Short Daily Exposure to Environmental Enrichment, Fluoxetine, or Their Combination Reverses Deterioration of the Coat and Anhedonia Behaviors with Differential Effects on Hippocampal Neurogenesis in Chronically Stressed Mice
Abstract
:1. Introduction
2. Results
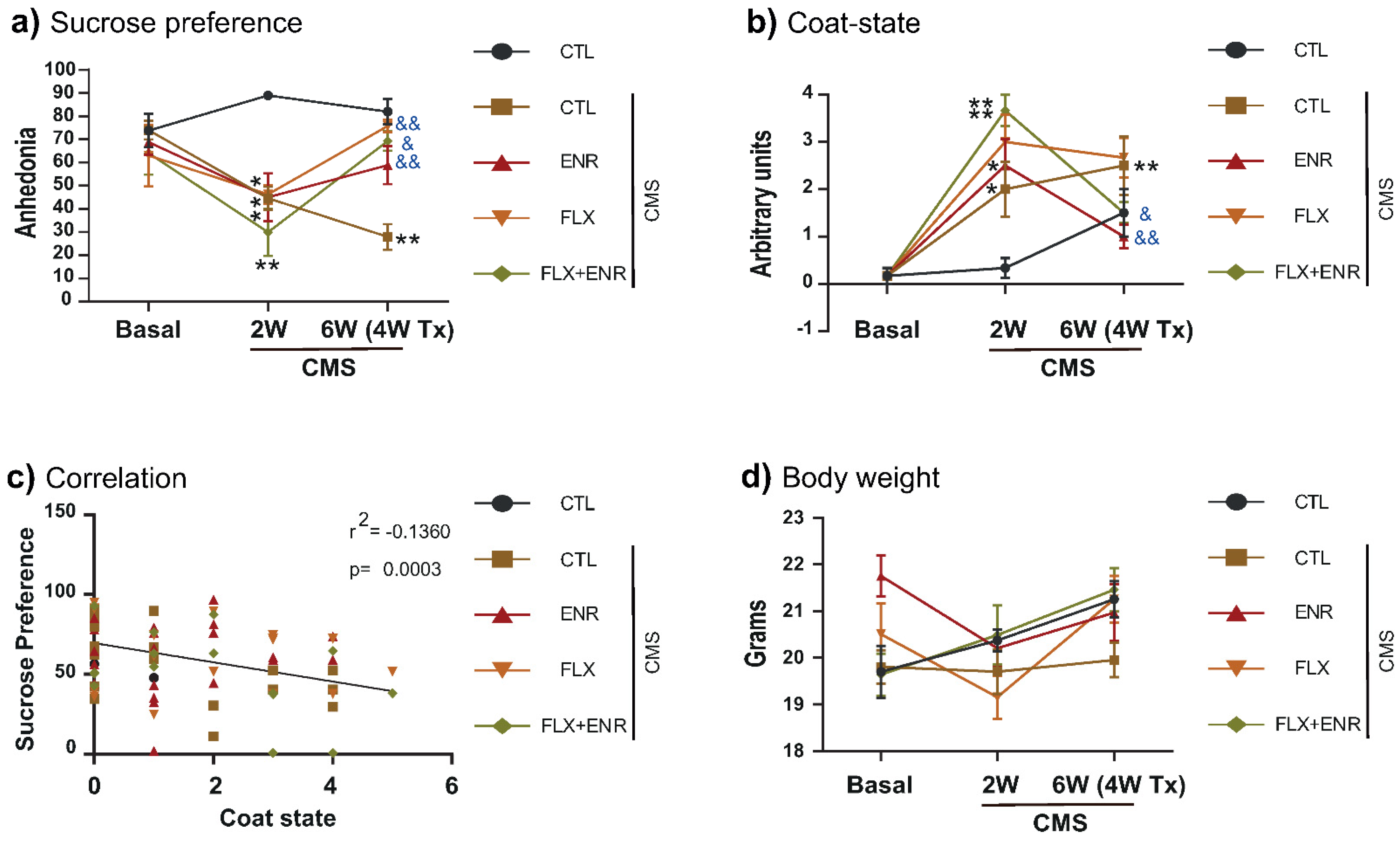
2.1. Coat-State and Sucrose Preference after Chronic Mild Stress
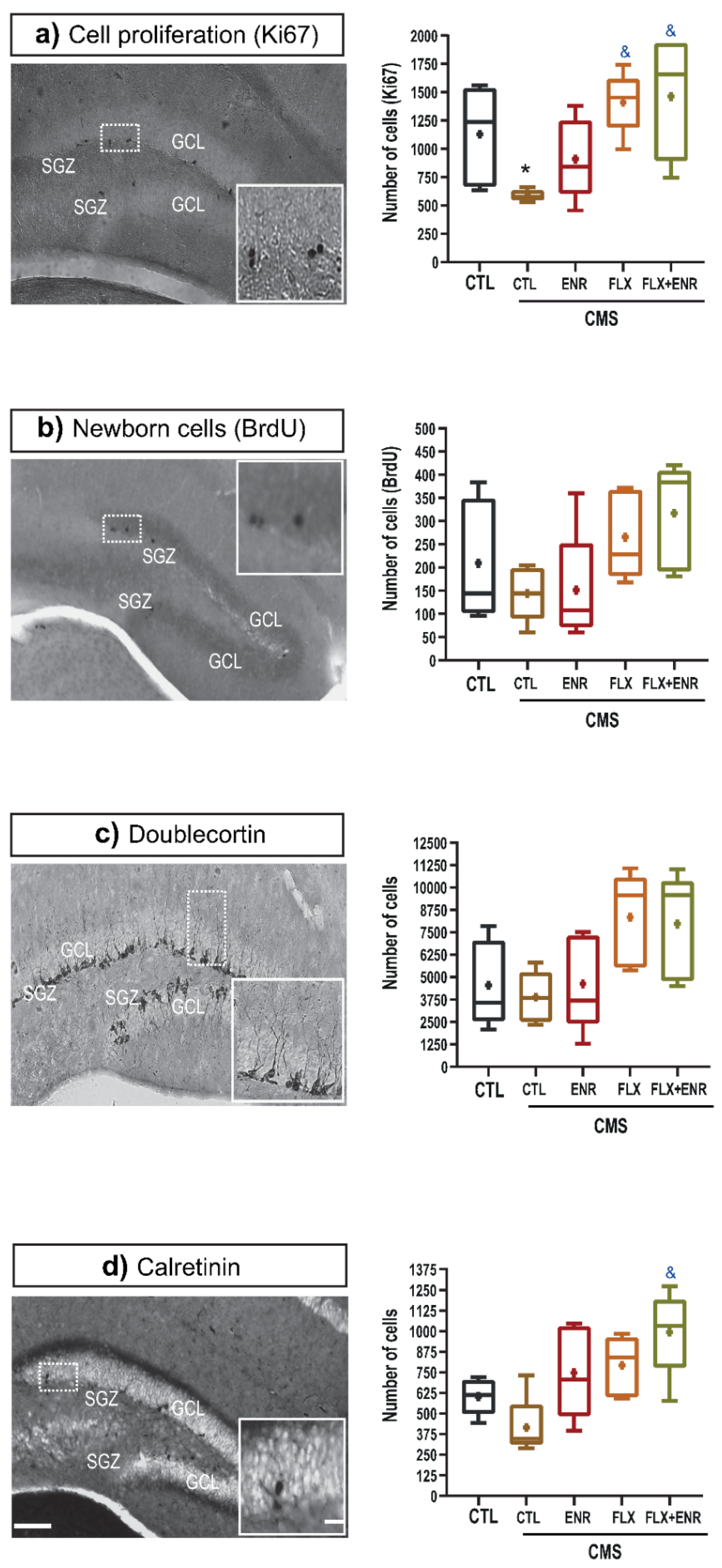
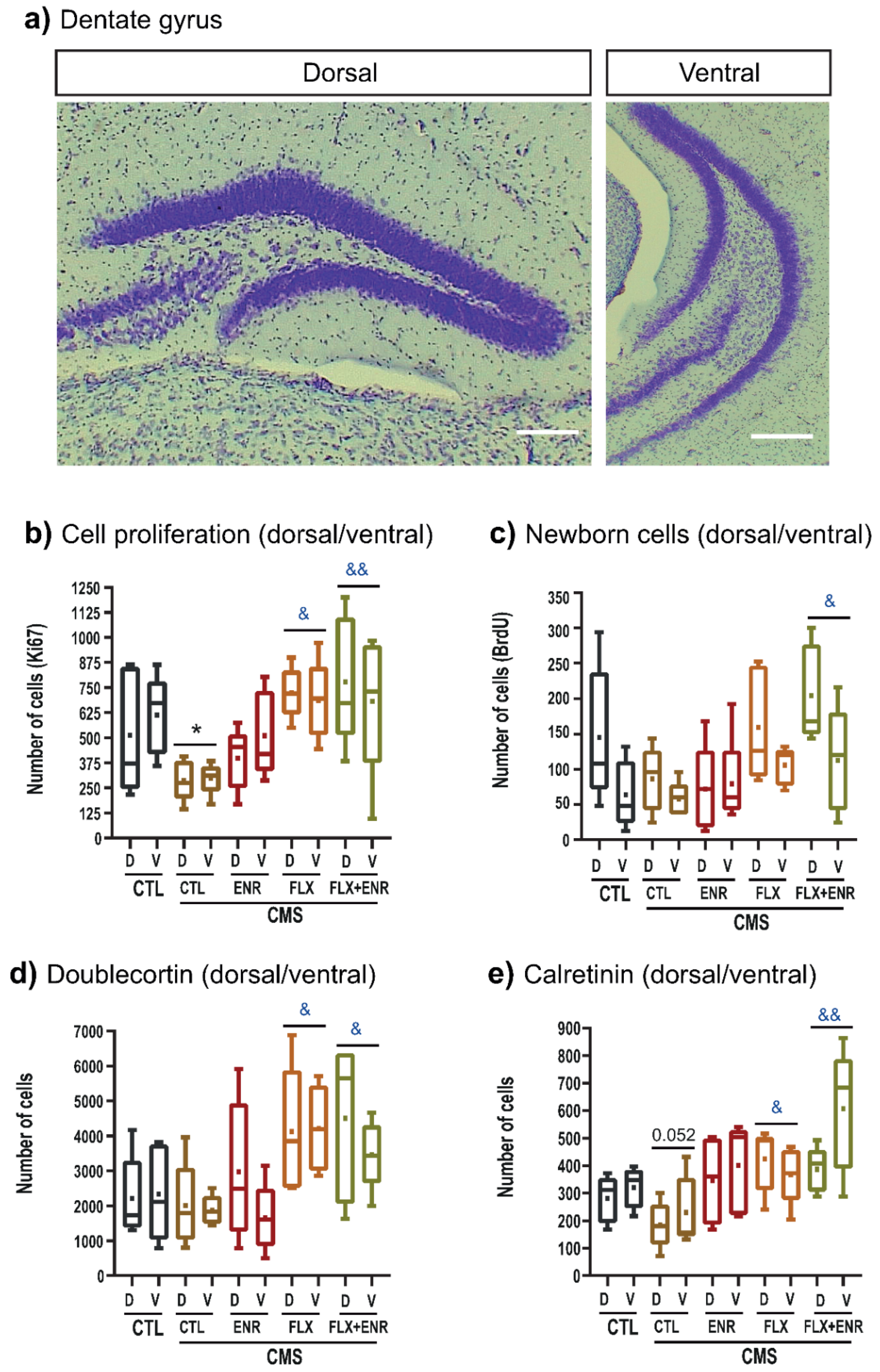
2.2. Hippocampal Neurogenic Associated Parameters
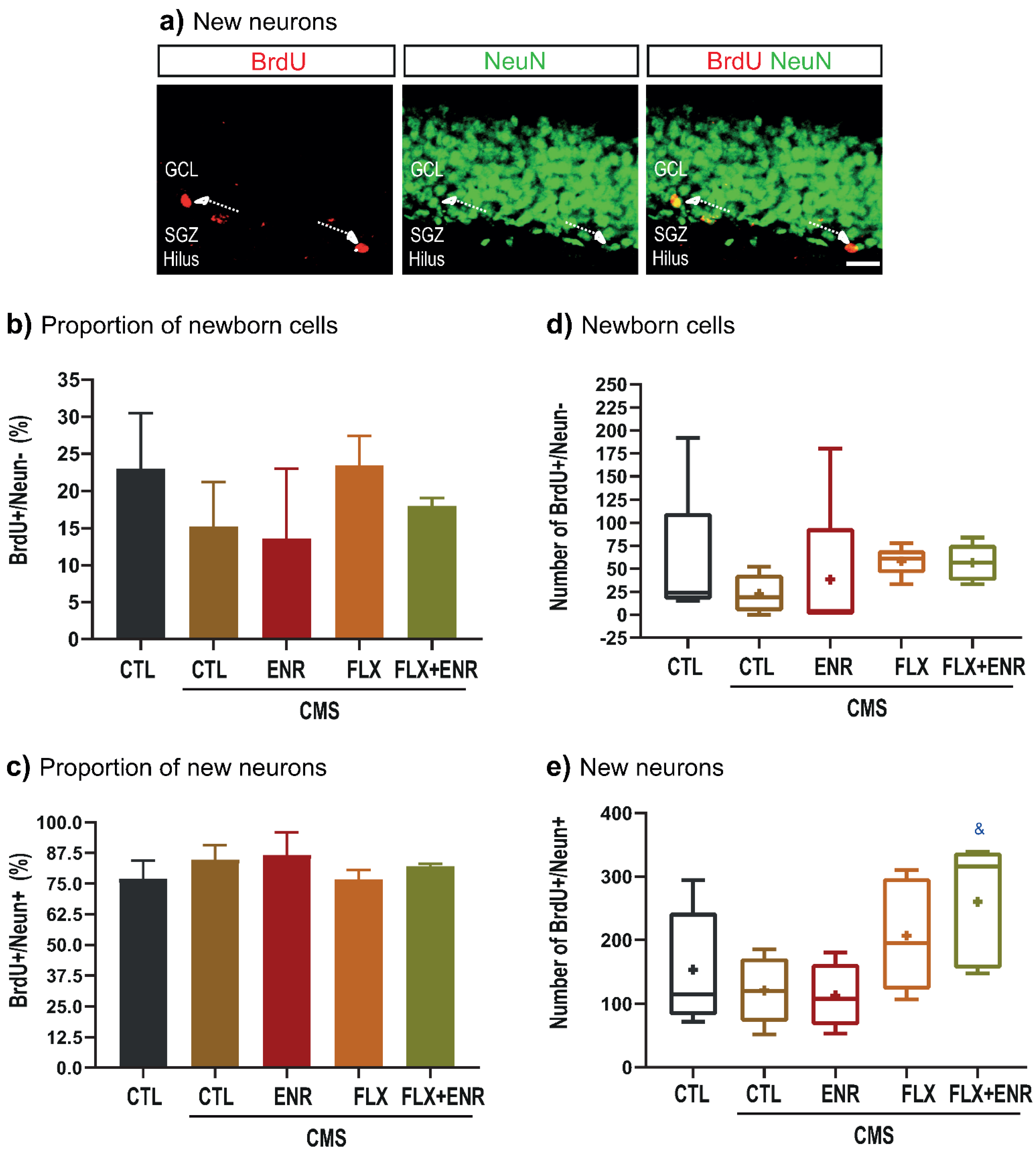
2.3. Net Neurogenesis after CMS
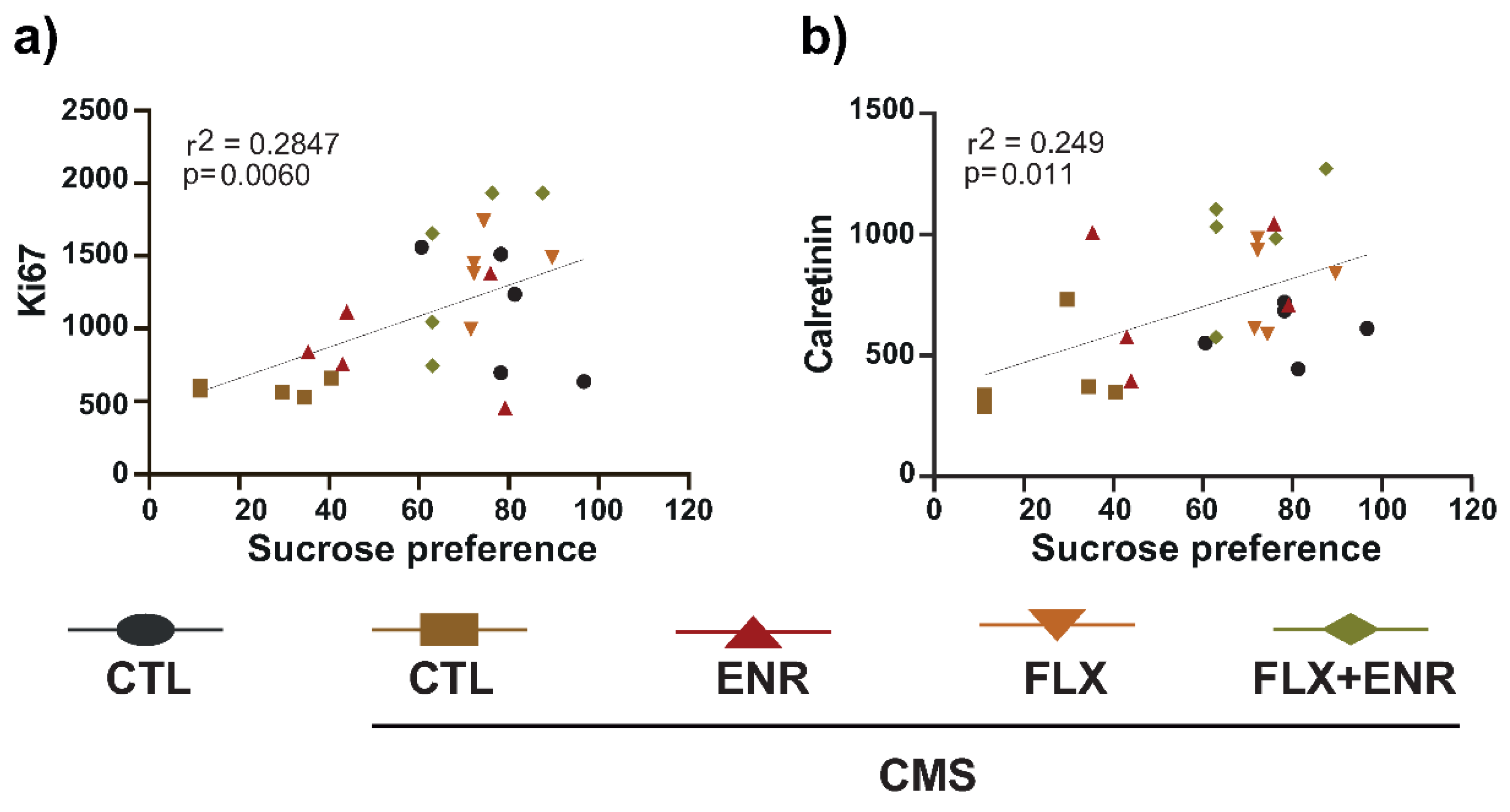
2.4. Correlations of Depressive-like Behavior with Parameters Related to Adult Hippocampal Neurogenesis
3. Discussion
3.1. Behavioral Changes in Response to Chronic Mild Stress and Pharmacological or Environmental Interventions
3.2. Neurogenic Changes in Response to Chronic Mild Stress and Pharmacological or Environmental Interventions
4. Materials and Methods
4.1. Animals
4.2. Chronic Mild Stress, Coat-State, and Anhedonia-like Behavior
4.3. BrdU-Labeling, Standard Housing, Environmental Enrichment, and Fluoxetine Administration
4.4. Tissue Processing for Immunohistochemistry
4.5. Immunohistochemistry
4.6. Quantification of Neurogenic Markers and Phenotypic Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSRIs | Selective serotonin reuptake inhibitors |
| BDNF | Brain-derived neurotrophic factor |
| CMS | Chronic mild stress |
| FLX | Fluoxetine |
| ENR | Environmental enrichment |
References
- Campbell, S.; Marriott, M.; Nahmias, C.; MacQueen, G.M. Lower Hippocampal Volume in Patients Suffering from Depression: A Meta-Analysis. Am. J. Psychiatry 2004, 161, 598–607. [Google Scholar] [CrossRef] [PubMed]
- MacQueen, G.M.; Campbell, S.; McEwen, B.S.; Macdonald, K.; Amano, S.; Joffe, R.T.; Nahmias, C.; Young, L.T. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. USA 2003, 100, 1387–1392. [Google Scholar] [CrossRef] [Green Version]
- Baumann, P. Clinical pharmacokinetics of citalopram and other selective serotonergic reuptake inhib-itors (SSRI). Int. Clin. Psychopharmacol. 1992, 6 (Suppl. 5), 13–20. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Arango, V. Antidepressants, age, and neuroprogenitors. Neuropsychopharmacology 2009, 35, 351–352. [Google Scholar] [CrossRef]
