Potential of Melt Electrowritten Scaffolds Seeded with Meniscus Cells and Mesenchymal Stromal Cells
Abstract
:1. Introduction
2. Results
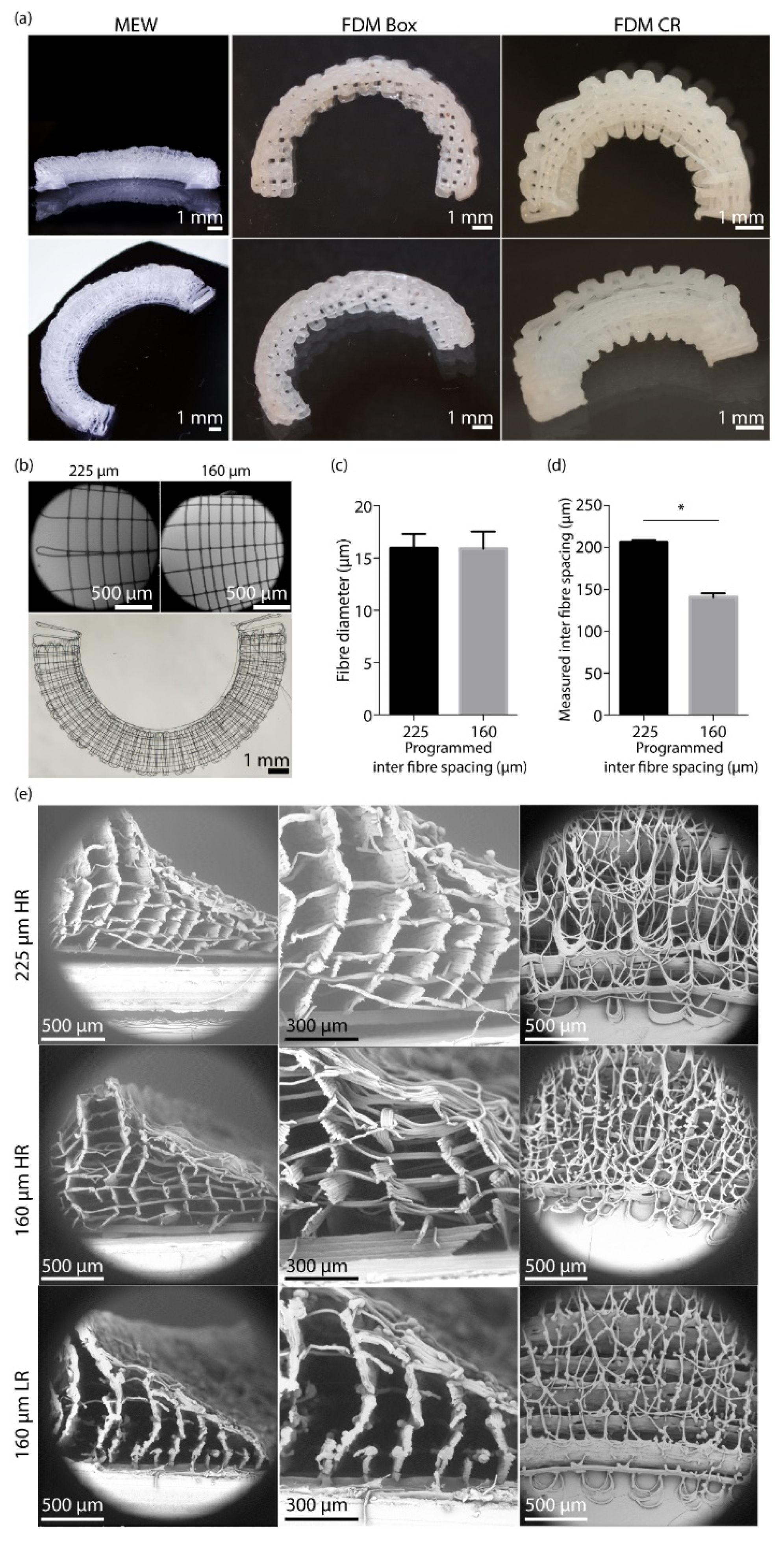
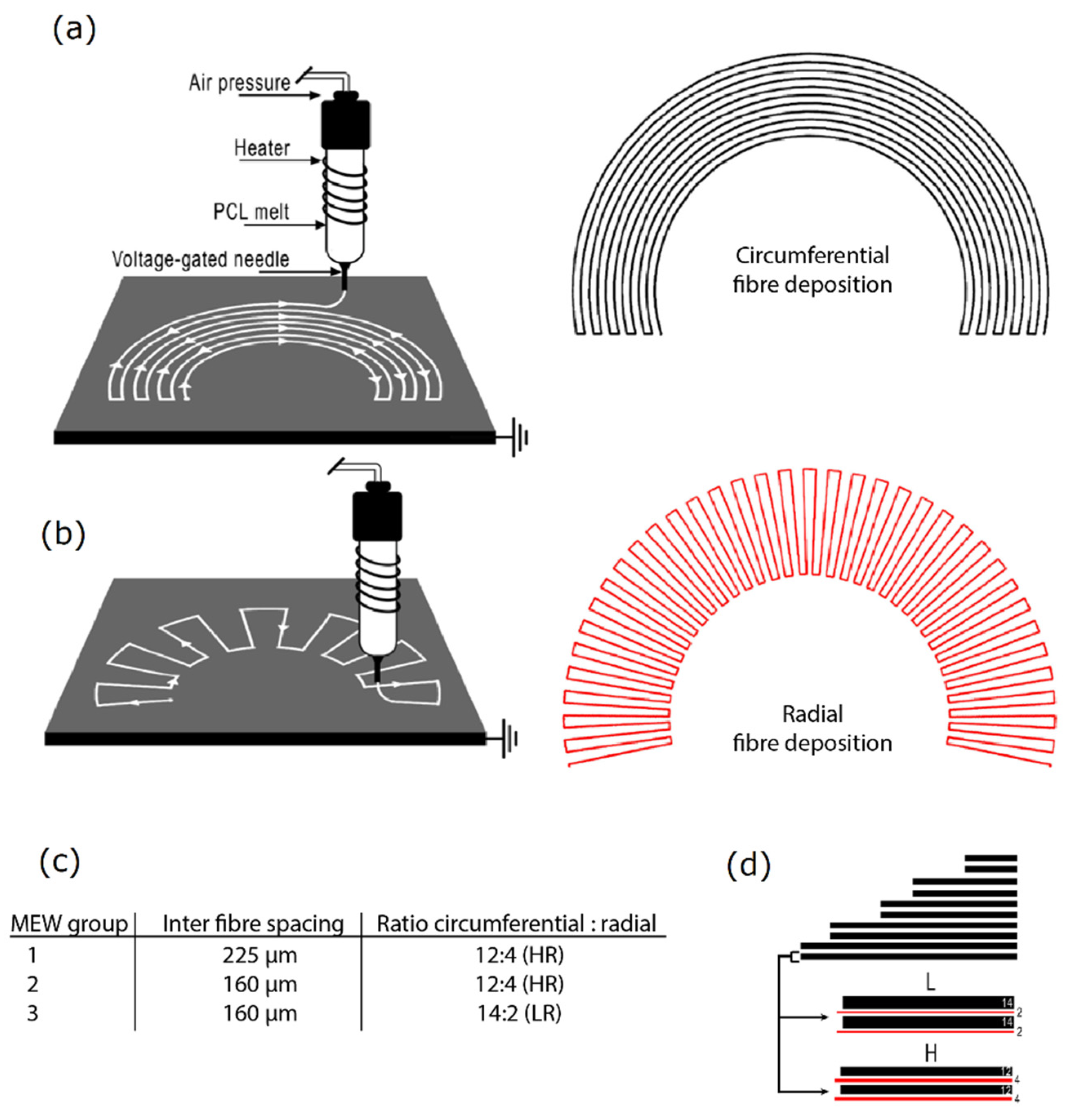
2.1. Scaffold Fabrication
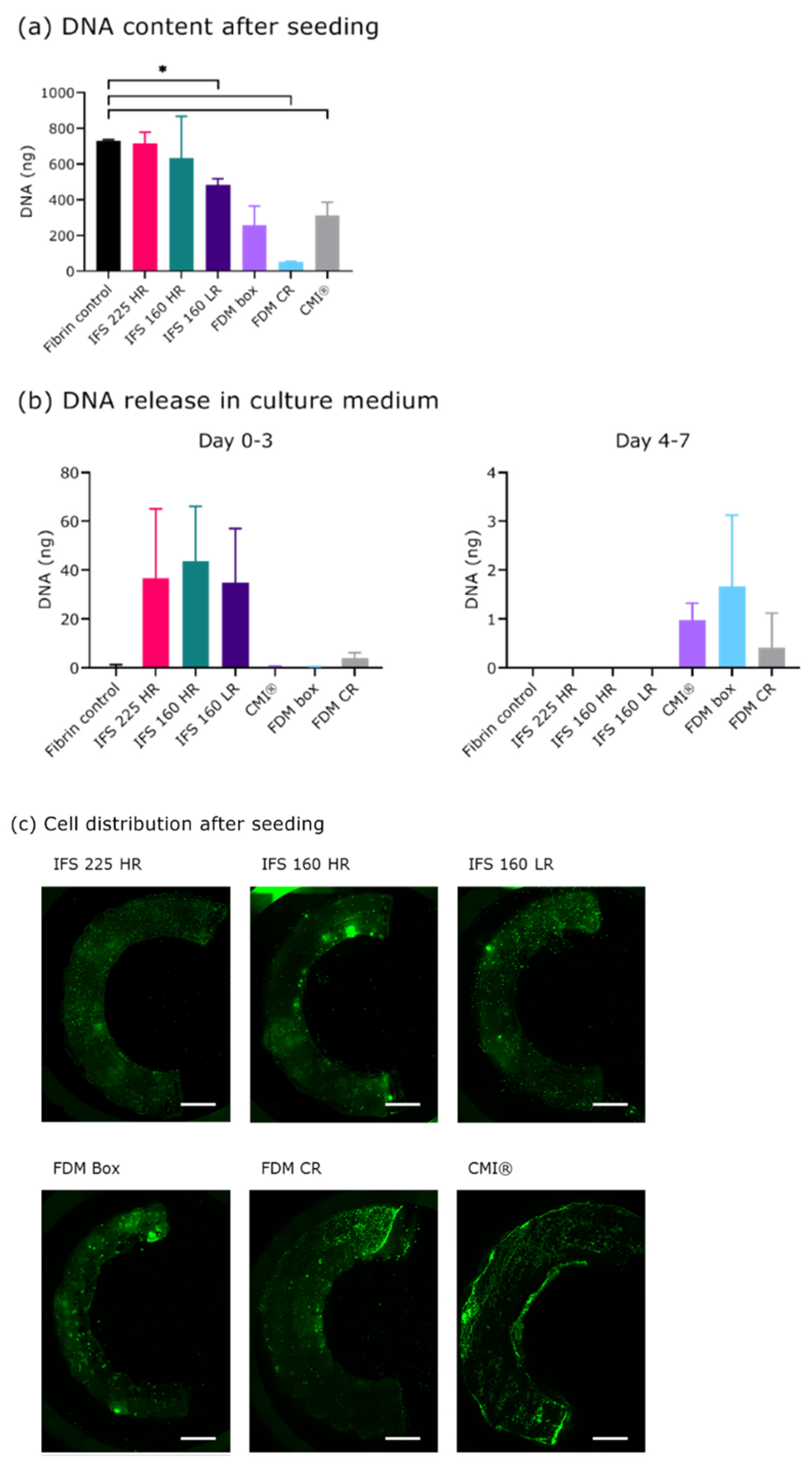
2.2. Seeding and Culture of Scaffolds
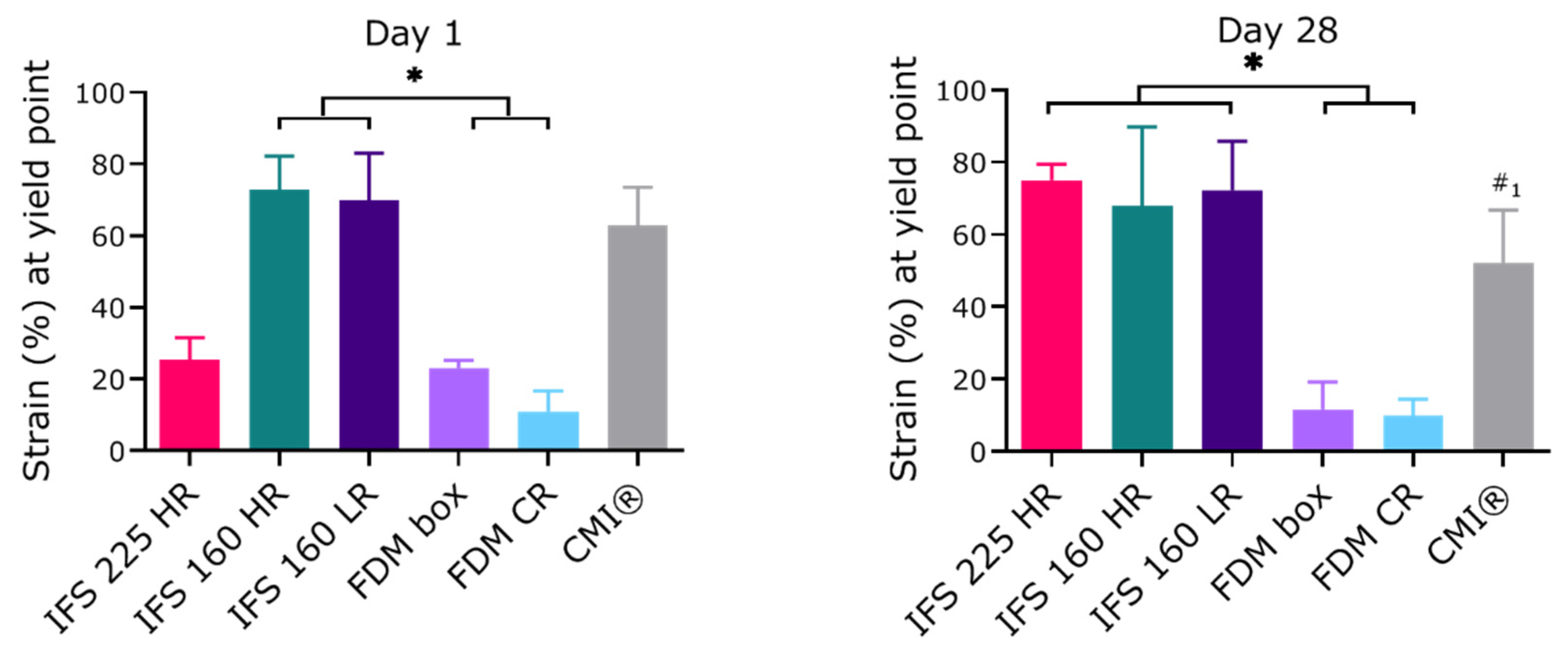
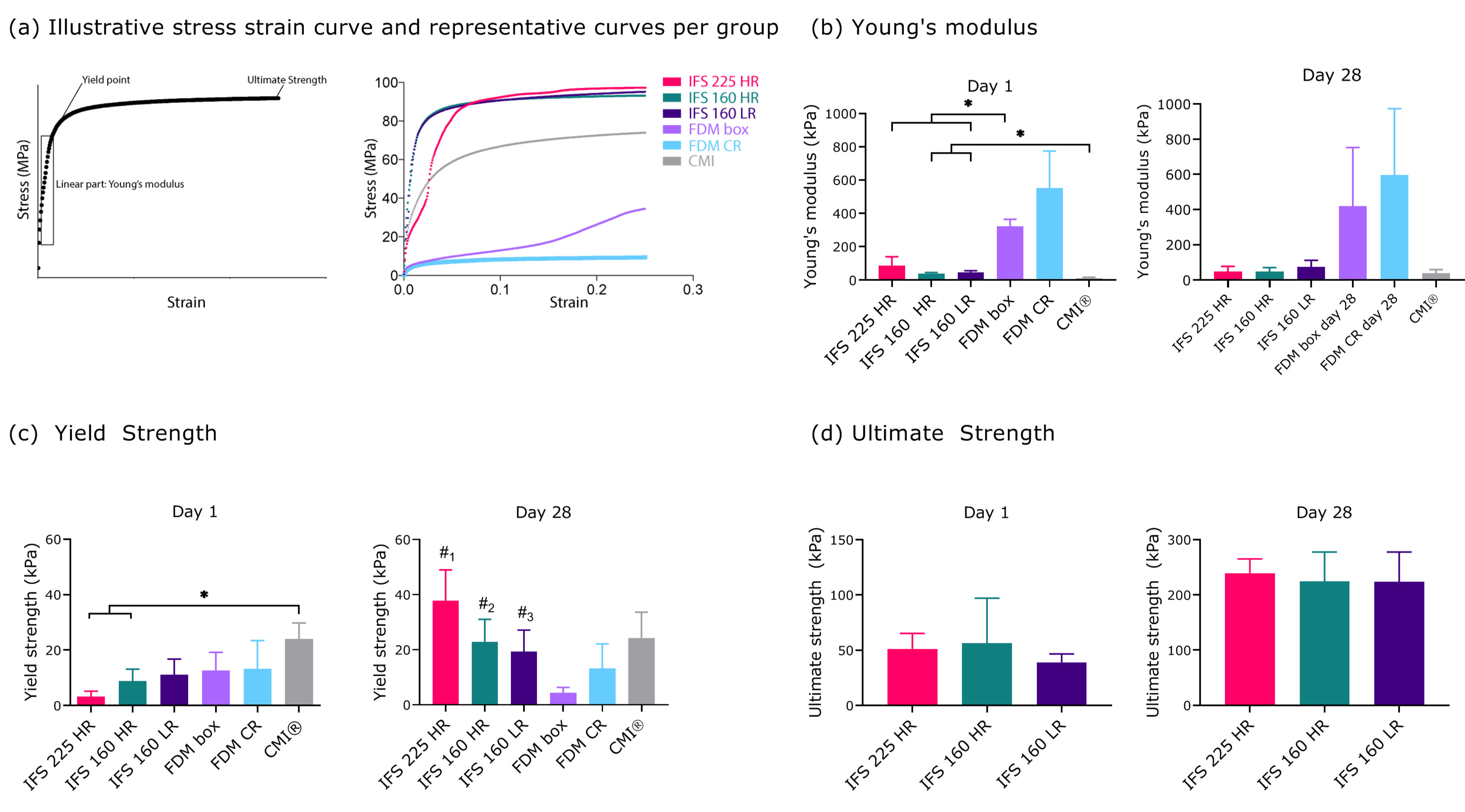
2.3. Mechanical Properties of In Vitro Cultured Scaffolds
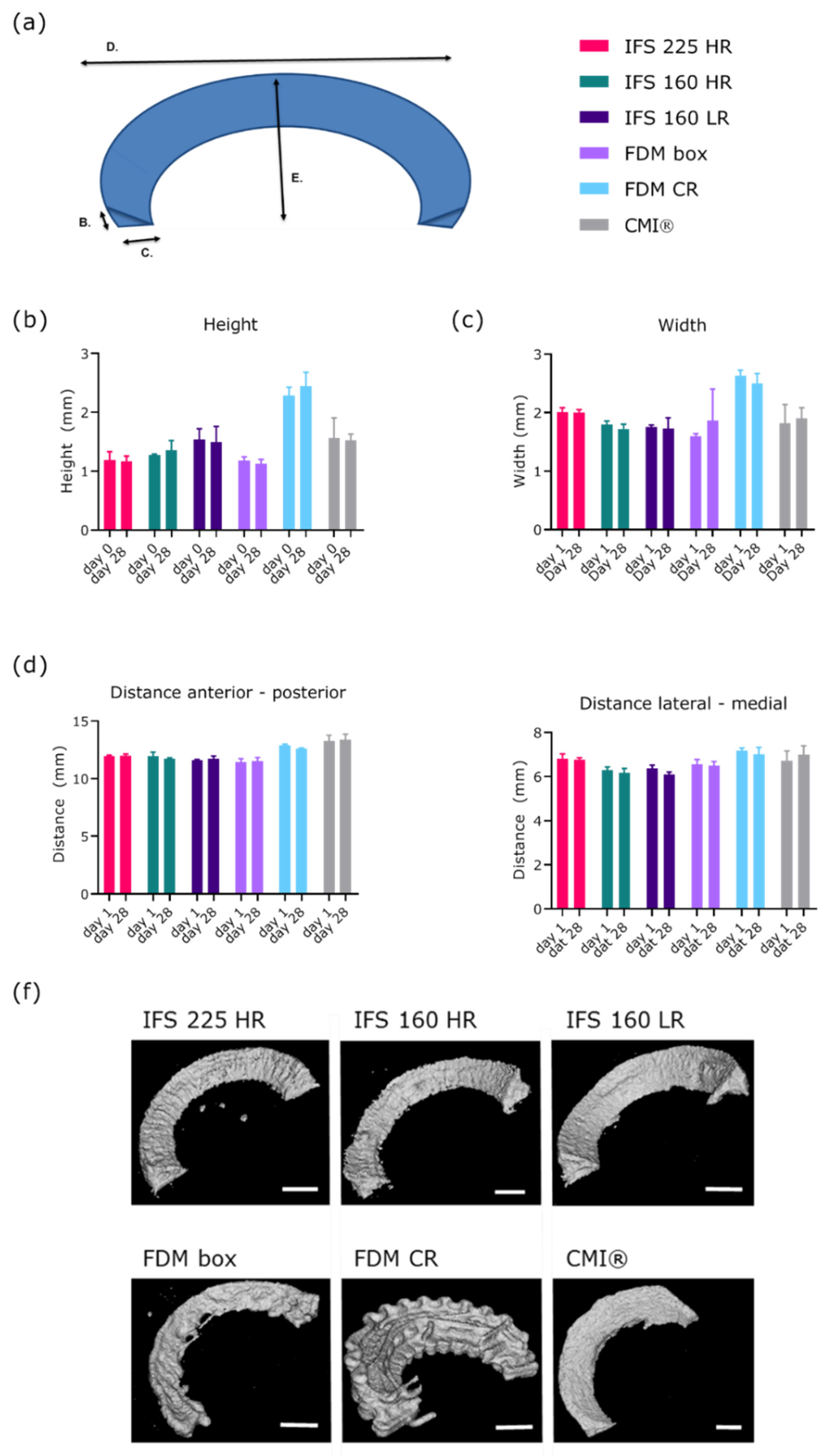
2.4. Scaffold Shape Fidelity
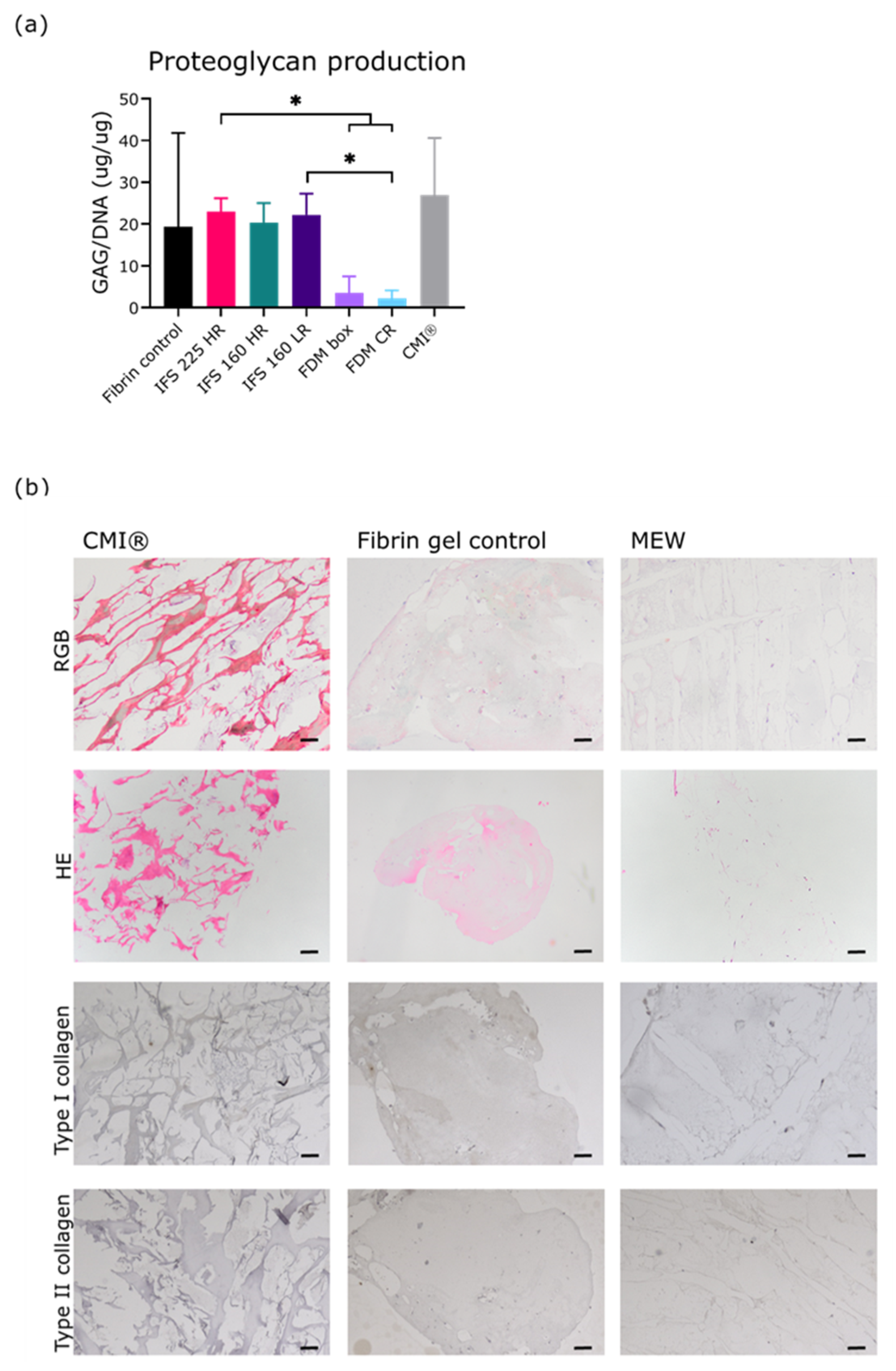
2.5. Extracellular Matrix Formation During 28 Days of Culture
3. Discussion
3.1. Limitations
3.2. Implications
4. Materials and Methods
4.1. Scaffold Design and Printing
4.2. Cell Isolation and Culturing
4.3. Scaffold and CMI® Preparation
4.4. Seeding of The Scaffolds
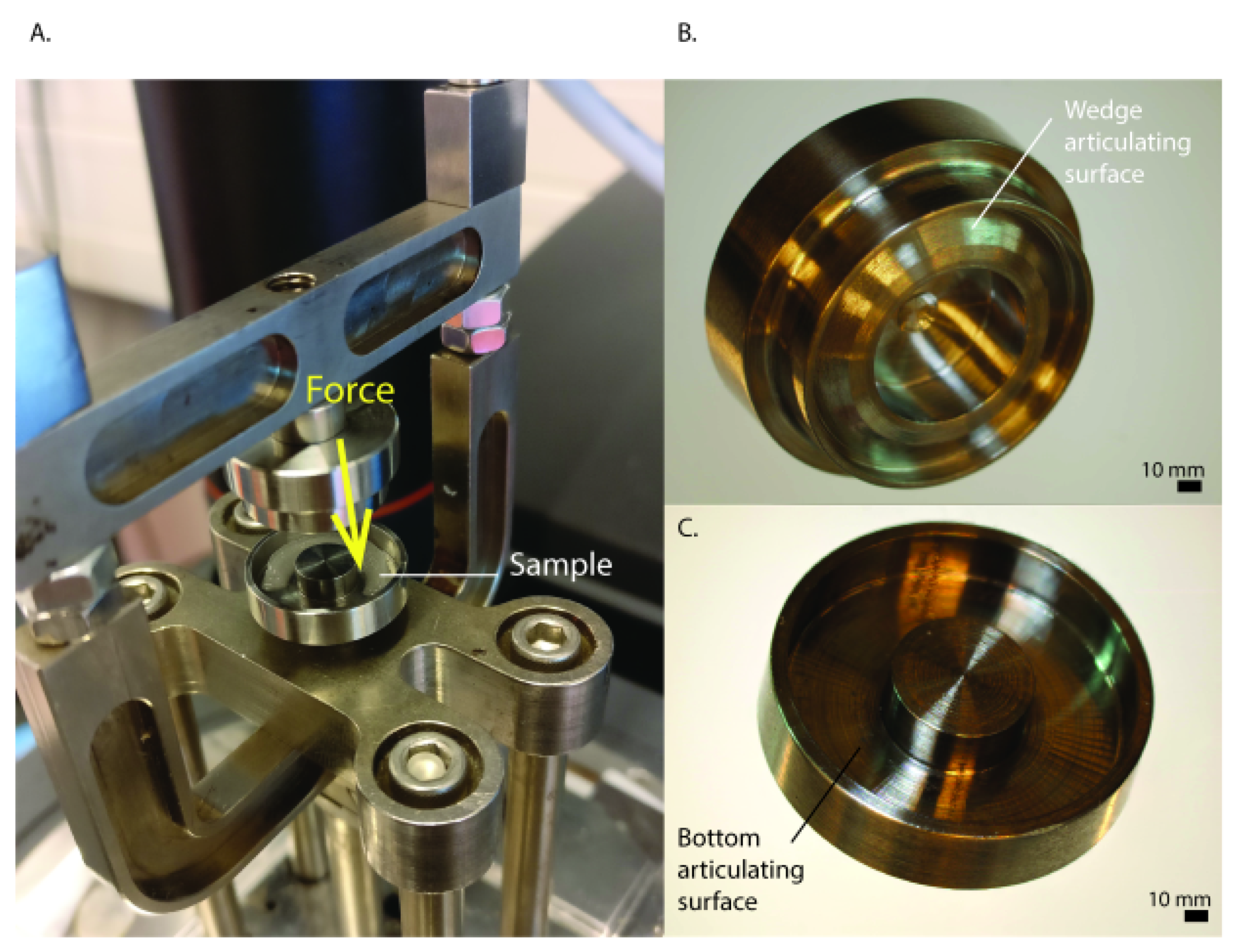
4.5. Mechanical Analysis
4.6. Computed Tomography
4.7. Extracellular Matrix Formation
4.8. Histology
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Melrose, J.; Smith, S.; Cake, M.; Read, R.; Whitelock, J. Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: An ageing study. Histochem. Cell Biol. 2005, 124, 225–235. [Google Scholar] [CrossRef]
- McDevitt, C.A.; Webber, R.J. The ultrastructure and biochemistry of meniscal cartilage. Clin. Orthop. Relat. Res. 1990, 252, 8–18. [Google Scholar] [CrossRef]
