Chronic Exposure to HIV-Derived Protein Tat Impairs Endothelial Function via Indirect Alteration in Fat Mass and Nox1-Mediated Mechanisms in Mice
Abstract
1. Introduction
2. Results
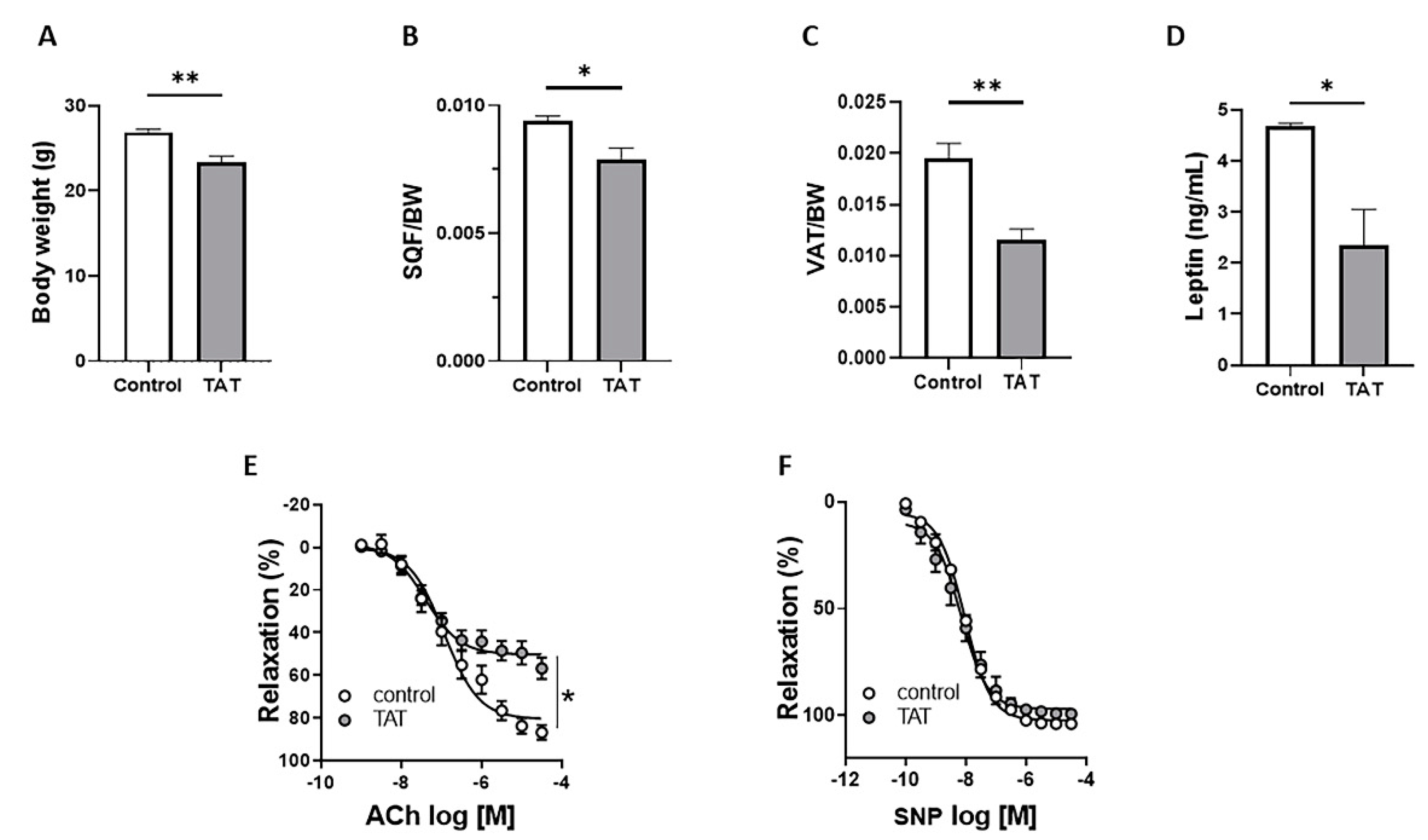
2.1. Tat Induces Endothelial Dysfunction