- Carvalho, L.; Gorenstein, C.; Moreno, R.; Pariante, C.; Markus, R. Effect of antidepressants on melatonin metabolite in depressed patients. J. Psychopharmacol. 2008, 23, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Nakagawa, S.; Malberg, J. Regulation of Adult Neurogenesis by Antidepressant Treatment. Neuropsychopharmacology 2001, 25, 836–844. [Google Scholar] [CrossRef]
- Fava, M.; Targum, S.D.; Nierenberg, A.A.; Bleicher, L.S.; Carter, T.A.; Wedel, P.C.; Hen, R.; Gage, F.H.; Barlow, C. An exploratory study of combination buspirone and melatonin SR in major depressive disorder (MDD): A possible role for neurogenesis in drug discovery. J. Psychiatr. Res. 2013, 46, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.A.; Fernandes, K.; Jha, S. Regulation of adult hippocampal neurogenesis: Relevance to depression. Expert Rev. Neurother. 2007, 7, 853–864. [Google Scholar] [CrossRef]
- Wang, J.-W.; David, D.J.; Monckton, J.E.; Battaglia, F.; Hen, R. Chronic Fluoxetine Stimulates Maturation and Synaptic Plasticity of Adult-Born Hippocampal Granule Cells. J. Neurosci. 2008, 28, 1374–1384. [Google Scholar] [CrossRef]
- Jaako-Movits, K.; Zharkovsky, T.; Pedersen, M.; Zharkovsky, A. Decreased Hippocampal Neurogenesis Following Olfactory Bulbectomy is Reversed by Repeated Citalopram Administration. Cell. Mol. Neurobiol. 2006, 26, 1557–1568. [Google Scholar] [CrossRef]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Tanti, A.; Belzung, C. Neurogenesis along the septotemporal axis of the hippocampus: Are depression and the action of antidepressants region-specific? Neuroscience 2013, 252, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Wiener, S.; McNaughton, B. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci. 1994, 14, 7347–7356. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.; Rawlins, J.; McHugh, S.; Deacon, R.; Yee, B.; Bast, T.; Zhang, W.-N.; Pothuizen, H.; Feldon, J. Regional dissociations within the hippocampus—Memory and anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef]
- Kjelstrup, K.G.; Tuvnes, F.A.; Steffenach, H.-A.; Murison, R.; Moser, E.I.; Moser, M.-B. Reduced fear expression after lesions of the ventral hippocampus. Proc. Natl. Acad. Sci. USA 2002, 99, 10825–10830. [Google Scholar] [CrossRef] [Green Version]
