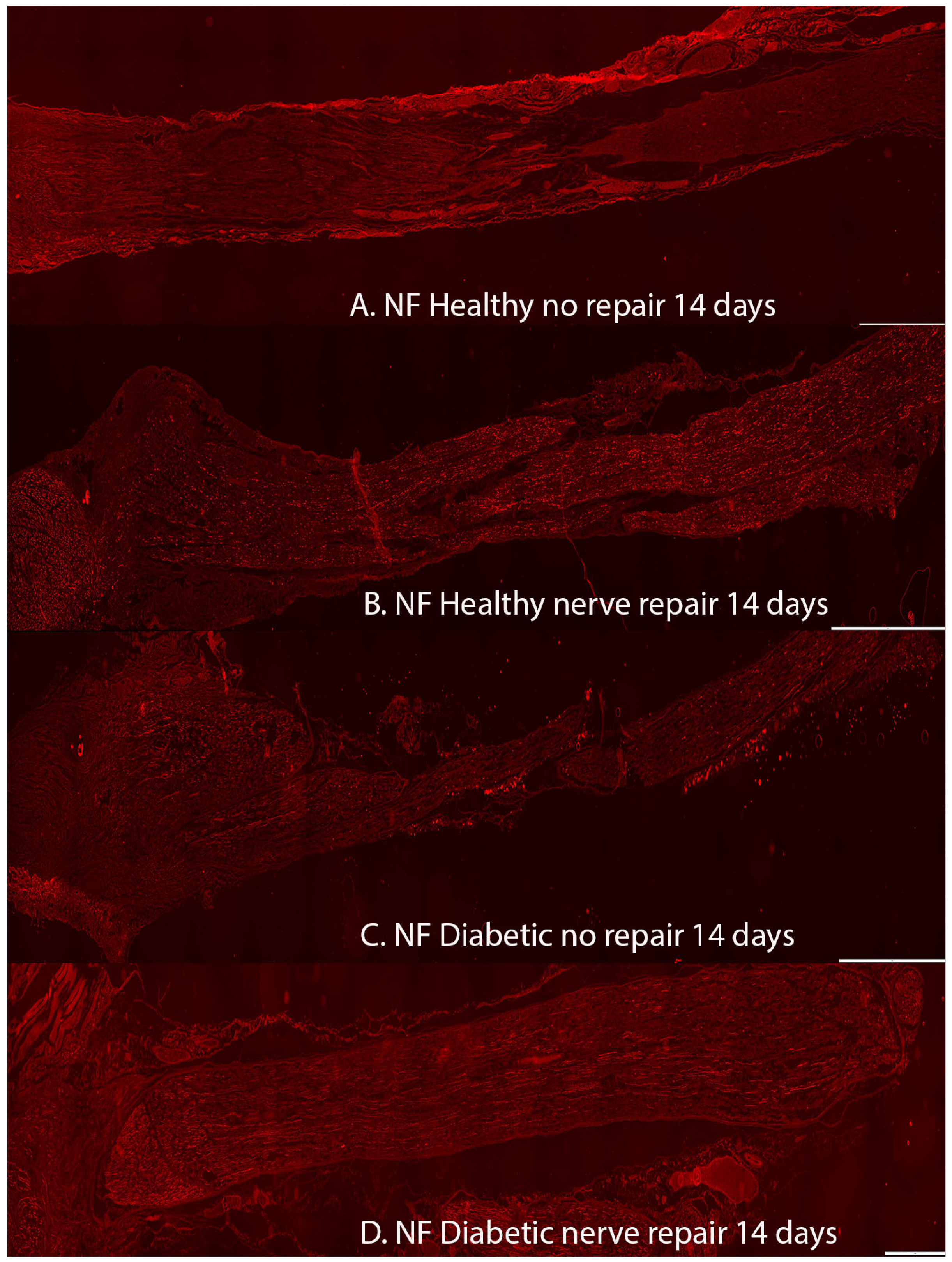
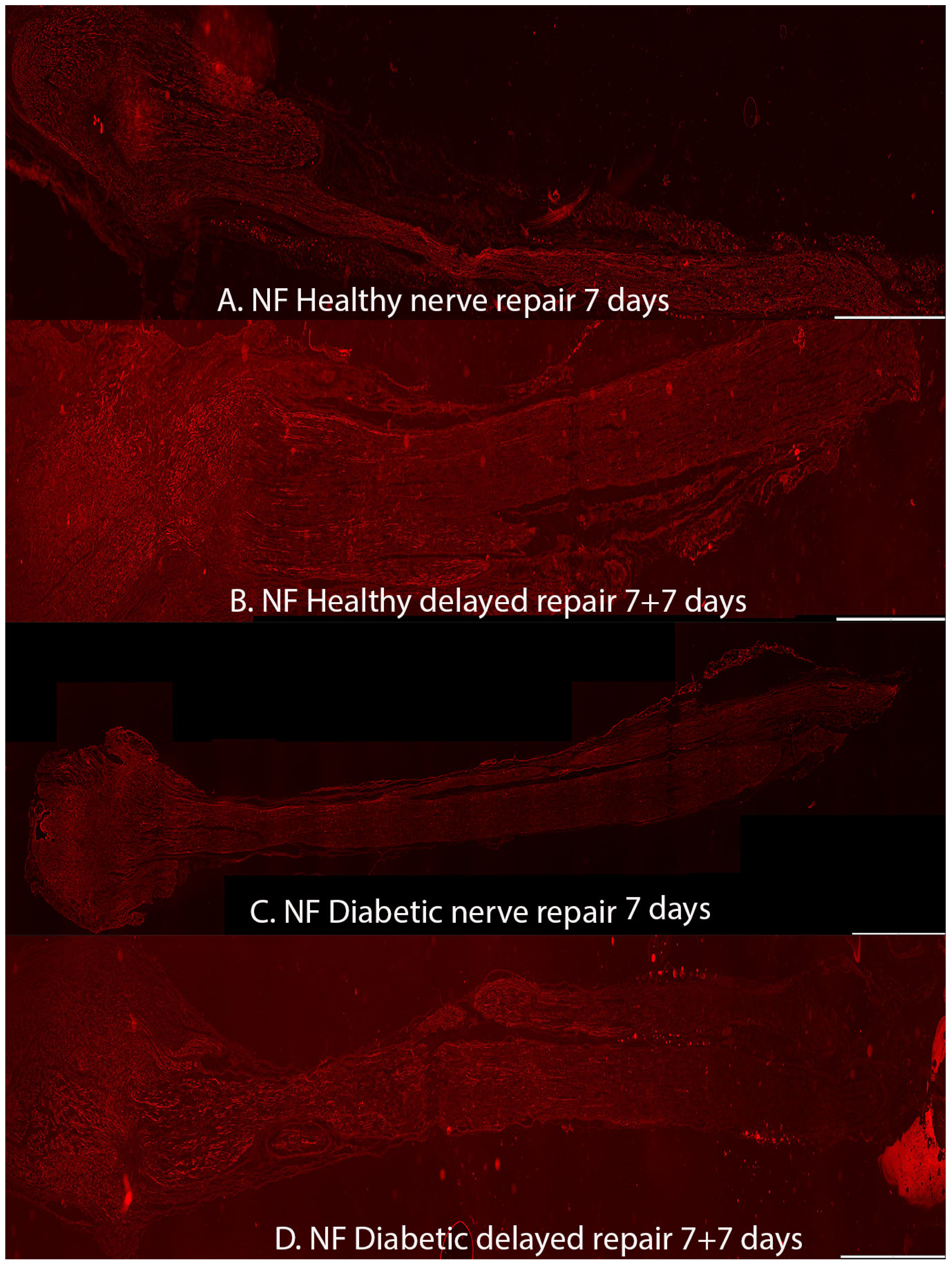
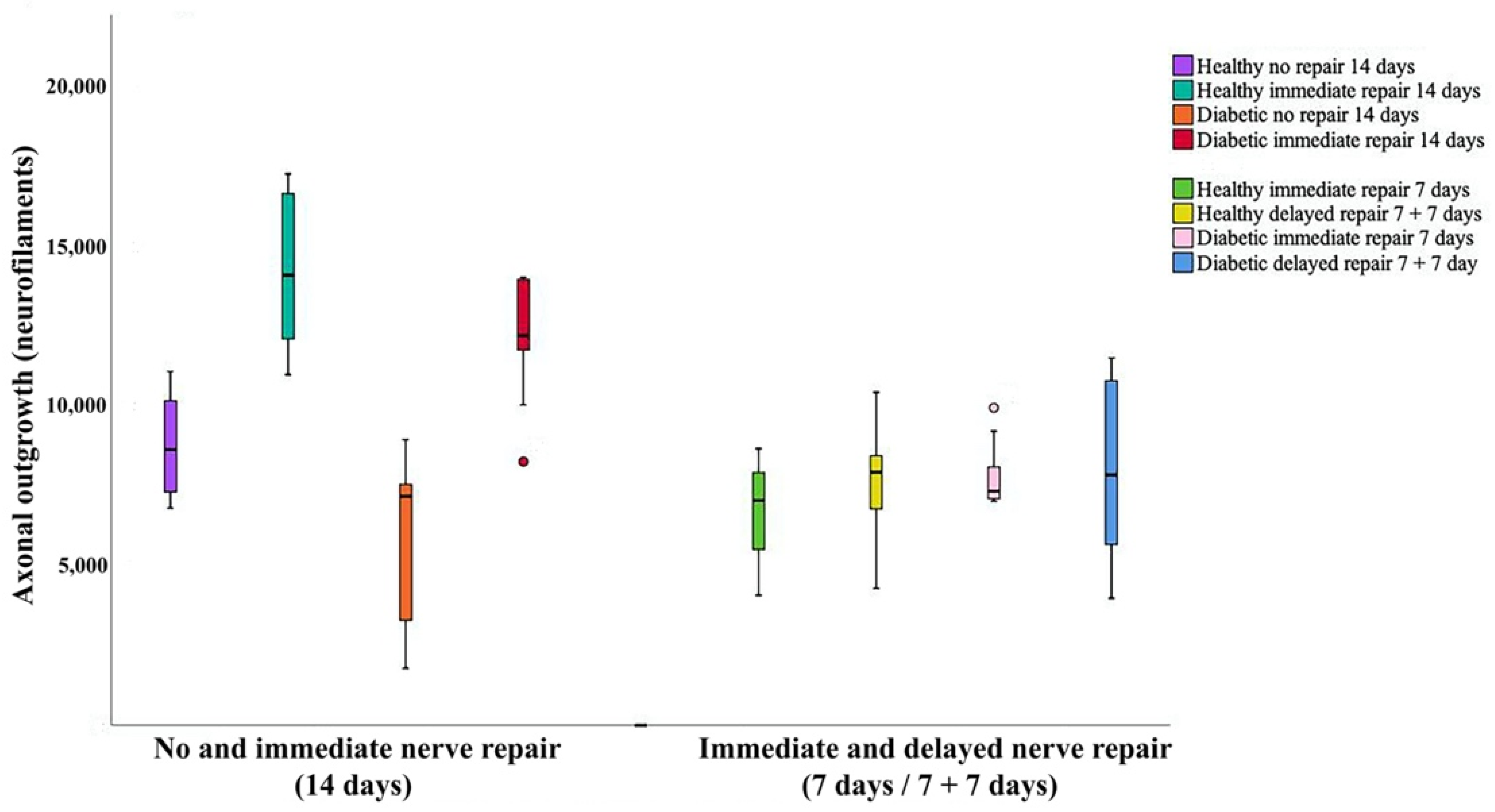
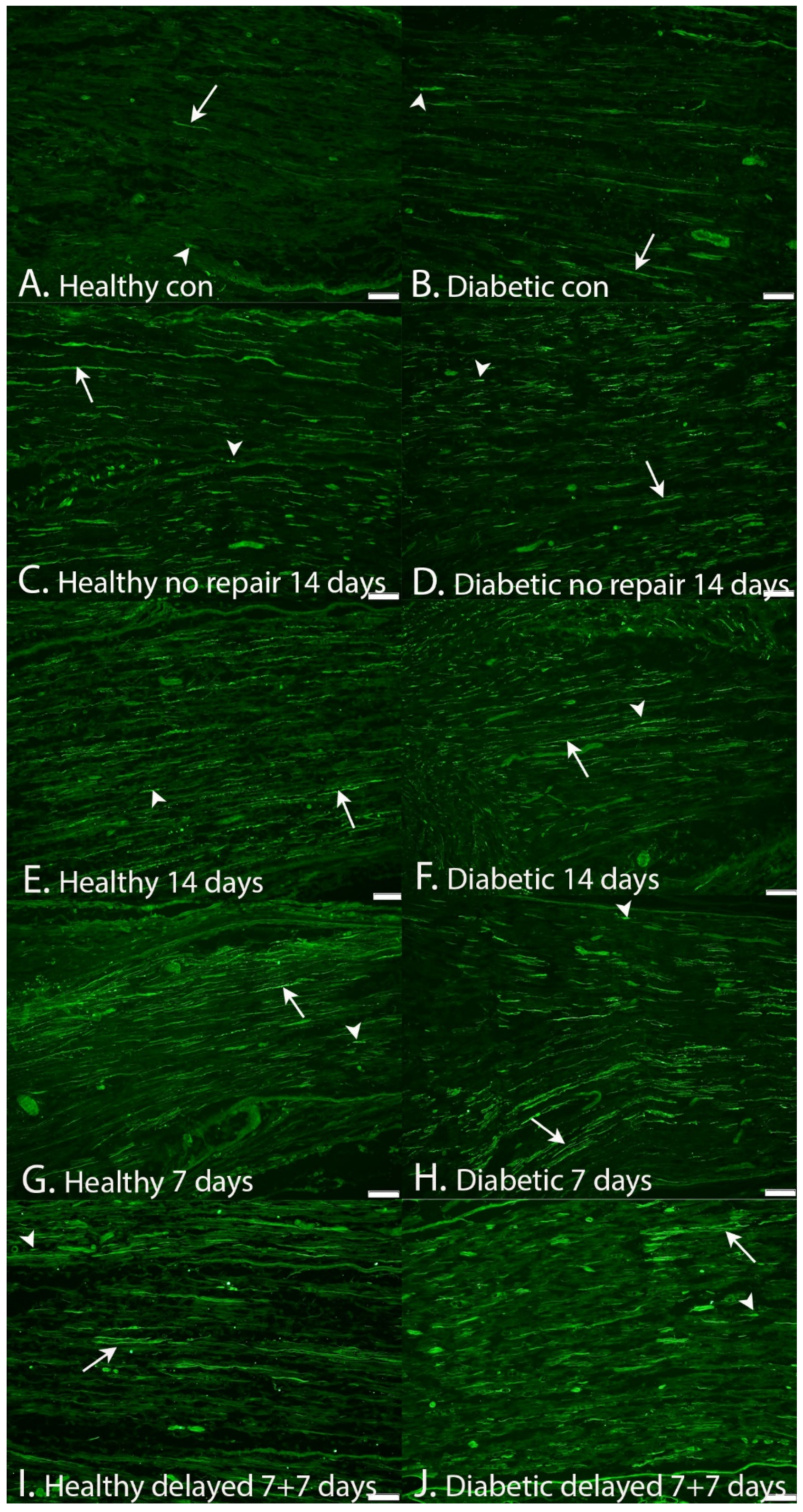
2.1. Axonal Outgrowth
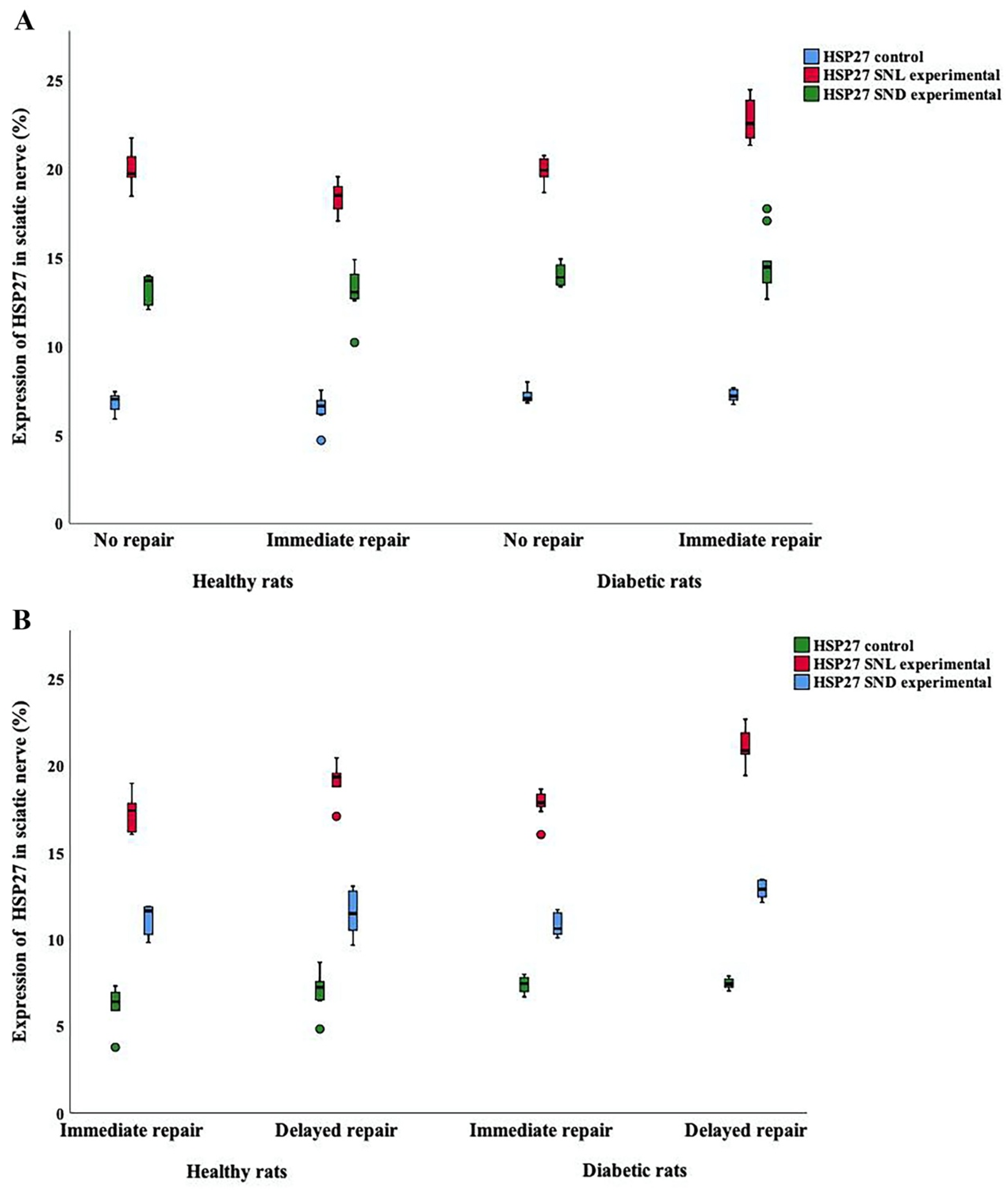
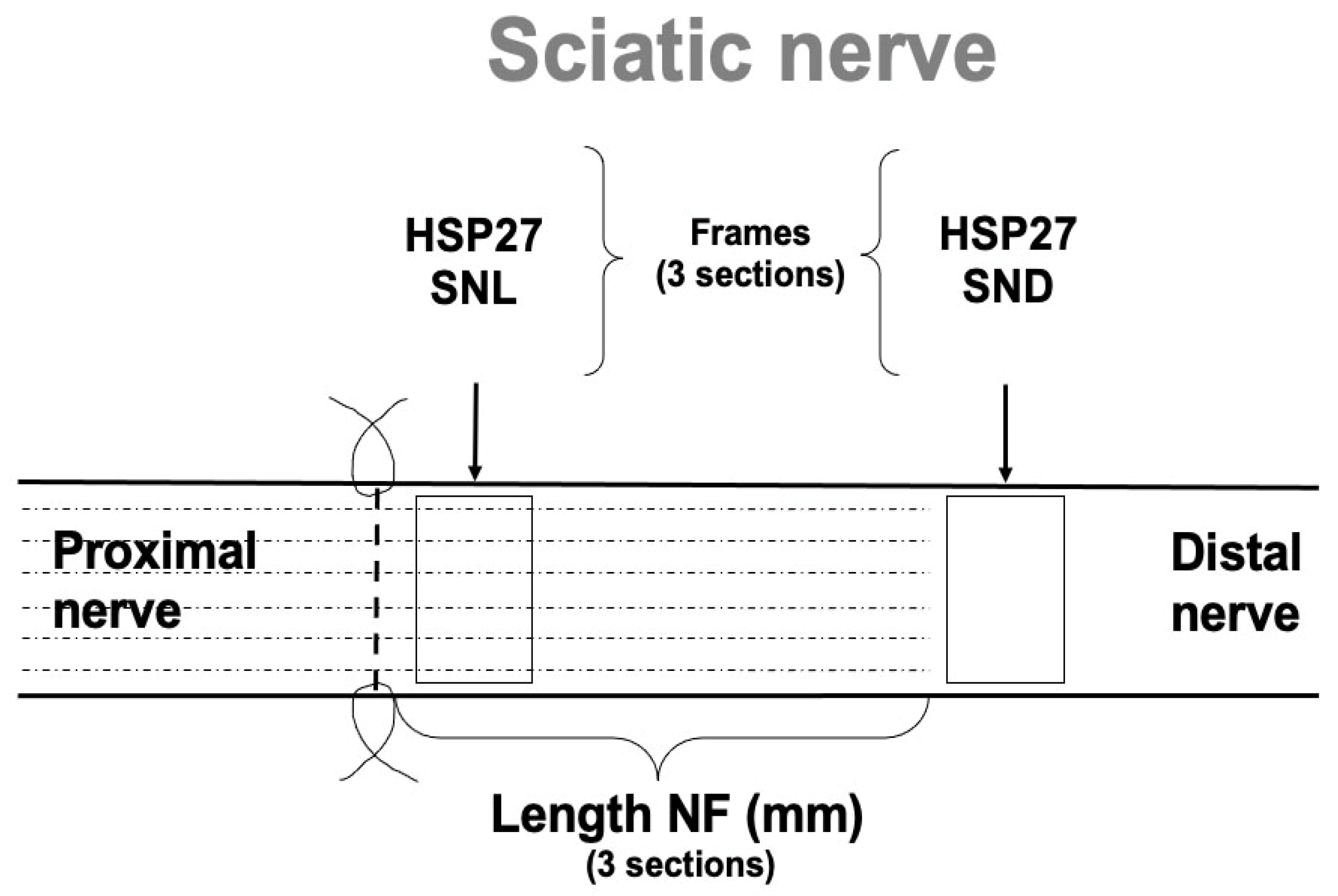
2.2. Expression of HSP27 in Sciatic Nerve
2.2.1. HSP27 Expression at the Contralateral Uninjured Side of the Sciatic Nerve
2.2.2. HSP27 Expression at the Site of Lesion (SNL) at the Experimental Side
2.2.3. HSP27 Expression in the Distal Sciatic Nerve (SND) in the Experimental Side
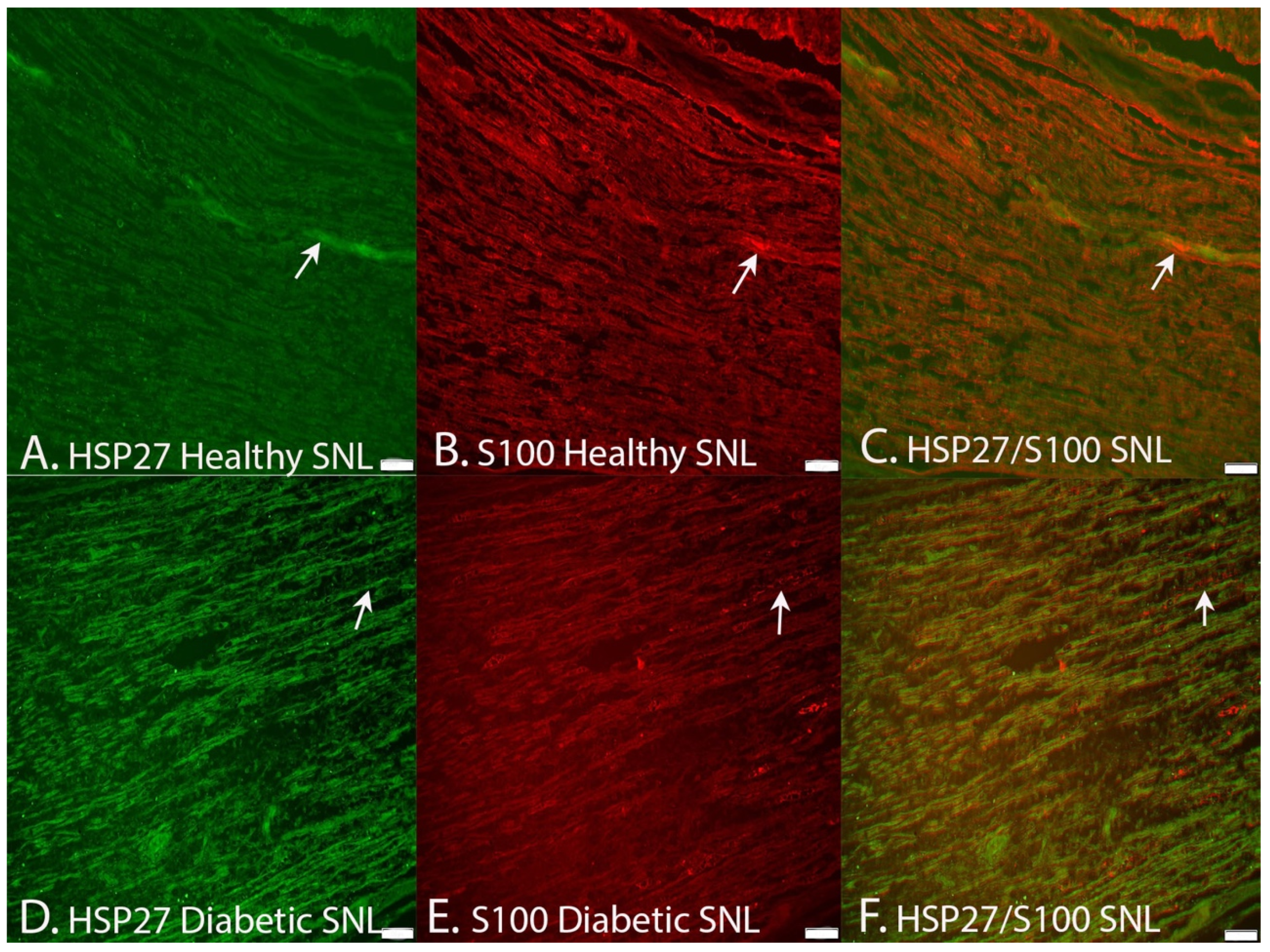
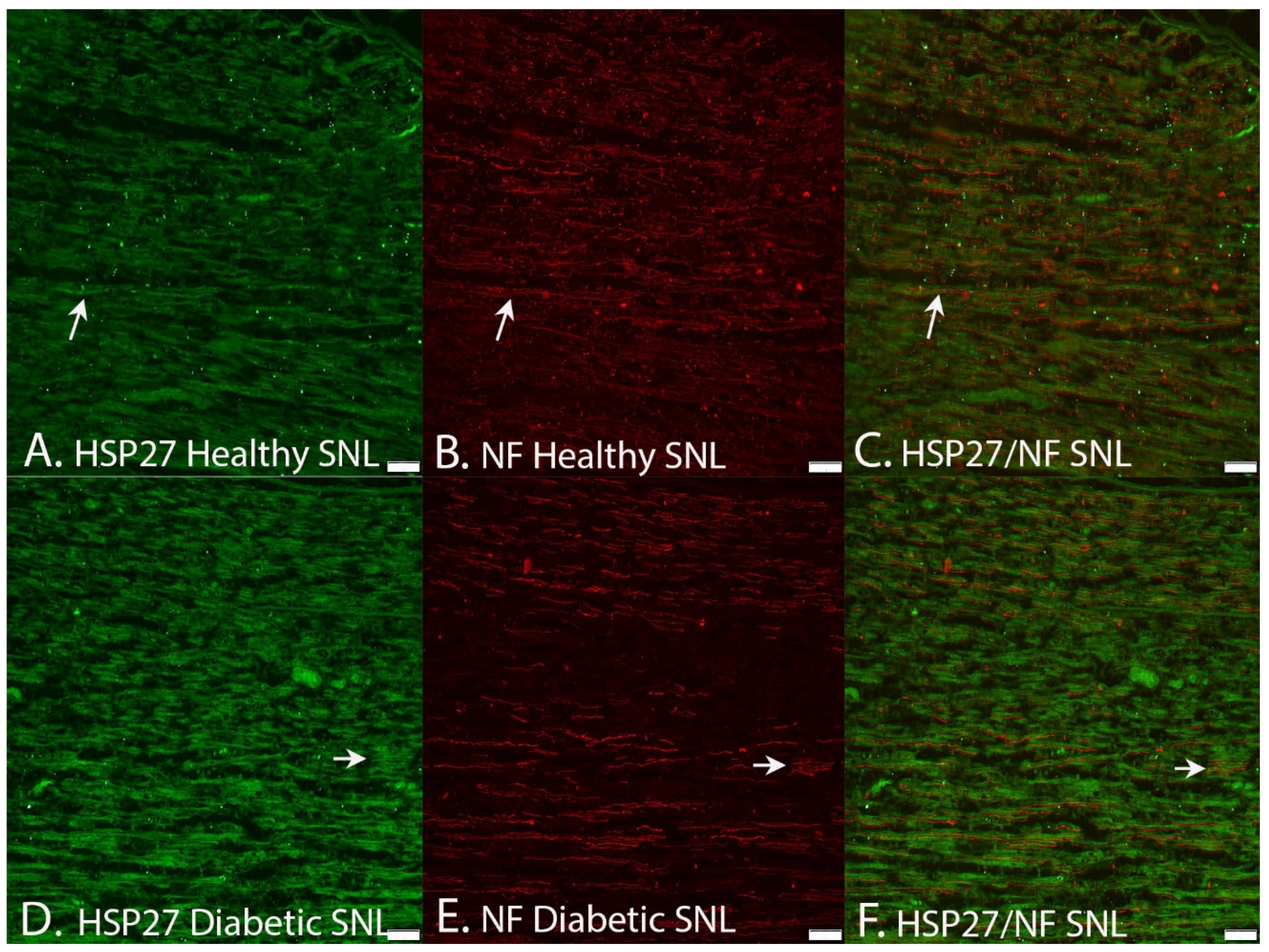
2.2.4. Co-Localization of HSP2 in Schwann Cells and Axons
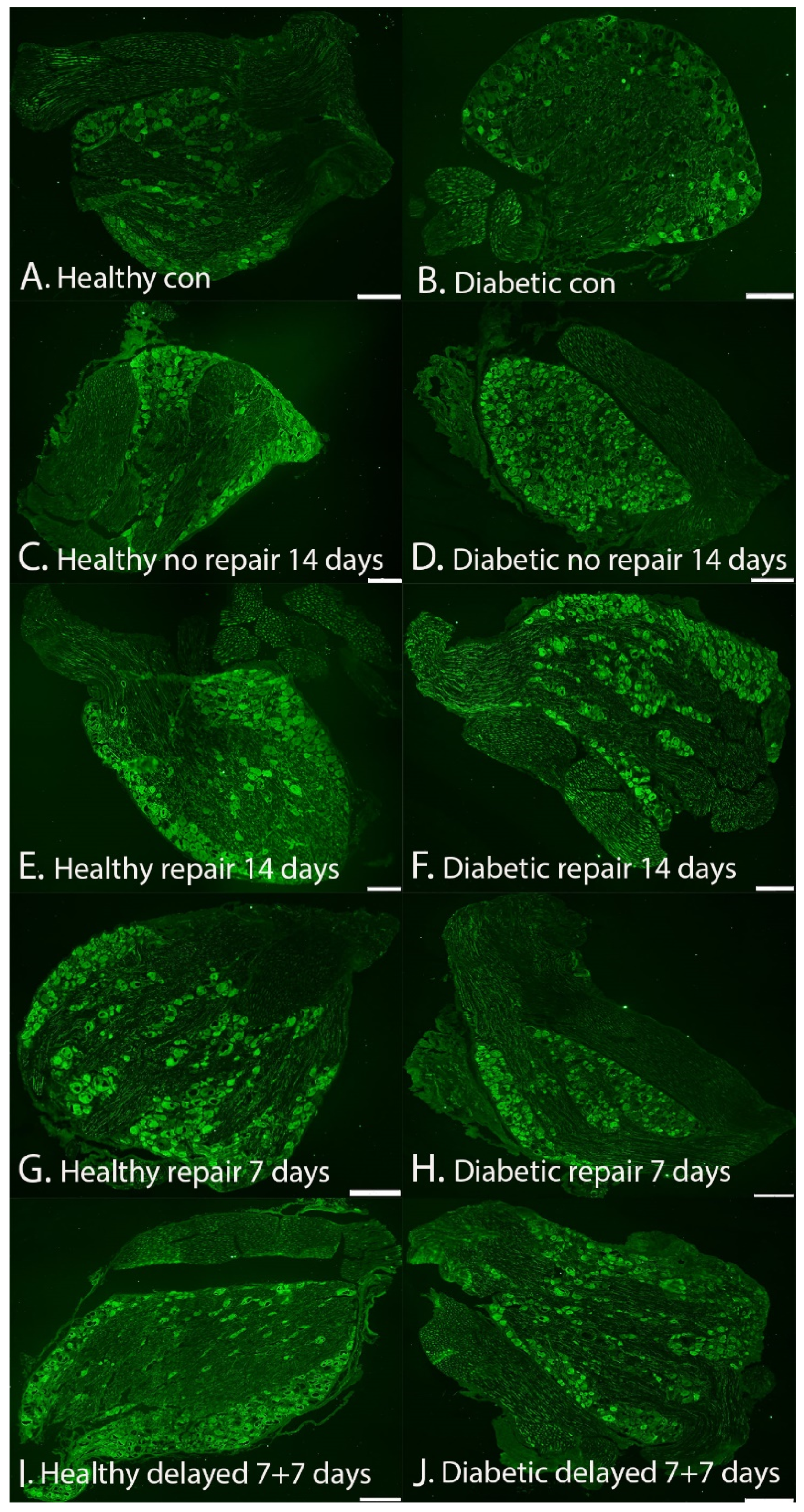
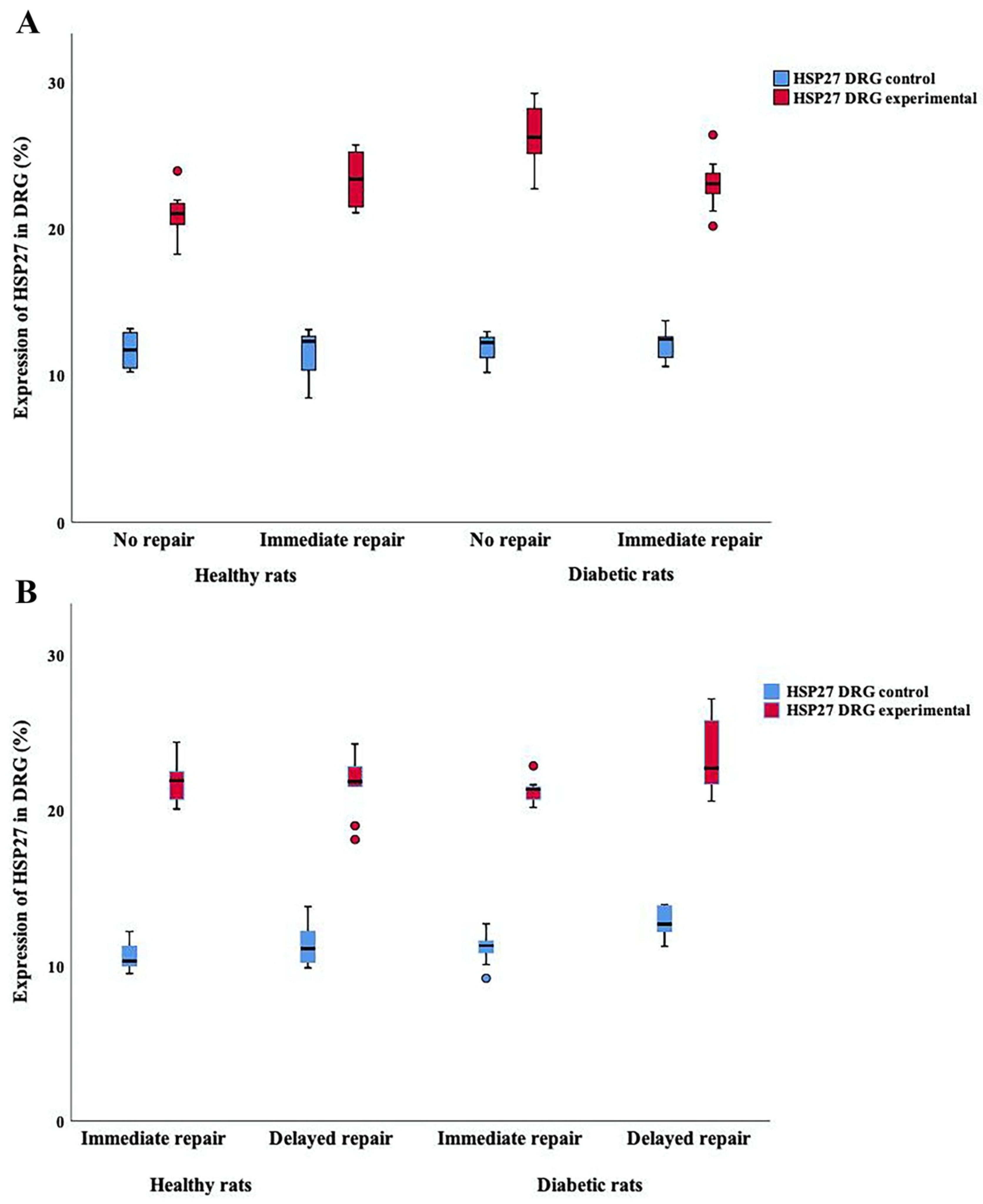
2.3. Expression of HSP27 in DRG
HSP27 Expression in DRG at Contralateral Uninjured and Experimental Sides (i.e., Sensory Neurons)
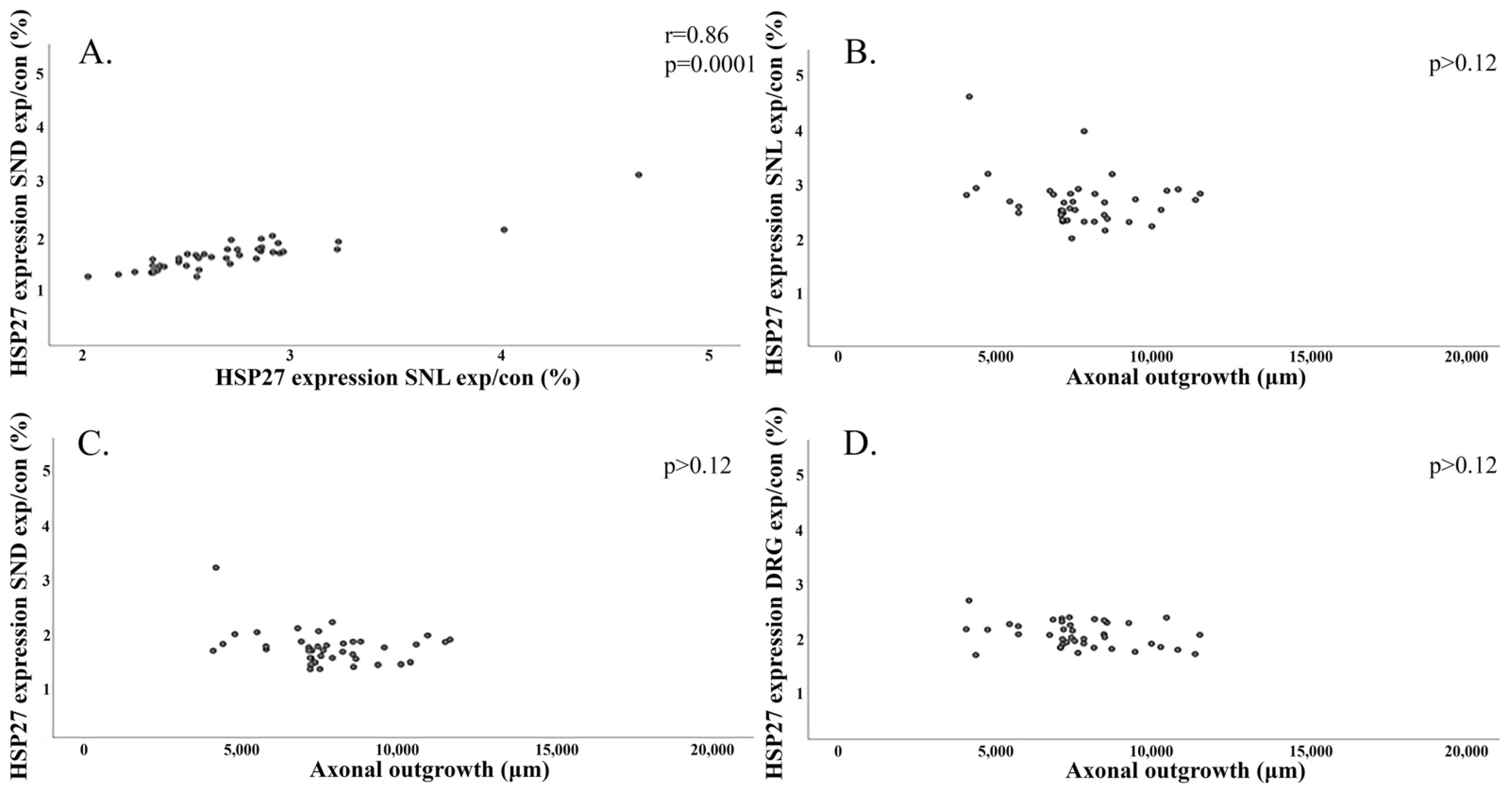
2.4. Regression Analyses and Correlations
2.4.1. Axonal Outgrowth in Association with Type of Nerve Repair, Diabetes Status, and Expression of HSP27
2.4.2. Expression of HSP27 in the Contralateral Uninjured Sciatic Nerve and in DRG Associated to Diabetes Status
2.4.3. Correlation between Axonal Outgrowth and Expression of HSP27 in the Sciatic Nerve and in DRG
3. Discussion
4. Materials and Methods
4.1. Animals and Surgery
4.2. Harvest of Specimens
4.3. Immunohistochemistry
4.4. Photography and Image Analysis
4.5. The Intra-Rater Reliability
4.6. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahlin, L.B.; Wiberg, M. Nerve injuries of the upper extremity and hand. EFORT Open Rev. 2017, 2, 158–170. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Hur, J.; Feldman, E.L. Diabetic neuropathy: One disease or two? Curr. Opin. Neurol. 2012, 25, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, L.; Dahlin, L.B. Gender differences in nerve regeneration after sciatic nerve injury and repair in healthy and in type 2 diabetic Goto-Kakizaki rats. BMC Neurosci. 2014, 15, 107. [Google Scholar] [CrossRef]
- Dahlin, L.B.; Rix, K.R.; Dahl, V.A.; Dahl, A.B.; Jensen, J.N.; Cloetens, P.; Pacureanu, A.; Mohseni, S.; Thomsen, N.O.B.; Bech, M. Three-dimensional architecture of human diabetic peripheral nerves revealed by X-ray phase contrast holographic nanotomography. Sci. Rep. 2020, 10, 7592. [Google Scholar] [CrossRef]
