Detection and Evaluation of Serological Biomarkers to Predict Osteoarthritis in Anterior Cruciate Ligament Transection Combined Medial Meniscectomy Rat Model
Abstract
1. Introduction
2. Results
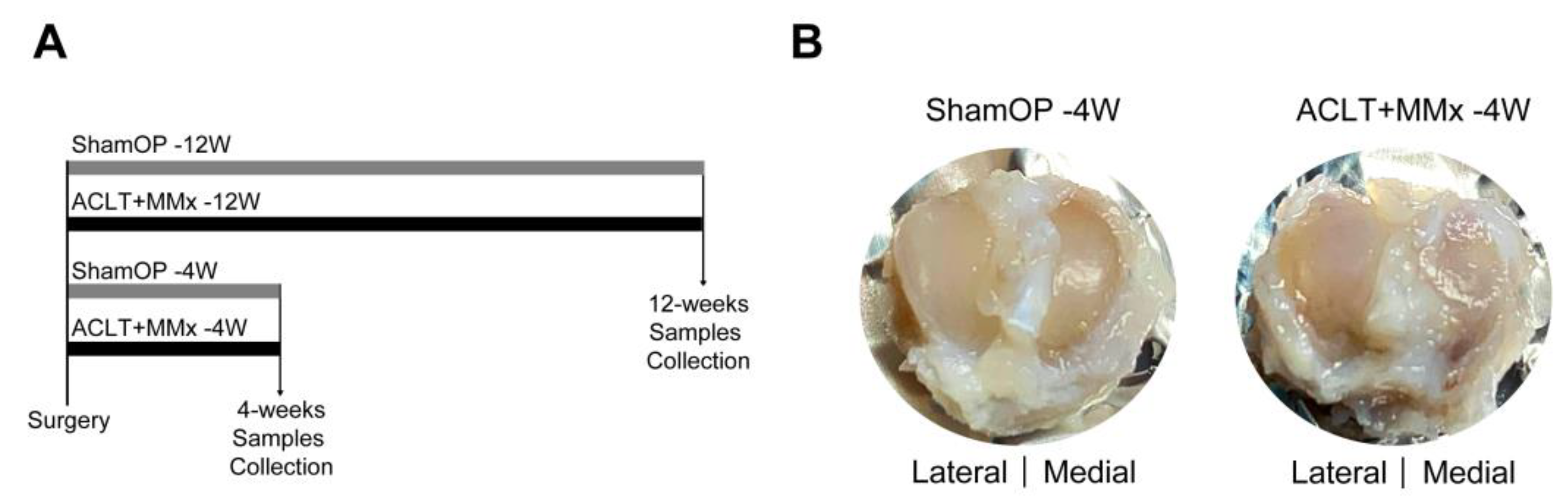
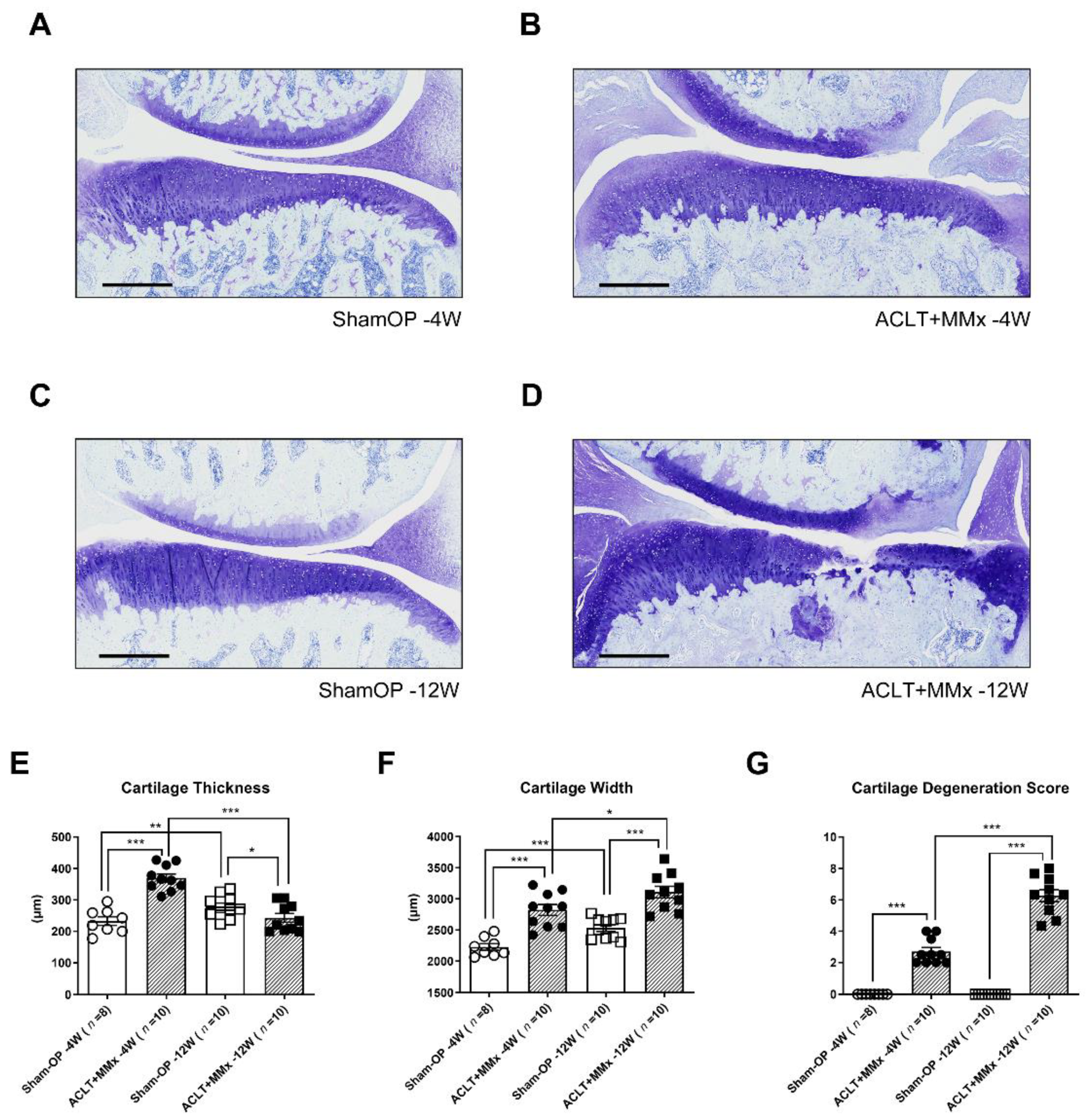
2.1. ACLT+MMx Induced Changes in Tibial Cartilage Morphology
2.2. Blood and Biochemical Composition of ACLT+MMx-Induced OA Rats
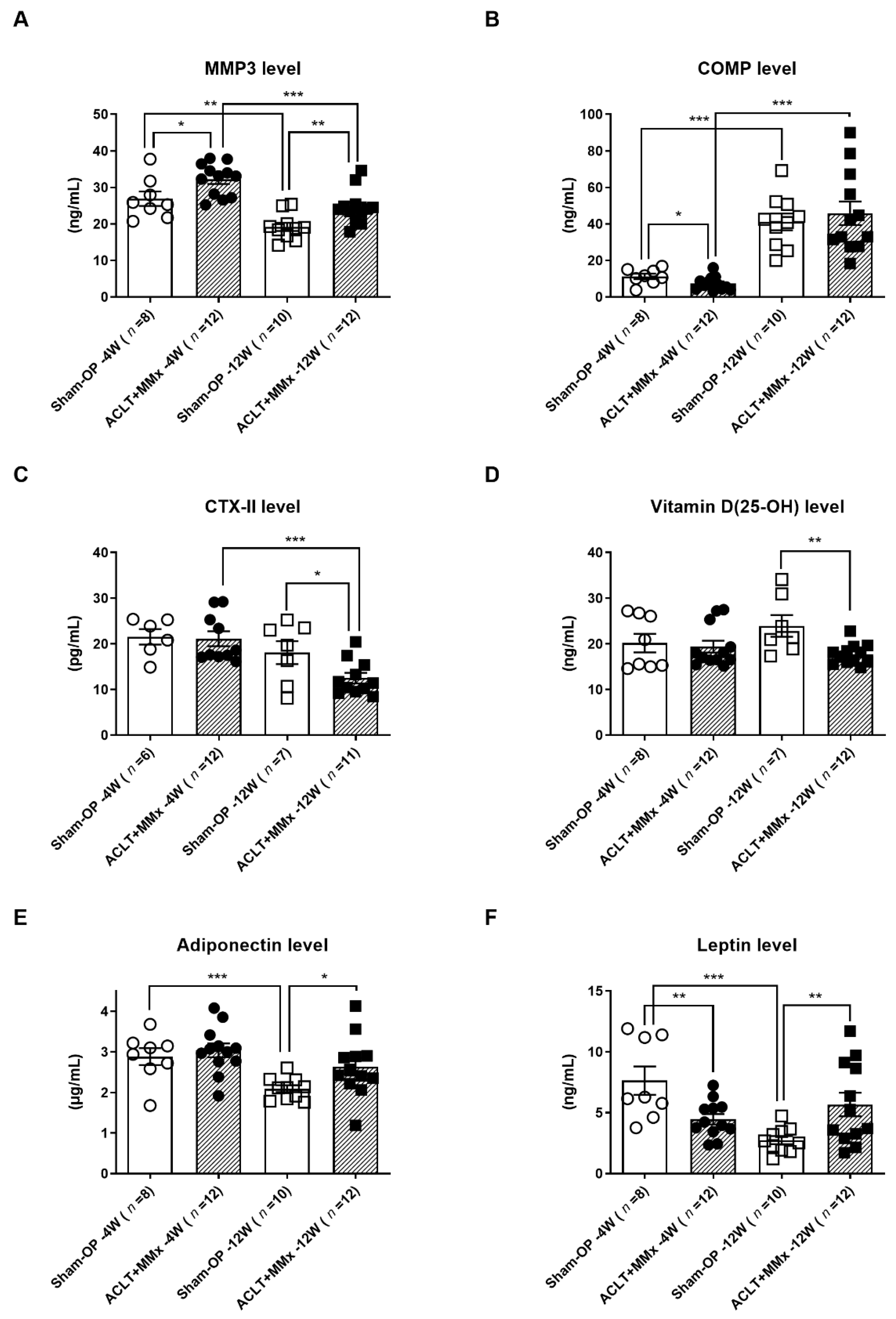
2.3. Serum Level OA Biomarkers’ Detection and Evaluation
3. Discussion
4. Materials and Methods
4.1. ACLT+MMx-Induced OA Animal Model
4.2. Histological Examination of the Operated Knee Joint
4.3. Complete Blood Count and Biochemical Assays
4.4. Enzyme-Linked Immunosorbent Assay (ELISA) and ElectroChemiluminescence Binding Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.; Murphy, E.; Dacre, J. Osteoarthritis. Postgrad. Med. J. 2003, 79, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Sakalauskiene, G.; Jauniskiene, D. Osteoarthritis: Etiology, epidemiology, impact on the individual and society and the main principles of management. Medicina 2010, 46, 790. [Google Scholar] [CrossRef]
- Corti, M.C.; Rigon, C. Epidemiology of osteoarthritis: Prevalence, risk factors and functional impact. Aging Clin. Exp. Res. 2003, 15, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Bodur, H. Current review on osteoarthritis in Turkey and the world; Epidemiology and socioeconomic aspect. Turk. J. Geriatr. 2011, 14, 7–14. [Google Scholar]
- Cleveland, R.J.; Alvarez, C.; Schwartz, T.A.; Losina, E.; Renner, J.B.; Jordan, J.M.; Callahan, L.F. The impact of painful knee osteoarthritis on mortality: A community-based cohort study with over 24 years of follow-up. Osteoarthr. Cartil. 2019, 27, 593–602. [Google Scholar] [CrossRef]
- Nelson, A.E. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef]
- Leite, A.A.; Costa, A.J.G.; de Lima, B.D.M.; Padilha, A.V.L.; de Albuquerque, E.C.; Marques, C.D.L. Comorbidities in patients with osteoarthritis: Frequency and impact on pain and physical function. Rev. Bras. Reumatol. 2011, 51, 113–123. [Google Scholar]