- Herwig, J.; Egner, E.; Buddecke, E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann. Rheum. Dis. 1984, 43, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Petersen, W.; Tillmann, B. Collagenous fibril texture of the human knee joint menisci. Anat. Embryol. 1998, 197, 317–324. [Google Scholar] [CrossRef]
- King, D. The healing of semilunar cartilages. Clin. Orthop. Relat. Res. 1990, 252, 4–7. [Google Scholar] [CrossRef]
- Starke, C.; Kopf, S.; Petersen, W.; Becker, R.; Stärke, C.; Kopf, S.; Petersen, W.; Becker, R. Meniscal repair. Arthroscopy 2009, 25, 1033–1044. [Google Scholar] [CrossRef]
- Espejo-Reina, A.; Aguilera, J.; Espejo-Reina, M.J.; Espejo-Reina, M.P.; Espejo-Baena, A. One-Third of Meniscal Tears Are Repairable: An Epidemiological Study Evaluating Meniscal Tear Patterns in Stable and Unstable Knees. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 857–863. [Google Scholar] [CrossRef]
- Englund, M.; Roemer, F.W.; Hayashi, D.; Crema, M.D.; Guermazi, A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat. Rev. Rheumatol. 2012, 8, 412–419. [Google Scholar] [CrossRef]
- McDermott, I.; Amis, A. The consequences of meniscectomy. J. Bone Joint Surg. Br. 2006, 88, 1549–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englund, M.; Roos, E.M.; Lohmander, L.S. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: A sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003, 48, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cantullera, A.N.; Scheffler, S.U.; Monllau, J.C. Meniscus allograft: Organization and regulation