Possibility of Venous Serum Cl− Concentration ([Cl−]s) as a Marker for Human Metabolic Status: Correlation of [Cl−]s to Age, Fasting Blood Sugar (FBS), and Glycated Hemoglobin (HbA1c)
Abstract
1. Introduction
2. Results
2.1. Age-Dependent Changes in Venous Serum Cl− Concentration ([Cl−]s)
2.2. Age-Dependent Changes in Venous Serum Fasting Blood Sugar Concentration (FBS)
2.3. Age-Dependent Changes in Venous Hemoglobin A1c (HbA1c)
2.4. Relationship among [Cl−]s, Age, FBS and HbA1c
| Coefficient | |||
|---|---|---|---|
| UL of 95% CI | 0.1226 | 0.2707 | 0.2870 |
| Mean | 0.1167 | 0.2649 | 0.2812 |
| LL of 95% CI | 0.1108 | 0.2591 | 0.2754 |
2.5. Relationship between [Cl−]s and FBS
2.6. Relationship between [Cl−]s and HbA1c
2.7. Relationship between FBS and HbA1c
2.8. Possible Mechanims of [Cl−]s Changes by Age, FBS, and HbA1c and Clincially Significant Meanings of [Cl−]s
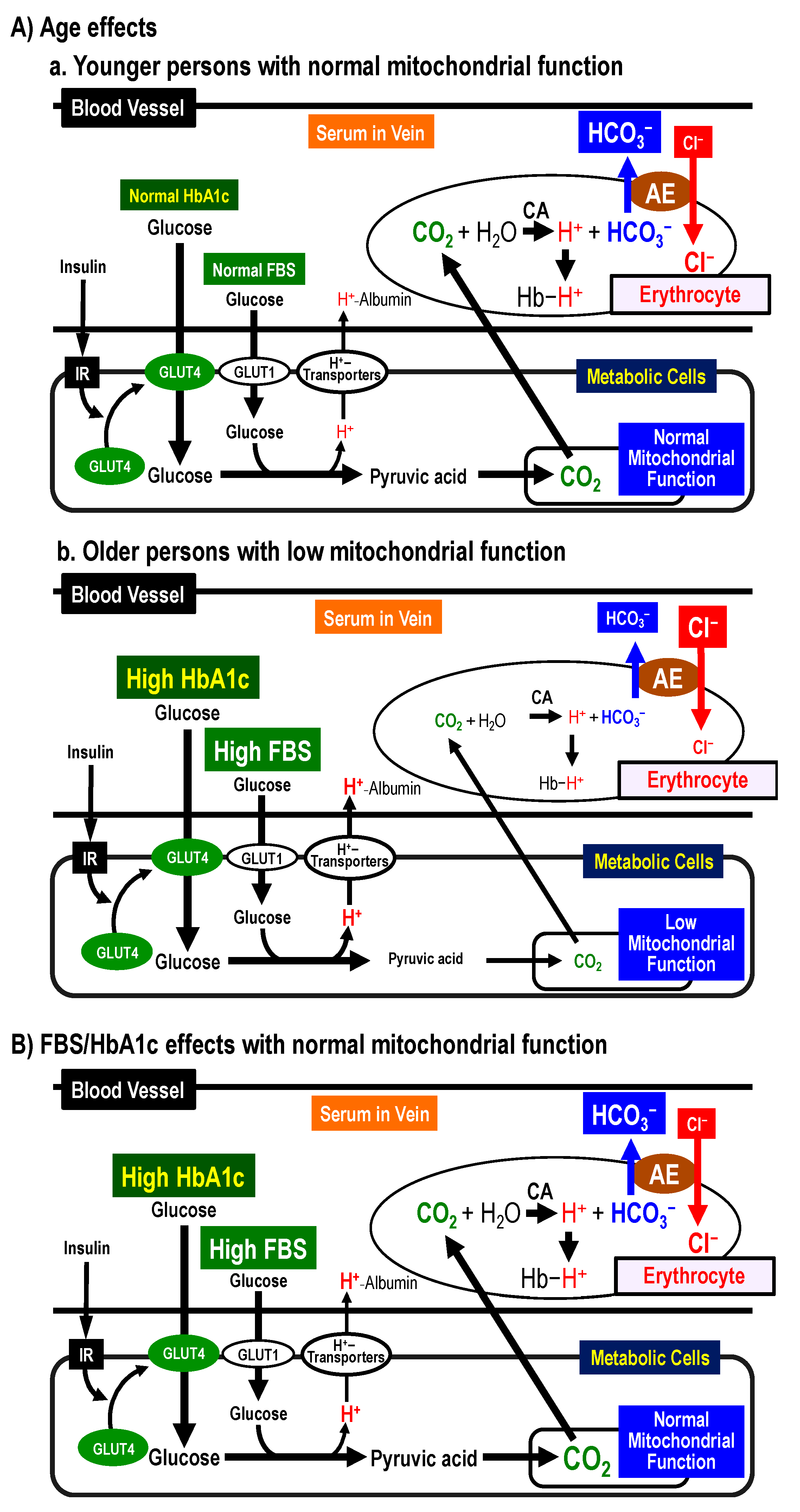
2.8.1. Possible Mechanisms of [Cl−]s Changes by Age
2.8.2. Possible Mechanisms of [Cl−]s Changes by FBS and HbA1c
2.8.3. Clinically Significant Meanings of [Cl−]s Values
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Fasting Blood Samples
4.3. Measurements of [Cl−]s, FBS and HbA1c
4.4. Statistical Analysis
4.5. Relationship among [Cl−]s, Age, FBS and HbA1c
4.6. The Relationship among Normalized Data, N[Cl−]s, NAge, NFBS, and NHbA1c
4.7. Correlation of [Cl−]s, FBS or HbA1c to Age Using the Normalized Data, N[Cl−]s, NFBS, NHbA1c, and NAge
4.8. Age-Dependent Factor and FBS/HbA1c-Dependent Factor Influencing N[Cl−]s
4.9. The Relationship between [Cl−]s and FBS
4.10. The Relationship between [Cl−]s and HbA1c
4.11. Relationship between FBS and HbA1c
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | anion exchanger |
| CA | carbonic anhydrase |
| CAI | carbonic anhydrase I |
| CAII | carbonic anhydrase II |
| CI | confidence interval |
| [Cl−]s | venous serum Cl− concentration |
| COPD | chronic obstructive pulmonary disease |
| DM | diabetes mellitus |
| FBS | fasting blood sugar |
| GFR | glomerular filtration rate |
| Hb | hemoglobin |
| HbA1c | glycated hemoglobin |
| [HCO3−]s | venous serum HCO3− concentration |
| HSD | honestly significant difference |
| LL | lower limit |
| PBS | postprandial blood sugar |
| RAA | renin-angiotensin-aldosterone |
| UL | upper limit |
References
