Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Toxicity Caused by Different Food-Derived Forms of Cadmium in Mice
Abstract
:1. Introduction
2. Results
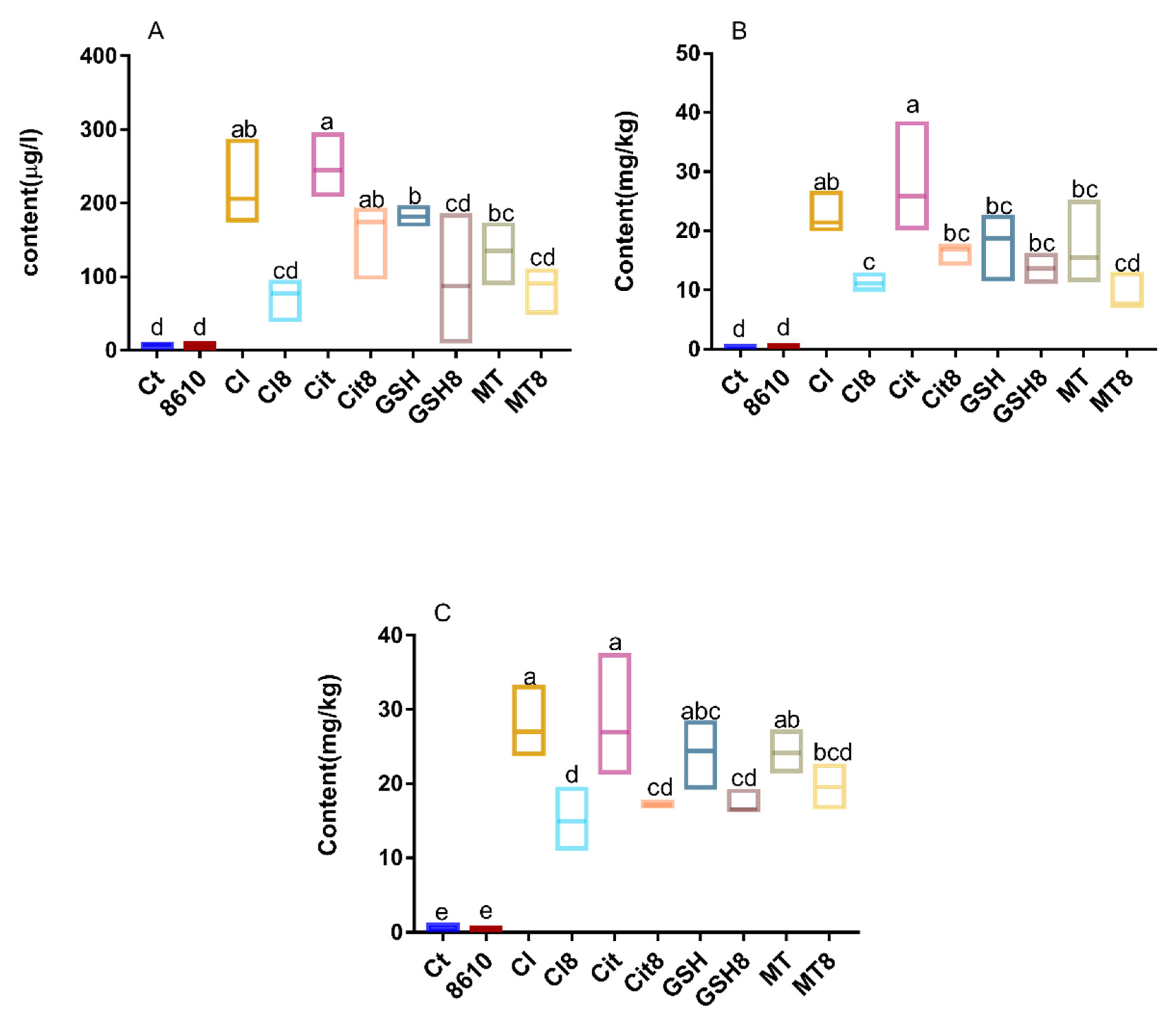
2.1. Changes in Cadmium Content and Antioxidant Indexes in Livers and Kidneys