- Païzanis, E.; Renoir, T.; Lelievre, V.; Saurini, F.; Melfort, M.; Gabriel, C.; Barden, N.; Mocaër, E.; Hamon, M.; Lanfumey, L. Behavioural and neuroplastic effects of the new-generation antidepressant agomelatine compared to fluoxetine in glucocorticoid receptor-impaired mice. Int. J. Neuropsychopharmacol. 2009, 13, 759–774. [Google Scholar] [CrossRef] [Green Version]
- Saarelainen, T.; Hendolin, P.; Lucas, G.; Koponen, E.; Sairanen, M.; MacDonald, E.; Agerman, K.; Haapasalo, A.; Nawa, H.; Aloyz, R.; et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 2003, 23, 349–357. [Google Scholar] [CrossRef]
- Sairanen, M.; Lucas, G.; Ernfors, P.; Castren, M.; Castren, E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005, 25, 1089–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldrini, M.; Underwood, M.; Hen, R.; Rosoklija, G.B.; Dwork, A.J.; Mann, J.J.; Arango, V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 2009, 34, 2376–2389. [Google Scholar] [CrossRef] [Green Version]
- Chiarotti, F.; Viglione, A.; Giuliani, A.; Branchi, I. Citalopram amplifies the influence of living conditions on mood in depressed patients enrolled in the STAR*D study. Transl. Psychiatry 2017, 7, e1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alboni, S.; Van Dijk, R.M.; Poggini, S.; Milior, G.; Perrotta, M.; Drenth, T.; Brunello, N.; Wolfer, D.P.; Limatola, C.; Amrein, I.; et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol. Psychiatry 2017, 22, 552–561. [Google Scholar] [CrossRef] [Green Version]
- Nithianantharajah, J.; Hannan, A.J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006, 7, 697–709. [Google Scholar] [CrossRef]
- Veena, J.; Srikumar, B.N.; Raju, T.R.; Shankaranarayana Rao, B.S. Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci. Lett. 2009, 455, 178–182. [Google Scholar] [CrossRef]
- Hutchinson, K.M.; McLaughlin, K.J.; Wright, R.L.; Ortiz, J.B.; Anouti, D.P.; Mika, A.; Diamond, D.M.; Conrad, C.D. Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity. Neurobiol. Learn. Mem. 2012, 97, 250–260. [Google Scholar] [CrossRef]
- Kempermann, G.; Kuhn, H.-G.; Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nat. Cell Biol. 1997, 386, 493–495. [Google Scholar] [CrossRef]