in europe and USA. In Surgery of the Meniscus; Hulet, C., Pereira, H., Peretti, G., Denti, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Waugh, N.; Mistry, H.; Metcalfe, A.; Loveman, E.; Colquitt, J.; Royle, P.; Smith, N.A.; Spalding, T. Meniscal allograft transplantation after meniscectomy: Clinical effectiveness and cost-effectiveness. Knee Surgery, Sport. Traumatol. Arthrosc. 2019, 27, 1825–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElAttar, M.; Dhollander, A.; Verdonk, R.; Almqvist, K.F.; Verdonk, P. Twenty-six years of meniscal allograft transplantation: Is it still experimental? A meta-analysis of 44 trials. Knee Surgery, Sport. Traumatol. Arthrosc. 2011, 19, 147–157. [Google Scholar] [CrossRef]
- Zaffagnini, S.; Giordano, G.; Vascellari, A.; Bruni, D.; Neri, M.P.; Iacono, F.; Kon, E.; Presti, M.L.; Marcacci, M. Arthroscopic collagen meniscus implant results at 6 to 8 years follow up. Knee Surgery, Sport. Traumatol. Arthrosc. 2007, 15, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Fortier, L.A.; MaoIchiro, J.J.; Sekiya, I.; Rodeo, S.A. 3D-printed artificial meniscus. In Bio-orthopaedics A New Approach; Springer: Berlin/Heidelberg, Germany, 2017; pp. 419–433. [Google Scholar]
- Lee, C.H.; Rodeo, S.A.; Fortier, L.A.; Lu, C.; Erisken, C.; Mao, J.J. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci. Transl. Med. 2014, 6, 266ra171. [Google Scholar] [CrossRef] [Green Version]