- Altobelli, E.; Angeletti, P.M.; Marziliano, C.; Mastrodomenico, M.; Giuliani, A.R.; Petrocelli, R. Potential therapeutic effects of curcumin on glycemic and lipid profile in uncomplicated type 2 diabetes-A meta-analysis of randomized controlled trial. Nutrients 2021, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.W.; Hayes, S.M.; Andrews, R.J.; Fonda, J.R.; Beck, B.M.; Hanlon, L.B.; Fortier, C.B.; Milberg, W.P.; McGlinchey, R.E. Cardiorespiratory fitness Is associated with better cardiometabolic health and lower PTSD severity in post-9/11 veterans. Mil. Med. 2020, 185, e592–e596. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fu, J.; Qiao, Y.; Cao, J.; Deehan, E.C.; Li, Z.; Jin, M.; Wang, X.; Wang, Y. Higher intake of microbiota-accessible carbohydrates and improved cardiometabolic risk factors: A meta-analysis and umbrella review of dietary management in patients with type 2 diabetes. Am. J. Clin. Nutr. 2021, 113, 1515–1530. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Guimaraes, J.P.; Guarino, M.P.; Timóteo, A.T.; Caires, I.; Sacramento, J.F.; Ribeiro, M.J.; Selas, M.; Santiago, J.C.P.; Mota-Carmo, M.; Conde, S.V. Carotid body chemosensitivity: Early biomarker of dysmetabolism in humans. Eur. J. Endocrinol. 2020, 182, 549–557. [Google Scholar] [CrossRef]
- Peng, C.C.; Tu, Y.K.; Lee, G.Y.; Chang, R.H.; Huang, Y.; Bukhari, K.; Tsai, Y.C.; Fu, Y.; Huang, H.K.; Munir, K.M. Effects of proton pump inhibitors on glycemic control and incident diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2021. [Google Scholar] [CrossRef]
- Marunaka, Y. Roles of interstitial fluid pH and weak organic acids in development and amelioration of insulin resistance. Biochem. Soc. Trans. 2021, 49, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Marunaka, Y. The importance of regulation of body fluid pH in the development and progression of metabolic diseases. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Publishers: Hauppauge, NY, USA, 2014; Volume 77, pp. 177–189. [Google Scholar]
- Aoi, W.; Marunaka, Y. Importance of pH homeostasis in metabolic health and diseases: Crucial role of membrane proton transport. BioMed Res. Int. 2014, 2014, 598986. [Google Scholar] [CrossRef]
- Aoi, W.; Iwasa, M.; Marunaka, Y. Metabolic functions of flavonoids: From human epidemiology to molecular mechanism. Neuropeptides 2021, 88, 102163. [Google Scholar] [CrossRef]
