Testing Hypoxia in Pig Meniscal Culture: Biological Role of the Vascular-Related Factors in the Differentiation and Viability of Neonatal Meniscus
Abstract
:1. Introduction
2. Results
2.1. Morphological Analyses
2.2. Molecular Analysis of Cell Viability
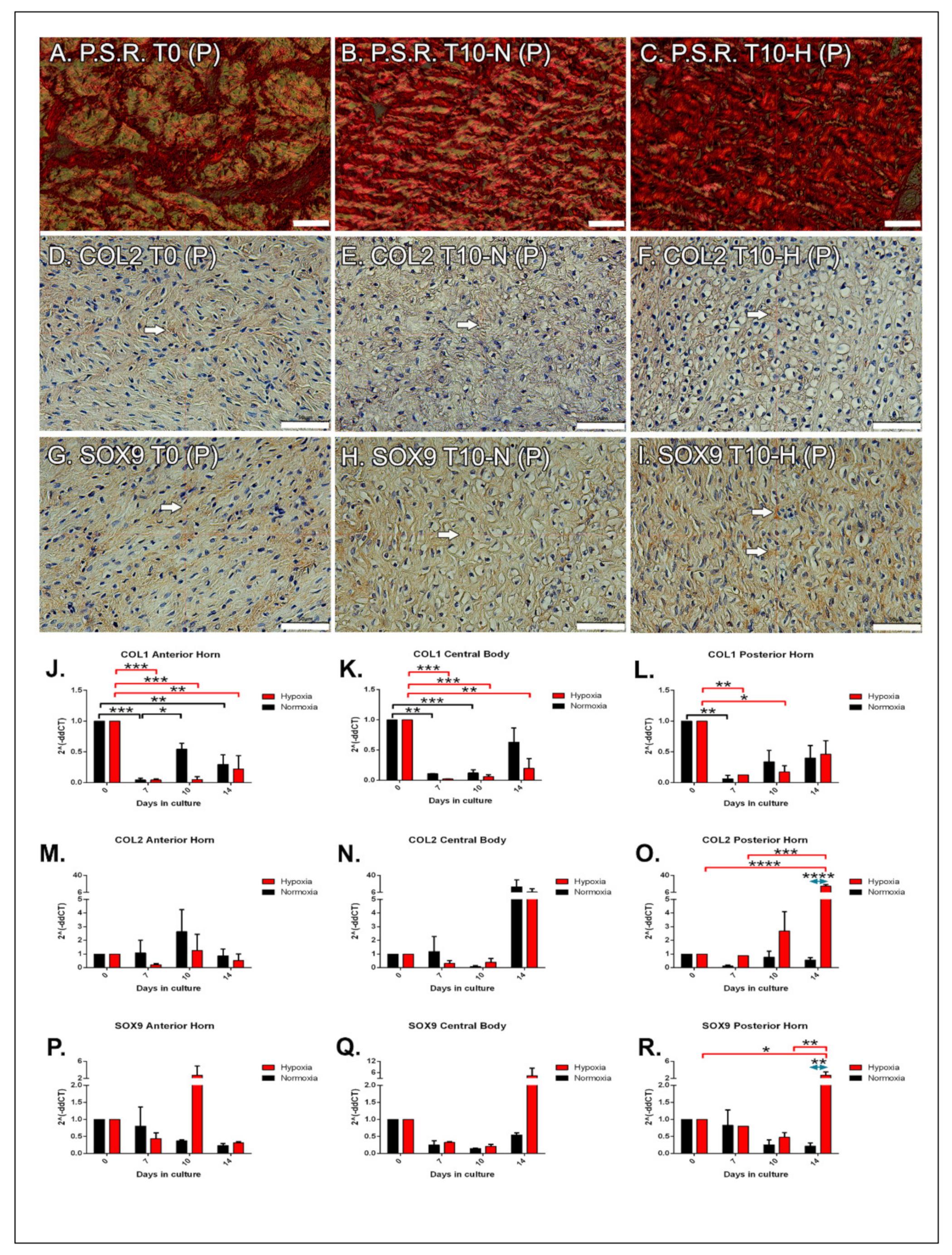
2.3. Histological Analysis: Type I Collagen
2.4. Immunohistochemical Analysis: Type II Collagen
2.5. Immunohistochemical Analysis: SOX9
2.6. Molecular Analysis
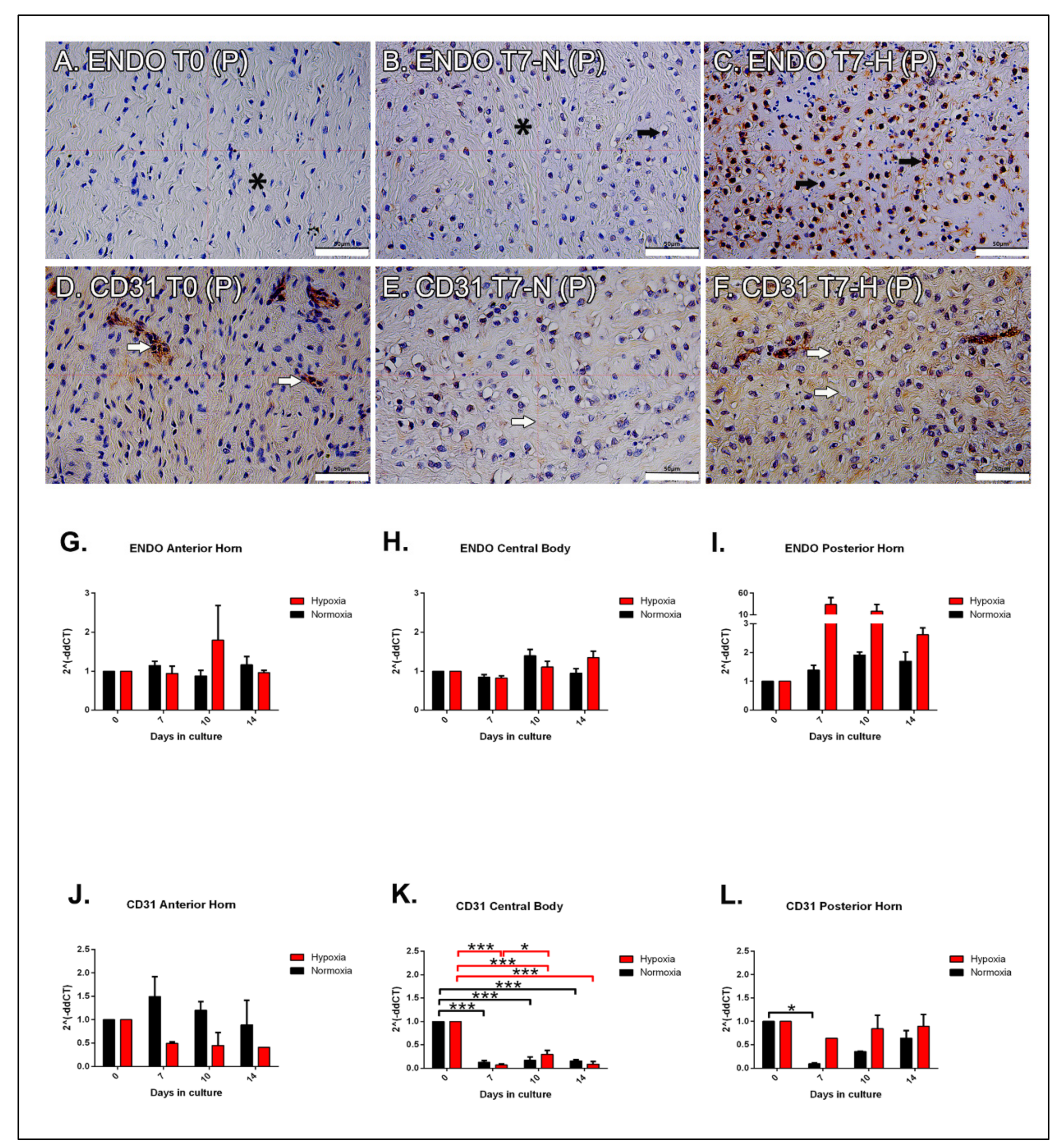
2.7. Immunohistochemical Analysis: Endostatin
2.8. Immunohistochemical Analysis: CD31
2.9. Molecular Analysis
3. Discussion
4. Materials and Methods
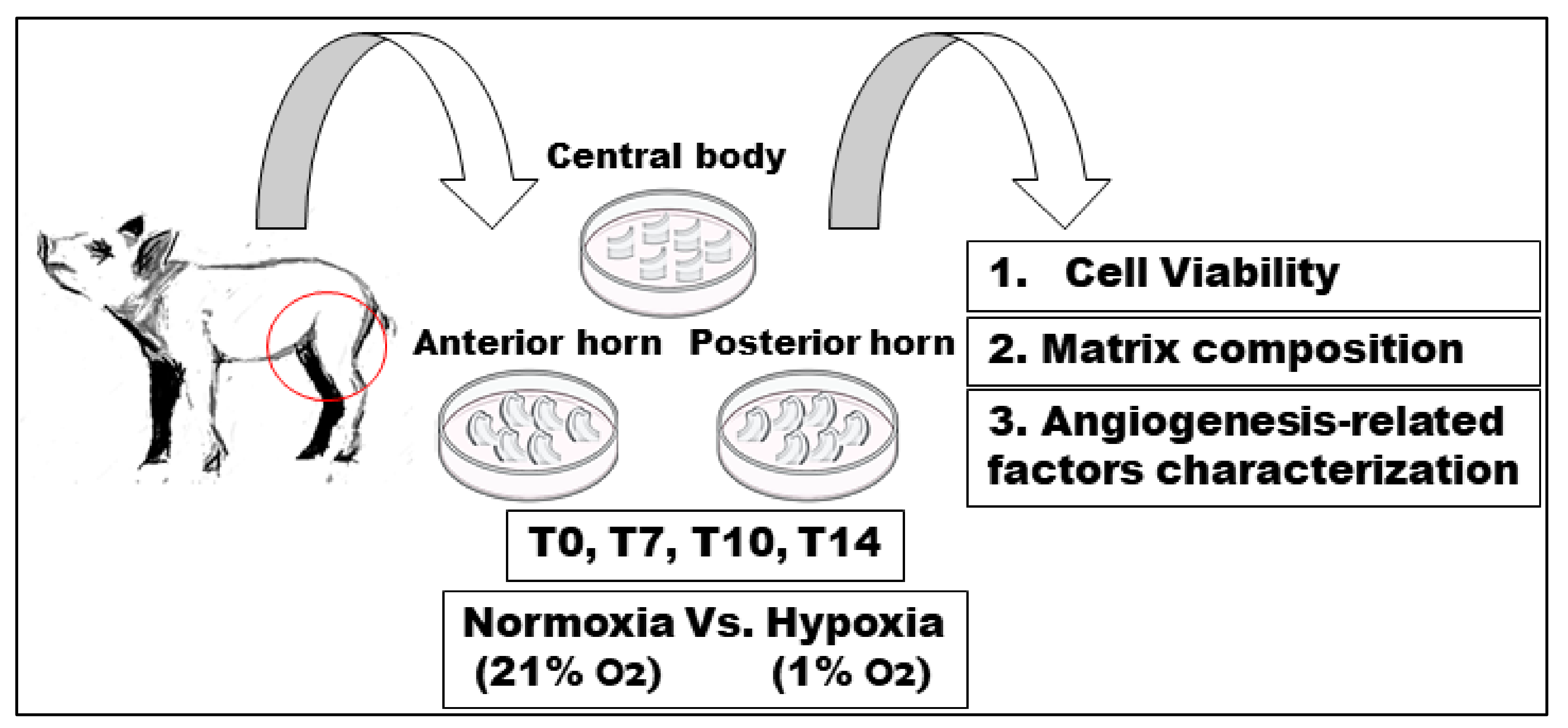
4.1. Preparation of Meniscal Tissue Cultures
4.2. Morpho-Functional Analyses
4.2.1. Histology
4.2.2. Quantitative Polymerase Chain Reaction (qPCR)
4.3. Matrix Characterization
4.3.1. Histology
4.3.2. Immunohistochemistry
4.4. qPCR
4.5. Vascular-Related Markers Characterization
Immunohistochemistry
4.6. qPCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, M.; Ralphs, J.R. Biology of fibrocartilage cells. Int. Rev. Cytol. 2004, 233, 1–45. [Google Scholar] [CrossRef]
- Makris, E.A.; Hadidi, P.; Athanasiou, K.A. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of human knee menisci: Structure, composition, and function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Adams, J.; Athanasiou, K.A. The Knee Meniscus: A Complex Tissue of Diverse Cells. Cell Mol. Bioeng. 2009, 2, 332–340. [Google Scholar] [CrossRef]
- McDevitt, C.A.; Webber, R.J. The ultrastructure and biochemistry of meniscal cartilage. Clin. Orthop. Relat. Res. 1990, 252, 8–18. [Google Scholar] [CrossRef]
- Hyde, G.; Boot-Handford, R.P.; Wallis, G.A. Col2a1 lineage tracing reveals that the meniscus of the knee joint has a complex cellular origin. J. Anat. 2008, 213, 531–538. [Google Scholar] [CrossRef]
- Nakano, T.; Dodd, C.M.; Scott, P.G. Glycosaminoglycans and proteoglycans from different zones of the porcine knee meniscus. J. Orthop. Res. 1997, 15, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.G.; Nakano, T.; Dodd, C.M. Isolation and characterization of small proteoglycans from different zones of the porcine knee meniscus. Biochim. Biophys. Acta 1997, 1336, 254–262. [Google Scholar] [CrossRef]