- Speisman, R.B.; Kumar, A.; Rani, A.; Pastoriza, J.M.; Severance, J.E.; Foster, T.C.; Ormerod, B.K. Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol. Aging 2013, 34, 263–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garthe, A.; Behr, J.; Kempermann, G. Adult-Generated Hippocampal Neurons Allow the Flexible Use of Spatially Precise Learning Strategies. PLoS ONE 2009, 4, e5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Rivera, N.M.; Fernández-Guasti, A.; Ramírez-Rodríguez, G.; Estrada-Camarena, E. Forced swim and chronic variable stress reduced hippocampal cell survival in OVX female rats. Behav. Brain Res. 2014, 270, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Rivera, N.M.; Ortiz-López, L.; Gómez-Sánchez, A.; Oikawa-Sala, J.; Estrada-Camarena, E.M.; Ramírez-Rodríguez, G.B. The neurogenic effects of an enriched environment and its protection against the behavioral consequences of chronic mild stress persistent after enrichment cessation in six-month-old female Balb/C mice. Behav. Brain Res. 2016, 301, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Griebel, G.; Pavone, G.; Stemmelin, J.; Le Fur, G.; Soubrie, P. Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol. Psychiatry 2004, 9, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Surget, A.; Saxe, M.; Leman, S.; Ibarguen-Vargas, Y.; Chalon, S.; Griebel, G.; Hen, R.; Belzung, C. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biol. Psychiatry 2008, 64, 293–301. [Google Scholar] [CrossRef]
- Ortiz-Lopez, L.; Perez-Beltran, C.; Ramirez-Rodriguez, G. Chronic administration of a melatonin mem-brane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness-like behavior in adult mice. Neuropharmacology 2016, 103, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gast, D.; Kronenberg, G.; Yamaguchi, M.; Gage, F.H. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 2003, 130, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Depression: A case of neuronal life and death? Biol. Psychiatry 2004, 56, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Bachis, A.; Mallei, A.; Cruz, M.I.; Wellstein, A.; Mocchetti, I. Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology 2008, 55, 1114–1120. [Google Scholar] [CrossRef] [Green Version]
