Water Activity Prediction in Sugar and Polyol Systems Using Theoretical Molecular Descriptors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Correlation of Water Activity Data
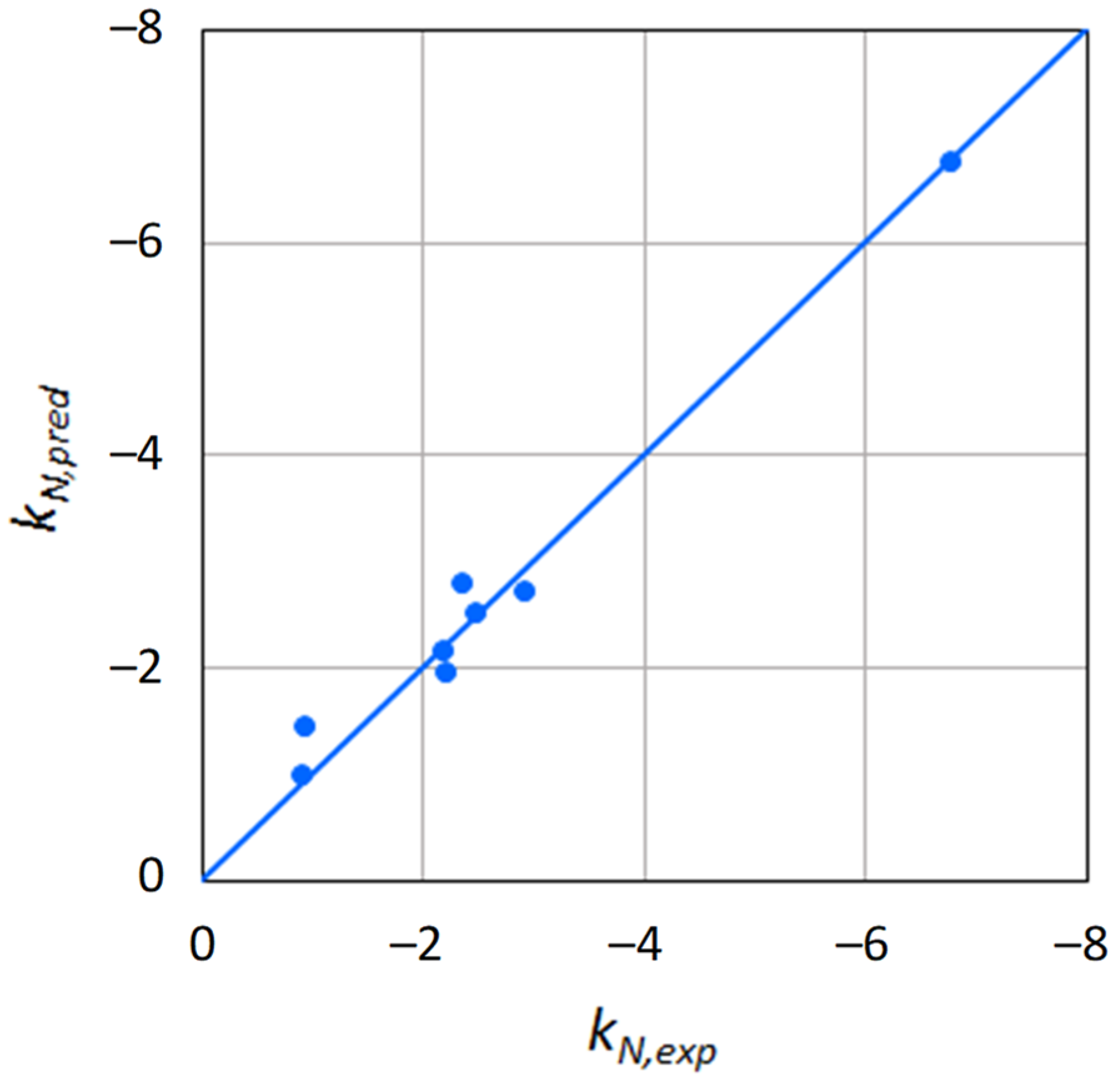
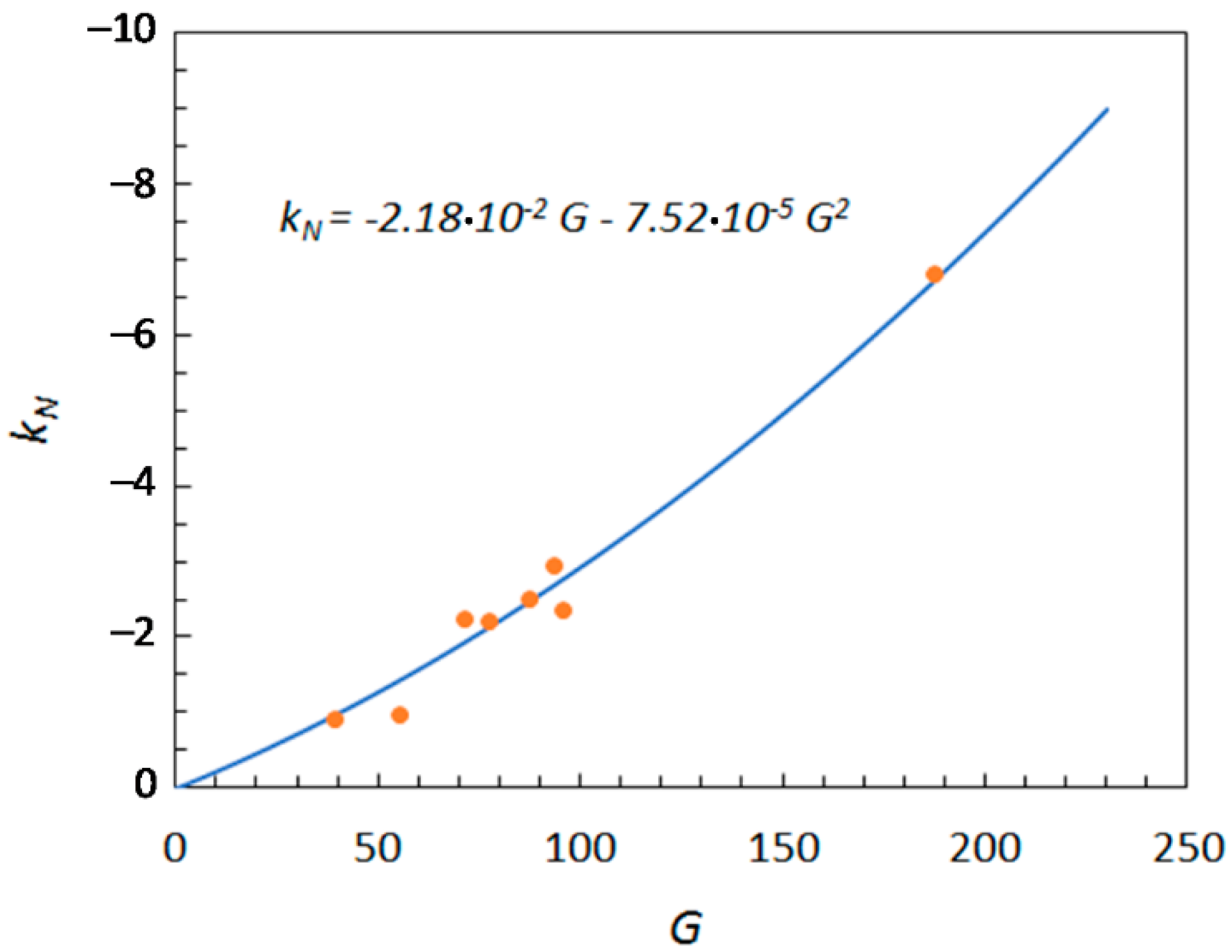
2.2. Use of Molecular Descriptors for the Prediction of the Norrish Constant
3. Methods
3.1. Studied Systems and Literature Data
3.2. Molecular Descriptors
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
Appendix A
References
- Jayaprakasha, H.M.; Jayaraj Rao, K.; Lokesh Kumar, W.A. Studies on the influence of water activity (aw) on the stability of foods—A critical appraisal. J. Food Sci. Technol. 1997, 34, 273–285. [Google Scholar]
- Kerdudo, A.; Fontaine-Vive, F.; Dingas, A.; Faure, C.; Fernandez, X. Optimization of cosmetic preservation: Water activity reduction. Int. J. Cosmet. Sci. 2015, 37, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Cundell, T. The role of water activity in the microbial stability of non-sterile drug products. Eur. Pharm. Rev. 2015, 20, 58–63. [Google Scholar]
- Escobedo-Avellaneda, Z.; Rodríguez-Martínez, V.; Serment-Moreno, V.; Velázquez, G.; Welti-Chanes, J.; Antonio Torre, J. Selected Applications of Water Activity Management in the Food Industry. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Barbosa-Cánovas, G.V., Fontana, A.J., Jr., Schmidt, S.J., Labuza, T.P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 465–482. [Google Scholar]