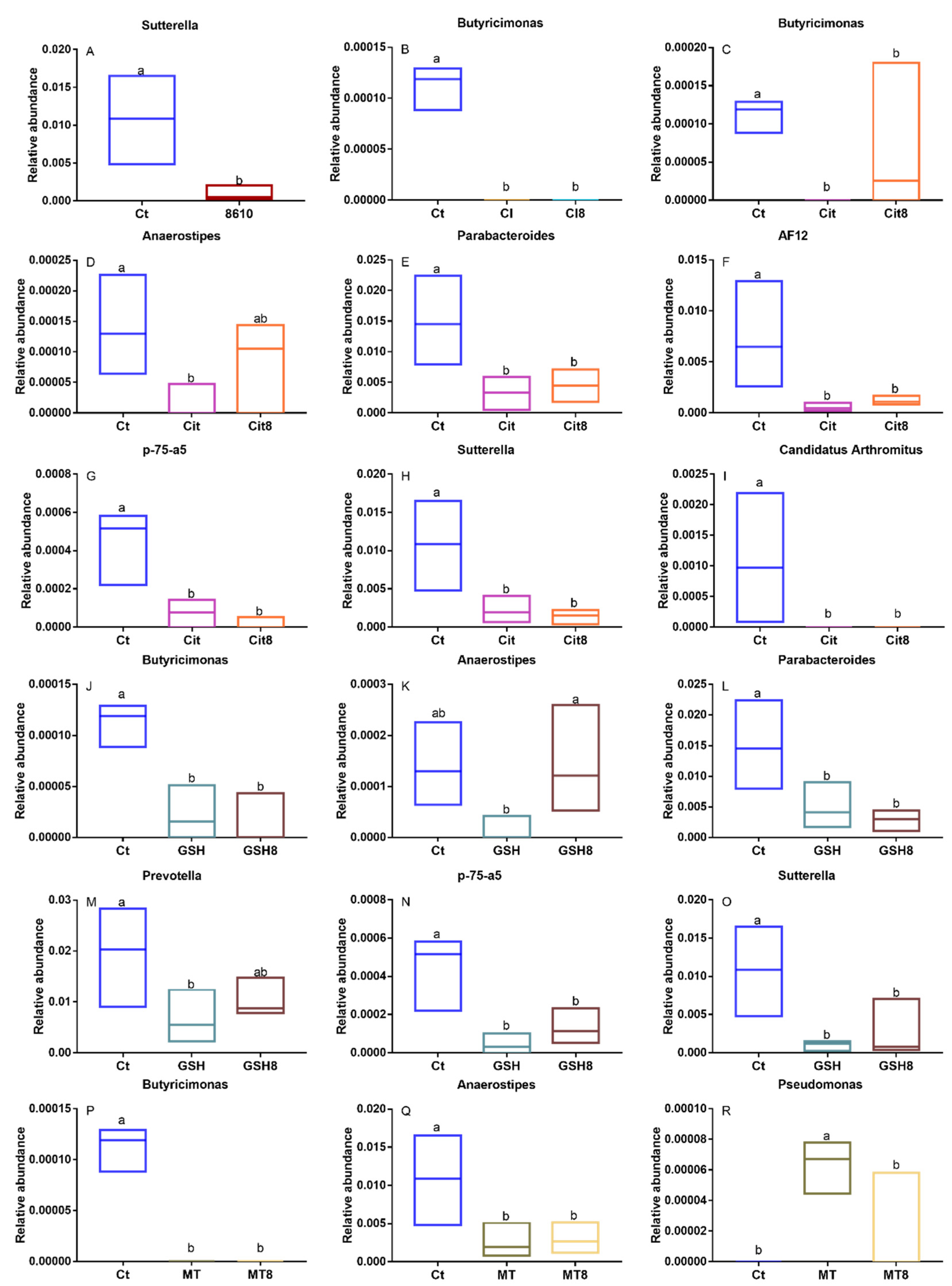
2.2. Changes in the Composition of the Intestinal Microbiota
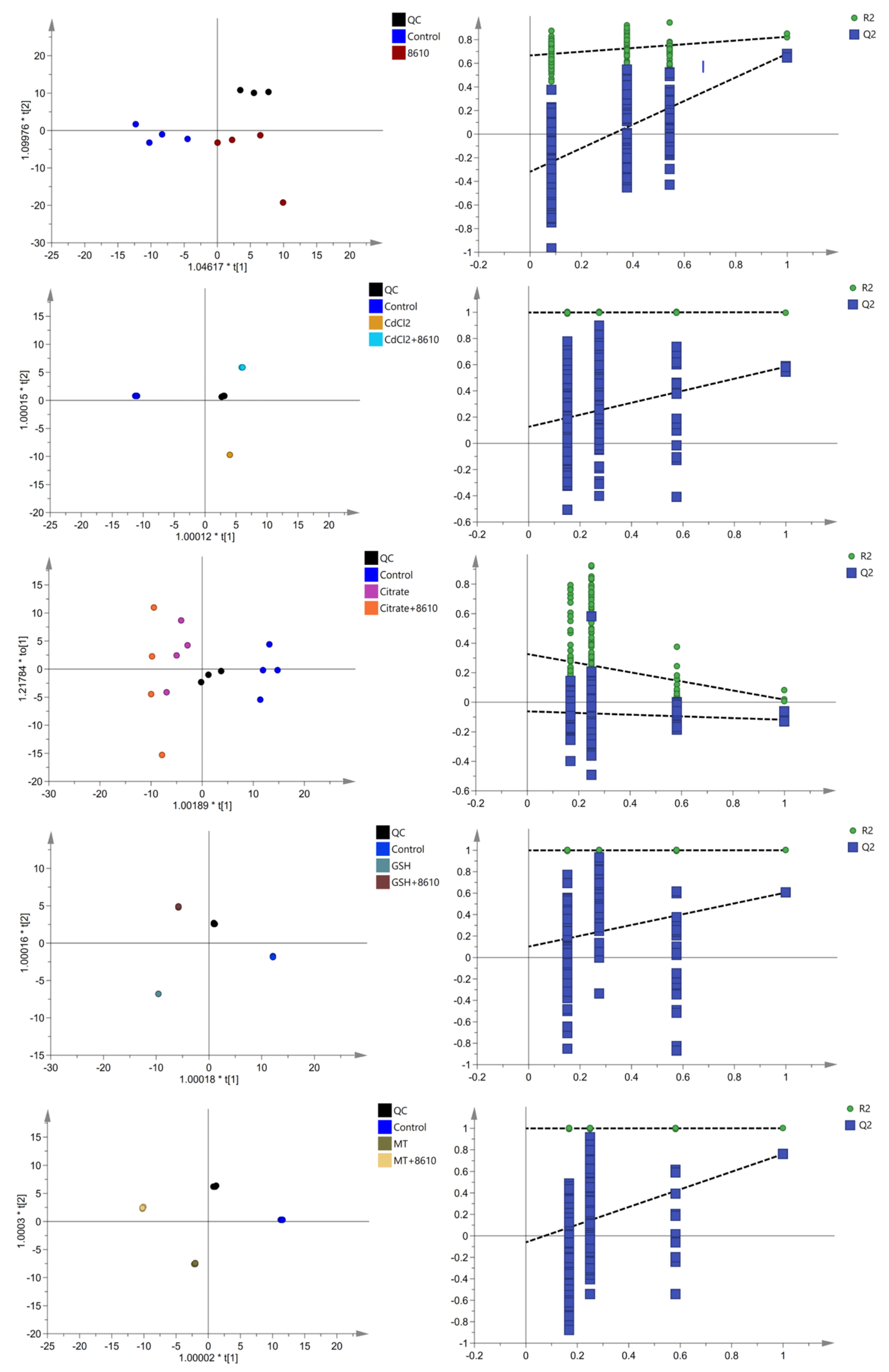
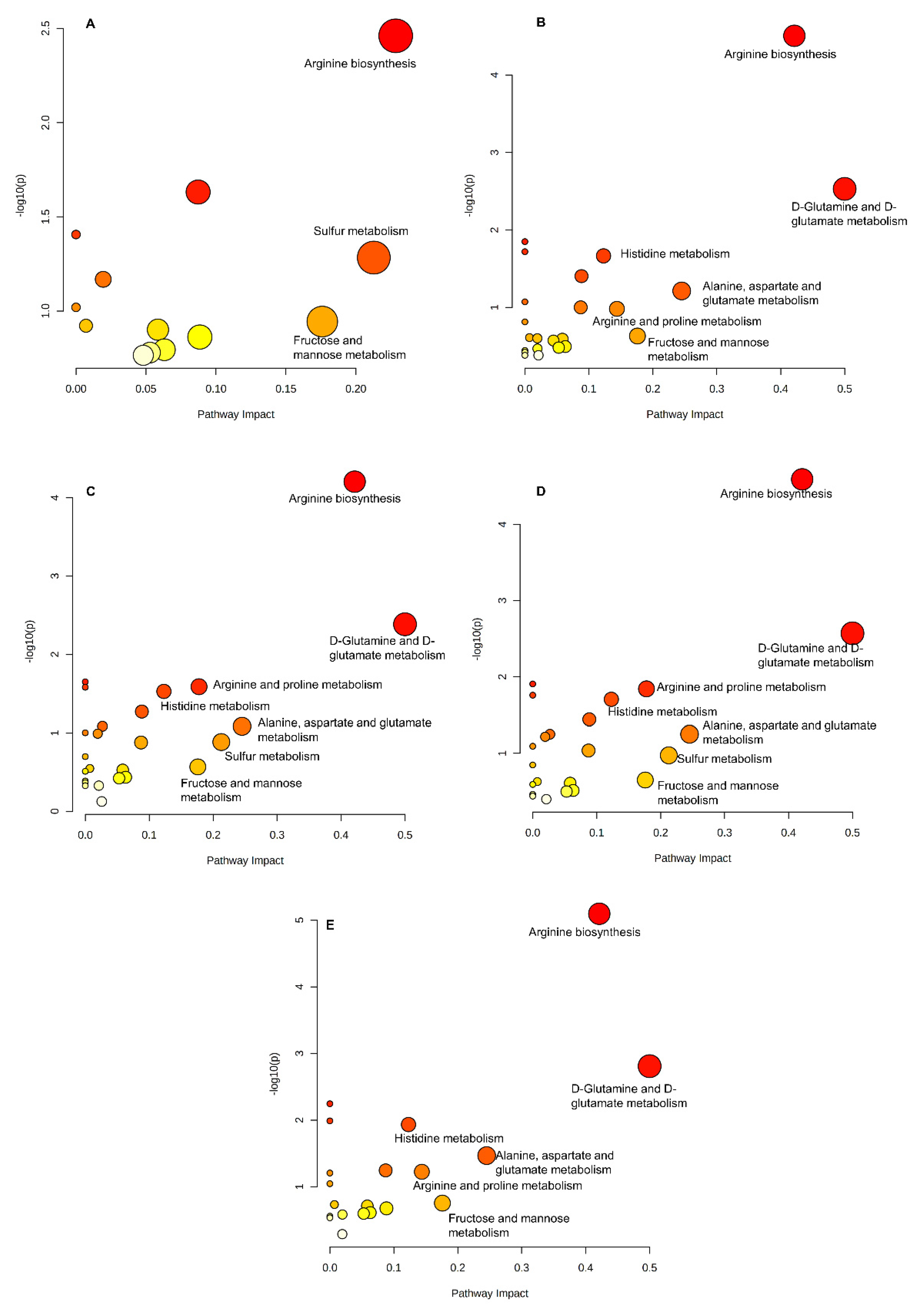
2.3. Changes in Serum Metabolites
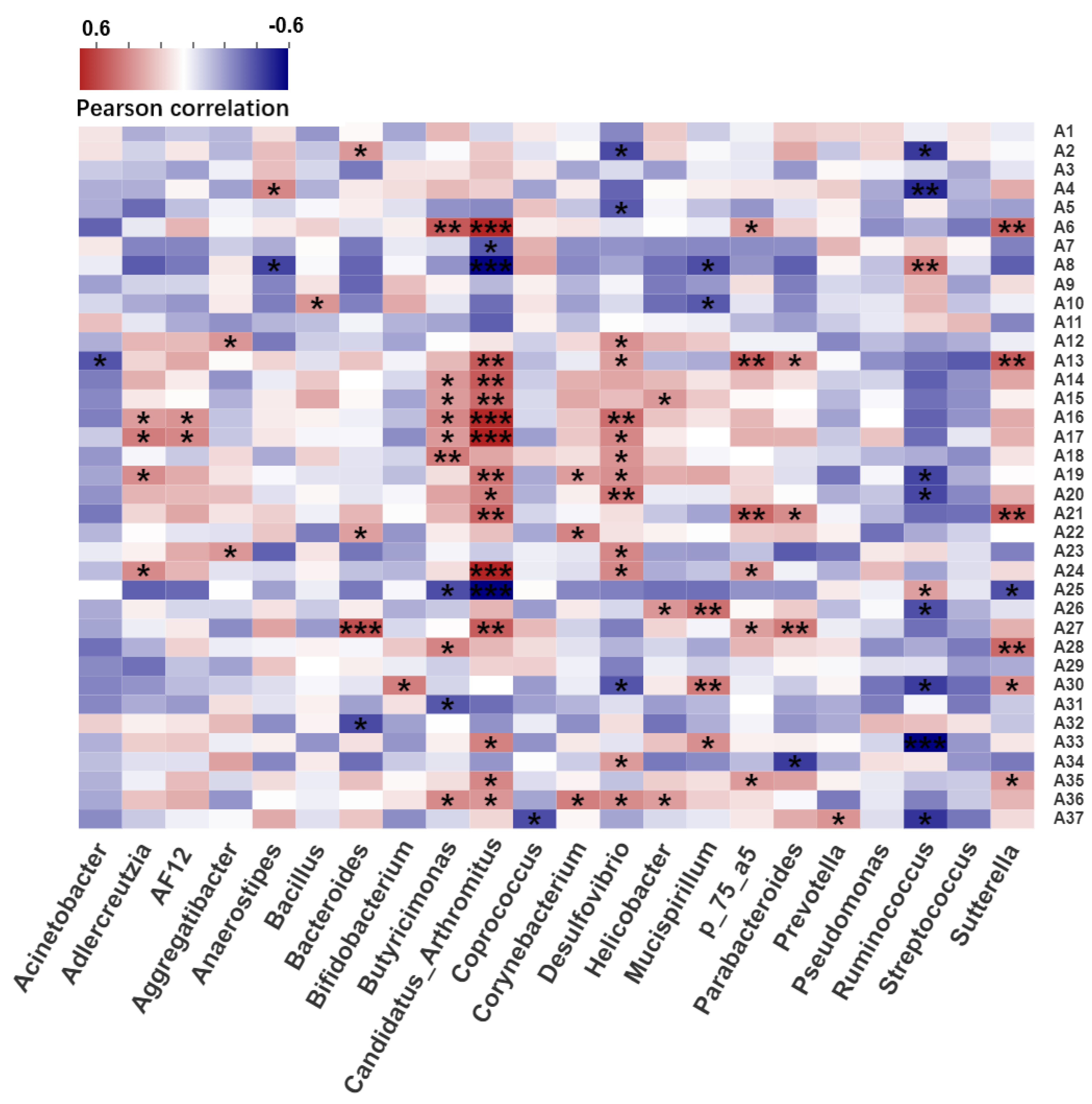
2.4. Correlation Analysis between Intestinal Microbiota and Serum Metabolites
3. Discussion
4. Materials and Methods
4.1. Preparation of Diverse Foodborne Forms of Cadmium
4.2. Preparation of L. plantarum CCFM8610
4.3. Animal Treatment
4.4. Determination of Cadmium Content
4.5. Analysis of Antioxidant Indexes in Livers and Kidneys
4.6. Analysis of Fecal Microbial Diversity
4.7. Metabolomics Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niño–savala, A.G.; Zhuang, Z.; Ma, X.; Fangmeier, A.; Li, H.; Tang, A.; Liu, X. Cadmium pollution from phosphate fertilizers in arable soils and crops: An overview. Front. Agric. Sci. Eng. 2019, 6, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Registry, A. The ATSDR 2019 Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 12 October 2021).
- International Agency for Research on Cancer Agents Classified by the IARC Monographs, Volumes 1–129. Available online: https://monographs.iarc.who.int/list–of–classifications (accessed on 12 October 2021).
- El–Sokkary, G.H.; Awadalla, E.A. The protective role of vitamin C against cerebral and pulmonary damage induced by cadmium chloride in male adult albino rat. Open Neuroendocrinol. J. 2011, 4, 1–8. [Google Scholar] [CrossRef]
- Nemmiche, S.; Chabane–Sari, D.; Guiraud, P. Role of α–tocopherol in cadmium–induced oxidative stress in Wistar rat’s blood, liver and brain. Chem.–Biol. Interact. 2007, 170, 221–230. [Google Scholar] [CrossRef]
- Halttunen, T.; Salminen, S.; Tahvonen, R. Rapid removal of lead and cadmium from water by specific lactic acid bacteria. Int. J. Food Microbiol. 2007, 114, 30–35. [Google Scholar] [CrossRef]
- Jafarpour, D.; Shekarforoush, S.S.; Ghaisari, H.R.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. Protective effects of synbiotic diets of Bacillus coagulans, Lactobacillus plantarum and inulin against acute cadmium toxicity in rats. BMC Complementary Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Ebbs, S.D.; Zambrano, M.C.; Spiller, S.M.; Newville, M. Cadmium sorption, influx, and efflux at the mesophyll layer of leaves from ecotypes of the Zn/Cd hyperaccumulator Thlaspi caerulescens. New Phytologist. 2009, 181, 626–636. [Google Scholar] [CrossRef]
- Ueno, D.; Ma, J.F.; Iwashita, T.; Zhao, F.-J.; McGrath, S.P. Identification of the form of Cd in the leaves of a superior Cd–accumulating ecotype of Thlaspi caerulescens using 113 Cd–NMR. Planta 2005, 221, 928–936. [Google Scholar] [CrossRef]
- Zhao, Y.; Shang, D.; Ning, J.; Zhai, Y. Speciation Analysis of Cadmium in Marine Bivalves by Size Exclusion Chromatography–High Performance Liquid Chromatography–Inductively Coupled Plasma Mass Spectrometry. Chin. J. Anal. Chem. 2012, 40, 681. [Google Scholar] [CrossRef]
- Yang, H.; Liu, W.; Li, B.; Wei, W.; Zhang, H.-J.; Chen, D. Speciation analysis of cadmium in Indian mustard (Brassica juncea) by size exclusion chromatography–high performance liquid chromatography–inductively coupled plasma mass spectrometry. Chin. J. Anal. Chem. 2009, 37, 1511–1514. [Google Scholar] [CrossRef]
- Koropatnick, J.; Cherian, M.G. Exposure to different forms of cadmium in mice: Differences in metallothionein and alphafetoprotein mRNA induction in liver and kidney. J. Biochem. Toxicol. 1988, 3, 159–172. [Google Scholar] [CrossRef]
- Zhu, J.; Hong, K.; Shen, X.; Gan, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. A new method for evaluating the bioaccessibility of different foodborne forms of cadmium. Toxicol. Lett. 2020, 319, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Wang, G.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.; Chen, W. Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl. Environ. Microbiol. 2013, 79, 1508–1515. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral administration of probiotics inhibits absorption of the heavy metal cadmium by protecting the intestinal barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Q.; Wang, G.; Zhao, J.; Liu, X.; Narbad, A.; Chen, Y.Q.; Zhang, H.; Tian, F.; Chen, W. Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl. Environ. Microbiol. 2014, 80, 4063–4071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Q.; Yin, R.; Yu, L.; Wang, G.; Tian, F.; Yu, R.; Zhao, J.; Liu, X.; Chen, Y.Q.; Zhang, H. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control. 