- Hart, O.R.; Uden, R.M.; McMullan, J.E.; Ritchie, M.S.; Williams, T.D.; Smith, B.H. A study of National Health Service management of chronic osteoarthritis and low back pain. Prim. Health Care Res. Dev. 2015, 16, 157–166. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Yerges-Armstrong, L.; Yau, M.; Mitchell, B.D. Genetic epidemiology of osteoarthritis: Recent developments and future directions. Curr. Opin. Rheumatol. 2013, 25, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, A.; Petersson, I.F.; Bjork, J.; Hawker, G.; Dahlberg, L.E.; Lohmander, L.S.; Englund, M. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthr. Cartil. 2014, 22, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Alabajos-Cea, A.; Herrero-Manley, L.; Suso-Marti, L.; Alonso-Perez-Barquero, J.; Viosca-Herrero, E. Are Psychosocial Factors Determinant in the Pain and Social Participation of Patients with Early Knee Osteoarthritis? A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 4575. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Metcalf, B.; Bennell, K.L.; Zhang, Y.Q.; Gross, K.D.; Mills, K.; Deveza, L.A.; Robbins, S.R.; Hunter, D.J. Is Heel Height Associated with Pain Exacerbations in Hip Osteoarthritis Patients?-Results from a Case-Crossover Study. J. Clin. Med. 2020, 9, 1872. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, S.; Konstantinidis, L. The Diagnosis of Osteoarthritis. Aktuelle Rheumatol. 2020, 45, 39–47. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef]
- Hayami, T.; Pickarski, M.; Zhuo, Y.; Wesolowski, G.A.; Rodan, G.A.; Duong, L.T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 2006, 38, 234–243. [Google Scholar] [CrossRef]
- Chen, I.J.; Lin, S.H.; Wong, C.S. Oral shea nut oil triterpene concentrate supplement ameliorates pain and histological assessment of articular cartilage deterioration in an ACLT injured rat knee osteoarthritis model. PLoS ONE 2019, 14, e0215812. [Google Scholar] [CrossRef]
- Chen, I.J.; Wong, C.S. Shea Nut Oil Extracts Enhance the Intra-Articular Sodium Hyaluronate Effectiveness on Surgically Induced OA Progression in Rats. Nutrients 2020, 12, 957. [Google Scholar] [CrossRef]
- Pickarski, M.; Hayami, T.; Zhuo, Y.; Duong, L.T. Molecular changes in articular cartilage and subchondral bone in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. BMC Musculoskelet. Disord. 2011, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Piskin, A.; Gulbahar, M.Y.; Tomak, Y.; Gulman, B.; Hokelek, M.; Kerimoglu, S.; Koksal, B.; Alic, T.; Kabak, Y.A. Osteoarthritis models after anterior cruciate ligament resection and medial meniscectomy in rats—A histological and immunohistochemical study. Saudi Med. J. 2007, 28, 1796–1802. [Google Scholar] [PubMed]