- Scott, W.J. Water relations of Staphylococcus aureus at 30 °C. Aust. J. Biol. Sci. 1953, 6, 549–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, D.S. Water Activity: Fundamentals and Relationships. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Barbosa-Cánovas, G.V., Fontana, A.J., Jr., Schmidt, S.J., Labuza, T.P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 13–26. [Google Scholar]
- Maneffa, A.J.; Stenner, R.; Matharu, A.S.; Clark, J.H.; Matubayasi, N.; Shimizu, S. Water activity in liquid food systems: A molecular scale interpretation. Food Chem. 2017, 237, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Labuza, T.P.; Altunakar, B. Water Activity Prediction and Moisture Sorption Isotherms. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Barbosa-Cánovas, G.V., Fontana, A.J., Jr., Schmidt, S.J., Labuza, T.P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 161–205. [Google Scholar]
- Caurie, M. Water activity of multicomponent mixture of solutes and non-solutes. Int. J. Food Sci. Technol. 2005, 40, 295–303. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Rogé, B.; Génotelle, J.; Mathlouthi, M. Relationships between hydration number, water activity and density of aqueous sugar solutions. Food Chem. 2008, 106, 1443–1453. [Google Scholar] [CrossRef]
- Chirife, J.; Fontana Jr, A.J. Introduction: Historical Highlights of Water Activity Research. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Barbosa-Cánovas, G.V., Fontana, A.J., Jr., Schmidt, S.J., Labuza, T.P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–11. [Google Scholar]