- Hellio Le Graverand, M.P.; Reno, C.; Hart, D.A. Gene expression in menisci from the knees of skeletally immature and mature female rabbits. J. Orthop. Res. 1999, 17, 738–744. [Google Scholar] [CrossRef]
- Verdonk, P.C.; Forsyth, R.G.; Wang, J.; Almqvist, K.F.; Verdonk, R.; Veys, E.M.; Verbruggen, G. Characterisation of human knee meniscus cell phenotype. Osteoarthr. Cartil. 2005, 13, 548–560. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Deponti, D.; Addis, A.; Domeneghini, C.; Peretti, G.M. Meniscus maturation in the swine model: Changes occurring along with anterior to posterior and medial to lateral aspect during growth. J. Cell Mol. Med. 2014, 18, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Deponti, D.; Di Giancamillo, A.; Scotti, C.; Peretti, G.M.; Martin, I. Animal models for meniscus repair and regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Arnoczky, S.P.; Warren, R.F. Microvasculature of the human meniscus. Am. J. Sports Med. 1982, 10, 90–95. [Google Scholar] [CrossRef]
- Arnoczky, S.P.; Warren, R.F. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am. J. Sports Med. 1983, 11, 131–141. [Google Scholar] [CrossRef]
- Petersen, W.; Tillmann, B. Age-related blood and lymph supply of the knee menisci. A cadaver study. Acta. Orthop. Scand. 1995, 66, 308–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, J.H.; Seon, J.K.; Song, E.K.; Choi, J.I.; Kim, M.C.; Lee, K.B.; Seo, H.Y. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. Am. J. Sports Med. 2013, 41, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.A.; Wilde, J.; Miller, B.S.; Bedi, A. Comparison of inside-out and all-inside techniques for the repair of isolated meniscal tears: A systematic review. Am. J. Sports Med. 2012, 40, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Pujol, N.; Bohu, Y.; Boisrenoult, P.; Macdes, A.; Beaufils, P. Clinical outcomes of open meniscal repair of horizontal meniscal tears in young patients. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1530–1533. [Google Scholar] [CrossRef]
- Kurzweil, P.R.; Lynch, N.M.; Coleman, S.; Kearney, B. Repair of horizontal meniscus tears: A systematic review. Arthroscopy 2014, 30, 1513–1519. [Google Scholar] [CrossRef]