- Rydberg, M.; Zimmerman, M.; Gottsäter, A.; Nilsson, P.M.; Melander, O.; Dahlin, L.B. Diabetes mellitus as a risk factor for compression neuropathy: A longitudinal cohort study from southern Sweden. BMJ Open Diabetes Res. Care 2020, 8, e001298. [Google Scholar] [CrossRef]
- Bolandghamat, S.; Behnam-Rassouli, M. Recent Findings on the Effects of Pharmacological Agents on the Nerve Regeneration after Peripheral Nerve Injury. Curr. Neuropharmacol. 2020, 18, 1154–1163. [Google Scholar] [CrossRef]
- Dahlin, L.B. The Role of Timing in Nerve Reconstruction. Int. Rev. Neurobiol. 2013, 109, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Jivan, S.; Kumar, N.; Wiberg, M.; Kay, S. The influence of pre-surgical delay on functional outcome after reconstruction of brachial plexus injuries. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 472–479. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef]
- Sulaiman, O.A.; Gordon, T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia 2000, 32, 234–246. [Google Scholar] [CrossRef]
- Michaelevski, I.; Segal-Ruder, Y.; Rozenbaum, M.; Medzihradszky, K.F.; Shalem, O.; Coppola, G.; Horn-Saban, S.; Ben-Yaakov, K.; Dagan, S.Y.; Rishal, I.; et al. Signaling to Transcription Networks in the Neuronal Retrograde Injury Response. Sci. Signal. 2010, 3, ra53. [Google Scholar] [CrossRef]
- Saito, H.; Dahlin, L.B. Expression of ATF3 and axonal outgrowth are impaired after delayed nerve repair. BMC Neurosci. 2008, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Kanje, M.; Dahlin, L.B. Delayed nerve repair increases number of caspase 3 stained Schwann cells. Neurosci. Lett. 2009, 456, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Kanje, M.; Dahlin, L.B. Axonal outgrowth is associated with increased ERK 1/2 activation but decreased caspase 3 linked cell death in Schwann cells after immediate nerve repair in rats. BMC Neurosci. 2011, 12, 12. [Google Scholar] [CrossRef]
- Jonsson, S.; Wiberg, R.; McGrath, A.; Novikov, L.; Wiberg, M.; Novikova, L.N.; Kingham, P.J. Effect of Delayed Peripheral Nerve Repair on Nerve Regeneration, Schwann Cell Function and Target Muscle Recovery. PLoS ONE 2013, 8, e56484. [Google Scholar] [CrossRef]