- Branchi, I.; Santarelli, S.; Capoccia, S.; Poggini, S.; D’Andrea, I.; Cirulli, F.; Alleva, E. Antidepressant Treatment Outcome Depends on the Quality of the Living Environment: A Pre-Clinical Investigation in Mice. PLoS ONE 2013, 8, e62226. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.-J.; Herbert, J. Stimulation of Neurogenesis in the Hippocampus of the Adult Rat by Fluoxetine Requires Rhythmic Change in Corticosterone. Biol. Psychiatry 2006, 59, 619–624. [Google Scholar] [CrossRef]
- Kohl, Z.; Winner, B.; Ubhi, K.; Rockenstein, E.; Mante, M.; Münch, M.; Barlow, C.; Carter, T.; Masliah, E.; Winkler, J. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur. J. Neurosci. 2012, 35, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef]
- Schloesser, R.J.; Lehmann, M.; Martinowich, K.; Manji, H.K.; Herkenham, M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry 2010, 15, 1152–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alboni, S.; Poggini, S.; Garofalo, S.; Milior, G.; El Hajj, H.; Lecours, C.; Girard, I.; Gagnon, S.; Boisjoly-Villeneuve, S.; Brunello, N.; et al. Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav. Immun. 2016, 58, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gu, J.Y.; Han, J.H.; Yan, F.L.; Li, Y.; Lv, T.T.; Zhao, L.Q.; Shao, Q.J.; Feng, Y.Y.; Zhang, X.Y.; et al. Enriched envi-ronment combined with fluoxetine ameliorates depression-like behaviors and hippocampal SYP expression in a rat CUS model. Brain Res. Bull. 2017, 135, 33–39. [Google Scholar] [CrossRef]
- Yalcin, I.; Belzung, C.; Surget, A. Mouse strain differences in the unpredictable chronic mild stress: A four-antidepressant survey. Behav. Brain Res. 2008, 193, 140–143. [Google Scholar] [CrossRef]
- Tang, M.; Lei, J.; Sun, X.; Liu, G.; Zhao, S. Stress-induced anhedonia correlates with lower hippocampal serotonin transporter protein expression. Brain Res. 2013, 1513, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, T.; Le Guisquet, A.M.; Brizard, B.; Hommet, C.; Minier, F.; Belzung, C. Fluoxetine induces paradoxical effects in C57BL6/J mice: Comparison with BALB/c mice. Behav. Pharmacol. 2017, 28, 466–476. [Google Scholar] [CrossRef]
- Du Preez, A.; Onorato, D.; Eiben, I.; Musaelyan, K.; Egeland, M.; Zunszain, P.A.; Fernandes, C.; Thuret, S.; Pariante, C.M. Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain Behav. Immun. 2021, 91, 24–47. [Google Scholar] [CrossRef]
- Stemmelin, J.; Cohen, C.; Yalcin, I.; Keane, P.; Griebel, G. Implication of beta3-adrenoceptors in the antidepressant-like effects of amibegron using Adrb3 knockout mice in the chronic mild stress. Behav. Brain Res. 2010, 206, 310–312. [Google Scholar] [CrossRef]
- Planchez, B.; Surget, A.; Belzung, C. Animal models of major depression: Drawbacks and challenges. J. Neural Transm. 2019, 126, 1383–1408. [Google Scholar] [CrossRef] [Green Version]
- Tanti, A.; Rainer, Q.; Minier, F.; Surget, A.; Belzung, C. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology 2012, 63, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.R.; Bennett, E.L.; Diamond, M.C. Effects of differential environments on brain anatomy and brain chemistry. Proc. Annu. Meet. Am. Psychopathol. Assoc. 1967, 56, 45–56. [Google Scholar]
- Widman, D.R.; Rosellini, R.A. Restricted daily exposure to environmental enrichment increases the diversity of exploration. Physiol. Behav. 1990, 47, 57–62. [Google Scholar] [CrossRef]
- Rojas-Carvajal, M.; Sequeira-Cordero, A.; Brenes, J.C. Neurobehavioral Effects of Restricted and Unpredictable Environmental Enrichment in Rats. Front. Pharmacol. 2020, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Khemissi, W.; Farooq, R.K.; Le Guisquet, A.M.; Sakly, M.; Belzung, C. Dysregulation of the hypothalamus-pituitary-adrenal axis predicts some aspects of the behavioral response to chronic fluoxetine: Association with hippocampal cell proliferation. Front. Behav. Neurosci. 2014, 8, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanti, A.; Westphal, W.P.; Girault, V.; Brizard, B.; Devers, S.; Leguisquet, A.M.; Surget, A.; Belzung, C. Region-dependent and stage-specific effects of stress, environmental enrichment, and antidepressant treatment on hippocampal neurogenesis. Hippocampus 2013, 23, 797–811. [Google Scholar] [CrossRef]
- García, A.; Steiner, B.; Kronenberg, G.; Bick-Sander, A.; Kempermann, G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell 2004, 3, 363–371. [Google Scholar] [CrossRef]
- Vega-Rivera, N.M.; Ortiz-López, L.; Granados-Juárez, A.; Estrada-Camarena, E.M.; Ramírez-Rodríguez, G.B. Melatonin Reverses the Depression-associated Behaviour and Regulates Microglia, Fractalkine Expression and Neurogenesis in Adult Mice Exposed to Chronic Mild Stress. Neuroscience 2020, 440, 316–336. [Google Scholar] [CrossRef]
- Kreisel, T.; Frank, M.G.; Licht, T.; Reshef, R.; Ben-Menachem-Zidon, O.; Baratta, M.V.; Maier, S.F.; Yirmiya, R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry 2014, 19, 699–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.-G.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Plümpe, T.; Ehninger, D.; Steiner, B.; Klempin, F.; Jessberger, S.; Brandt, M.; Römer, B.; Rodriguez, G.R.; Kronenberg, G.; Kempermann, G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Goh, E.; Sailor, K.; Kitabatake, Y.; Ming, G.-L.; Song, H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nat. Cell Biol. 2005, 439, 589–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.D.; Jessberger, S.; Steiner, B.; Kronenberg, G.; Reuter, K.; Bick-Sander, A.; von der Behrens, W.; Kempermann, G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell. Neurosci. 2003, 24, 603–613. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ikeda, Y.; Sakai, A.; Yamasaki, N.; Haneda, E.; Miyakawa, T.; Suzuki, H. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc. Natl. Acad. Sci. USA 2010, 107, 8434–8439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaGamma, C.T.; Tang, W.W.; Morgan, A.A.; McGowan, J.C.; Brachman, R.A.; Denny, C.A. Antidepressant but Not Prophylactic Ketamine Administration Alters Calretinin and Calbindin Expression in the Ventral Hippocampus. Front. Mol. Neurosci. 2018, 11, 404. [Google Scholar] [CrossRef] [Green Version]
- Bellush, L. Caloric Restriction and Spatial Learning in Old Mice. Physiol. Behav. 1996, 60, 549–553. [Google Scholar] [CrossRef]
- Wagenmaker, E.R.; Moenter, S.M. Exposure to Acute Psychosocial Stress Disrupts the Luteinizing Hormone Surge Independent of Estrous Cycle Alterations in Female Mice. Endocrinology 2017, 158, 2593–2602. [Google Scholar] [CrossRef] [Green Version]
- Viau, V.; Meaney, M.J. Variations in the Hypothalamic-Pituitary-Adrenal Response to Stress during the Estrous Cycle in the Rat. Endocrinology 1991, 129, 2503–2511. [Google Scholar] [CrossRef] [Green Version]
- Scheggi, S.; De Montis, M.G.; Gambarana, C. Making Sense of Rodent Models of Anhedonia. Int. J. Neuropsychopharmacol. 2018, 21, 1049–1065. [Google Scholar] [CrossRef] [PubMed]