- Marunaka, Y. The proposal of molecular mechanisms of weak organic acids intake-induced improvement of insulin resistance in diabetes mellitus via elevation of interstitial fluid pH. Int. J. Mol. Sci. 2018, 19, 3244. [Google Scholar] [CrossRef]
- Lee, D.; Hong, J.H. The Fundamental role of bicarbonate transporters and associated carbonic anhydrase enzymes in maintaining ion and pH homeostasis in non-secretory organs. Int. J. Mol. Sci. 2020, 21, 339. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Marino, A. Band 3 protein function and oxidative stress in erythrocytes. J. Cell Physiol. 2021, 236, 6225–6234. [Google Scholar] [CrossRef] [PubMed]
- Hosogi, S.; Miyazaki, H.; Nakajima, K.; Ashihara, E.; Niisato, N.; Kusuzaki, K.; Marunaka, Y. An inhibitor of Na+/H+ exchanger (NHE), ethyl-isopropyl amiloride (EIPA), diminishes proliferation of MKN28 human gastric cancer cells by decreasing the cytosolic Cl− concentration via DIDS-sensitive pathways. Cell. Physiol. Biochem. 2012, 30, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, A.; Hikami, S.; Ichikawa, D.; Kosuga, T.; Shimizu, H.; Kudou, M.; Yamazato, Y.; Kobayashi, T.; Shoda, K.; Arita, T.; et al. Anion exchanger 2 suppresses cellular movement and has prognostic significance in esophageal squamous cell carcinoma. Oncotarget 2018, 9, 25993–26006. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, A.; Kudou, M.; Ichikawa, D.; Shimizu, H.; Arita, T.; Kosuga, T.; Konishi, H.; Komatsu, S.; Fujiwara, H.; Okamoto, K.; et al. Expression and role of anion exchanger 1 in esophageal squamous cell carcinoma. Oncotarget 2017, 8, 17921–17935. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, A.; Ichikawa, D.; Otsuji, E.; Marunaka, Y. Cellular physiological approach for treatment of gastric cancer. World J. Gastroenterol. 2014, 20, 11560–11566. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kaczmarska, M.; Szczesny-Malysiak, E.; Wajda, A.; Bulat, K.; Alcicek, F.C.; Zygmunt, M.; Sacha, T.; Marzec, K.M. An insight into the stages of ion leakage during red blood cell storage. Int. J. Mol. Sci. 2021, 22, 2885. [Google Scholar] [CrossRef] [PubMed]
- Perez Ruiz de Garibay, A.; Kellum, J.A.; Honigschnabel, J.; Kreymann, B. Respiratory and metabolic acidosis correction with the ADVanced Organ Support system. Intensive Care Med. Exp. 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, Y. Roles of interstitial fluid pH in diabetes mellitus: Glycolysis and mitochondrial function. World J. Diabetes 2015, 6, 125–135. [Google Scholar] [CrossRef]