2015, 54, 23–30. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Panwar, R.; Ram, C. Efficacy of indigenous probiotic Lactobacillus strains to reduce cadmium bioaccessibility–an in vitro digestion model. Environ. Sci. Pollut. Res. 2017, 24, 1241–1250. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, A.K.; Ghosh, A.R.; Mandal, B.K. Probiotic Pediococcus pentosaceus GS 4 shields brush border membrane and alleviates liver toxicity imposed by chronic cadmium exposure in Swiss albino mice. J. Appl. Microbiol. 2019, 126, 1233–1244. [Google Scholar] [CrossRef]
- Breton, J.; Daniel, C.; Dewulf, J.; Pothion, S.; Froux, N.; Sauty, M.; Thomas, P.; Pot, B.; Foligné, B. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol. Lett. 2013, 222, 132–138. [Google Scholar] [CrossRef]
- Lu, K.; Abo, R.P.; Schlieper, K.A.; Graffam, M.E.; Levine, S.; Wishnok, J.S.; Swenberg, J.A.; Tannenbaum, S.R.; Fox, J.G. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: An integrated metagenomics and metabolomics analysis. Environ. Health Perspect. 2014, 122, 284–291. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Liu, K.; Shen, J. Exposing to cadmium stress cause profound toxic effect on microbiota of the mice intestinal tract. PLoS ONE 2014, 9, e85323. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Y.; Zeng, Z.; Liu, Z.; Fu, Z. Subchronic exposure of mice to cadmium perturbs their hepatic energy metabolism and gut microbiome. Chem. Res. Toxicol. 2015, 28, 2000–2009. [Google Scholar] [CrossRef]
- Fazeli, M.; Hassanzadeh, P.; Alaei, S. Cadmium chloride exhibits a profound toxic effect on bacterial microflora of the mice gastrointestinal tract. Hum. Exp. Toxicol. 2011, 30, 152–159. [Google Scholar] [CrossRef]
- Shi, Y.; Zhai, Q.; Li, D.; Mao, B.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Restoration of cefixime–induced gut microbiota changes by Lactobacillus cocktails and fructooligosaccharides in a mouse model. Microbiol. Res. 2017, 200, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHardy, I.H.; Li, X.; Tong, M.; Ruegger, P.; Jacobs, J.; Borneman, J.; Anton, P.; Braun, J. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome 2013, 1, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinen, E.; Rinttilä, T.; Kajander, K.; Mättö, J.; Kassinen, A.; Krogius, L.; Saarela, M.; Korpela, R.; Palva, A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real–time PCR. Off. J. Am. Coll. Gastroenterol. ACG 2005, 100, 373–382. [Google Scholar] [CrossRef]
- Rajilić–Stojanović, M.; Biagi, E.; Heilig, H.G.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef]
- Kato, T.; Yamazaki, K.; Nakajima, M.; Date, Y.; Kikuchi, J.; Hase, K.; Ohno, H.; Yamazaki, K. Oral administration of Porphyromonas gingivalis alters the gut microbiome and serum metabolome. Msphere 2018, 3, e00460-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; Guan, T.; Zhang, X.; Liu, Y.; Liu, Y.; Zhao, X. Serum metabonomics analysis of quercetin against the toxicity induced by cadmium in rats. J. Biochem. Mol. Toxicol. 2020, 34, e22448. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X. Gut microbiome and serum metabolome alterations in obesity and after weight–loss intervention. Nat. Med. 2017, 23, 859. [Google Scholar] [CrossRef]