- Sereno, A.M.; Hubinger, M.D.; Comesaña, J.F.; Correa, A. Prediction of water activity of osmotic solutions. J. Food Eng. 2001, 49, 103–114. [Google Scholar] [CrossRef]
- Norrish, R.S. An equation for the activity coefficients and equilibrium relative humidities of water in confectionery syrups. Int. J. Food Sci. Technol. 1966, 1, 25–39. [Google Scholar] [CrossRef]
- Mauri, A.; Consonni, V.; Todeschini, R. Molecular Descriptors. In Handbook of Computational Chemistry; Leszczynski, J., Kaczmarek-Kedziera, A., Puzyn, T., Papadopoulos, M.G., Reis, H., Shukla, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 2065–2093. [Google Scholar]
- Grisoni, F.; Consonni, V.; Todeschini, R. Impact of molecular descriptors on computational models. Methods Mol. Biol. 2018, 1825, 171–209. [Google Scholar]
- Sahoo, S.; Adhikari, C.; Kuanar, M.; Mishra, B.K. A short review of the generation of molecular descriptors and their applications in quantitative structure property/activity relationships. Curr. Comput.-Aided Drug Des. 2016, 12, 181–250. [Google Scholar] [CrossRef]
- Grisoni, F.; Ballabio, D.; Todeschini, R.; Consonni, V. Molecular descriptors for structure–activity applications: A hands-on approach. Methods Mol. Biol. 2018, 1800, 3–53. [Google Scholar] [PubMed]
- Barbiroli, A.; Marengo, M.; Fessas, D.; Ragg, E.; Renzetti, S.; Bonomi, F.; Iametti, S. Stabilization of beta-lactoglobulin by polyols and sugars against temperature-induced denaturation involves diverse and specific structural regions of the protein. Food Chem. 2017, 234, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Rockinger, U.; Funk, M.; Winter, G. Current approaches of preservation of cells during (freeze-) drying. J. Pharm. Sci. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- López-Malo, A.; Alzamora, S.M. Water activity and microorganism control: Past and future. In Water Stress in Biological, Chemical, Pharmaceutical and Food Systems; Gutiérrez-López, G.F., Alamilla-Beltrán, L., del Pilar Buera, M., Welti-Chanes, J., Parada-Arias, E., Barbosa-Cánovas, G.V., Eds.; Springer: New York, NY, USA, 2015; pp. 245–262. [Google Scholar]
- Bonchev, D.; Trinajstic, N. Chemical information theory: Structural aspects. Int. J. Quantum Chem. 1982, 16, 463–480. [Google Scholar] [CrossRef]
- Barigye, S.J.; Marrero-Ponce, Y.; Pérez-Giménez, F.; Bonchev, D. Trends in information theory-based chemical structure codification. Mol. Divers. 2014, 18, 673–686. [Google Scholar] [CrossRef]
- Stanton, D.T. On the importance of topological descriptors in understanding structure–property relationships. J. Comput. Aided Mol. Des. 2008, 22, 441–460. [Google Scholar] [CrossRef]
- Bueso-Bordils, J.I.; Alemán-López, P.A.; Suay-García, B.; Martín-Algarra, R.; Duart, M.J.; Falcó, A.; Antón-Fos, G.M. Molecular topology for the discovery of new broad-spectrum antibacterial drugs. Biomolecules 2020, 10, 1343. [Google Scholar] [CrossRef]
- Basant, N.; Gupta, S.; Singh, K.P. Predicting toxicities of diverse chemical pesticides in multiple avian species using tree-based QSAR approaches for regulatory purposes. J. Chem. Inf. Model. 2015, 55, 1337–1348. [Google Scholar] [CrossRef]