- Mohebbi, N.; Perna, A.; van der Wijst, J.; Becker, H.M.; Capasso, G.; Wagner, C.A. Regulation of two renal chloride transporters, AE1 and pendrin, by electrolytes and aldosterone. PLoS ONE 2013, 8, e55286. [Google Scholar] [CrossRef]
- Jennings, M.L. Stoichiometry of a half-turnover of band 3, the chloride transport protein of human erythrocytes. J. Gen. Physiol. 1982, 79, 169–185. [Google Scholar] [CrossRef]
- Falke, J.J.; Pace, R.J.; Chan, S.I. Chloride binding to the anion transport binding sites of band 3. A 35Cl NMR study. J. Biol. Chem. 1984, 259, 6472–6480. [Google Scholar] [CrossRef]
- Wolpaw, E.W.; Martin, D.L. A membrane protein in LRM55 glial cells cross-reacts with antibody to the anion exchange carrier of human erythrocytes. Neurosci. Lett. 1986, 67, 42–47. [Google Scholar] [CrossRef]
- Kim, H.R.; Yew, N.S.; Ansorge, W.; Voss, H.; Schwager, C.; Vennström, B.; Zenke, M.; Engel, J.D. Two different mRNAs are transcribed from a single genomic locus encoding the chicken erythrocyte anion transport proteins (band 3). Mol. Cell. Biol. 1988, 8, 4416–4424. [Google Scholar] [CrossRef][Green Version]
- Verlander, J.W.; Madsen, K.M.; Low, P.S.; Allen, D.P.; Tisher, C.C. Immunocytochemical localization of band 3 protein in the rat collecting duct. Am. J. Physiol. Cell Physiol. 1988, 255, F115–F125. [Google Scholar] [CrossRef]
- Grassi, B. Oxygen uptake kinetics: Old and recent lessons from experiments on isolated muscle in situ. Eur. J. Appl. Physiol. 2003, 90, 242–249. [Google Scholar] [CrossRef]
- Murias, J.M.; Paterson, D.H. Slower O2 kinetics in older individuals: Is it inevitable? Med. Sci. Sports Exerc. 2015, 47, 2308–2318. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Boveris, A. The mitochondrial energy transduction system and the aging process. Am. J. Physiol. Cell Physiol. 2007, 292, C670–C686. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Vinod, V.; Symons, J.D.; Boudina, S. Protein and mitochondria quality control mechanisms and cardiac aging. Cells 2020, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Zou, X.; Xiao, J.B.; Marunaka, Y. Body fluid pH balance in metabolic health and possible benefits of dietary alkaline foods. eFood 2020, 1, 12–23. [Google Scholar] [CrossRef]