- Hirata, K.; He, J.; Hirakawa, Y.; Liu, W.; Wang, S.; Kawabuchi, M. HSP27 is markedly induced in Schwann cell columns and associated regenerating axons. Glia 2003, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Costigan, M.; Mannion, R.J.; Kendall, G.; Lewis, S.E.; Campagna, J.A.; Coggeshall, R.E.; Meridith-Middleton, J.; Tate, S.; Woolf, C.J. Heat Shock Protein 27: Developmental Regulation and Expression after Peripheral Nerve Injury. J. Neurosci. 1998, 18, 5891–5900. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The Heat-Shock Proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Garrido, C.; Gurbuxani, S.; Ravagnan, L.; Kroemer, G. Heat shock proteins: Endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 2001, 286, 433–442. [Google Scholar] [CrossRef]
- Jakob, U.; Gaestel, M.; Engel, K.; Buchner, J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993, 268, 1517–1520. [Google Scholar] [CrossRef]
- Pourhamidi, K.; Dahlin, L.B.; Boman, K.; Rolandsson, O. Heat shock protein 27 is associated with better nerve function and fewer signs of neuropathy. Diabetologia 2011, 54, 3143–3149. [Google Scholar] [CrossRef]
- Pourhamidi, K.; Skärstrand, H.; Dahlin, L.B.; Rolandsson, O. HSP27 Concentrations Are Lower in Patients with Type 1 Diabetes and Correlate with Large Nerve Fiber Dysfunction. Diabetes Care 2014, 37, 49–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Korngut, L.; Ma, C.H.E.; Martinez, J.; Toth, C.; Guo, G.; Singh, V.; Woolf, C.; Zochodne, D. Overexpression of human HSP27 protects sensory neurons from diabetes. Neurobiol. Dis. 2012, 47, 436–443. [Google Scholar] [CrossRef]
- Lindwall, C.; Kanje, M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol. Cell. Neurosci. 2005, 29, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, S.; Suzuki, Y.; Namikawa, K.; Kiryu-Seo, S.; Kiyama, H. Expression of the Activating Transcription Factor 3 Prevents c-Jun N-Terminal Kinase-Induced Neuronal Death by Promoting Heat Shock Protein 27 Expression and Akt Activation. J. Neurosci. 2003, 23, 5187–5196. [Google Scholar] [CrossRef]
- Lewis, S.E.; Mannion, R.J.; White, F.A.; Coggeshall, R.E.; Beggs, S.; Costigan, M.; Martin, J.L.; Dillmann, W.H.; Woolf, C.J. A Role for HSP27 in Sensory Neuron Survival. J. Neurosci. 1999, 19, 8945–8953. [Google Scholar] [CrossRef]