- Gutman, I. Degree-based topological indices. Croat. Chem. Acta 2013, 86, 351–361. [Google Scholar] [CrossRef]
- Bonchev, D. Information Theoretic Indices for Characterization of Chemical Structures, 3rd ed.; Research Studies Press: Chichester, UK, 1983; pp. 185–194. [Google Scholar]
- Hawkins, D.M. The problem of overfitting. J. Chem. Inf. Comput. Sci. 2004, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chirife, J.; Ferro Fontán, C.; Benmergui, E.A. The prediction of water activity in aqueous solutions in connection with intermediate moisture foods. IV. aw prediction in aqueous nonelectrolyte solution. J. Food Technol. 1980, 15, 59–70. [Google Scholar] [CrossRef]
- Alzamora, S.M.; Chirife, J.; Gerschenson, L.N. Determination and correlation of the water activity of propylene glycol solutions. Food Res. Int. 1994, 27, 65–67. [Google Scholar] [CrossRef]
- Miyawaki, O.; Saito, A.; Matsuo, T.; Nakamura, K. Activity and activity coefficient of water in aqueous solutions and their relationships with solution structure parameters. Biosci. Biotechnol. Biochem. 1997, 61, 466–469. [Google Scholar] [CrossRef]
- Baeza, R.; Pérez, A.; Sánchez, V.; Zamora, M.C.; Chirife, J. Evaluation of Norrish’s equation for correlating the water activity of highly concentrated solutions of sugars, polyols, and polyethylene glycols. Food Bioprocess Technol. 2010, 3, 87–92. [Google Scholar] [CrossRef]