- Marunaka, Y.; Niisato, N.; Zou, X.; Xiao, J.B.; Nakahari, T. Food intake targeting and improving acidity in diabetes and cancer. Food Front. 2020, 1, 9–12. [Google Scholar] [CrossRef]
- Chadda, K.R.; Cheng, T.S.; Ong, K.K. GLP-1 agonists for obesity and type 2 diabetes in children: Systematic review and meta-analysis. Obes. Rev. 2021, 22, e13177. [Google Scholar] [CrossRef]
- Carlson, A.L.; Criego, A.B.; Martens, T.W.; Bergenstal, R.M. HbA1c: The glucose management indicator, time in range, and standardization of continuous glucose monitoring reports in clinical practice. Endocrinol. Metab. Clin. N. Am. 2020, 49, 95–107. [Google Scholar] [CrossRef]
- Chehregosha, H.; Khamseh, M.E.; Malek, M.; Hosseinpanah, F.; Ismail-Beigi, F. A view beyond HbA1c: Role of continuous glucose monitoring. Diabetes Ther. 2019, 10, 853–863. [Google Scholar] [CrossRef]
- Alim, Z. 1H-indazole molecules reduced the activity of human erythrocytes carbonic anhydrase I and II isoenzymes. J. Biochem. Mol. Toxicol. 2018, 32, e22194. [Google Scholar] [CrossRef] [PubMed]
- Fais, S.; Marunaka, Y. The acidic microenvironment: Is it a phenotype of all cancers? A focus on multiple myeloma and some analogies with diabetes mellitus. Cancers 2020, 12, 3226. [Google Scholar] [CrossRef]
- Gillies, R.J.; Pilot, C.; Marunaka, Y.; Fais, S. Targeting acidity in cancer and diabetes. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.R.; Damaghi, M.; Marunaka, Y.; Spugnini, E.P.; Fais, S.; Gillies, R.J. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019, 38, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Hosogi, S.; Ohsawa, M.; Kato, I.; Kuwahara, A.; Inui, T.; Inui, A.; Marunaka, Y. Improvement of diabetes mellitus symptoms by intake of ninjin’yoeito. Front. Nutr. 2018, 5, 112. [Google Scholar] [CrossRef]
- Hayata, H.; Miyazaki, H.; Niisato, N.; Yokoyama, N.; Marunaka, Y. Lowered extracellular pH is involved in the pathogenesis of skeletal muscle insulin resistance. Biochem. Biophys. Res. Commun. 2014, 445, 170–174. [Google Scholar] [CrossRef]
- Aoi, W.; Hosogi, S.; Niisato, N.; Yokoyama, N.; Hayata, H.; Miyazaki, H.; Kusuzaki, K.; Fukuda, T.; Fukui, M.; Nakamura, N.; et al. Improvement of insulin resistance, blood pressure and interstitial pH in early developmental stage of insulin resistance in OLETF rats by intake of propolis extracts. Biochem. Biophys. Res. Commun. 2013, 432, 650–653. [Google Scholar] [CrossRef]