- Fillingham, Y.A.; Riboh, J.C.; Erickson, B.J.; Bach, B.R., Jr.; Yanke, A.B. Inside-Out Versus All-Inside Repair of Isolated Meniscal Tears: An Updated Systematic Review. Am. J. Sports Med. 2017, 45, 234–242. [Google Scholar] [CrossRef]
- Pufe, T.; Petersen, W.J.; Miosge, N.; Goldring, M.B.; Mentlein, R.; Varoga, D.J.; Tillmann, B.N. Endostatin/collagen XVIII—An inhibitor of angiogenesis—Is expressed in cartilage and fibrocartilage. Matrix Biol. 2004, 23, 267–276. [Google Scholar] [CrossRef]
- Meller, R.; Schiborra, F.; Brandes, G.; Knobloch, K.; Tschernig, T.; Hankemeier, S.; Haasper, C.; Schmiedl, A.; Jagodzinski, M.; Krettek, C.; et al. Postnatal maturation of tendon, cruciate ligament, meniscus and articular cartilage: A histological study in sheep. Ann. Anat. 2009, 191, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Esparza, R.; Gortazar, A.R.; Forriol, F. Cell study of the three areas of the meniscus: Effect of growth factors in an experimental model in sheep. J. Orthop. Res. 2012, 30, 1647–1651. [Google Scholar] [CrossRef]
- Kawanishi, Y.; Nakasa, T.; Shoji, T.; Hamanishi, M.; Shimizu, R.; Kamei, N.; Usman, M.A.; Ochi, M. Intra-articular injection of synthetic microRNA-210 accelerates avascular meniscal healing in rat medial meniscal injured model. Arthritis Res. Ther. 2014, 16, 488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, W.; Pufe, T.; Starke, C.; Fuchs, T.; Kopf, S.; Raschke, M.; Becker, R.; Tillmann, B. Locally applied angiogenic factors—A new therapeutic tool for meniscal repair. Ann. Anat 2005, 187, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.R.; Ogden, J.A. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. J. Bone Jt. Surg. Am. 1983, 65, 538–547. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Mentlein, R.; Held-Feindt, J. Angiogenesis factors in gliomas: A new key to tumour therapy? Naturwissenschaften 2003, 90, 385–394. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Deponti, D.; Modina, S.; Tessaro, I.; Domeneghini, C.; Peretti, G.M. Age-related modulation of angiogenesis-regulating factors in the swine meniscus. J. Cell Mol. Med. 2017, 21, 3066–3075. [Google Scholar] [CrossRef]