- Watt, F.E. Osteoarthritis biomarkers: Year in review. Osteoarthr. Cartil. 2018, 26, 312–318. [Google Scholar] [CrossRef]
- Bauer, D.C.; Hunter, D.J.; Abramson, S.B.; Attur, M.; Corr, M.; Felson, D.; Heinegard, D.; Jordan, J.M.; Kepler, T.B.; Lane, N.E.; et al. Classification of osteoarthritis biomarkers: A proposed approach. Osteoarthr. Cartil. 2006, 14, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.W.; Zhang, H.; Yager, T.D.; Nossova, N.; Dempsey, A.; Zheng, R.; Han, M.; Tang, H.; Chao, S.; Liew, C.C. Blood-based biomarkers for detecting mild osteoarthritis in the human knee. Osteoarthr. Cartil. 2005, 13, 861–871. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.W.; Kawcak, C.E.; Frisbie, D.D.; Little, C.B.; Clegg, P.D.; Peffers, M.J.; Karsdal, M.A.; Ekman, S.; Laverty, S.; Slayden, R.A.; et al. Biomarkers for equine joint injury and osteoarthritis. J. Orthop. Res. 2018, 36, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Sanchez, C.; Bay-Jensen, A.C.; Mobasheri, A. Osteoarthritis biomarkers derived from cartilage extracellular matrix: Current status and future perspectives. Ann. Phys. Rehabil. Med. 2016, 59, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Legrand, C.B.; Lambert, C.J.; Comblain, F.V.; Sanchez, C.; Henrotin, Y.E. Review of Soluble Biomarkers of Osteoarthritis: Lessons From Animal Models. Cartilage 2017, 8, 211–233. [Google Scholar] [CrossRef]
- Patra, D.; Sandell, L.J. Recent advances in biomarkers in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 465–470. [Google Scholar] [CrossRef]
- Ravalli, S.; Szychlinska, M.A.; Lauretta, G.; di Rosa, M.; Musumeci, G. Investigating lubricin and known cartilage-based biomarkers of osteoarthritis. Expert Rev. Mol. Diagn. 2020, 20, 443–452. [Google Scholar] [CrossRef]
- Schmitz, N.; Laverty, S.; Kraus, V.B.; Aigner, T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartil. 2010, 18, S113–S116. [Google Scholar] [CrossRef] [PubMed]
- Van Spil, W.E.; Szilagyi, I.A. Osteoarthritis year in review 2019: Biomarkers (biochemical markers). Osteoarthr. Cartil. 2020, 28, 296–315. [Google Scholar] [CrossRef]
- Ma, T.W.; Zhang, Z.H.; Song, X.P.; Bai, H.; Li, Y.; Li, X.R.; Zhao, J.H.; Ma, Y.Q.; Gao, L. Combined detection of COMP and CS846 biomarkers in experimental rat osteoarthritis: A potential approach for assessment and diagnosis of osteoarthritis. J. Orthop. Surg. Res. 2018, 13, 230. [Google Scholar] [CrossRef]