- Starzak, M.; Peacock, S.D.; Mathlouthi, M. Hydration number and water activity models for the sucrose-water system: A critical review. Crit. Rev. Food Sci. Nutr. 2000, 40, 327–367. [Google Scholar] [CrossRef]
- Sharp, K.A.; Vanderkooi, J.M. Water in the half shell: Structure of water, focusing on angular structure and solvation. Acc. Chem. Res. 2010, 43, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S. Sucrose-water mixture: From thermodynamics to solution structure. Chem. Phys. Lett. 2013, 582, 129–133. [Google Scholar] [CrossRef]
- Subbiah, B.; Blank, U.K.M.; Morison, K.R. A review, analysis and extension of water activity data of sugars and model honey solutions. Food Chem. 2020, 326, 126981. [Google Scholar] [CrossRef]
- Politi, R.; Sapir, L.; Harries, F. The impact of polyols on water structure in solution: A computational study. J. Phys. Chem. A 2009, 113, 7548–7555. [Google Scholar] [CrossRef]
- Sundar, S.; Sandilya, A.A.; Priya, M.H. Unraveling the influence of osmolytes on water hydrogen-bond network: From local structure to graph theory analysis. J. Chem. Inf. Model. 2021, 61, 3927–3944. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Omote, C.; Miyawaki, O. Effect of intermolecular interactions on water activity of multicomponent aqueous solutions. Japan J. Food Eng. 2015, 16, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Comesaña, J.F.; Correa, A.; Sereno, A.M. Water activity at 35 °C in ‘sugar’ + water and ‘sugar’ + sodium chloride + water systems. Int. J. Food Sci. Technol. 2001, 36, 655–661. [Google Scholar] [CrossRef]