- Becker, R.; Pufe, T.; Kulow, S.; Giessmann, N.; Neumann, W.; Mentlein, R.; Petersen, W. Expression of vascular endothelial growth factor during healing of the meniscus in a rabbit model. J. Bone Jt. Surg. Br. 2004, 86, 1082–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipani, E.; Ryan, H.E.; Didrickson, S.; Kobayashi, T.; Knight, M.; Johnson, R.S. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001, 15, 2865–2876. [Google Scholar] [CrossRef]
- Aro, E.; Khatri, R.; Gerard-O’Riley, R.; Mangiavini, L.; Myllyharju, J.; Schipani, E. Hypoxia-inducible factor-1 (HIF-1) but not HIF-2 is essential for hypoxic induction of collagen prolyl 4-hydroxylases in primary newborn mouse epiphyseal growth plate chondrocytes. J. Biol. Chem. 2012, 287, 37134–37144. [Google Scholar] [CrossRef] [Green Version]
- Mangiavini, L.; Merceron, C.; Araldi, E.; Khatri, R.; Gerard-O’Riley, R.; Wilson, T.L.; Rankin, E.B.; Giaccia, A.J.; Schipani, E. Loss of VHL in mesenchymal progenitors of the limb bud alters multiple steps of endochondral bone development. Dev. Biol. 2014, 393, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Schipani, E.; Mangiavini, L.; Merceron, C. HIF-1alpha and growth plate development: What we really know. Bonekey Rep. 2015, 4, 730. [Google Scholar] [CrossRef] [Green Version]
- Adesida, A.B.; Grady, L.M.; Khan, W.S.; Millward-Sadler, S.J.; Salter, D.M.; Hardingham, T.E. Human meniscus cells express hypoxia inducible factor-1alpha and increased SOX9 in response to low oxygen tension in cell aggregate culture. Arthritis Res. Ther. 2007, 9, R69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattappa, G.; Johnstone, B.; Zellner, J.; Docheva, D.; Angele, P. The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response. Int. J. Mol. Sci. 2019, 20, 484. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Khan, M.P.; Merceron, C.; LaGory, E.L.; Tata, Z.; Mangiavini, L.; Hu, J.; Vemulapalli, K.; Chandel, N.S.; Giaccia, A.J.; et al. Suppressing Mitochondrial Respiration Is Critical for Hypoxia Tolerance in the Fetal Growth Plate. Dev. Cell 2019, 49, 748–763. [Google Scholar] [CrossRef]
- Herrera Millar, V.R.; Mangiavini, L.; Polito, U.; Canciani, B.; Nguyen, V.T.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Modina, S.C.; Di Giancamillo, A. Hypoxia as a Stimulus for the Maturation of Meniscal Cells: Highway to Novel Tissue Engineering Strategies? Int. J. Mol. Sci. 2021, 22, 6905. [Google Scholar] [CrossRef]