- Warren, P.B.; Huebner, P.; Spang, J.T.; Shirwaiker, R.A.; Fisher, M.B. Engineering 3D-Bioplotted scaffolds to induce aligned extracellular matrix deposition for musculoskeletal soft tissue replacement. Connect. Tissue Res. 2017, 58, 342–354. [Google Scholar] [CrossRef]
- Visser, J.; Melchels, F.P.W.; Jeon, J.E.; Van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.A.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sooriyaarachchi, D.; Wu, J.; Feng, A.; Islam, M.; Tan, G.Z. Hybrid Fabrication of Biomimetic Meniscus Scaffold by 3D Printing and Parallel Electrospinning. Procedia Manuf. 2019, 34, 528–534. [Google Scholar] [CrossRef]
- de Ruijter, M.; Ribeiro, A.; Dokter, I.; Castilho, M.; Malda, J. Simultaneous Micropatterning of Fibrous Meshes and Bioinks for the Fabrication of Living Tissue Constructs. Adv. Healthc. Mater. 2019, 8, 1800418. [Google Scholar] [CrossRef] [Green Version]
- de Windt, T.; Vonk, L.; Slaper-Crtenbach, I.; Nizak, R.; van Rijen, M.H.P.; Saris, D.B.F. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: A first-in-man trial in 35 patients. Stem Cells 2017, 35, 1984–1993. [Google Scholar] [CrossRef] [Green Version]
- Hagmeijer, M.H.; Vonk, L.A.; Fenu, M.; Van Keep, Y.W.A.M.; Krych, A.J.; Saris, D.B.F. Meniscus regeneration combining meniscus and mesenchymal stromal cells in a degradable meniscus implant: An in vitro study. Eur. Cells Mater. 2019, 38, 51–62. [Google Scholar] [CrossRef]