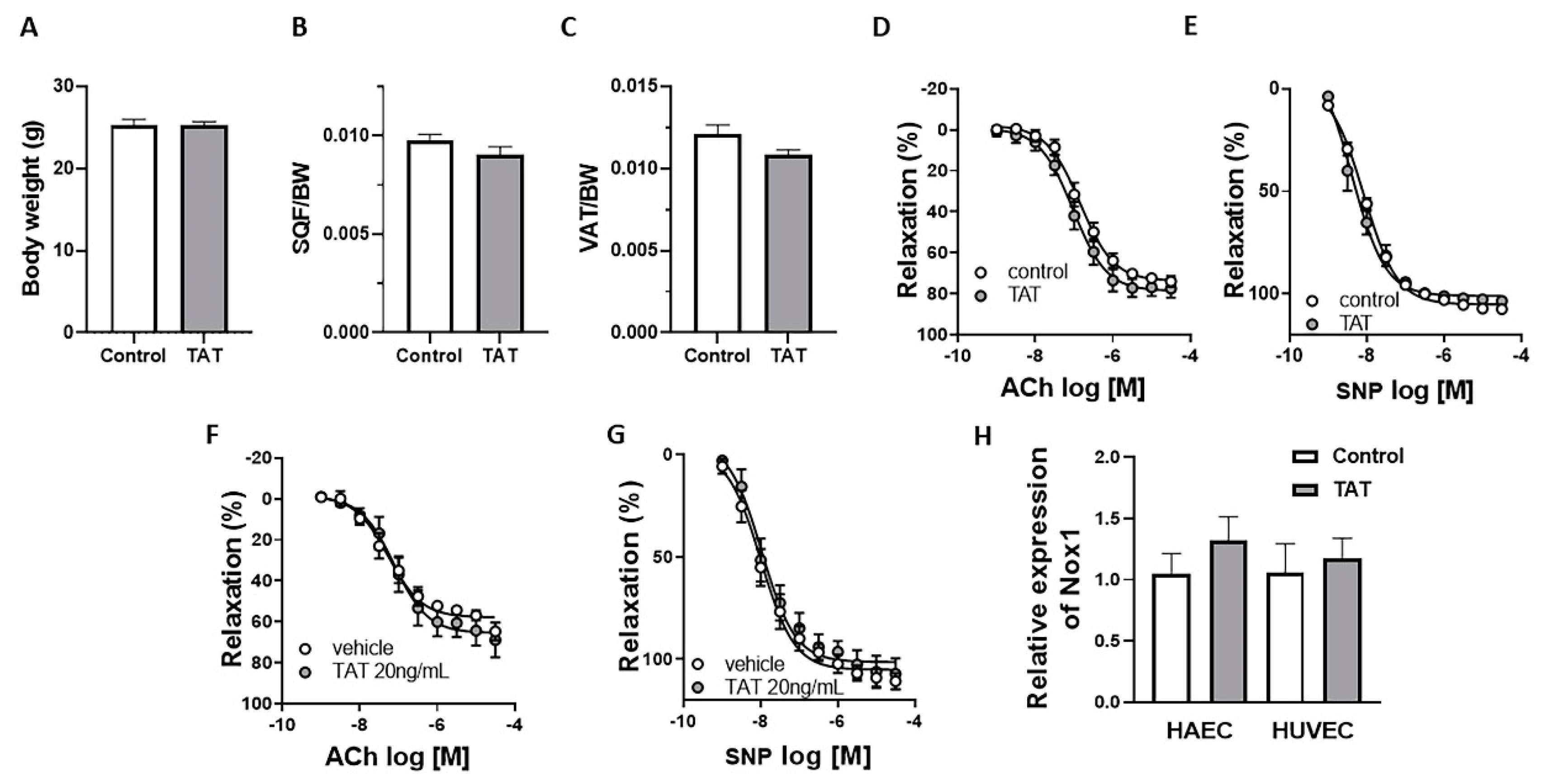
2.2. Tat Does Not Have Direct Effect on Endothelial Function
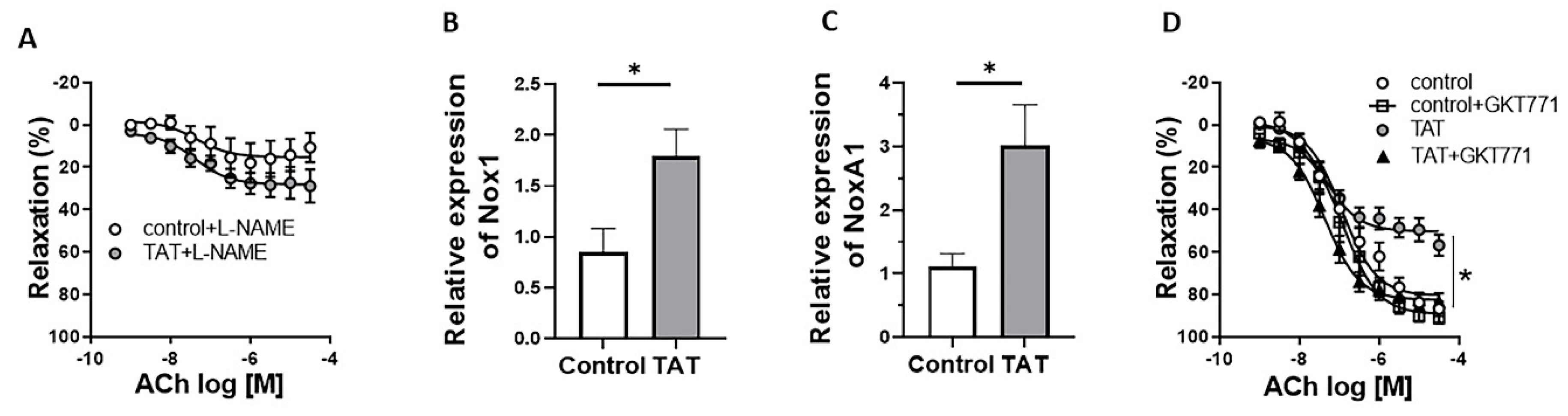
2.3. Tat-Induced Endothelial Dysfunction Is Mediated by Nox1
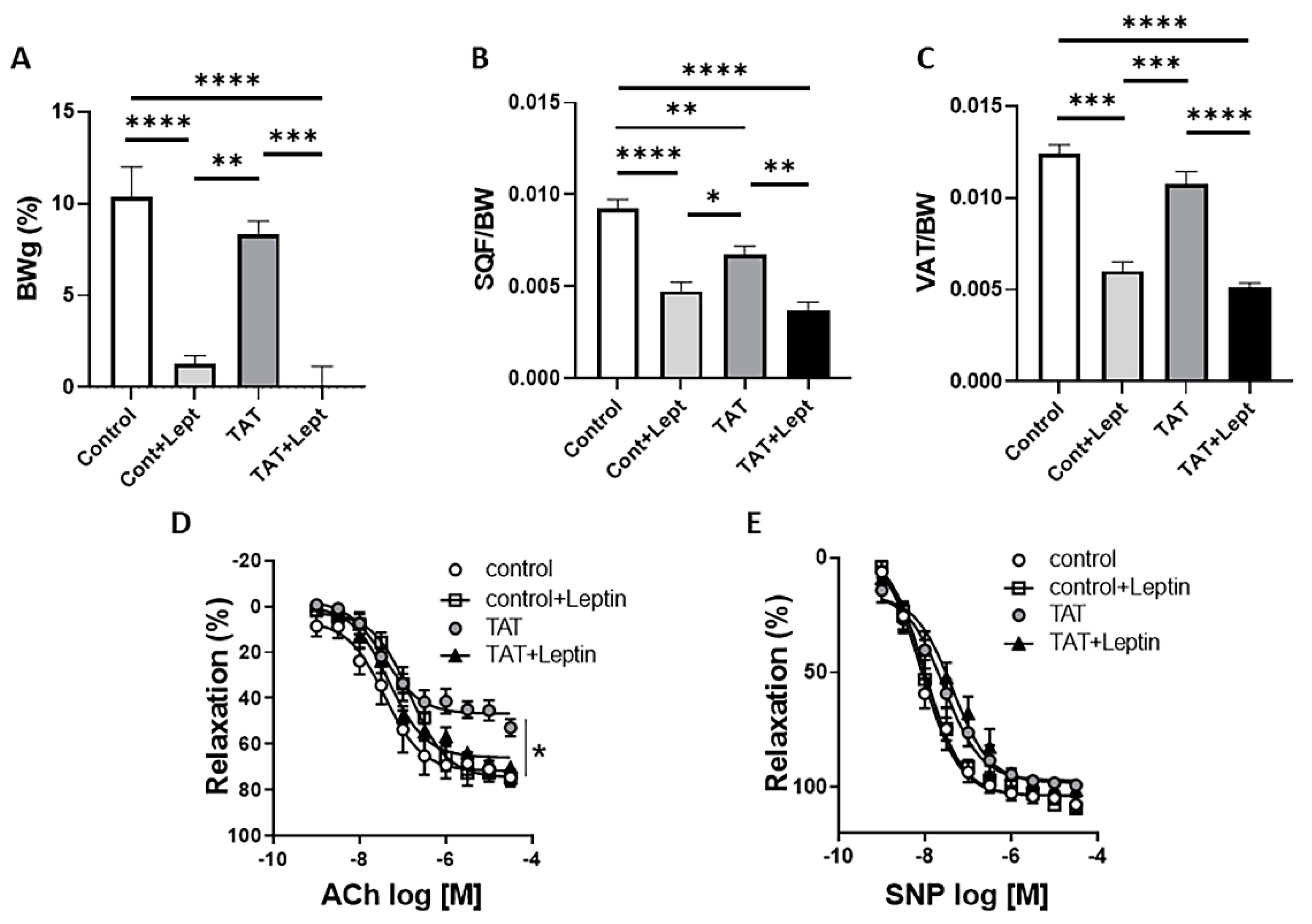
2.4. Leptin Restores Endothelial Function in Tat-Treated Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Human Aortic Endothelial Cells (HAEC) and Human Umbilical Vein Endothelial Cells (HUVEC)
4.3. Treatments
4.4. Metabolic Characterization
4.5. Vascular Function Studies
4.6. Real-Time PCR
4.7. HIV-Derived Tat Protein
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNAIDS. UNAIDS FactSheet. 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 14 July 2021).