| Stressors and Exposure Time | |
|---|---|
| Grouping housing (6 mice per cage) | 8 h |
| Water deprivation | 18 h |
| Food deprivation | 18 h |
| Continous light | 24 h |
| Cold room (4 °C) | 15 min |
| Stroboscopic light | 3 h |
| Constant motion (100 rpm) | 30 min |
| White noise | 12 h |
| Movement restriction | 1 h |
| Dirty or wet cage | 12 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Rodríguez, G.B.; Vega-Rivera, N.M.; Meneses-San Juan, D.; Ortiz-López, L.; Estrada-Camarena, E.M.; Flores-Ramos, M. Short Daily Exposure to Environmental Enrichment, Fluoxetine, or Their Combination Reverses Deterioration of the Coat and Anhedonia Behaviors with Differential Effects on Hippocampal Neurogenesis in Chronically Stressed Mice. Int. J. Mol. Sci. 2021, 22, 10976. https://doi.org/10.3390/ijms222010976
Ramírez-Rodríguez GB, Vega-Rivera NM, Meneses-San Juan D, Ortiz-López L, Estrada-Camarena EM, Flores-Ramos M. Short Daily Exposure to Environmental Enrichment, Fluoxetine, or Their Combination Reverses Deterioration of the Coat and Anhedonia Behaviors with Differential Effects on Hippocampal Neurogenesis in Chronically Stressed Mice. International Journal of Molecular Sciences. 2021; 22(20):10976. https://doi.org/10.3390/ijms222010976
Chicago/Turabian StyleRamírez-Rodríguez, Gerardo Bernabé, Nelly Maritza Vega-Rivera, David Meneses-San Juan, Leonardo Ortiz-López, Erika Montserrat Estrada-Camarena, and Mónica Flores-Ramos. 2021. "Short Daily Exposure to Environmental Enrichment, Fluoxetine, or Their Combination Reverses Deterioration of the Coat and Anhedonia Behaviors with Differential Effects on Hippocampal Neurogenesis in Chronically Stressed Mice" International Journal of Molecular Sciences 22, no. 20: 10976. https://doi.org/10.3390/ijms222010976
APA StyleRamírez-Rodríguez, G. B., Vega-Rivera, N. M., Meneses-San Juan, D., Ortiz-López, L., Estrada-Camarena, E. M., & Flores-Ramos, M. (2021). Short Daily Exposure to Environmental Enrichment, Fluoxetine, or Their Combination Reverses Deterioration of the Coat and Anhedonia Behaviors with Differential Effects on Hippocampal Neurogenesis in Chronically Stressed Mice. International Journal of Molecular Sciences, 22(20), 10976. https://doi.org/10.3390/ijms222010976