- Daisley, B.A.; Monachese, M.; Trinder, M.; Bisanz, J.E.; Chmiel, J.A.; Burton, J.P.; Reid, G. Immobilization of cadmium and lead by Lactobacillus rhamnosus GR–1 mitigates apical–to–basolateral heavy metal translocation in a Caco–2 model of the intestinal epithelium. Gut Microbes 2019, 10, 321–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Q.; Yu, L.; Li, T.; Zhu, J.; Zhang, C.; Zhao, J.; Zhang, H.; Chen, W. Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure. Antonie Leeuwenhoek 2017, 110, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Brejnrod, A.D.; Ernst, M.; Rykær, M.; Herschend, J.; Olsen, N.M.C.; Dorrestein, P.C.; Rensing, C.; Sørensen, S.J. Heavy metal exposure causes changes in the metabolic health–associated gut microbiome and metabolites. Environ. Int. 2019, 126, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Janda, T.; Majláth, I.; Szalai, G.; Pál, M. Comparative study on the effects of putrescine and spermidine pre–treatment on cadmium stress in wheat. Ecotoxicol. Environ. Saf. 2018, 148, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menegário, A.A.; Tonello, P.S.; Biscaro, P.A.; Brossi-Garcia, A.L. Determination of Cd (II) and Cd–metallothioneins in biological extracts using baker’s yeast and inductively coupled plasma optical emission spectrometry. Microchim. Acta 2007, 159, 247–254. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Wang, Y.; Liu, N. Protective effects of blueberry anthocyanin extracts on hippocampal neuron damage induced by extremely low–frequency electromagnetic field. Food Sci. Hum. Wellness 2020, 9, 264–271. [Google Scholar] [CrossRef]
- Ruan, M.; Bu, Y.; Wu, F.; Zhang, S.; Chen, R.; Li, N.; Liu, Z.; Wang, H. Chronic consumption of thermally processed palm oil or canola oil modified gut microflora of rats. Food Sci. Hum. Wellness 2021, 10, 94–102. [Google Scholar] [CrossRef]
- He, M.; Sun, M.; Koval, S.; Van Wijk, R.; Hankemeier, T.; Van der Greef, J.; Van Wijk, E.P.; Wang, M. Traditional Chinese medicine–based subtyping of early–stage type 2 diabetes using plasma metabolomics combined with ultra–weak photon emission. Engineering 2019, 5, 916–923. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, J.; Gao, N.; Sun, X.; Meng, X.; Liu, R.; Liu, Y.; Wang, W.; Li, B.; Wang, Y. Blueberry malvidin–3–galactoside modulated gut microbial dysbiosis and microbial TCA cycle KEGG pathway disrupted in a liver cancer model induced by HepG2 cells. Food Sci. Hum. Wellness 2020, 9, 245–255. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Yu, L.; Shen, X.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Toxicity Caused by Different Food-Derived Forms of Cadmium in Mice. Int. J. Mol. Sci. 2021, 22, 11045. https://doi.org/10.3390/ijms222011045
Zhu J, Yu L, Shen X, Tian F, Zhao J, Zhang H, Chen W, Zhai Q. Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Toxicity Caused by Different Food-Derived Forms of Cadmium in Mice. International Journal of Molecular Sciences. 2021; 22(20):11045. https://doi.org/10.3390/ijms222011045
Chicago/Turabian StyleZhu, Jiamin, Leilei Yu, Xudan Shen, Fengwei Tian, Jianxin Zhao, Hao Zhang, Wei Chen, and Qixiao Zhai. 2021. "Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Toxicity Caused by Different Food-Derived Forms of Cadmium in Mice" International Journal of Molecular Sciences 22, no. 20: 11045. https://doi.org/10.3390/ijms222011045
APA StyleZhu, J., Yu, L., Shen, X., Tian, F., Zhao, J., Zhang, H., Chen, W., & Zhai, Q. (2021). Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Toxicity Caused by Different Food-Derived Forms of Cadmium in Mice. International Journal of Molecular Sciences, 22(20), 11045. https://doi.org/10.3390/ijms222011045