- Forsythe, S.S.; McGreevey, W.; Whiteside, A.; Shah, M.; Cohen, J.; Hecht, R.; Bollinger, L.A.; Kinghorn, A. Twenty Years Of Antiretroviral Therapy For People Living With HIV: Global Costs, Health Achievements, Economic Benefits. Health Aff. 2019, 38, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Barnes, A.E.; Guest, J.L.; Shah, A.; Shao, I.Y.; Marconi, V. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. J. Am. Heart Assoc. 2019, 8, e012241. [Google Scholar] [CrossRef] [PubMed]
- Post, W.S.; Budoff, M.; Kingsley, L.; Palella, F.J., Jr.; Witt, M.D.; Li, X.; George, R.T.; Brown, T.T.; Jacobson, L.P. Associations between HIV infection and subclinical coronary atherosclerosis. Ann. Intern. Med. 2014, 160, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.; Gordon, J.; Burdo, T.H.; Qin, X. HIV-1-Associated Atherosclerosis: Unraveling the Missing Link. J. Am. Coll. Cardiol. 2017, 69, 3084–3098. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Stelzle, D.; Lee, K.K.; Beck, E.J.; Alam, S.; Clifford, S.; Longenecker, C.T.; Strachan, F.; Bagchi, S.; Whiteley, W.; et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living with HIV: Systematic Review and Meta-Analysis. Circulation 2018, 138, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Kline, E.R.; Sutliff, R.L. The roles of HIV-1 proteins and antiretroviral drug therapy in HIV-1-associated endothelial dysfunction. J. Investig. Med. 2008, 56, 752–769. [Google Scholar] [CrossRef]
- Marincowitz, C.; Genis, A.; Goswami, N.; De Boever, P.; Nawrot, T.S.; Strijdom, H. Vascular endothelial dysfunction in the wake of HIV and ART. FEBS J. 2019, 286, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, U.; Bonadies, G.; Apuzzi, V.; Foggia, M.; Bosso, G.; Nappa, S.; Valvano, A.; Leonardi, E.; Borgia, G.; Castello, G.; et al. Human immunodeficiency virus per se exerts atherogenic effects. Atherosclerosis 2009, 204, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Hsue, P.Y.; Hunt, P.W.; Schnell, A.; Kalapus, S.C.; Hoh, R.; Ganz, P.; Martin, J.N.; Deeks, S.G. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009, 23, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, F.; Lo, J.; Triant, V.A.; Wei, J.; Buzon, M.J.; Fitch, K.V.; Hwang, J.; Campbell, J.H.; Burdo, T.H.; Williams, K.C.; et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012, 26, 2409–2412. [Google Scholar] [CrossRef]
- Anand, A.R.; Rachel, G.; Parthasarathy, D. HIV Proteins and Endothelial Dysfunction: Implications in Cardiovascular Disease. Front. Cardiovasc. Med. 2018, 5, 185. [Google Scholar] [CrossRef]
- Hansen, L.; Parker, I.; Sutliff, R.L.; Platt, M.O.; Gleason, R.L., Jr. Endothelial dysfunction, arterial stiffening, and intima-media thickening in large arteries from HIV-1 transgenic mice. Ann. Biomed. Eng. 2013, 41, 682–693. [Google Scholar] [CrossRef]
- Matzen, K.; Dirkx, A.E.; oude Egbrink, M.G.; Speth, C.; Gotte, M.; Ascherl, G.; Grimm, T.; Griffioen, A.W.; Sturzl, M. HIV-1 Tat increases the adhesion of monocytes and T-cells to the endothelium in vitro and in vivo: Implications for AIDS-associated vasculopathy. Virus Res. 2004, 104, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Debaisieux, S.; Rayne, F.; Yezid, H.; Beaumelle, B. The ins and outs of HIV-1 Tat. Traffic 2012, 13, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Carosio, R.; Fenoglio, D.; Brenci, S.; Murdaca, G.; Setti, M.; Indiveri, F.; Scabini, S.; Ferrero, E.; Zocchi, M.R. Migration of V delta 1 and V delta 2 T cells in response to CXCR3 and CXCR4 ligands in healthy donors and HIV-1-infected