- Shargorodsky, M.; Boaz, M.; Goldberg, Y.; Matas, Z.; Gavish, D.; Fux, A.; Wolfson, N. Adiponectin and vascular properties in obese patients: Is it a novel biomarker of early atherosclerosis? Int. J. Obes. 2009, 33, 553–558. [Google Scholar] [CrossRef]
- Alonso, B.; Bravo, B.; Mediavilla, L.; Gortazar, A.R.; Forriol, F.; Vaquero, J.; Guisasola, M.C. Osteoarthritis-related biomarkers profile in chronic anterior cruciate ligament injured knee. Knee 2020, 27, 51–60. [Google Scholar] [CrossRef]
- Convill, J.G.; Tawy, G.F.; Freemont, A.J.; Biant, L.C. Clinically Relevant Molecular Biomarkers for Use in Human Knee Osteoarthritis: A Systematic Review. Cartilage 2020. [Google Scholar] [CrossRef] [PubMed]
- Bernotiene, E.; Bagdonas, E.; Kirdaite, G.; Bernotas, P.; Kalvaityte, U.; Uzieliene, I.; Thudium, C.S.; Hannula, H.; Lorite, G.S.; Dvir-Ginzberg, M.; et al. Emerging Technologies and Platforms for the Immunodetection of Multiple Biochemical Markers in Osteoarthritis Research and Therapy. Front. Med. 2020, 7, 572977. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B. Osteoarthritis year 2010 in review: Biochemical markers. Osteoarthr. Cartil. 2011, 19, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Acta Med. Port. 2015, 28, 99–106. [Google Scholar] [CrossRef]
- Frisbie, D.D.; Al-Sobayil, F.; Billinghurst, R.C.; Kawcak, C.E.; McLlwraith, C.W. Changes in synovial fluid and serum biomarkers with exercise and early osteoarthritis in horses. Osteoarthr. Cartil. 2008, 16, 1196–1204. [Google Scholar] [CrossRef]
- Attur, M.; Krasnokutsky-Samuels, S.; Samuels, J.; Abramson, S.B. Prognostic biomarkers in osteoarthritis. Curr. Opin. Rheumatol. 2013, 25, 136–144. [Google Scholar] [CrossRef]
- Fernandez-Puente, P.; Mateos, J.; Fernandez-Costa, C.; Oreiro, N.; Fernandez-Lopez, C.; Ruiz-Romero, C.; Blanco, F.J. Identification of a Panel of Novel Serum Osteoarthritis Biomarkers. J. Proteome Res. 2011, 10, 5095–5101. [Google Scholar] [CrossRef]
- McAlindon, T.; Bartnik, E.; Ried, J.S.; Teichert, L.; Herrmann, M.; Flechsenhar, K. Determination of serum biomarkers in osteoarthritis patients: A previous interventional imaging study revisited. J. Biomed. Res. 2017, 31, 25–30. [Google Scholar] [CrossRef]
- Goode, A.P.; Nelson, A.E.; Kraus, V.B.; Renner, J.B.; Jordan, J.M. Biomarkers reflect differences in osteoarthritis phenotypes of the lumbar spine: The Johnston County Osteoarthritis Project. Osteoarthr. Cartil. 2017, 25, 1672–1679. [Google Scholar] [CrossRef]