- Hagmeijer, M.H.; Vonk, L.A.; Kouwenhoven, J.W.; Custers, R.J.H.; Bleys, R.L.; Krych, A.J.; Saris, D.B.F. Surgical feasibility of a one-stage cell-based arthroscopic procedure for meniscus regeneration: A cadaveric study. Tissue Eng. Part C Methods 2018, 24, 688–696. [Google Scholar] [CrossRef] [PubMed]
- de Windt, T.S.; Sorel, J.C.; Vonk, L.A.; Kip, M.M.A.; Ijzerman, M.J.; Saris, D.B.F. Early health economic modelling of single-stage cartilage repair. Guiding implementation of technologies in regenerative medicine. J. Tissue Eng. Regen. Med. 2017, 11, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D. Melt electrowriting with additive manufacturing principles. Curr. Opin. Biomed. Eng. 2017, 2, 49–57. [Google Scholar] [CrossRef]
- Robinson, T.M.; Hutmacher, D.W.; Dalton, P.D. The Next Frontier in Melt Electrospinning: Taming the Jet. Adv. Funct. Mater. 2019, 29, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Hochleitner, G.; Jüngst, T.; Brown, T.D.; Hahn, K.; Moseke, C.; Jakob, F.; Dalton, P.D.; Groll, J. Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication 2015, 7, 035002. [Google Scholar] [CrossRef]
- Hochleitner, G.; Youssef, A.; Hrynevich, A.; Haigh, J.N.; Jungst, T.; Groll, J.; Dalton, P.D. Fibre pulsing during melt electrospinning writing. BioNanoMaterials 2016, 17, 159–171. [Google Scholar] [CrossRef]
- Kim, J.; Bakirci, E.; O’Neill, K.L.; Hrynevich, A.; Dalton, P.D. Fiber Bridging during Melt Electrowriting of Poly(ε-Caprolactone) and the Influence of Fiber Diameter and Wall Height. Macromol. Mater. Eng. 2021, 306, 2000685. [Google Scholar] [CrossRef]