- Malandrucco, I.; Russo, B.; Picconi, F.; Menduni, M.; Frontoni, S. Glycemic status assessment by the latest glucose monitoring technologies. Int. J. Mol. Sci. 2020, 21, 8243. [Google Scholar] [CrossRef]
- Byrne, F.L.; Martin, A.R.; Kosasih, M.; Caruana, B.T.; Farrell, R. The role of hyperglycemia in endometrial cancer pathogenesis. Cancers 2020, 12, 1191. [Google Scholar] [CrossRef]
- Long, B.; Lentz, S.; Koyfman, A.; Gottlieb, M. Euglycemic diabetic ketoacidosis: Etiologies, evaluation, and management. Am. J. Emerg. Med. 2021, 44, 157–160. [Google Scholar] [CrossRef]
- Persson, B. Determination of plasma acetoacetate and D-beta-hydroxybutyrate in new-born infants by an enzymatic fluorometric micro-method. Scand. J. Clin. Lab. Investig. 1970, 25, 9–18. [Google Scholar] [CrossRef]
- Gosmanov, A.R.; Gosmanova, E.O.; Dillard-Cannon, E. Management of adult diabetic ketoacidosis. Diabetes Metab. Syndr. Obes. 2014, 7, 255–264. [Google Scholar] [CrossRef]
- Fenves, A.Z.; Emmett, M. Approach to patients with high anion gap metabolic acidosis: Core curriculum 2021. Am. J. Kidney Dis. 2021, 78, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Madias, N.E. Serum anion gap: Its uses and limitations in clinical medicine. Clin. J. Am. Soc. Nephrol. 2007, 2, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Nye, P.C.; Torrance, R.W. Studies on the localization of pulmonary carbonic anhydrase in the cat. J. Physiol. 1981, 319, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Lossow, K.; Hermans-Borgmeyer, I.; Behrens, M.; Meyerhof, W. Genetic labeling of Car4-expressing cells reveals subpopulations of Type III taste cells. Chem. Senses 2017, 42, 747–758. [Google Scholar] [CrossRef]
- Bayrak, B.B.; Tunali, S.; Bal-Demirci, T.; Ulkuseven, B.; Yanardag, R. Glycoprotein levels and oxidative lung injury in experimental diabetes: Effect of oxovanadium(IV) complex based on thiosemicarbazone. Toxicol. Mech. Methods 2021, 31, 581–588. [Google Scholar] [CrossRef]
- Easter, M.; Bollenbecker, S.; Barnes, J.W.; Krick, S. Targeting aging pathways in chronic obstructive pulmonary disease. Int. J. Mol. Sci. 2020, 21, 6924. [Google Scholar] [CrossRef]
- Fuschillo, S.; Paris, D.; Tramice, A.; Ambrosino, P.; Palomba, L.; Maniscalco, M.; Motta, A. Metabolomic profiling of exhaled breath condensate and plasma/serum in chronic obstructive pulmonary disease. Curr. Med. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Balnis, J.; Korponay, T.C.; Jaitovich, A. AMP-activated protein kinase (AMPK) at the crossroads between CO2 retention and skeletal muscle dysfunction in chronic obstructive pulmonary disease (COPD). Int. J. Mol. Sci. 2020, 21, 955. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.M.; Wysham, N.G.; Xie, J.; Qin, X.; Giovacchini, C.X.; Ekström, M.; MacIntyre, N.R. Hypercapnia in advanced chronic obstructive pulmonary disease: A secondary analysis of the National Emphysema Treatment Trial. Chronic Obstr. Pulm. Dis. 2020, 7, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Ennis, J.L.; Luo, D.; Asplin, J.R.; Coe, F.L. A laboratory-based algorithm to predict future kidney function decline in older adults with reduced estimated glomerular filtration rate. Clin. Nephrol. 2019, 92, 113–122. [Google Scholar] [CrossRef]
- Schmitt, R.; Melk, A. Molecular mechanisms of renal aging. Kidney Int. 2017, 92, 569–579. [Google Scholar] [CrossRef] [PubMed]
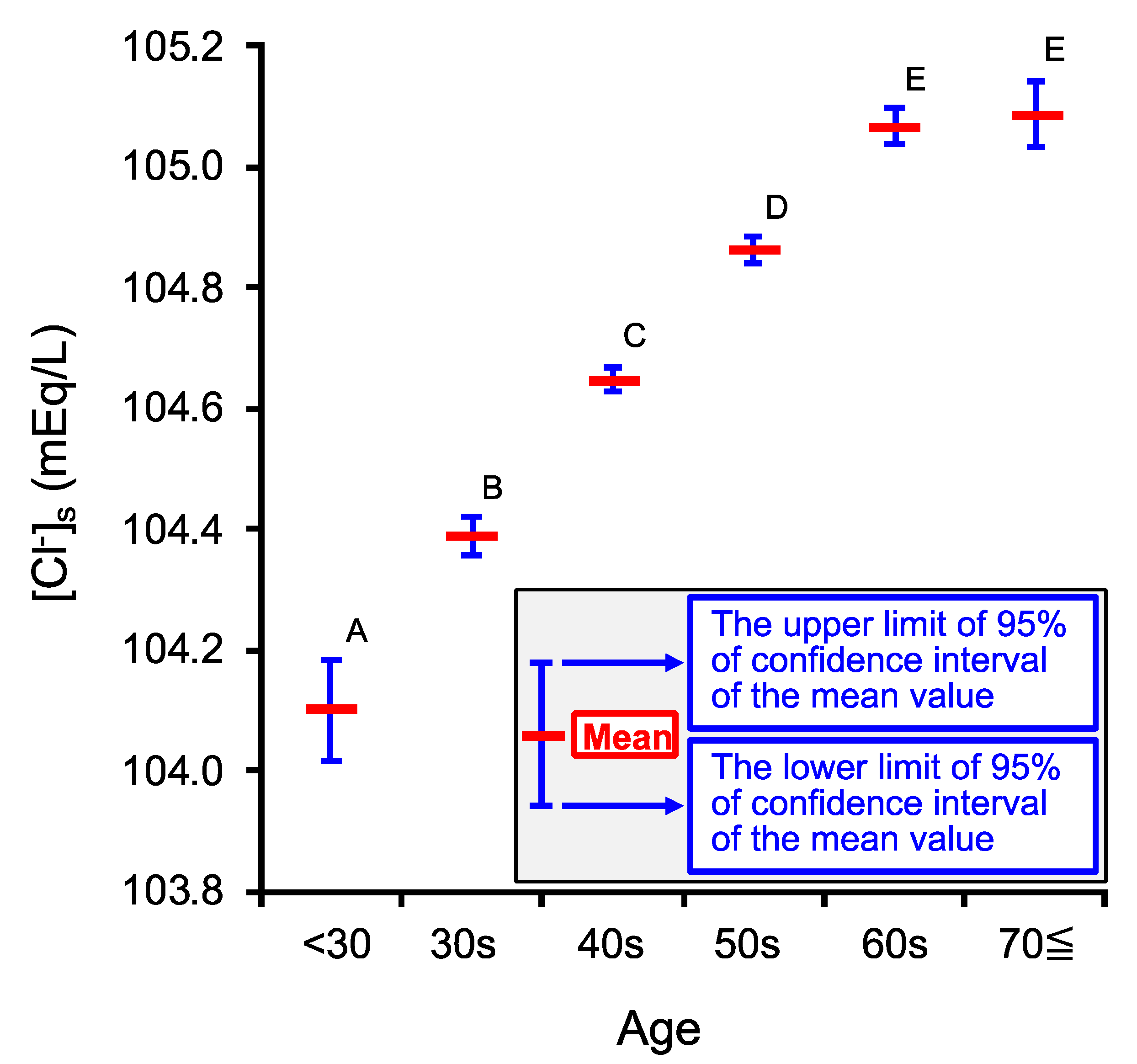
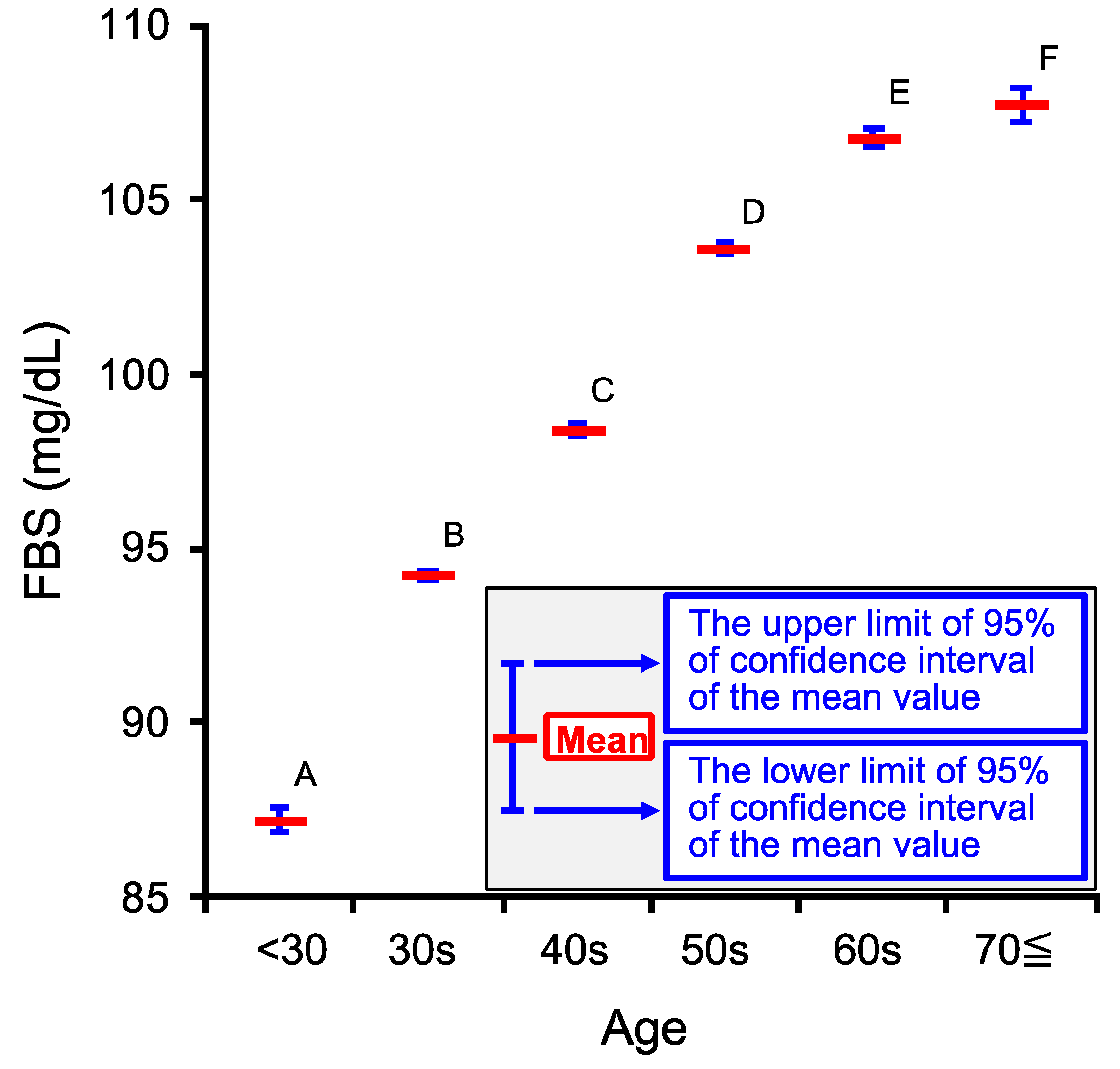
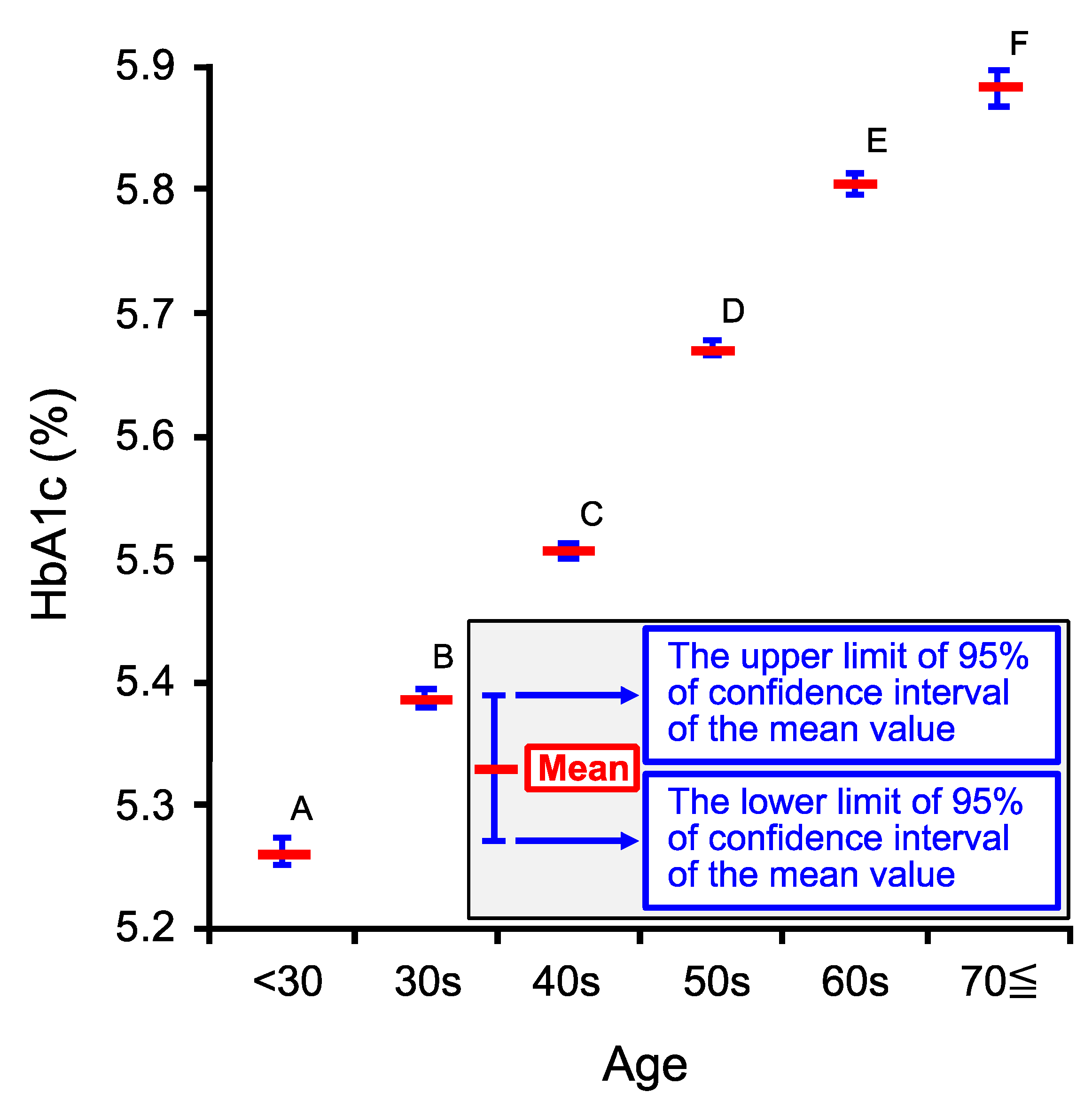
| Age Category | <30 | 30s | 40s | 50s | 60s | 70≦ |
|---|---|---|---|---|---|---|
| Meaning of Age Category (years old) | Age < 30 | 30 ≦ Age < 40 | 40 ≦ Age < 50 | 50 ≦ Age < 60 | 60 ≦ Age < 70 | 70 ≦ Age |
| Number of persons (n) | 1878 | 14,300 | 35,457 | 29,175 | 20,344 | 6476 |
| Coefficient | (mEq/L/year) | (mEq/L/>mg/dL) | (mEq/L/%) | (mEq/L) |
|---|---|---|---|---|
| UL of 95% CI | 0.0312 | −0.00727 | −0.311 | 106.1 |
| Mean | 0.0300 | −0.00837 | −0.345 | 106.0 |
| LL of 95% CI | 0.0289 | −0.00947 | −0.379 | 105.9 |
| Coefficient | (mEq/L/year) | (mEq/L/mg/dL) | (mEq/L/%) | (mEq/L) |
|---|---|---|---|---|
| UL of 95% CI | 0.1693 | −0.0626 | −0.0875 | 0.0059 |
| Mean | 0.1631 | −0.0721 | −0.0970 | 0.0000 |
| LL of 95% CI | 0.1569 | −0.0816 | −0.1065 | −0.0059 |
| FBS (mg/dL) | FBS < 100 | 100 ≦ FBS < 126 | 126 ≦ FBS | |
|---|---|---|---|---|
| n | 59,922 | 41,633 | 6075 | |
| UL of 95% CI | 0.0281 | −0.0128 | −0.0186 | |
| Mean | 0.0252 | −0.0161 | −0.0202 | |
| LL of 95% CI | 0.0222 | −0.0194 | −0.0219 | |
| HbA1c (%) | HbA1c < 5.6 | 5.6 ≦ HbA1c < 6.5 | 6.5 ≦ HbA1c | |
|---|---|---|---|---|
| n | 57,189 | 44,699 | 5742 | |
| UL of 95% CI | −0.0188 | −0.1394 | −0.5794 | |
| Mean | −0.1082 | −0.2332 | −0.6312 | |
| LL of 95% CI | −0.1976 | −0.3269 | −0.6830 | |
| Coefficient | ||