- Ninni, L.; Camargo, M.S.; Meirelles, A.J.A. Water activity in polyol systems. J. Chem. Eng. Data 2000, 45, 654–660. [Google Scholar] [CrossRef]
- Kirkwood, J.G.; Buff, F.P. The statistical mechanical theory of solutions. I. J. Chem. Phys. 1951, 19, 774–777. [Google Scholar] [CrossRef]
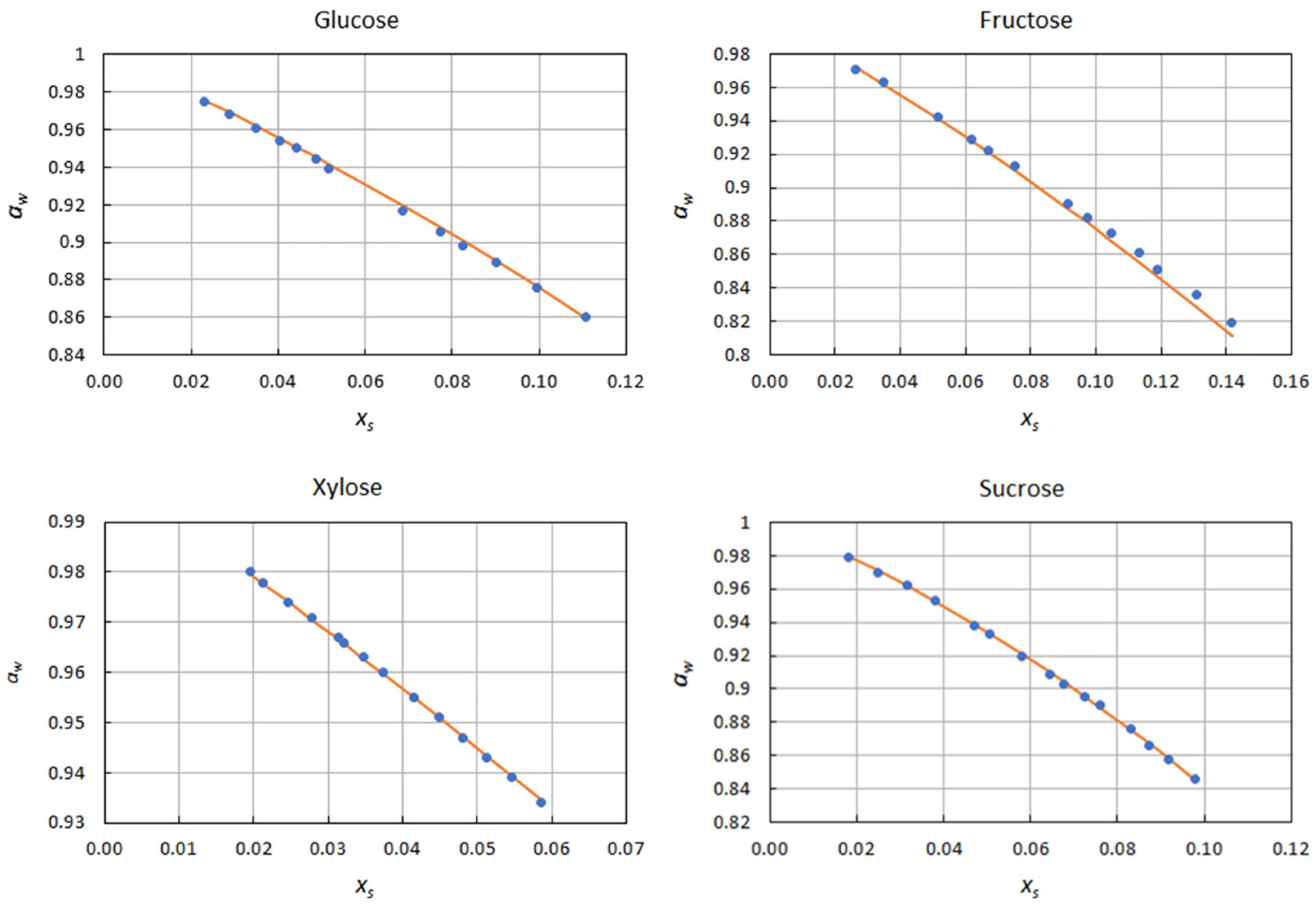
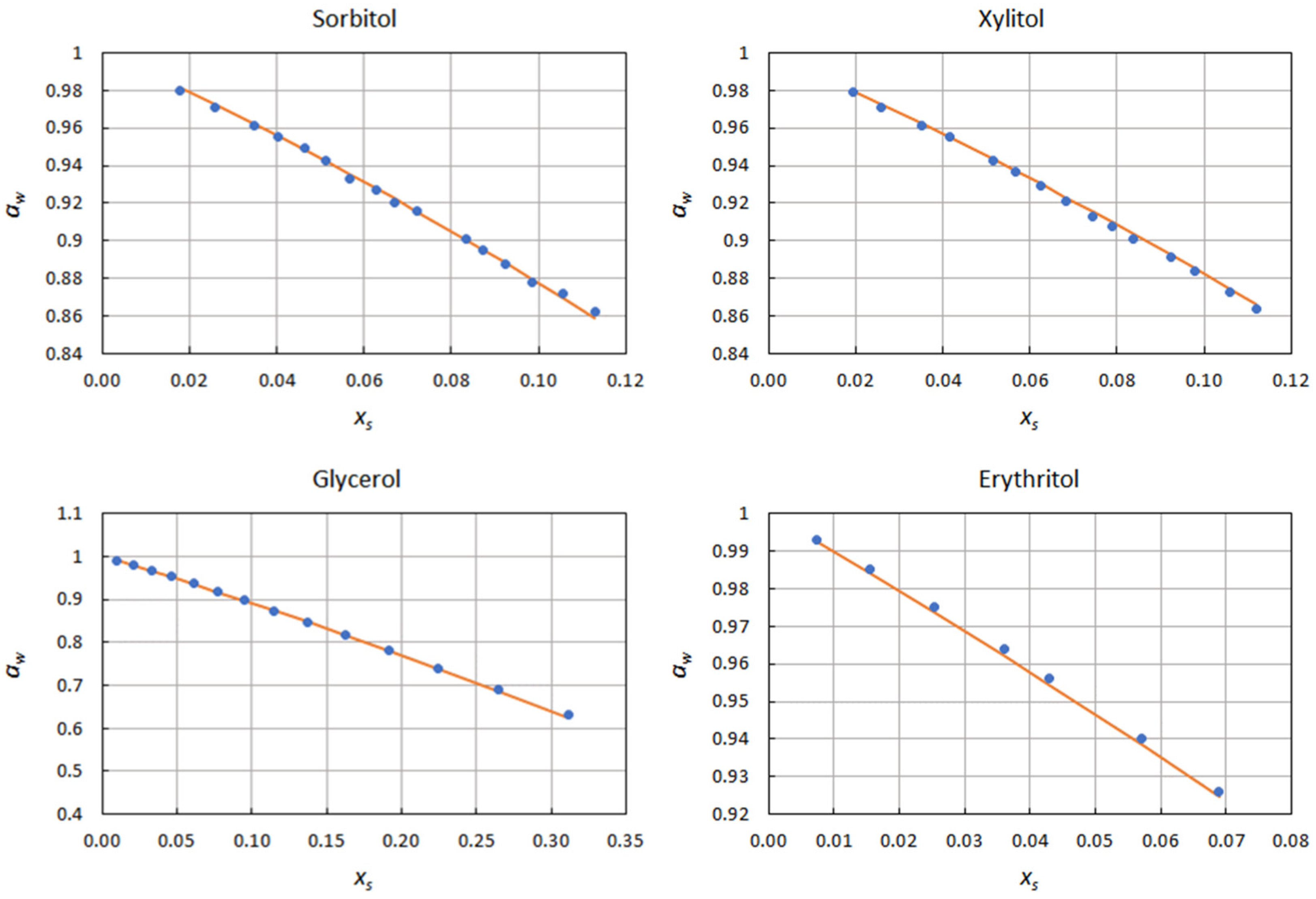
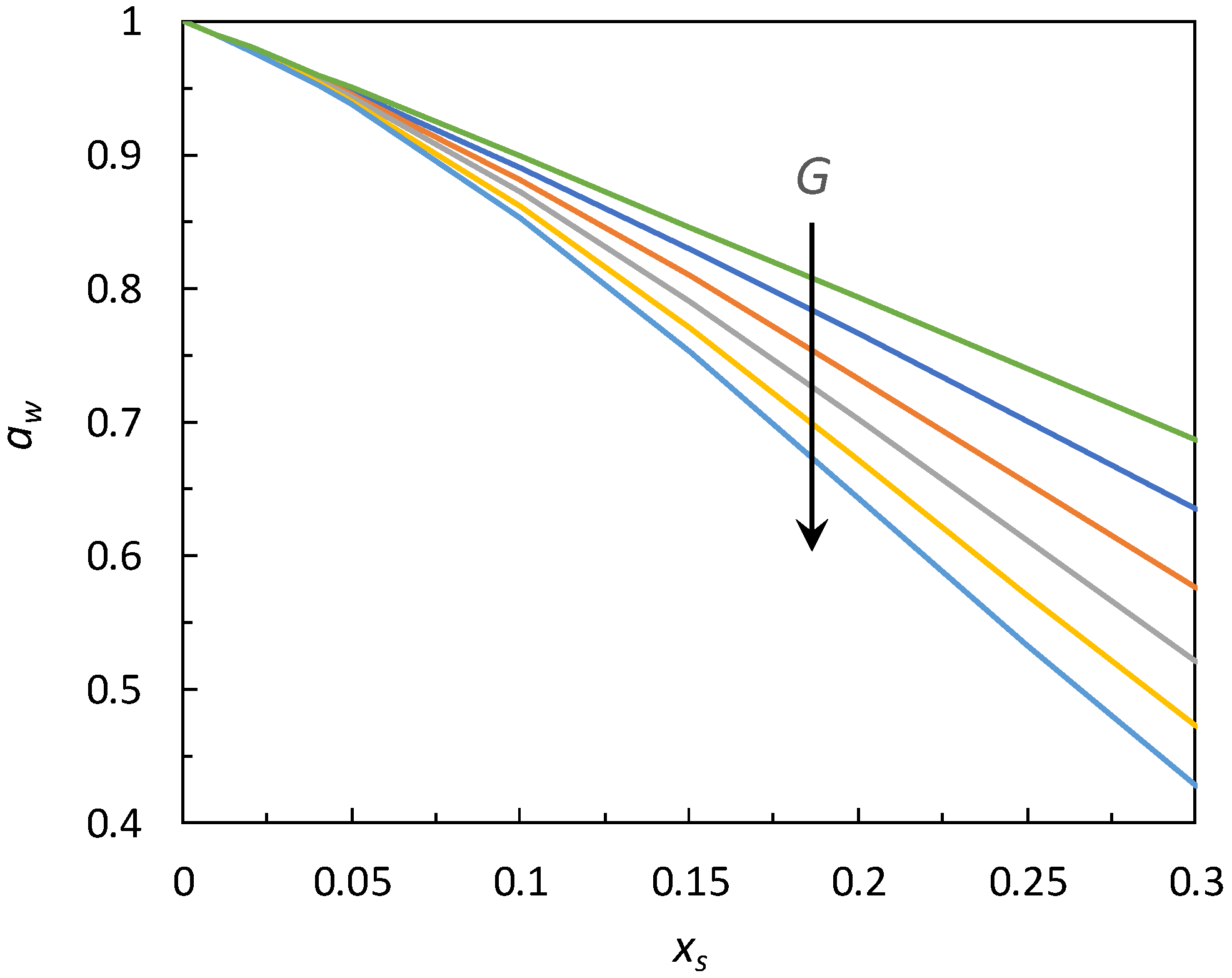
| Solute | ∆xs | kN | Φmin | ε |
|---|---|---|---|---|
| Glucose | 0.023–0.111 | −2.920 | 1.71 × 10−5 | 1.00 × 10−3 |
| Fructose | 0.026–0.142 | −2.351 | 6.76 × 10−6 | 6.64 × 10−4 |
| Xylose | 0.020–0.059 | −2.196 | 1.30 × 10−6 | 2.71 × 10−4 |
| Sucrose | 0.018–0.098 | −6.777 | 6.87 × 10−6 | 5.85 × 10−4 |
| Sorbitol | 0.018–0.113 | −2.494 | 4.18 × 10−5 | 1.35 × 10−3 |
| Xylitol | 0.020–0.112 | −2.221 | 1.12 × 10−6 | 7.46 × 10−4 |
| Glycerol | 0.010–0.312 | −0.908 | 1.65 × 10−5 | 9.70 × 10−4 |
| Erythritol | 0.007–0.069 | −0.950 | 4.28 × 10−6 | 7.08 × 10−4 |
| Model | Parameters | Θ | Φ |
|---|---|---|---|
| a11 = 1.333 a12 = −8.39 × 10−2 a13 = −1.87 × 10−2 | 4.15 × 10−1 | 8.29 × 10−2 | |
| a21 = −1.44 × 10−2 a22 = 1.026 a23 = 3.81 × 10−1 | 2.67 × 10−1 | 5.35 × 10−2 | |
| b11 = 8.90 × 10−1 b12 = −3.98 × 10−2 | 4.98 × 10−1 | 8.29 × 10−2 | |
| b21 = −7.65 × 10−3 b22 = 1.295 | 3.35 × 10−1 | 5.58 × 10−2 | |
| b31 = −2.18 × 10−2 b32 = −7.52 × 10−5 | 3.10 × 10−1 | 5.17 × 10−2 |
| Solute | Formula | MW | NNHA | NB | NR |
|---|---|---|---|---|---|
| Glucose | C6H12O6 | 180.18 | 12 | 24 | 1 |
| Fructose | C6H12O6 | 180.18 | 12 | 24 | 1 |