patients: Competition by HIV-1 Tat. Blood 2004, 103, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Neuveut, C.; Tiffany, H.L.; Benkirane, M.; Rich, E.A.; Murphy, P.M.; Jeang, K.T. Selective CXCR4 antagonism by Tat: Implications for in vivo expansion of coreceptor use by HIV-1. Proc. Natl Acad Sci USA 2000, 97, 11466–11471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xue, M.; Qin, D.; Zhu, X.; Wang, C.; Zhu, J.; Hao, T.; Cheng, L.; Chen, X.; Bai, Z.; et al. HIV-1 Tat promotes Kaposi’s sarcoma-associated herpesvirus (KSHV) vIL-6-induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK-3beta signaling pathway. PLoS ONE 2013, 8, e53145. [Google Scholar] [CrossRef] [PubMed]
- Dickens, A.M.; Yoo, S.W.; Chin, A.C.; Xu, J.; Johnson, T.P.; Trout, A.L.; Hauser, K.F.; Haughey, N.J. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci. Rep. 2017, 7, 7748. [Google Scholar] [CrossRef]
- Fang, Q.; Kan, H.; Lewis, W.; Chen, F.; Sharma, P.; Finkel, M.S. Dilated cardiomyopathy in transgenic mice expressing HIV Tat. Cardiovasc. Toxicol. 2009, 9, 39–45. [Google Scholar] [CrossRef]
- Raidel, S.M.; Haase, C.; Jansen, N.R.; Russ, R.B.; Sutliff, R.L.; Velsor, L.W.; Day, B.J.; Hoit, B.D.; Samarel, A.M.; Lewis, W. Targeted myocardial transgenic expression of HIV Tat causes cardiomyopathy and mitochondrial damage. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1672–H1678. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109 (Suppl. 23), III27–III32. [Google Scholar] [CrossRef] [PubMed]
- Toborek, M.; Lee, Y.W.; Pu, H.; Malecki, A.; Flora, G.; Garrido, R.; Hennig, B.; Bauer, H.C.; Nath, A. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J. Neurochem. 2003, 84, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Price, T.O.; Ercal, N.; Nakaoke, R.; Banks, W.A. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005, 1045, 57–63. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Wang, J.L.; Chen, P.; Jeyaseelan, R.; Hofman, F. Human immunodeficiency virus Tat protein induces interleukin 6 mRNA expression in human brain endothelial cells via protein kinase C- and cAMP-dependent protein kinase pathways. AIDS Res. Hum. Retrovir. 1998, 14, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Eum, S.Y.; Nath, A.; Toborek, M. Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovasc. Res. 2004, 63, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chi, D.S.; Li, C.; Hall, H.K.; Milhorn, D.M.; Krishnaswamy, G. HIV-1 Tat protein-induced VCAM-1 expression in human pulmonary artery endothelial cells and its signaling. Am. J. Physiology. Lung Cell. Mol. Physiol. 2005, 289, L252–L260. [Google Scholar] [CrossRef]
- Dhawan, S.; Puri, R.K.; Kumar, A.; Duplan, H.; Masson, J.M.; Aggarwal, B.B. Human immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cells. Blood 1997, 90, 1535–1544. [Google Scholar]
- Duan, M.; Yao, H.; Hu, G.; Chen, X.; Lund, A.K.; Buch, S. HIV Tat induces expression of ICAM-1 in HUVECs: Implications for miR-221/-222 in HIV-associated cardiomyopathy. PLoS ONE 2013, 8, e60170. [Google Scholar] [CrossRef]
- Paladugu, R.; Fu, W.; Conklin, B.S.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Hiv Tat protein causes endothelial dysfunction in porcine coronary arteries. J. Vasc. Surg. 2003, 38, 549–555; discussion 555–556. [Google Scholar] [CrossRef]
- Baker, J.; Quick, H.; Hullsiek, K.H.; Tracy, R.; Duprez, D.; Henry, K.; Neaton, J.D. Interleukin-6 and d-dimer levels are associated with vascular dysfunction in patients with untreated HIV infection. HIV Med. 2010, 11, 608–609. [Google Scholar] [CrossRef]