- de Ruijter, M.; Hrynevich, A.; Haigh, J.N.; Hochleitner, G.; Castilho, M.; Groll, J.; Malda, J.; Dalton, P.D. Out-of-Plane 3D-Printed Microfibers Improve the Shear Properties of Hydrogel Composites. Small 2018, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hrynevich, A.; Bilge, S.; Haigh, J.N.; Mcmaster, R.; Youssef, A.; Blum, C.; Blunk, T.; Hochleitner, G.; Groll, J.; Dalton, P.D. Dimension-Based Design of Melt Electrowritten Scaffolds. Small 2018, 14, 1–6. [Google Scholar] [CrossRef]
- Liashenko, I.; Hrynevich, A.; Dalton, P.D. Designing Outside the Box: Unlocking the Geometric Freedom of Melt Electrowriting using Microscale Layer Shifting. Adv. Mater. 2020, 32, e2001874. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New English J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Cui, X.; Hasegawa, A.; Lotz, M.; D’Lima, D. Structured three-dimensional co-culture of mesenchymal stem cells with meniscus cells promotes meniscal phenotype without hypertrophy. Biotechnol. Bioeng. 2012, 109, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. A 3D printed PCL/hydrogel construct with zone-specific biochemical composition mimicking that of the meniscus. Biofabrication 2019, 11, 025002. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Mandal, B.B. A three-dimensional printed silk-based biomimetic tri-layered meniscus for potential patient-specific implantation. Biofabrication 2020, 12, 015003. [Google Scholar] [CrossRef] [PubMed]
- Diloksumpan, P.; Abinzano, F.; de Ruijter, M.; Mensinga, A.; Plomp, S.; Khan, I.; Brommer, H.; Smit, I.; Dias Castilho, M.; van Weeren, P.R.; et al. The Complexity of Joint Regeneration: How an Advanced Implant could Fail by Its In Vivo Proven Bone Component. J. Trial Error 2021, 1–18. [Google Scholar]
- Wunner, F.M.; Wille, M.L.; Noonan, T.G.; Bas, O.; Dalton, P.D.; De-Juan-Pardo, E.M.; Hutmacher, D.W. Melt Electrospinning Writing of Highly Ordered Large Volume Scaffold Architectures. Adv. Mater. 2018, 30, 1706570. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.B.; Williams, J.M.; Hallab, N.; Virdi, A.; Yanke, A.; Cole, B.J. Multiple Freeze-Thaw Cycled Meniscal Allograft Tissue: A Biomechanical, Biochemical, and Histologic Analysis. J. Orthop. Res. 2008, 26, 49–55. [Google Scholar] [CrossRef]
- Pauli, C.; Grogan, S.P.; Patil, S.; Otsuki, S.; Hasegawa, A.; Koziol, J.; Lotz, M.K. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Federa Human Tissue and Medical Research: Code of Conduct for Responsible Use. Available online: https://www.federa.org/code-goed-gebruik (accessed on 29 April 2020).
- Hedrich, H.C.; Simunek, M.; Reisinger, S.; Ferguson, J.; Gulle, H.; Goppelt, A.; Redl, H. Fibrin chain cross-linking, fibrinolysis, and in vivo sealing efficacy of differently structured fibrin sealants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1507–1512. [Google Scholar] [CrossRef]
- Gaytan, F.; Morales, C.; Reymundo, C.; Tena-Sempere, M. A novel RGB-trichrome staining method for routine histological analysis of musculoskeletal tissues. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korpershoek, J.V.; Ruijter, M.d.; Terhaard, B.F.; Hagmeijer, M.H.; Saris, D.B.F.; Castilho, M.; Malda, J.; Vonk, L.A. Potential of Melt Electrowritten Scaffolds Seeded with Meniscus Cells and Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2021, 22, 11200. https://doi.org/10.3390/ijms222011200
Korpershoek JV, Ruijter Md, Terhaard BF, Hagmeijer MH, Saris DBF, Castilho M, Malda J, Vonk LA. Potential of Melt Electrowritten Scaffolds Seeded with Meniscus Cells and Mesenchymal Stromal Cells. International Journal of Molecular Sciences. 2021; 22(20):11200. https://doi.org/10.3390/ijms222011200
Chicago/Turabian StyleKorpershoek, Jasmijn V., Mylène de Ruijter, Bastiaan F. Terhaard, Michella H. Hagmeijer, Daniël B.F. Saris, Miguel Castilho, Jos Malda, and Lucienne A. Vonk. 2021. "Potential of Melt Electrowritten Scaffolds Seeded with Meniscus Cells and Mesenchymal Stromal Cells" International Journal of Molecular Sciences 22, no. 20: 11200. https://doi.org/10.3390/ijms222011200
APA StyleKorpershoek, J. V., Ruijter, M. d., Terhaard, B. F., Hagmeijer, M. H., Saris, D. B. F., Castilho, M., Malda, J., & Vonk, L. A. (2021). Potential of Melt Electrowritten Scaffolds Seeded with Meniscus Cells and Mesenchymal Stromal Cells. International Journal of Molecular Sciences, 22(20), 11200. https://doi.org/10.3390/ijms222011200