| Xylose | C5H10O5 | 150.15 | 10 | 20 | 1 |
| Sucrose | C12H22O11 | 342.34 | 23 | 46 | 2 |
| Sorbitol | C6H14O6 | 182.20 | 12 | 25 | 0 |
| Xylitol | C5H12O5 | 152.17 | 10 | 21 | 0 |
| Glycerol | C3H8O3 | 92.11 | 6 | 13 | 0 |
| Erythritol | C4H10O4 | 122.14 | 8 | 17 | 0 |
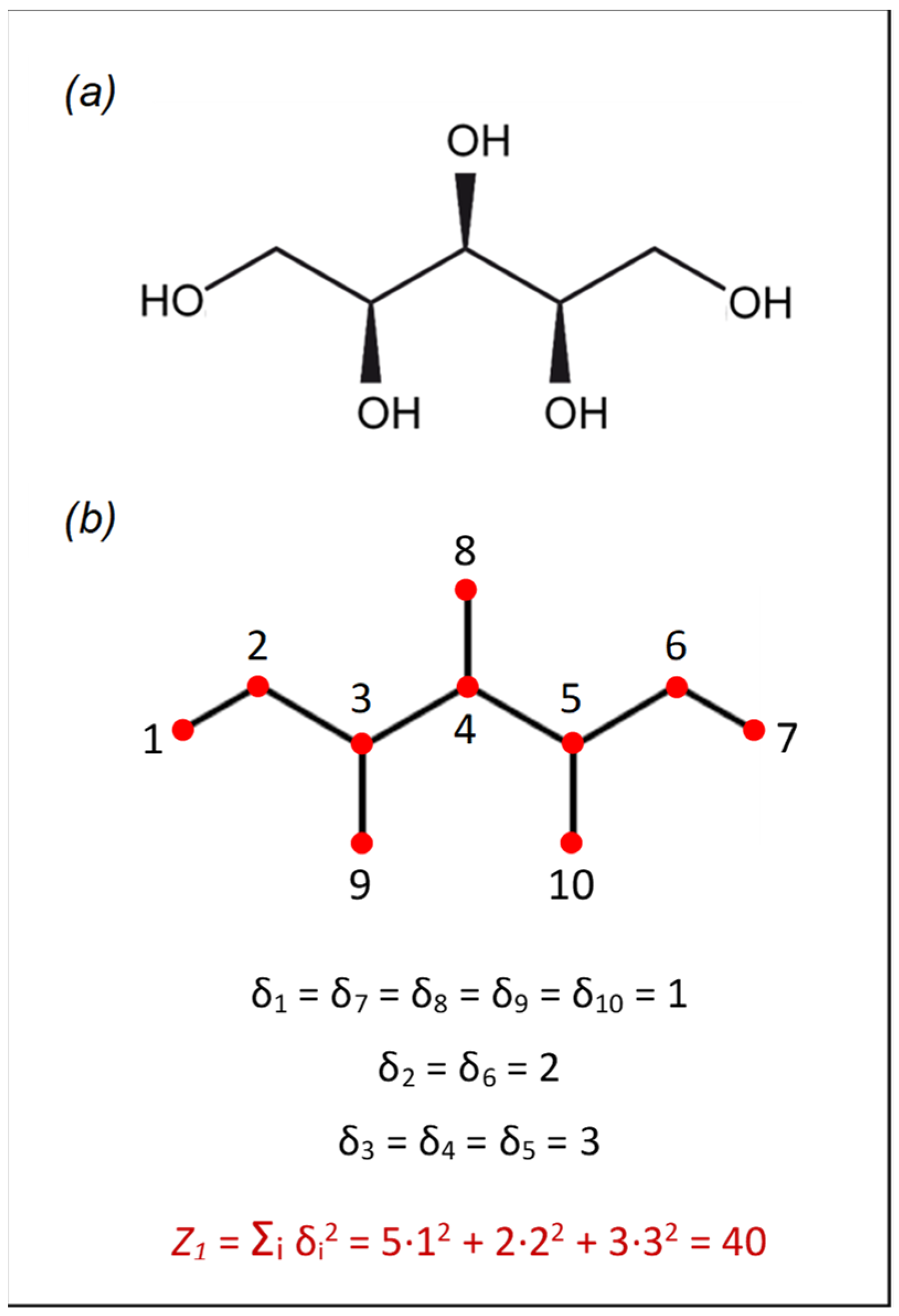
| Solute | IAC | Z1 | G |
|---|---|---|---|
| Glucose | 36.00 | 58 | 94.00 |
| Fructose | 36.00 | 60 | 96.00 |
| Xylose | 30.00 | 48 | 78.00 |
| Sucrose | 67.95 | 120 | 187.95 |
| Sorbitol | 37.89 | 50 | 87.89 |
| Xylitol | 31.87 | 40 | 71.87 |
| Glycerol | 19.79 | 20 | 39.79 |
| Erythritol | 25.84 | 30 | 55.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuorro, A. Water Activity Prediction in Sugar and Polyol Systems Using Theoretical Molecular Descriptors. Int. J. Mol. Sci. 2021, 22, 11044. https://doi.org/10.3390/ijms222011044
Zuorro A. Water Activity Prediction in Sugar and Polyol Systems Using Theoretical Molecular Descriptors. International Journal of Molecular Sciences. 2021; 22(20):11044. https://doi.org/10.3390/ijms222011044
Chicago/Turabian StyleZuorro, Antonio. 2021. "Water Activity Prediction in Sugar and Polyol Systems Using Theoretical Molecular Descriptors" International Journal of Molecular Sciences 22, no. 20: 11044. https://doi.org/10.3390/ijms222011044
APA StyleZuorro, A. (2021). Water Activity Prediction in Sugar and Polyol Systems Using Theoretical Molecular Descriptors. International Journal of Molecular Sciences, 22(20), 11044. https://doi.org/10.3390/ijms222011044