- Islam, F.M.; Wu, J.; Jansson, J.; Wilson, D.P. Relative risk of cardiovascular disease among people living with HIV: A systematic review and meta-analysis. HIV Med. 2012, 13, 453–468. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Waters, D.D. HIV infection and coronary heart disease: Mechanisms and management. Nat. Rev. Cardiol. 2019, 16, 745–759. [Google Scholar] [CrossRef]
- Stein, J.H.; Kime, N.; Korcarz, C.E.; Ribaudo, H.; Currier, J.S.; Delaney, J.C. Effects of HIV Infection on Arterial Endothelial Function: Results From a Large Pooled Cohort Analysis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 512–522. [Google Scholar] [PubMed]
- Burdo, T.H.; Lentz, M.R.; Autissier, P.; Krishnan, A.; Halpern, E.; Letendre, S.; Rosenberg, E.S.; Ellis, R.J.; Williams, K.C. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J. Infect. Dis 2011, 204, 154–163. [Google Scholar] [CrossRef]
- Baril, J.G.; Junod, P.; Leblanc, R.; Dion, H.; Therrien, R.; Laplante, F.; Falutz, J.; Cote, P.; Hebert, M.N.; Lalonde, R.; et al. HIV-associated lipodystrophy syndrome: A review of clinical aspects. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Troll, J.G. Approach to dyslipidemia, lipodystrophy, and cardiovascular risk in patients with HIV infection. Curr. Atheroscler. Rep. 2011, 13, 51–56. [Google Scholar] [CrossRef]
- Hussain, I.; Patni, N.; Garg, A. Lipodystrophies, dyslipidaemias and atherosclerotic cardiovascular disease. Pathology 2019, 51, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.S.; Tsiodras, S.; Martin, L.D.; Avihingsanon, A.; Gavrila, A.; Hsu, W.C.; Karchmer, A.W.; Mantzoros, C.S. Human immunodeficiency virus type 1-related lipoatrophy and lipohypertrophy are associated with serum concentrations of leptin. Clin. Infect. Dis. 2003, 36, 795–802. [Google Scholar] [CrossRef]
- Visnegarwala, F.; Raghavan, S.S.; Mullin, C.M.; Bartsch, G.; Wang, J.; Kotler, D.; Gibert, C.L.; Shlay, J.; Grunfeld, C.; Carr, A.; et al. Sex differences in the associations of HIV disease characteristics and body composition in antiretroviral-naive persons. Am. J. Clin. Nutr. 2005, 82, 850–856. [Google Scholar]
- Agarwal, N.; Iyer, D.; Patel, S.G.; Sekhar, R.V.; Phillips, T.M.; Schubert, U.; Oplt, T.; Buras, E.D.; Samson, S.L.; Couturier, J.; et al. HIV-1 Vpr induces adipose dysfunction in vivo through reciprocal effects on PPAR/GR co-regulation. Sci. Transl. Med. 2013, 5, 213ra164. [Google Scholar] [CrossRef]
- Freeman, L.M.; Mansfield, K.G.; Goldin, B.; Woods, M.; Gualtieri, L.; Li, W.; Bussell, S.; Lackner, A.; Gorbach, S.L. Body-composition changes in the simian immunodeficiency virus-infected juvenile rhesus macaque. J. Infect. Dis. 2004, 189, 2010–2015. [Google Scholar] [CrossRef]
- Caron-Debarle, M.; Lagathu, C.; Boccara, F.; Vigouroux, C.; Capeau, J. HIV-associated lipodystrophy: From fat injury to premature aging. Trends Mol. Med. 2010, 16, 218–229. [Google Scholar] [CrossRef]
- Diaz-Delfin, J.; Domingo, P.; Wabitsch, M.; Giralt, M.; Villarroya, F. HIV-1 Tat protein impairs adipogenesis and induces the expression and secretion of proinflammatory cytokines in human SGBS adipocytes. Antivir. Ther. 2012, 17, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Vidal, F.; Domingo, P.; Villarroya, F.; Giralt, M.; Lopez-Dupla, M.; Gutierrez, M.; Gallego-Escuredo, J.M.; Peraire, J.; Vilades, C.; Veloso, S.; et al. Adipogenic/lipid, inflammatory, and mitochondrial parameters in subcutaneous adipose tissue of untreated HIV-1-infected long-term nonprogressors: Significant alterations despite low viral burden. J. Acquir. Immune Defic. Syndr. 2012, 61, 131–137. [Google Scholar] [CrossRef]