- Phillips, B.S.; Kass, P.H.; Naydan, D.K.; Winthrop, M.D.; Griffey, S.M.; Madewell, B.R. Apoptotic and proliferation indexes in canine lymphoma. J. Vet. Diagn Investig. 2000, 12, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von der Mark, K.; Gauss, V.; von der Mark, H.; Muller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1977, 267, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Trepman, E.; Folkman, J. Cell shape and phenotypic expression in chondrocytes. Proc. Soc. Exp. Biol Med. 1983, 172, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.L.; Polak, J.M. Control of human articular chondrocyte differentiation by reduced oxygen tension. J. Cell Physiol. 2004, 199, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Domm, C.; Schunke, M.; Christesen, K.; Kurz, B. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthr. Cartil. 2002, 10, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Nakata, K.; Shino, K.; Hamada, M.; Mae, T.; Miyama, T.; Shinjo, H.; Horibe, S.; Tada, K.; Ochi, T.; Yoshikawa, H. Human meniscus cell: Characterization of the primary culture and use for tissue engineering. Clin. Orthop. Relat. Res. 2001, 391, S208–S218. [Google Scholar] [CrossRef]
- Di Giancamillo, A.; Mangiavini, L.; Tessaro, I.; Marmotti, A.; Scurati, R.; Peretti, G.M. The meniscus vascularization: The direct correlation with tissue composition for tissue engineering purposes. J. Biol. Regul. Homeost. Agents 2016, 30, 85–90. [Google Scholar]
- Howell, R.; Kumar, N.S.; Patel, N.; Tom, J. Degenerative meniscus: Pathogenesis, diagnosis, and treatment options. World J. Orthop. 2014, 5, 597–602. [Google Scholar] [CrossRef]
- Fisher, J.N.; Tessaro, I.; Bertocco, T.; Peretti, G.M.; Mangiavini, L. The Application of Stem Cells from Different Tissues to Cartilage Repair. Stem Cells Int. 2017, 2017, 2761678. [Google Scholar] [CrossRef]
- Biswas, S.; Roy, S.; Banerjee, J.; Hussain, S.R.; Khanna, S.; Meenakshisundaram, G.; Kuppusamy, P.; Friedman, A.; Sen, C.K. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc. Natl. Acad. Sci. USA 2010, 107, 6976–6981. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef]
- Eliasson, P.; Jonsson, J.I. The hematopoietic stem cell niche: Low in oxygen but a nice place to be. J. Cell Physiol. 2010, 222, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, P.; Rehn, M.; Hammar, P.; Larsson, P.; Sirenko, O.; Flippin, L.A.; Cammenga, J.; Jonsson, J.I. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term-reconstituting hematopoietic stem cells during in vitro culture. Exp. Hematol. 2010, 38, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, L.B.; Li, Q.; Park, M.S.; Flanagan, W.M.; Semenza, G.L.; Dang, C.V. Hypoxia inhibits G1/S transition through regulation of p27 expression. J. Biol. Chem. 2001, 276, 7919–7926. [Google Scholar] [CrossRef] [Green Version]
- Goda, N.; Ryan, H.E.; Khadivi, B.; McNulty, W.; Rickert, R.C.; Johnson, R.S. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol. Cell Biol. 2003, 23, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Gordan, J.D.; Thompson, C.B.; Simon, M.C. HIF and c-Myc: Sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell 2007, 12, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Hubbi, M.E.; Hu, H.; Kshitiz; Ahmed, I.; Levchenko, A.; Semenza, G.L. Chaperone-mediated autophagy targets hypoxia-inducible factor-1alpha (HIF-1alpha) for lysosomal degradation. J. Biol. Chem. 2013, 288, 10703–10714. [Google Scholar] [CrossRef] [Green Version]
- Koshiji, M.; Kageyama, Y.; Pete, E.A.; Horikawa, I.; Barrett, J.C.; Huang, L.E. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004, 23, 1949–1956. [Google Scholar] [CrossRef] [Green Version]
- Takubo, K.; Goda, N.; Yamada, W.; Iriuchishima, H.; Ikeda, E.; Kubota, Y.; Shima, H.; Johnson, R.S.; Hirao, A.; Suematsu, M.; et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 2010, 7, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Merceron, C.; Ranganathan, K.; Wang, E.; Tata, Z.; Makkapati, S.; Khan, M.P.; Mangiavini, L.; Yao, A.Q.; Castellini, L.; Levi, B.; et al. Hypoxia-inducible factor 2α is a negative regulator of osteoblastogenesis and bone mass accrual. Bone Res. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Das, F.; Bera, A.; Ghosh-Choudhury, N.; Abboud, H.E.; Kasinath, B.S.; Choudhury, G.G. TGFbeta-induced deptor suppression recruits mTORC1 and not mTORC2 to enhance collagen I (alpha2) gene expression. PLoS ONE 2014, 9, e109608. [Google Scholar] [CrossRef]
- Hubbi, M.E.; Luo, W.; Baek, J.H.; Semenza, G.L. MCM proteins are negative regulators of hypoxia-inducible factor 1. Mol. Cell 2011, 42, 700–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.Q.; Jackson, D.A.; Blow, J.J. Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev. 2007, 21, 3331–3341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyrien, O.; Marheineke, K.; Goldar, A. Paradoxes of eukaryotic DNA replication: MCM proteins and the random completion problem. Bioessays 2003, 25, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, A.; Schwob, E.; Mendez, J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc. Natl. Acad. Sci. USA 2008, 105, 8956–8961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, A.M.; Gohler, T.; Luciani, M.G.; Oehlmann, M.; Ge, X.; Gartner, A.; Jackson, D.A.; Blow, J.J. Excess Mcm2-7 license dormant origins of replication that can be used under conditions of replicative stress. J. Cell Biol. 2006, 173, 673–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafont, J.E.; Talma, S.; Hopfgarten, C.; Murphy, C.L. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J. Biol. Chem. 2008, 283, 4778–4786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Wu, Y.P.; Zhu, X.D.; Zhang, Y.H.; Ma, Q.J. Endostatin promotes the anabolic program of rabbit chondrocyte. Cell Res. 2005, 15, 201–206. [Google Scholar] [CrossRef]
- Ergun, S.; Kilic, N.; Wurmbach, J.H.; Ebrahimnejad, A.; Fernando, M.; Sevinc, S.; Kilic, E.; Chalajour, F.; Fiedler, W.; Lauke, H.; et al. Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis 2001, 4, 193–206. [Google Scholar] [CrossRef]
- Cerejo, R.; Dunlop, D.D.; Cahue, S.; Channin, D.; Song, J.; Sharma, L. The influence of alignment on risk of knee osteoarthritis progression according to baseline stage of disease. Arthritis Rheum 2002, 46, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|
| Actin Beta (ACTB) | CAA GGA GAA GCT CTG CTA CG | AGA GGT CCT TCC TGA TGT CC | 245 |
| Caspase 3 (CASP3) | TGG GAT TGA GAC GGA CAG TG | CGC TGC ACA AAG TGA CTG GA | 121 |
| Collagen Type I Alpha 1 Chain (COL1A1) | CCA ACA AGG CCA AGA AGA AG | ATG GTA CCT GAG GCC GTT CT | 64 |