- Kung, L.H.W.; Zaki, S.; Ravi, V.; Rowley, L.; Smith, M.M.; Bell, K.M.; Bateman, J.F.; Little, C.B. Utility of circulating serum miRNAs as biomarkers of early cartilage degeneration in animal models of post-traumatic osteoarthritis and inflammatory arthritis. Osteoarthr. Cartil. 2017, 25, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.D.; Messier, S.P.; Legault, C.; Lenz, M.E.; Thonar, E.; Loeser, R.F. Effect of an exercise and dietary intervention on serum biomarkers in overweight and obese adults with osteoarthritis of the knee. Osteoarthr. Cartil. 2008, 16, 1047–1053. [Google Scholar] [CrossRef]
- Kraus, V.B.; Burnett, B.; Coindreau, J.; Cottrell, S.; Eyre, D.; Gendreau, M.; Gardiner, J.; Garnero, P.; Hardin, J.; Henrotin, Y.; et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 515–542. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y. Osteoarthritis year 2011 in review: Biochemical markers of osteoarthritis: An overview of research and initiatives. Osteoarthr. Cartil. 2012, 20, 215–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joseph, G.B.; Nevitt, M.C.; McCulloch, C.E.; Neumann, J.; Lynch, J.A.; Heilmeier, U.; Lane, N.E.; Link, T.M. Associations between molecular biomarkers and MR-based cartilage composition and knee joint morphology: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2018, 26, 1070–1077. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Raynauld, J.P.; Mineau, F.; Abram, F.; Paiement, P.; Delorme, P.; Pelletier, J.P. Levels of serum biomarkers from a two-year multicentre trial are associated with treatment response on knee osteoarthritis cartilage loss as assessed by magnetic resonance imaging: An exploratory study. Arthritis Res. Ther. 2017, 19, 169. [Google Scholar] [CrossRef]
- De Bakker, E.; Broeckx, B.; Demeyere, K.; Stroobants, V.; van Ryssen, B.; Meyer, E. Detection of osteoarthritis in dogs by metabolic, pro-inflammatory and degenerative synovial fluid biomarkers and traditional radiographic screening: A pilot study. Vet. Immunol. Immunopathol. 2021, 237, 110252. [Google Scholar] [CrossRef]
- Van den Berg, R.; Jongbloed, E.M.; de Schepper, E.I.T.; Bierma-Zeinstra, S.M.A.; Koes, B.W.; Luijsterburg, P.A.J. The association between pro-inflammatory biomarkers and nonspecific low back pain: A systematic review. Spine J. 2018, 18, 2140–2151. [Google Scholar] [CrossRef]
- Mundermann, A.; Geurts, J.; Hugle, T.; Nickel, T.; Schmidt-Trucksass, A.; Halle, M.; Hanssen, H. Marathon performance but not BMI affects post-marathon pro-inflammatory and cartilage biomarkers. J. Sports Sci. 2017, 35, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Bertuglia, A.; Pagliara, E.; Grego, E.; Ricci, A.; Brkljaca-Bottegaro, N. Pro-inflammatory cytokines and structural biomarkers are effective to categorize osteoarthritis phenotype and progression in Standardbred racehorses over five years of racing career. BMC Vet. Res. 2016, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Rajandran, S.N.; Ma, C.A.; Tan, J.R.; Liu, J.; Wong, S.B.S.; Leung, Y.Y. Exploring the Association of Innate Immunity Biomarkers With MRI Features in Both Early and Late Stages Osteoarthritis. Front. Med. 2020, 7, 554669. [Google Scholar] [CrossRef] [PubMed]
- Heard, B.J.; Rosvold, J.M.; Fritzler, M.J.; El-Gabalawy, H.; Wiley, J.P.; Krawetz, R.J. A computational method to differentiate normal individuals, osteoarthritis and rheumatoid arthritis patients using serum biomarkers. J. R. Soc. Interface 2014, 11, 20140428. [Google Scholar] [CrossRef]
- Georgiev, T.; Ivanova, M.; Kopchev, A.; Velikova, T.; Miloshov, A.; Kurteva, E.; Yuzeir, K.; Penkov, M.; Kabakchieva, P.; Rashkov, R.; et al. Cartilage oligomeric protein, matrix metalloproteinase-3, and Coll2-1 as serum biomarkers in knee osteoarthritis: A cross-sectional study. Rheumatol. Int. 2018, 38, 821–830. [Google Scholar] [CrossRef]
- Sharif, M.; Granell, R.; Johansen, J.; Clarke, S.; Elson, C.; Kirwan, J.R. Serum cartilage oligomeric matrix protein and other biomarker profiles in tibiofemoral and patellofemoral osteoarthritis of the knee. Rheumatology 2006, 45, 522–526. [Google Scholar] [CrossRef]
- Ni, G.X.; Zhan, L.Q.; Gao, M.Q.; Lei, L.; Zhou, Y.Z.; Pan, Y.X. Matrix metalloproteinase-3 inhibitor retards treadmill running-induced cartilage degradation in rats. Arthritis Res. Ther. 2011, 13, R192. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, K.; Xu, J.; Ruan, G.; Zhu, Q.; Cai, J.; Ren, J.; Zheng, S.; Zhu, Z.; Otahal, P.; et al. Associations between serum ghrelin and knee symptoms, joint structures and cartilage or bone biomarkers in patients with knee osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1428–1435. [Google Scholar] [CrossRef]
- Abd-Allah, S.H.; Shalaby, S.M.; Pasha, H.F.; El-Shal, A.S.; Abou El-Saoud, A.M. Variation of Matrix Metalloproteinase 1 and 3 Haplotypes and Their Serum Levels in Patients with Rheumatoid Arthritis and Osteoarthritis. Genet. Test. Mol. Biomark. 2012, 16, 15–20. [Google Scholar] [CrossRef]
- Oldenburg, J.; Zimmermann, R.; Katsarou, O.; Zanon, E.; Kellermann, E.; Lundin, B.; Ellinghaus, P. Potential biomarkers of haemophilic arthropathy: Correlations with compatible additive magnetic resonance imaging scores. Haemophilia 2016, 22, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Appleton, C.T.G.; Usmani, S.E.; Pest, M.A.; Pitelka, V.; Mort, J.S.; Beier, F. Reduction in Disease Progression by Inhibition of Transforming Growth Factor alpha-CCL2 Signaling in Experimental Posttraumatic Osteoarthritis. Arthritis Rheumatol. 2015, 67, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, J.H.; Kim, J.; Baek, W.K.; Rhee, J.; Kim, T.H.; Kim, S.H.; Kim, K.P.; Son, C.N.; Kim, J.S. Proteomic analysis of human synovial fluid reveals potential diagnostic biomarkers for ankylosing spondylitis. Clin. Proteom. 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Gao, Y.H.; Liu, B.; Zhao, C.W.; Yang, C.; Li, S.Q.; Liu, J.G.; Qi, X. Differential expression of adipokines in knee osteoarthritis patients with and without metabolic syndrome. Int. Orthop. 2018, 42, 1283–1289. [Google Scholar] [CrossRef]
| Sham-OP-12W | ACLT+MMx-12W | |
|---|---|---|
| Complete Blood Count (CBC) | ||
| WBC (103 cells/µL) | 6.59 ± 0.44 | 5.42 ± 0.35 |
| RBC (106 cells/µL) | 8.17 ± 0.12 | 8.32 ± 0.12 |
| HGB (g/dL) | 13.83 ± 0.18 | 13.92 ± 0.11 |
| HCT (%) | 41.40 ± 0.52 | 41.28 ± 0.37 |
| PLT (103 cells/µL) | 1189 ± 32 | 1066 ± 28 |
| Metabolic Profile | ||
| Glucose (mg/dL) | 159.70 ± 3.62 | 160.52 ± 5.96 |
| Total Cholesterol (mg/dL) | 85.41 ± 3.05 | 83.26 ± 5.51 |
| TG (mg/dL) | 58.98 ± 7.35 | 66.61 ± 8.09 |
| HDL (mg/dL) | 48.39 ± 2.10 | 47.83 ± 4.11 |
| LDL (mg/dL) | 20.53 ± 1.66 | 16.83 ± 1.67 |
| Bone Electrolytes | ||
| Calcium (mg/dL) | 10.43 ± 0.01 | 10.68 ± 0.01 |
| Phosphorus (mg/dL) | 6.60 ± 0.13 | 6.28 ± 0.12 |
| Magnesium (mg/dL) | 2.26 ± 0.02 | 2.19 ± 0.04 |
| Sham-OP-4W | ACLT+MMx-4W | Sham-OP-12W | ACLT+MMx-12W | |
|---|---|---|---|---|
| Inflammatory Marker | ||||
| IL-1β (pg/mL) | ND | ND | Below Detection Limit | Below Detection Limit |
| TNF-α (pg/mL) | ND | ND | Below Detection Limit | Below Detection Limit |
| IL-6 (pg/mL) | ND | ND | Below Detection Limit | Below Detection Limit |