- Koethe, J.R. Adipose Tissue in HIV Infection. Compr. Physiol. 2017, 7, 1339–1357. [Google Scholar] [PubMed]
- Gorwood, J.; Bourgeois, C.; Mantecon, M.; Atlan, M.; Pourcher, V.; Pourcher, G.; Le Grand, R.; Desjardins, D.; Feve, B.; Lambotte, O.; et al. Impact of HIV/simian immunodeficiency virus infection and viral proteins on adipose tissue fibrosis and adipogenesis. AIDS 2019, 33, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Gori, T.; Munzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Jiang, Y.; Chai, L.; Fasae, M.B.; Bai, Y. The role of HIV Tat protein in HIV-related cardiovascular diseases. J. Transl. Med. 2018, 16, 121. [Google Scholar] [CrossRef]
- Watanabe, L.M.; Barbosa Junior, F.; Jordao, A.A.; Navarro, A.M. Influence of HIV infection and the use of antiretroviral therapy on selenium and selenomethionine concentrations and antioxidant protection. Nutrition 2016, 32, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- El-Amine, R.; Germini, D.; Zakharova, V.V.; Tsfasman, T.; Sheval, E.V.; Louzada, R.A.N.; Dupuy, C.; Bilhou-Nabera, C.; Hamade, A.; Najjar, F.; et al. HIV-1 Tat protein induces DNA damage in human peripheral blood B-lymphocytes via mitochondrial ROS production. Redox. Biol. 2018, 15, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Capone, C.; Cervelli, M.; Angelucci, E.; Colasanti, M.; Macone, A.; Mariottini, P.; Persichini, T. A role for spermine oxidase as a mediator of reactive oxygen species production in HIV-Tat-induced neuronal toxicity. Free Radic. Biol. Med. 2013, 63, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Flores, S.C.; Marecki, J.C.; Harper, K.P.; Bose, S.K.; Nelson, S.K.; McCord, J.M. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proc. Natl. Acad. Sci. USA 1993, 90, 7632–7636. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.F.; Ma, Z.; Myers, D.P.; Terada, L.S. HIV-1 Tat activates dual Nox pathways leading to independent activation of ERK and JNK MAP kinases. J. Biol. Chem. 2007, 282, 37412–37419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Sang, W.W.; Ruan, Z.; Wang, Y.O. Akt/Nox2/NF-kappaB signaling pathway is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation. Arch. Biochem. Biophys. 2011, 505, 266–272. [Google Scholar] [CrossRef]
- Youn, G.S.; Cho, H.; Kim, D.; Choi, S.Y.; Park, J. Crosstalk between HDAC6 and Nox2-based NADPH oxidase mediates HIV-1 Tat-induced pro-inflammatory responses in astrocytes. Redox Biol. 2017, 12, 978–986. [Google Scholar] [CrossRef]
- Bruder-Nascimento, T.; Kress, T.C.; Kennard, S.; Belin de Chantemele, E.J. HIV Protease Inhibitor Ritonavir Impairs Endothelial Function Via Reduction in Adipose Mass and Endothelial Leptin Receptor-Dependent Increases in NADPH Oxidase 1 (Nox1), C-C Chemokine Receptor Type 5 (CCR5), and Inflammation. J. Am. Heart Assoc. 2020, 9, e018074. [Google Scholar] [CrossRef]
- Bobbert, P.; Jenke, A.; Bobbert, T.; Kuhl, U.; Rauch, U.; Lassner, D.; Scheibenbogen, C.; Poller, W.; Schultheiss, H.P.; Skurk, C. High leptin and resistin expression in chronic heart failure: Adverse outcome in patients with dilated and inflammatory cardiomyopathy. Eur. J. Heart Fail. 2012, 14, 1265–1275. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Whincup, P.H.; Lennon, L.; Sattar, N. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: Does leptin have a role? J. Am. Coll. Cardiol. 2011, 58, 1870–1877. [Google Scholar] [CrossRef]
- Huby, A.C.; Antonova, G.; Groenendyk, J.; Gomez-Sanchez, C.E.; