Dye-Mediated Photo-Oxidation Biomaterial Fixation: Analysis of Bioinductivity and Mechanical Properties of Bovine Pericardium for Use in Cardiac Surgery
Abstract
:1. Introduction
2. Results
2.1. DMPO-Fixed ECM Bioscaffold Modulates Transcription of Vasculogenic, Fibrogenic, And Extracellular Matrix Remodeling Pathways in Human Cardiac Fibroblasts
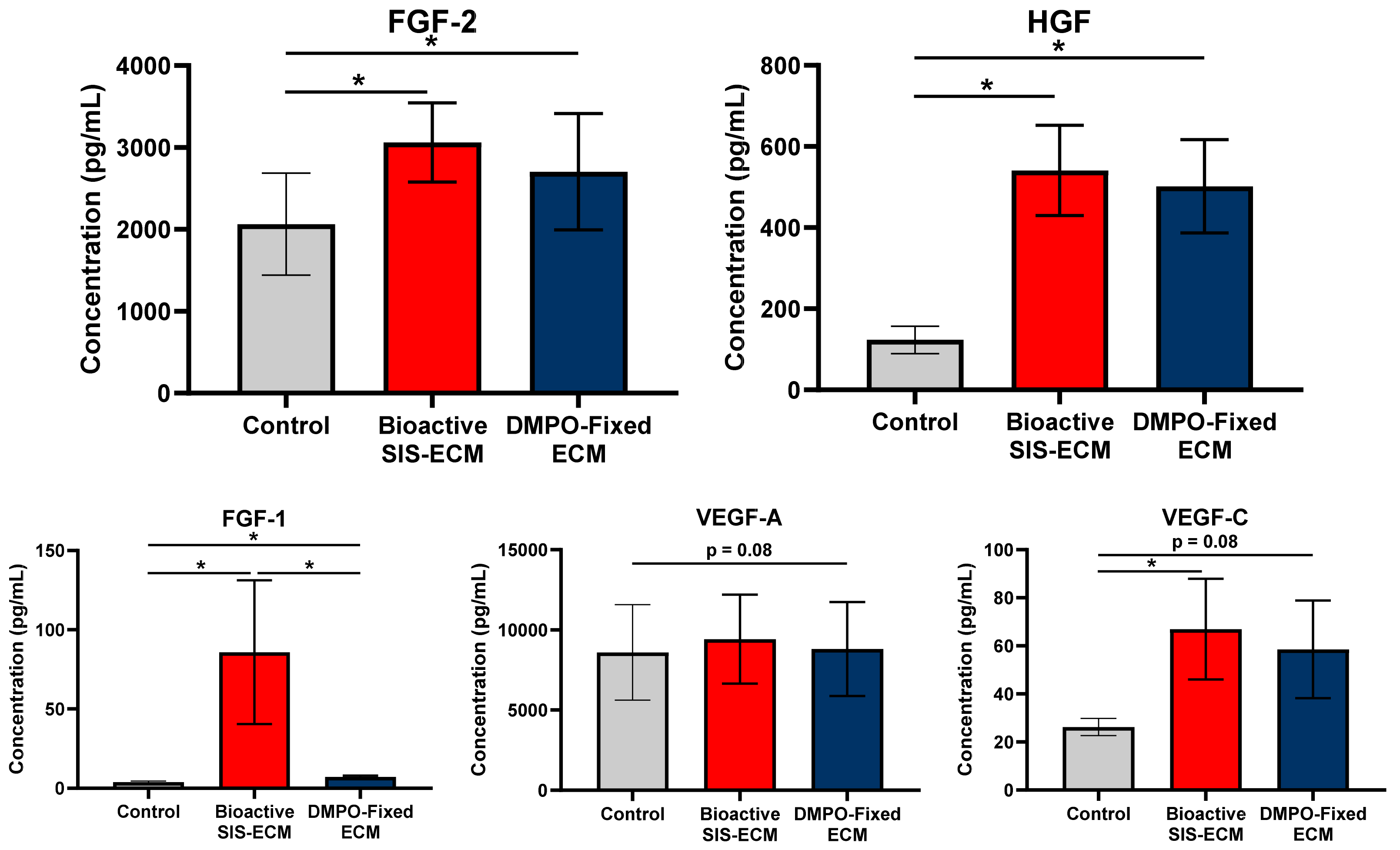
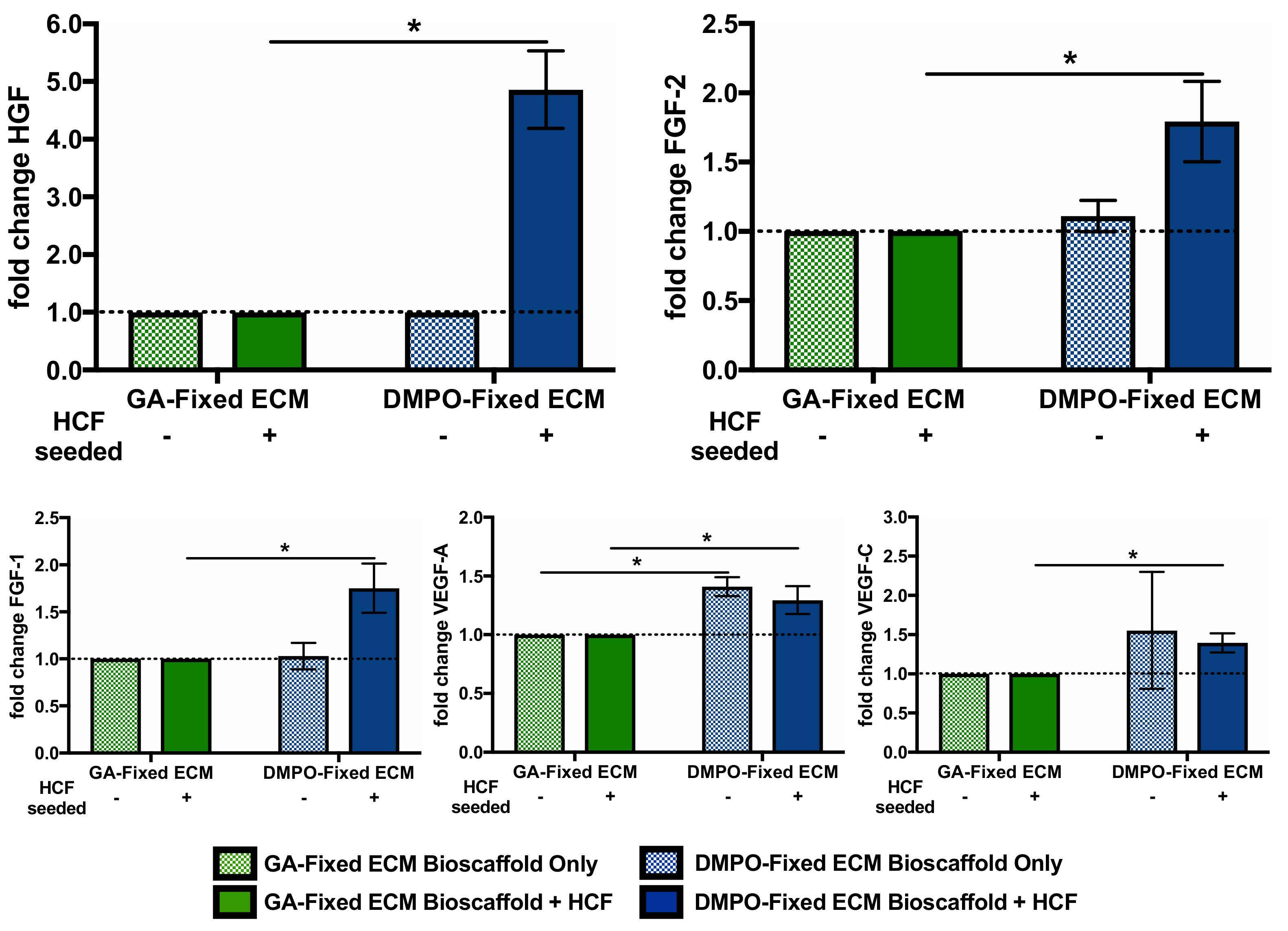
2.2. DMPO-Fixed ECM Alters the Secretome of Human Cardiac Fibroblasts to Promote the Release of Vasculogenic Factors
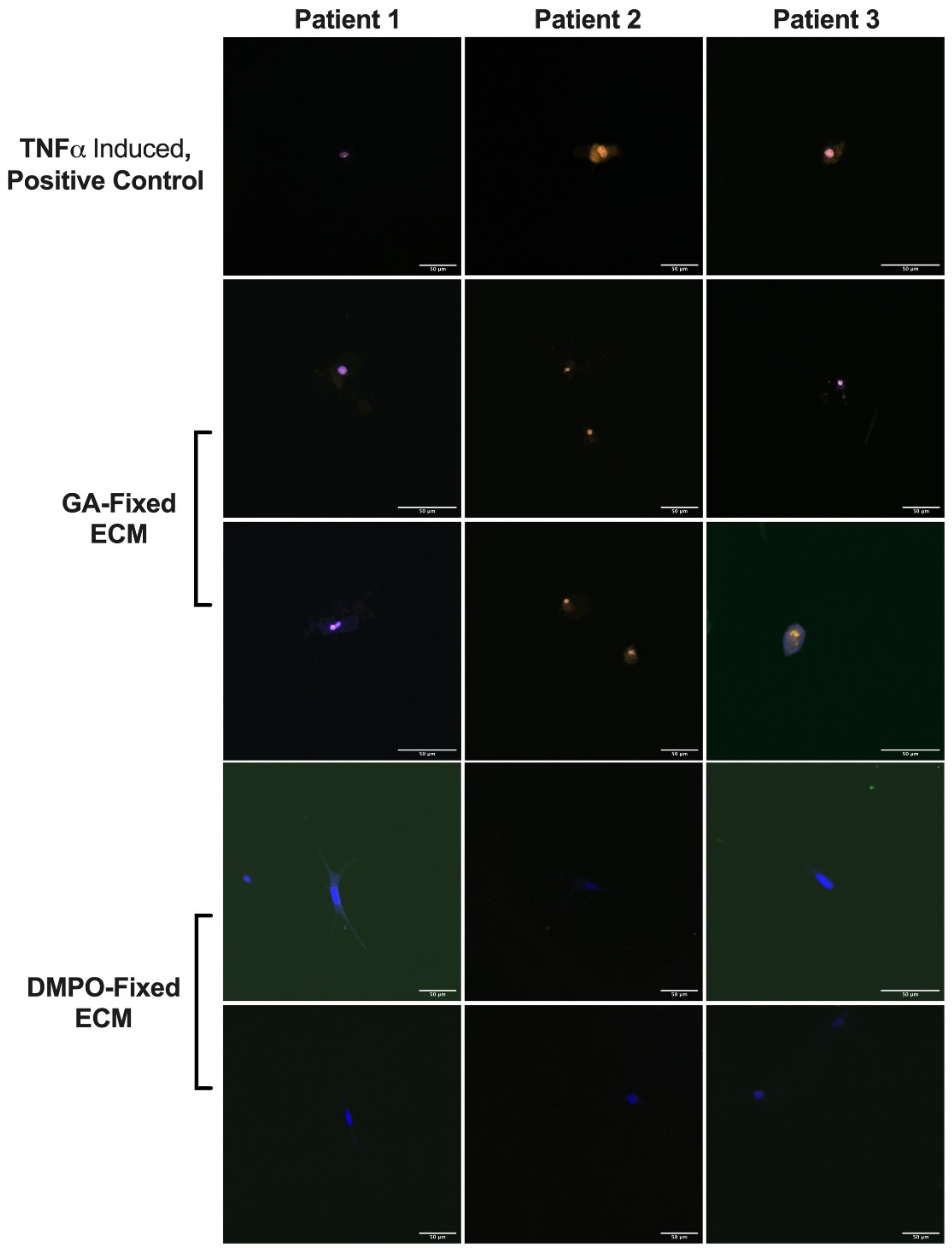
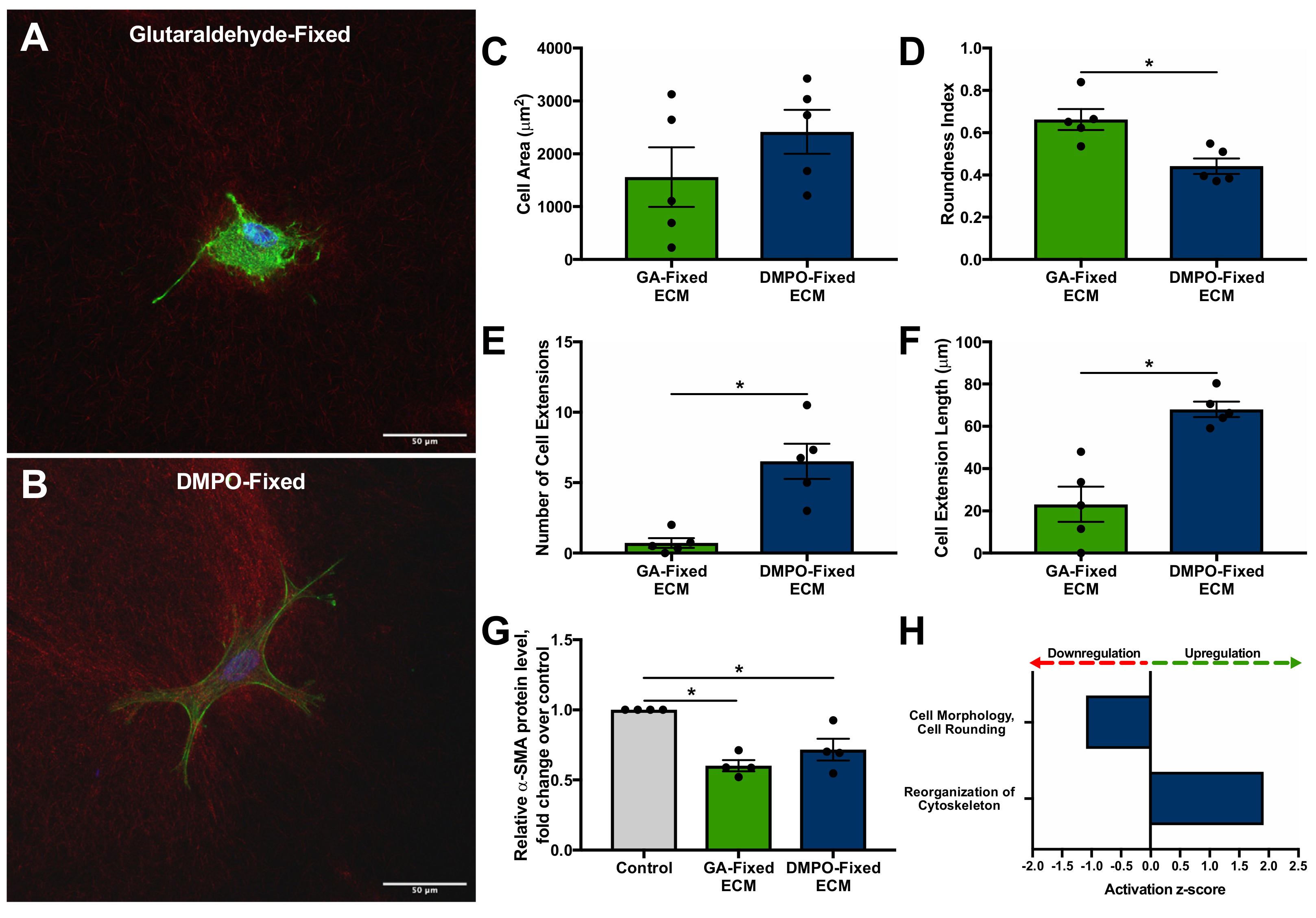
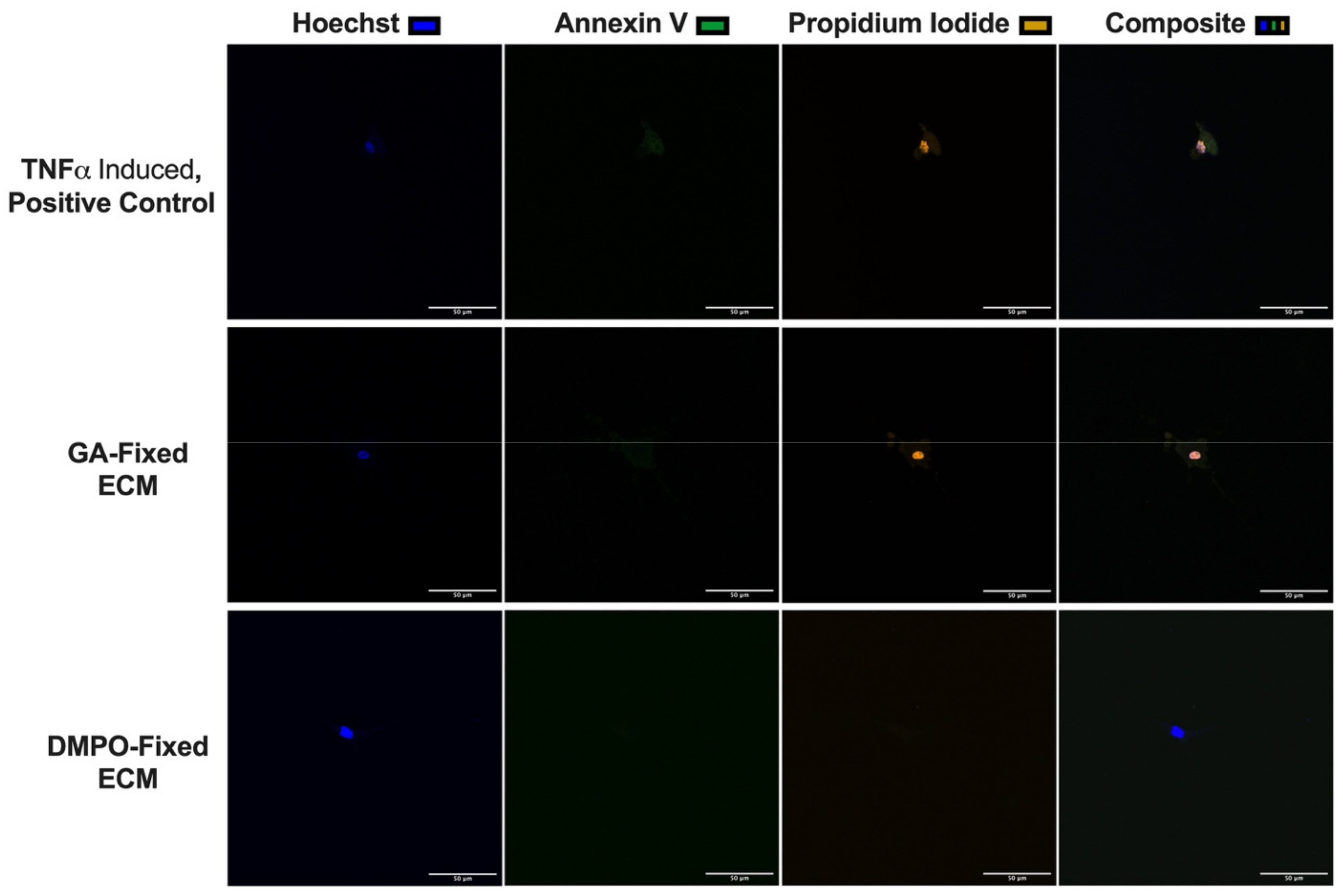
2.3. DMPO-Fixed ECM Preserves Cardiac Fibroblast Phenotype and Promotes Cellular Viability
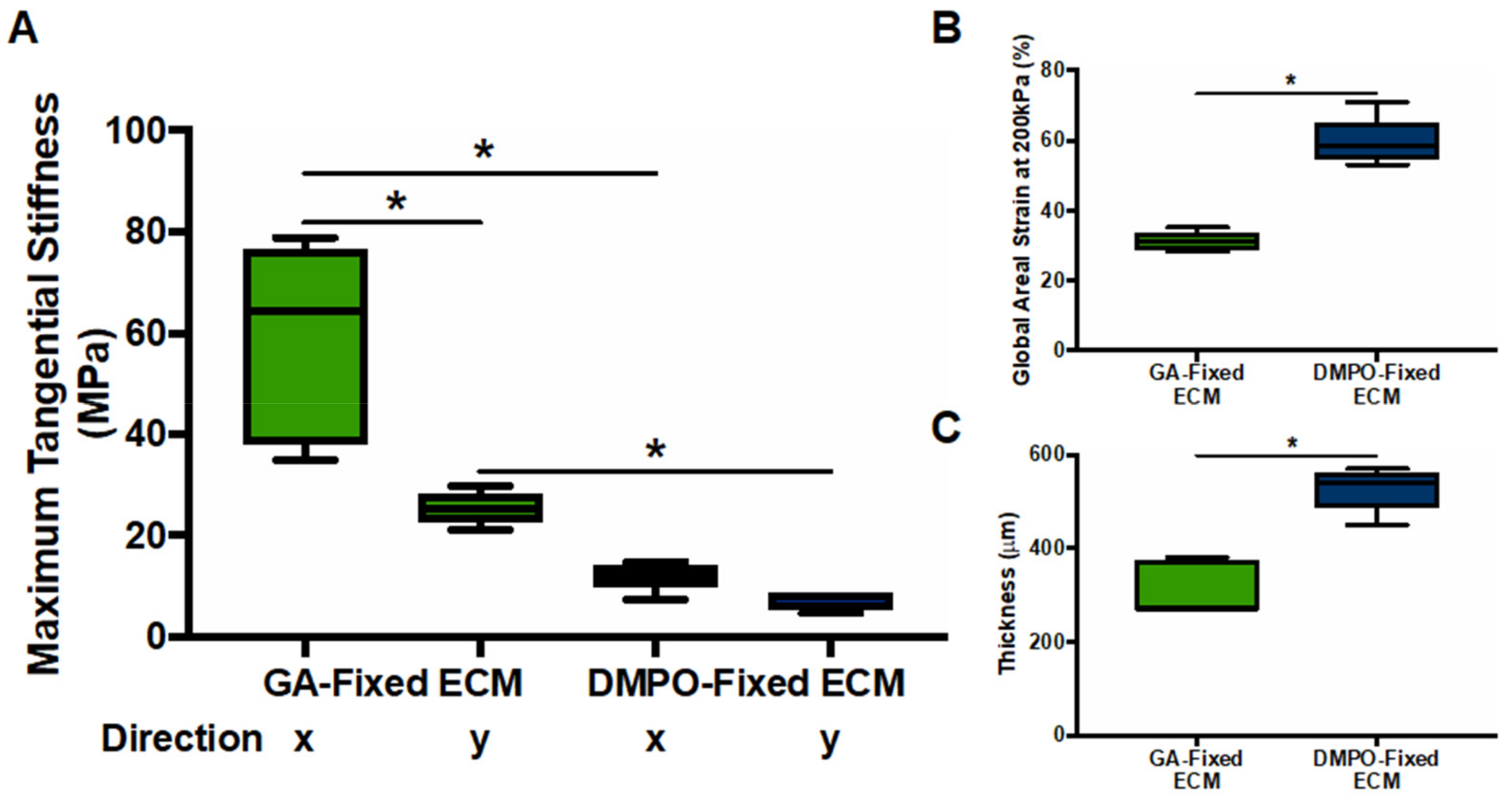
2.4. DMPO-Fixed ECM Demonstrates Greater Compliance Compared to GA-Fixed ECM
3. Discussion
4. Materials and Methods
4.1. Biomaterials
4.2. Isolation and Expansion of Human Cardiac Fibroblasts
4.3. RNA Sequencing Analysis of Human Cardiac Fibroblasts on Bioscaffolds
4.4. Biochemical Characterization of Bioscaffolds
4.5. Characterization of the Human Cardiac Fibroblast Paracrine Response to Bioscaffolds
4.6. Characterization of Human Cardiac Fibroblast Cell Morphology and Viability Using a Bioscaffold-Microgel Model
4.7. Western Blot Analysis of Alpha-Smooth Muscle Actin of Human Cardiac Fibroblast on Bioscaffolds
4.8. Biomechanical Characterization of Bioscaffolds
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Patient Demographics | N = 13 | |
|---|---|---|
| Sex | M | 10 (76.9%) |
| F | 3 (23.1%) | |
| Age (years) | Average Age | 64.3 |
| >65 | 6 (46.2%) | |
| Surgical Procedure | CABG | 6 (46.2%) |
| CABG + AVR | 2 (15.4%) | |
| CABG + MVR | 0 (0.0%) | |
| AVR | 5 (38.5%) | |
| MVR | 0 (0.0%) | |
| Cardiovascular Risk Factors | HTN | 8 (61.5%) |
| Dyslipidemia | 8 (61.5%) | |
| DM | 4 (30.8%) | |
| Smoking History | 4 (30.8%) | |
| Co-existing Conditions | CAD | 8 (61.5%) |