- Wagstaff, M.J.D.; Collaço-Moraes, Y.; Smith, J.; de Belleroche, J.S.; Coffin, R.S.; Latchman, D.S. Protection of Neuronal Cells from Apoptosis by Hsp27 Delivered with a Herpes Simplex Virus-based Vector. J. Biol. Chem. 1999, 274, 5061–5069. [Google Scholar] [CrossRef]
- Williams, K.L.; Rahimtula, M.; Mearow, K.M. Heat shock protein 27 is involved in neurite extension and branching of dorsal root ganglion neurons in vitro. J. Neurosci. Res. 2006, 84, 716–723. [Google Scholar] [CrossRef] [PubMed]
- He, J.-W.; Hirata, K.; Wang, S.; Kawabuchi, M. Expression of nitric oxide synthase and 27-kD heat shock protein in motor neurons of ventral root-avulsed rats. Arch. Histol. Cytol. 2003, 66, 83–93. [Google Scholar] [CrossRef][Green Version]
- Benn, S.C.; Perrelet, D.; Kato, A.C.; Scholz, J.; Decosterd, I.; Mannion, R.J.; Bakowska, J.C.; Woolf, C.J. Hsp27 Upregulation and Phosphorylation Is Required for Injured Sensory and Motor Neuron Survival. Neuron 2002, 36, 45–56. [Google Scholar] [CrossRef]
- Ma, C.H.E.; Omura, T.; Cobos, E.J.; Latrémolière, A.; Ghasemlou, N.; Brenner, G.; van Veen, J.E.; Barrett, L.; Sawada, T.; Gao, F.; et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J. Clin. Investig. 2011, 121, 4332–4347. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Yono, M.; Pouresmail, M.; Takahashi, W.; Flanagan, J.F.; Weiss, R.M.; Latifpour, J. Effect of insulin treatment on tissue size of the genitourinary tract in BB rats with spontaneously developed and streptozotocin-induced diabetes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2005, 372, 251–255. [Google Scholar] [CrossRef]
- Portha, B.; Lacraz, G.; Kergoat, M.; Homo-Delarche, F.; Giroix, M.-H.; Bailbé, D.; Gangnerau, M.-N.; Dolz, M.; Tourrel-Cuzin, C.; Movassat, J. The GK rat beta-cell: A prototype for the diseased human beta-cell in type 2 diabetes? Mol. Cell. Endocrinol. 2009, 297, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.M.; Terenghi, G.; Wiberg, M. Neuronal death after peripheral nerve injury and experimental strategies for neuroprotection. Neurol. Res. 2008, 30, 999–1011. [Google Scholar] [CrossRef]
- Read, D.E.; Gorman, A.M. Heat shock protein 27 in neuronal survival and neurite outgrowth. Biochem. Biophys. Res. Commun. 2009, 382, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.L.; Wormald, J.C.R.; Jain, A. Outcome of surgical repair of adult digital nerve injury: A systematic review. BMJ Open 2019, 9, e025443. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Dunlop, R.; Hems, T.; Tang, J.B. Outcomes of surgical repair of a single digital nerve in adults. J. Hand Surg. 2019, 44, 560–565. [Google Scholar] [CrossRef]
- Meyer, C.; Stenberg, L.; Gonzalez-Perez, F.; Wrobel, S.; Ronchi, G.; Udina, E.; Suganuma, S.; Geuna, S.; Navarro, X.; Dahlin, L.B.; et al. Chitosan-film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials 2016, 76, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, L.; Stößel, M.; Ronchi, G.; Geuna, S.; Yin, Y.; Mommert, S.; Mårtensson, L.; Metzen, J.; Grothe, C.; Dahlin, L.B.; et al. Regeneration of long-distance peripheral nerve defects after delayed reconstruction in healthy and diabetic rats is supported by immunomodulatory chitosan nerve guides. BMC Neurosci. 2017, 18, 53. [Google Scholar] [CrossRef]
- Poppler, L.H.; Parikh, R.P.; Bichanich, M.J.; Rebehn, K.; Bettlach, C.R.; MacKinnon, S.E.; Moore, A.M. Surgical interventions for the treatment of painful neuroma: A comparative meta-analysis. Pain 2018, 159, 214–223. [Google Scholar] [CrossRef]
- Doron-Mandel, E.; Fainzilber, M.; Terenzio, M. Growth control mechanisms in neuronal regeneration. FEBS Lett. 2015, 589, 1669–1677. [Google Scholar] [CrossRef]
- Hebb, M.O.; Myers, T.L.; Clarke, D.B. Enhanced expression of heat shock protein 27 is correlated with axonal regeneration in mature retinal ganglion cells. Brain Res. 2006, 1073–1074, 146–150. [Google Scholar] [CrossRef]
- Tsubouchi, H.; Ikeda, K.; Sugimoto, N.; Tomita, K. Local application of olprinone for promotion of peripheral nerve regeneration. J. Orthop. Sci. 2009, 14, 801–810. [Google Scholar] [CrossRef]
- Williams, K.L.; Rahimtula, M.; Mearow, K.M. Hsp27 and axonal growth in adult sensory neurons in vitro. BMC Neurosci. 2005, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Chine, V.B.; Au, N.P.B.; Ma, C.H.E. Therapeutic benefits of maintaining mitochondrial integrity and calcium homeostasis by forced expression of Hsp27 in chemotherapy-induced peripheral neuropathy. Neurobiol. Dis. 2019, 130, 104492. [Google Scholar] [CrossRef]
- Brussee, V.; Guo, G.; Dong, Y.; Cheng, C.; Martinez, J.A.; Smith, D.; Glazner, G.W.; Fernyhough, P.; Zochodne, D.W. Distal Degenerative Sensory Neuropathy in a Long-Term Type 2 Diabetes Rat Model. Diabetes 2008, 57, 1664–1673. [Google Scholar] [CrossRef]
- Blom, C.L.; Mårtensson, L.B.; Dahlin, L.B. Nerve Injury-Induced c-Jun Activation in Schwann Cells Is JNK Independent. BioMed Res. Int. 2014, 2014, 392971. [Google Scholar] [CrossRef] [PubMed]
- Ramji, N.; Toth, C.; Kennedy, J.; Zochodne, D.W. Does diabetes mellitus target motor neurons? Neurobiol. Dis. 2007, 26, 301–311. [Google Scholar] [CrossRef]
- Kamiya, H.; Zhangm, W.; Sima, A.A. Apoptotic Stress Is Counterbalanced by Survival Elements Preventing Programmed Cell Death of Dorsal Root Ganglions in Subacute Type 1 Diabetic BB/Wor Rats. Diabetes 2005, 54, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Kerns, J.M.; Danielsen, N.; Holmquist, B.; Kanje, M.; Lundborg, G. The Influence of Predegeneration on Regeneration through Peripheral Nerve Grafts in the Rat. Exp. Neurol. 1993, 122, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, N.; Kerns, J.M.; Holmquist, B.; Zhao, Q.; Lundborg, G.; Kanje, M. Pre-degenerated nerve grafts enhance regeneration by shortening the initial delay period. Brain Res. 1994, 666, 250–254. [Google Scholar] [CrossRef]
- EU. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, L 276, 33–79. [Google Scholar]
- Stenberg, L.; Kodama, A.; Lindwall-Blom, C.; Dahlin, L.B. Nerve regeneration in chitosan conduits and in autologous nerve grafts in healthy and in type 2 diabetic Goto-Kakizaki rats. Eur. J. Neurosci. 2015, 43, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Fredricson, J. Signalling and Heat Shock Protein 27 in Sensory Neurons after Injury. Master’s Thesis, Lund University, Lund, Sweden, 2005. [Google Scholar]
- Stenberg, L.; Kanje, M.; Dolezal, K.; Dahlin, L.B. Expression of Activating Transcription Factor 3 (ATF 3) and caspase 3 in Schwann cells and axonal outgrowth after sciatic nerve repair in diabetic BB rats. Neurosci. Lett. 2012, 515, 34–38. [Google Scholar] [CrossRef]
- Stenberg, L.; Kanje, M.; Martensson, L.; Dahlin, L.B. Injury-induced activation of ERK 1/2 in the sciatic nerve of healthy and diabetic rats. Neuroreport 2011, 22, 73–77. [Google Scholar] [CrossRef]
- Fisher, R.A. Combining independent tests of significance. Am. Stat. 1948, 2, 30. [Google Scholar]


| Healthy Wistar Rats | Diabetic Goto-Kakizaki Rats | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | No Repair (14 Days) (n = 10) | Immediate Repair (14 Days) (n = 8) | No Repair (14 Days) (n = 12) | Immediate Repair (14 Days) (n = 10) | p-Values (KW a) | Fisher’s Method b | |
| No Repair/Immediate Repair | Healthy Rats/ Diabetic Rats | ||||||
| Axonal outgrowth (mm) | 8.6 (7.3–10.4) | 14.1 (11.8–16.8) | 7.2 (3.0–7.6) | 12.2 (11.3–13.9) | 0.0001 | <0.0001 | 0.011 |
| HSP27 Sciatic nerve Contralateral control (%) | 7.0 (6.3–7.2) | 6.6 (6.1–7.0) | 7.0 (6.9–7.4) | 7.2 (6.9–7.6) | 0.06 | NA | NA |
| HSP27 SNL Experimental (%) | 19.7 (19.5–20.7) | 18.5 (17.4–19.2) | 19.9 (19.4–20.6) | 22.6 (21.7–23.9) | 0.0001 | <0.0001 | 0.001 |
| HSP27 SNL Exp/control ratio | 2.8 (2.7–3.2) | 2.7 (2.5–3.0) | 2.8 (2.7–2.9) | 3.2 (3.0–3.2) | 0.006 | 0.0005 | 0.07 |
| HSP27 SND Experimental (%) | 13.7 (12.3–13.9) | 13.0 (12.6–14.3) | 13.9 (13.4–14.6) | 14.5 (13.4–15.4) | 0.048 | 0.81 | 0.05 |
| HSP27 SND Exp/control ratio | 1.9 (1.8–2.0) | 2.1 (1.8–2.2) | 1.9 (1.8–2.0) | 2.0 (1.8–2.2) | 0.66 | NA | NA |
| HSP27 DRG Contralateral control (%) | 11.7 (10.4–12.9) | 12.3 (10.4–12.8) | 12.3 (11.2–12.6) | 12.5 (11.1–12.8) | 0.85 | NA | NA |
| HSP27 DRG Experimental (%) | 21.1 (20.0–21.8) | 23.4 (21.3–25.3) | 26.3 (25.0–28.3) | 23.1 (22.1–23.9) | 0.0001 | 0.0004 | 0.0008 |
| HSP27 DRG Exp/Control ratio | 1.7 (1.6–2.1) | 2.0 (1.9–2.1) | 2.2 (2.0–2.4) | 1.9 (1.8–2.1) | 0.008 | 0.01 | 0.004 |
| Healthy Wistar Rats | Diabetic Goto-Kakizaki Rats | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Immediate Repair (7 Days) (n = 8) | Delayed Repair (7 + 7 Days) (n = 10) | Immediate Repair (7 Days) (n = 12) | Delayed Repair (7 + 7 Days) (n = 10) | p-Values (KW a) | Fisher’s Method b | |
| Immediate Repair/Delayed Repair | Healthy Rats/ Diabetic Rats | ||||||
| Axonal outgrowth (mm) | 7.0 (5.4–8.2) | 7.9 (6.7–8.9) | 7.3 (7.1–8.2) | 7.8 (5.4–10.9) | 0.43 | NA | NA |
| HSP27 Sciatic nerve Contralateral control (%) | 6.4 (5.9–7.0) | 7.2 (6.5–7.6) | 7.5 (7.0–7.8) | 7.4 (7.2–7.7) | 0.008 | 0.26 | 0.006 |
| HSP27 SNL Experimental (%) | 17.4 (16.2–17.9) | 19.3 (18.8–19.6) | 17.9 (17.6–18.4) | 20.9 (20.4–21.9) | 0.0001 | <0.0001 | 0.0002 |
| HSP27 SNL Exp/control ratio | 2.6 (2.4–3.1) | 2.8 (2.5–2.9) | 2.4 (2.3–2.6) | 2.8 (2.7–2.9) | 0.005 | 0.001 | 0.12 |
| HSP27 SND Experimental (%) | 11.6 (10.2–11.9) | 11.5 (10.5–12.8) | 10.6 (10.3–11.5) | 12.9 (12.4–13.4) | 0.0001 | 0.0009 | 0.0003 |
| HSP27 SND Exp/control ratio | 1.7 (1.5–2.0) | 1.7 (1.4–1.8) | 1.5 (1.3–1.6) | 1.7 (1.6–1.8) | 0.009 | 0.0006 | 0.04 |
| HSP27 DRG Contralateral control (%) | 10.3 (10.0–11.6) | 11.1 (10.2–12.3) | 11.3 (10.8–11.6) | 12.7 (12.2–13.9) | 0.001 | 0.0002 | 0.012 |
| HSP27 DRG Experimental (%) | 21.9 (20.6–22.5) | 21.9 (20.9–23.0) | 21.4 (20.7–21.5) | 22.7 (21.5–26.0) | 0.047 | 0.047 | 0.27 |
| HSP27 DRG Exp/Control ratio | 2.1 (1.8–2.2) | 1.9 (1.7–2.2) | 1.9 (1.8–2.0) | 1.9 (1.6–2.0) | 0.32 | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenberg, L.; Hazer Rosberg, D.B.; Kohyama, S.; Suganuma, S.; Dahlin, L.B. Injury-Induced HSP27 Expression in Peripheral Nervous Tissue Is Not Associated with Any Alteration in Axonal Outgrowth after Immediate or Delayed Nerve Repair. Int. J. Mol. Sci. 2021, 22, 8624. https://doi.org/10.3390/ijms22168624
Stenberg L, Hazer Rosberg DB, Kohyama S, Suganuma S, Dahlin LB. Injury-Induced HSP27 Expression in Peripheral Nervous Tissue Is Not Associated with Any Alteration in Axonal Outgrowth after Immediate or Delayed Nerve Repair. International Journal of Molecular Sciences. 2021; 22(16):8624. https://doi.org/10.3390/ijms22168624
Chicago/Turabian StyleStenberg, Lena, Derya Burcu Hazer Rosberg, Sho Kohyama, Seigo Suganuma, and Lars B. Dahlin. 2021. "Injury-Induced HSP27 Expression in Peripheral Nervous Tissue Is Not Associated with Any Alteration in Axonal Outgrowth after Immediate or Delayed Nerve Repair" International Journal of Molecular Sciences 22, no. 16: 8624. https://doi.org/10.3390/ijms22168624
APA StyleStenberg, L., Hazer Rosberg, D. B., Kohyama, S., Suganuma, S., & Dahlin, L. B. (2021). Injury-Induced HSP27 Expression in Peripheral Nervous Tissue Is Not Associated with Any Alteration in Axonal Outgrowth after Immediate or Delayed Nerve Repair. International Journal of Molecular Sciences, 22(16), 8624. https://doi.org/10.3390/ijms22168624