|---|---|---|
| UL of 95% CI | 0.021725 | 0.787159 |
| Mean | 0.021462 | 0.783447 |
| LL of 95% CI | 0.021199 | 0.779735 |
| FBS (mg/dL) | FBS < 100 | 100 ≦ FBS < 126 | 126 ≦ FBS | |
|---|---|---|---|---|
| n | 59,922 | 41,633 | 6075 | |
| UL of 95% CI | 0.0114 | 0.0266 | 0.0278 | |
| Mean | 0.0110 | 0.0261 | 0.0273 | |
| LL of 95% CI | 0.0106 | 0.0256 | 0.0267 | |
| UL of 95% CI | 0.3500 | 0.8146 | 0.8518 | |
| Mean | 0.3374 | 0.7985 | 0.8351 | |
| LL of 95% CI | 0.3247 | 0.7824 | 0.8185 | |
| Low [Cl−]s | High [Cl−]s | |||
|---|---|---|---|---|
| Normal HbA1c | High HbA1c | Normal HbA1c * | High HbA1c | |
| Glucose metabolism (Mitochondrial function) | Normal | Normal | Low | Low |
| Insulin resistance | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marunaka, Y.; Yagi, K.; Imagawa, N.; Kobayashi, H.; Murayama, M.; Minamibata, A.; Takanashi, Y.; Nakahari, T. Possibility of Venous Serum Cl− Concentration ([Cl−]s) as a Marker for Human Metabolic Status: Correlation of [Cl−]s to Age, Fasting Blood Sugar (FBS), and Glycated Hemoglobin (HbA1c). Int. J. Mol. Sci. 2021, 22, 11111. https://doi.org/10.3390/ijms222011111
Marunaka Y, Yagi K, Imagawa N, Kobayashi H, Murayama M, Minamibata A, Takanashi Y, Nakahari T. Possibility of Venous Serum Cl− Concentration ([Cl−]s) as a Marker for Human Metabolic Status: Correlation of [Cl−]s to Age, Fasting Blood Sugar (FBS), and Glycated Hemoglobin (HbA1c). International Journal of Molecular Sciences. 2021; 22(20):11111. https://doi.org/10.3390/ijms222011111
Chicago/Turabian StyleMarunaka, Yoshinori, Katsumi Yagi, Noboru Imagawa, Hironori Kobayashi, Masaru Murayama, Asami Minamibata, Yoshiaki Takanashi, and Takashi Nakahari. 2021. "Possibility of Venous Serum Cl− Concentration ([Cl−]s) as a Marker for Human Metabolic Status: Correlation of [Cl−]s to Age, Fasting Blood Sugar (FBS), and Glycated Hemoglobin (HbA1c)" International Journal of Molecular Sciences 22, no. 20: 11111. https://doi.org/10.3390/ijms222011111
APA StyleMarunaka, Y., Yagi, K., Imagawa, N., Kobayashi, H., Murayama, M., Minamibata, A., Takanashi, Y., & Nakahari, T. (2021). Possibility of Venous Serum Cl− Concentration ([Cl−]s) as a Marker for Human Metabolic Status: Correlation of [Cl−]s to Age, Fasting Blood Sugar (FBS), and Glycated Hemoglobin (HbA1c). International Journal of Molecular Sciences, 22(20), 11111. https://doi.org/10.3390/ijms222011111