| ACS | 4 (30.8%) | |
| Renal Insufficiency | 2 (15.4%) |
Appendix B
References
- Golomb, G.; Schoen, F.J.; Smith, M.S.; Linden, J.; Dixon, M.; Levy, R.J. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprostheses. Am. J. Pathol. 1987, 127, 122–130. [Google Scholar]
- Schoen, F.J.; Tsao, J.W.; Levy, R.J. Calcification of bovine pericardium used in cardiac valve bioprostheses. Implications for the mechanisms of bioprosthetic tissue mineralization. Am. J. Pathol. 1986, 123, 134–145. Available online: https://www.ncbi.nlm.nih.gov/pubmed/2421577 (accessed on 1 February 2019). [PubMed]
- Schoen, F.J.; Levy, R.J. Calcification of Tissue Heart Valve Substitutes: Progress Toward Understanding and Prevention. Ann. Thorac. Surg. 2005, 79, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- David, T.E.; Feindel, C.M.; Armstrong, S.; Sun, Z. Reconstruction of the mitral anulus: A ten-year experience. J. Thorac. Cardiovasc. Surg. 1995, 110, 1323–1332. [Google Scholar] [CrossRef] [Green Version]
- Manji, R.A.; Zhu, L.F.; Nijjar, N.K.; Rayner, D.C.; Korbutt, G.S.; Churchill, T.A.; Rajotte, R.V.; Koshal, A.; Ross, D.B. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation 2006, 114, 318–327. [Google Scholar] [CrossRef]
- Umashankar, P.; Kumari, T.; Mohanan, P. Glutaraldehyde treatment elicits toxic response compared to decellularization in bovine pericardium. Toxicol. Int. 2012, 19, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Pattar, S.S.; Hassanabad, A.F.; Fedak, P.W.M. Acellular Extracellular Matrix Bioscaffolds for Cardiac Repair and Regeneration. Front. Cell Dev. Biol. 2019, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, V.; Hassanabad, A.F.; Pattar, S.; Niklewski, P.; Wagner, K.; Fedak, P.W.M. Promoting Cardiac Regeneration and Repair Using Acellular Biomaterials. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Svystonyuk, D.A.; Mewhort, H.E.M.; Hassanabad, A.F.; Heydari, B.; Mikami, Y.; Turnbull, J.D.; Teng, G.; Belke, D.D.; Wagner, K.T.; Tarraf, S.A.; et al. Acellular bioscaffolds redirect cardiac fibroblasts and promote functional tissue repair in rodents and humans with myocardial injury. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Mewhort, H.E.; Svystonyuk, D.A.; Turnbull, J.D.; Teng, G.; Belke, D.D.; Guzzardi, D.G.; Park, D.S.; Kang, S.; Hollenberg, M.D.; Fedak, P.W. Bioactive Extracellular Matrix Scaffold Promotes Adaptive Cardiac Remodeling and Repair. JACC Basic Transl. Sci. 2017, 2, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Mewhort, H.E.; Turnbull, J.D.; Satriano, A.; Chow, K.; Flewitt, J.A.; Andrei, A.-C.; Guzzardi, D.G.; Svystonyuk, D.A.; White, J.A.; Fedak, P.W. Epicardial infarct repair with bioinductive extracellular matrix promotes vasculogenesis and myocardial recovery. J. Hear. Lung Transplant. 2016, 35, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, A.H.; Nathan, M.; Emani, S.; Baird, C.; Pedro, J.; Gauvreau, K.; Harris, M.; Sanders, S.P.; Padera, R.F. Preliminary experience with porcine intestinal submucosa (CorMatrix) for valve reconstruction in congenital heart disease: Histologic evaluation of explanted valves. J. Thorac. Cardiovasc. Surg. 2014, 148, 2216–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosario-Quinones, F.; Magid, M.S.; Yau, J.; Pawale, A.; Nguyen, K. Tissue reaction to porcine intestinal Submucosa (CorMatrix) implants in pediatric cardiac patients: A single-center experience. Ann. Thorac. Surg. 2015, 99, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, Z.M.; Poncelet, A.; de Kerchove, L.; Gianello, P.; Fervaille, C.; El Khoury, G. Small intestinal submucosa extracellular matrix (CorMatrix®) in cardiovascular surgery: A systematic review. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinde, A.V.; Frangogiannis, N.G. Fibroblasts in myocardial infarction: A role in inflammation and repair. J. Mol. Cell. Cardiol. 2013, 70, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Fedak, P.W.; Verma, S.; Weisel, R.D.; Li, R.