| TGF-β1 (ng/mL) | ND | ND | 96.13 ± 14.47 | 82.70 ± 7.80 |
| C-Reactive Protein (μg/mL) | ND | ND | 333.71 ± 13.27 | 353.86 ± 12.60 |
| Bone and Cartilage Marker | ||||
| Vitamin D (25-OH) (ng/mL) | 20.17 ± 2.03 | 19.38 ± 1.32 | 23.92 ± 2.38 | 17.69 ± 0.65 ** |
| CTX-II (pg/mL) | 21.50 ± 1.69 | 22.05 ± 1.92 | 18.08 ± 2.51 | 12.48 ± 1.14 * |
| PINP (ng/mL) | 46.61 ± 4.24 | 53.59 ± 2.87 | 21.63 ± 1.87 | 19.51 ± 1.32 |
| MMP3 (ng/mL) | 26.91 ± 1.98 | 32.19 ± 1.28 * | 19.20 ± 1.15 | 24.57 ± 1.36 ** |
| COMP (ng/mL) | 11.28 ± 1.48 | 7.42 ± 1.07 * | 41.14 ± 4.57 | 45.79 ± 6.50 |
| Lipid Marker | ||||
| Adiponectin (μg/mL) | 2.88 ± 0.21 | 3.04 ± 0.17 | 2.09 ± 0.09 | 2.63 ± 0.21 * |
| Leptin (ng/mL) | 7.64 ± 1.17 | 4.47 ± 0.43 ** | 2.95 ± 0.36 | 5.68 ± 0.97 ** |
| Resistin (ng/mL) | 18.93 ± 1.38 | 18.53 ± 0.86 | 18.93 ± 1.38 | 18.24 ± 1.05 |
| Visfatin (ng/mL) | ND | ND | Below Detection Limit | Below Detection Limit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, N.-C.; Yang, T.-S.; Busa, P.; Lin, C.-L.; Fang, Y.-C.; Chen, I.-J.; Wong, C.-S. Detection and Evaluation of Serological Biomarkers to Predict Osteoarthritis in Anterior Cruciate Ligament Transection Combined Medial Meniscectomy Rat Model. Int. J. Mol. Sci. 2021, 22, 10179. https://doi.org/10.3390/ijms221910179
Huang N-C, Yang T-S, Busa P, Lin C-L, Fang Y-C, Chen I-J, Wong C-S. Detection and Evaluation of Serological Biomarkers to Predict Osteoarthritis in Anterior Cruciate Ligament Transection Combined Medial Meniscectomy Rat Model. International Journal of Molecular Sciences. 2021; 22(19):10179. https://doi.org/10.3390/ijms221910179
Chicago/Turabian StyleHuang, Nian-Cih, Tsorng-Shyang Yang, Prabhakar Busa, Ching-Ling Lin, Ya-Chieh Fang, Ing-Jung Chen, and Chih-Shung Wong. 2021. "Detection and Evaluation of Serological Biomarkers to Predict Osteoarthritis in Anterior Cruciate Ligament Transection Combined Medial Meniscectomy Rat Model" International Journal of Molecular Sciences 22, no. 19: 10179. https://doi.org/10.3390/ijms221910179
APA StyleHuang, N.-C., Yang, T.-S., Busa, P., Lin, C.-L., Fang, Y.-C., Chen, I.-J., & Wong, C.-S. (2021). Detection and Evaluation of Serological Biomarkers to Predict Osteoarthritis in Anterior Cruciate Ligament Transection Combined Medial Meniscectomy Rat Model. International Journal of Molecular Sciences, 22(19), 10179. https://doi.org/10.3390/ijms221910179