| Collagen Type II Alpha 1 Chain (COL2A1) | CAC GGA TGG TCC CAA AGG | ATA CCA GCA GCT CCC CTC T | 102 |
| Endostatin (COL18A1) | GCC GAC TTC CAG TGC TTC | CGT CGC ACG ATG CTG TAG | 104 |
| Platelet And Endothelial Cell Adhesion Molecule 1 (CD31) | CAC CGA GGT CTG GGA | CGG AGC CTT CCG TTC | 103 |
| Proliferating Cell Nuclear Antigen (PCNA) | GCA GAG CAT GGA CTC GTC TC | TTG GAC ATG CTG GTG AGG TT | 120 |
| SRY-Box Transcription Factor 9 (SOX9) | CCG GTG CGC GTC AAC | TGC AGG TGC GGG TAC TGAT | 119 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canciani, B.; Herrera Millar, V.R.; Pallaoro, M.; Aidos, L.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Modina, S.C.; Mangiavini, L.; Di Giancamillo, A. Testing Hypoxia in Pig Meniscal Culture: Biological Role of the Vascular-Related Factors in the Differentiation and Viability of Neonatal Meniscus. Int. J. Mol. Sci. 2021, 22, 12465. https://doi.org/10.3390/ijms222212465
Canciani B, Herrera Millar VR, Pallaoro M, Aidos L, Cirillo F, Anastasia L, Peretti GM, Modina SC, Mangiavini L, Di Giancamillo A. Testing Hypoxia in Pig Meniscal Culture: Biological Role of the Vascular-Related Factors in the Differentiation and Viability of Neonatal Meniscus. International Journal of Molecular Sciences. 2021; 22(22):12465. https://doi.org/10.3390/ijms222212465
Chicago/Turabian StyleCanciani, Barbara, Valentina Rafaela Herrera Millar, Margherita Pallaoro, Lucia Aidos, Federica Cirillo, Luigi Anastasia, Giuseppe Maria Peretti, Silvia Clotilde Modina, Laura Mangiavini, and Alessia Di Giancamillo. 2021. "Testing Hypoxia in Pig Meniscal Culture: Biological Role of the Vascular-Related Factors in the Differentiation and Viability of Neonatal Meniscus" International Journal of Molecular Sciences 22, no. 22: 12465. https://doi.org/10.3390/ijms222212465
APA StyleCanciani, B., Herrera Millar, V. R., Pallaoro, M., Aidos, L., Cirillo, F., Anastasia, L., Peretti, G. M., Modina, S. C., Mangiavini, L., & Di Giancamillo, A. (2021). Testing Hypoxia in Pig Meniscal Culture: Biological Role of the Vascular-Related Factors in the Differentiation and Viability of Neonatal Meniscus. International Journal of Molecular Sciences, 22(22), 12465. https://doi.org/10.3390/ijms222212465