-K. Cardiac remodeling and failure. Cardiovasc. Pathol. 2005, 14, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Eghbali-Webb, M. Release of pro- and anti-angiogenic factors by human cardiac fibroblasts: Effects on DNA synthesis and protection under hypoxia in human endothelial cells. Biochim. Biophys. Acta (BBA) Bioenerg. 2001, 1538, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Ubil, E.; Duan, J.; Pillai, I.C.; Rosa-Garrido, M.; Wu, Y.; Bargiacchi, F.; Lu, Y.; Stanbouly, S.; Huang, J.; Rojas, M.; et al. Mesenchymal–endothelial transition contributes to cardiac neovascularization. Nature 2014, 514, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 2012, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Detillieux, K.A.; Sheikh, F.; Kardami, E.; Cattini, P.A. Biological activities of fibroblast growth factor-2 in the adult myocardium. Cardiovasc. Res. 2003, 57, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Laham, R.J.; Sellke, F.W.; Edelman, E.R.; Pearlman, J.D.; Ware, J.A.; Brown, D.L.; Gold, J.P.; Simons, M. Local Perivascular Delivery of Basic Fibroblast Growth Factor in Patients Undergoing Coronary Bypass Surgery. Circulation 1999, 100, 1865–1871. [Google Scholar] [CrossRef]
- Symes, J.F.; Losordo, D.; Vale, P.R.; Lathi, K.G.; Esakof, D.D.; Mayskiy, M.; Isner, J.M. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann. Thorac. Surg. 1999, 68, 830–836. [Google Scholar] [CrossRef]
- Hartikainen, J.; Hassinen, I.; Hedman, A.; Kivelä, A.; Saraste, A.; Knuuti, J.; Husso, M.; Mussalo, H.; Hedman, M.; Rissanen, T.T.; et al. Adenoviral intramyocardial VEGF-DΔNΔC gene transfer increases myocardial perfusion reserve in refractory angina patients: A phase I/IIa study with 1-year follow-up. Eur. Heart J. 2017, 38, 2547–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, X.; Yang, S.; Ingle, G.; Zlot, C.; Rangell, L.; Kowalski, J.; Schwall, R.; Ferrara, N.; Gerritsen, M.E. Hepatocyte Growth Factor Enhances Vascular Endothelial Growth Factor-Induced Angiogenesis in Vitro and in Vivo. Am. J. Pathol. 2001, 158, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Makarevich, P.I.; Dergilev, K.; Tsokolaeva, Z.I.; Boldyreva, M.A.; Shevchenko, E.K.; Gluhanyuk, E.V.; Gallinger, J.O.; Menshikov, M.Y.; Parfyonova, Y. Angiogenic and pleiotropic effects of VEGF165 and HGF combined gene therapy in a rat model of myocardial infarction. PLoS ONE 2018, 13, e0197566. [Google Scholar] [CrossRef] [PubMed]
- Ahmet, I.; Sawa, Y.; Yamaguchi, T.; Matsuda, H. Gene transfer of hepatocyte growth factor improves angiogenesis and function of chronic ischemic myocardium in canine heart. Ann. Thorac. Surg. 2003, 75, 1283–1287. [Google Scholar] [CrossRef]
- Aoki, M.; Morishita, R.; Taniyama, Y.; Kida, I.; Moriguchi, A.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; Higaki, J.; Ogihara, T. Angiogenesis induced by hepatocyte growth factor in non-infarcted myocardium and infarcted myocardium: Up-regulation of essential transcription factor for angiogenesis, ets. Gene Ther. 2000, 7, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Uchinaka, A.; Kawaguchi, N.; Mori, S.; Hamada, Y.; Miyagawa, S.; Saito, A.; Sawa, Y.; Matsuura, N. Tissue Inhibitor of Metalloproteinase-1 and -3 Improves Cardiac Function in an Ischemic Cardiomyopathy Model Rat. Tissue Eng. Part A 2014, 20, 3073–3084. [Google Scholar] [CrossRef] [Green Version]
- Ngu, J.M.; Teng, G.; Meijndert, H.C.; Mewhort, H.E.; Turnbull, J.D.; Stetler-Stevenson, W.G.; Fedak, P.W. Human cardiac fibroblast extracellular matrix remodeling: Dual effects of tissue inhibitor of metalloproteinase-2. Cardiovasc. Pathol. 2014, 23, 335–343. [Google Scholar] [CrossRef]
- Ramani, R.; Nilles, K.; Gibson, G.; Burkhead, B.; Mathier, M.; McNamara, D.; McTiernan, C.F. Tissue Inhibitor of Metalloproteinase-2 Gene Delivery Ameliorates Postinfarction Cardiac Remodeling. Clin. Transl. Sci. 2011, 4, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Van Steenberghe, M.; Schubert, T.; Guiot, Y.; Bouzin, C.; Bollen, X.; Gianello, P. Enhanced vascular biocompatibility of decellularized xeno-/allogeneic matrices in a rodent model. Cell Tissue Bank. 2017, 18, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Dahm, M.; Lyman, W.D.; Schwell, A.B.; Factor, S.M.; Frater, R.W. Immunogenicity of glutaraldehyde-tanned bovine pericardium. J. Thorac. Cardiovasc. Surg. 1990, 99, 1082–1090. [Google Scholar] [CrossRef]
- Saleeb, S.F.; Newburger, J.W.; Geva, T.; Baird, C.W.; Gauvreau, K.; Padera, R.F. Accelerated degeneration of a bovine pericardial bioprosthetic aortic valve in children and young adults. Circulation 2014, 130, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Levy, R.J.; Schoen, F.J.; Sherman, F.S.; Nichols, J.; Hawley, M.A.; Lund, S.A. Calcification of subcutaneously implanted type I collagen sponges. Effects of formaldehyde and glutaraldehyde pretreatments. Am. J. Pathol. 1986, 122, 71–82. [Google Scholar]
- Melman, L.; Jenkins, E.D.; Hamilton, N.A.; Bender, L.C.; Brodt, M.D.; Deeken, C.R.; Greco, S.C.; Frisella, M.M.; Matthews, B.D. Early biocompatibility of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral hernia repair. Hernia 2011, 15, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Bianco, R.; Phillips, R.; Mrachek, J.; Witson, J. Feasibility evaluation of a new pericardial bioprosthesis with dye mediated photo-oxidized bovine pericardial tissue. J. Heart Valve Dis. 1996, 5, 317–322. [Google Scholar]
- Moore, M.A.; Phillips, R.E. Biocompatibility and immunologic properties of pericardial tissue stabilized by dye-mediated photooxidation. J. Heart Valve Dis. 1997, 6, 307–315. [Google Scholar]
- Moore, M.A.; Phillips, R.E.; McIlroy, B.K.; Walley, V.M.; Hendry, P.J. Evaluation of porcine valves prepared by dye-mediated photooxidation. Ann. Thorac. Surg. 1998, 66, S245–S248. [Google Scholar] [CrossRef]
- Baird, C.W.; Myers, P.O.; Piekarski, B.; Borisuk, M.; Majeed, A.; Emani, S.M.; Sanders, S.P.; Nathan, M.; Del Nido, P.J. Photo-oxidized bovine pericardium in congenital cardiac surgery: Single-centre experience. Interact. Cardiovasc. Thorac. Surg. 2016, 24, 240–244. [Google Scholar] [CrossRef]
- Marsano, A.; Maidhof, R.; Luo, J.; Fujikara, K.; Konofagou, E.E.; Banfi, A.; Vunjak-Novakovic, G. The effect of controlled expression of VEGF by transduced myoblasts in a cardiac patch on vascularization in a mouse model of myocardial infarction. Biomaterials 2012, 34, 393–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, D.; Zigras, T.; Cartier, R.; Leduc, L.; Butany, J.; Mongrain, R.; Leask, R.L. A Comparison of Mechanical Properties of Materials Used in Aortic Arch Reconstruction. Ann. Thorac. Surg. 2009, 88, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Diaz-Rodriguez, P.; Balouch, B.; Paulsen, S.; Wu, S.; Miller, J.; Hahn, M.; Cosgriff-Hernandez, E. Elucidating the role of graft compliance mismatch on intimal hyperplasia using an ex vivo organ culture model. Acta Biomater. 2019, 89, 84–94. [Google Scholar] [CrossRef]
- Christman, K.L.; Lee, R.J. Biomaterials for the Treatment of Myocardial Infarction. J. Am. Coll. Cardiol. 2006, 48, 907–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mewhort, H.E.; Turnbull, J.D.; Meijndert, H.C.; Ngu, J.M.; Fedak, P.W. Epicardial infarct repair with basic fibroblast growth factor–enhanced CorMatrix-ECM biomaterial attenuates postischemic cardiac remodeling. J. Thorac. Cardiovasc. Surg. 2014, 147, 1650–1659. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2015, 44, D733–D745. [Google Scholar] [CrossRef] [Green Version]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Pimentel, H.; Bray, N.L.; Puente, S.; Melsted, P.; Pachter, L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat. Methods 2017, 14, 687–690. [Google Scholar] [CrossRef]
- Mohammadi, H.; Janmey, P.A.; McCulloch, C.A. Lateral boundary mechanosensing by adherent cells in a collagen gel system. Biomaterials 2014, 35, 1138–1149. [Google Scholar] [CrossRef]
- Yuda, A.; McCulloch, C.A. A Screening System for Evaluating Cell Extension Formation, Collagen Compaction, and Degradation in Drug Discovery. SLAS Discov. Adv. Life Sci. R&D 2017, 23, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.; Moody, W.E.; Umar, F.; Edwards, N.C.; Taylor, T.J.; Stegemann, B.; Townend, J.; Hor, K.N.; Steeds, R.; Mazur, W.; et al. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: Normal values. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 871–881. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattar, S.S.; Vasanthan, V.; Teng, G.; Wagner, K.T.; Jeon, K.; Kang, S.; Fatehi Hassanabad, A.; Fedak, P.W.M. Dye-Mediated Photo-Oxidation Biomaterial Fixation: Analysis of Bioinductivity and Mechanical Properties of Bovine Pericardium for Use in Cardiac Surgery. Int. J. Mol. Sci. 2021, 22, 10768. https://doi.org/10.3390/ijms221910768
Pattar SS, Vasanthan V, Teng G, Wagner KT, Jeon K, Kang S, Fatehi Hassanabad A, Fedak PWM. Dye-Mediated Photo-Oxidation Biomaterial Fixation: Analysis of Bioinductivity and Mechanical Properties of Bovine Pericardium for Use in Cardiac Surgery. International Journal of Molecular Sciences. 2021; 22(19):10768. https://doi.org/10.3390/ijms221910768
Chicago/Turabian StylePattar, Simranjit S., Vishnu Vasanthan, Guoqi Teng, Karl T. Wagner, Kristina Jeon, Sean Kang, Ali Fatehi Hassanabad, and Paul W. M. Fedak. 2021. "Dye-Mediated Photo-Oxidation Biomaterial Fixation: Analysis of Bioinductivity and Mechanical Properties of Bovine Pericardium for Use in Cardiac Surgery" International Journal of Molecular Sciences 22, no. 19: 10768. https://doi.org/10.3390/ijms221910768
APA StylePattar, S. S., Vasanthan, V., Teng, G., Wagner, K. T., Jeon, K., Kang, S., Fatehi Hassanabad, A., & Fedak, P. W. M. (2021). Dye-Mediated Photo-Oxidation Biomaterial Fixation: Analysis of Bioinductivity and Mechanical Properties of Bovine Pericardium for Use in Cardiac Surgery. International Journal of Molecular Sciences, 22(19), 10768. https://doi.org/10.3390/ijms221910768






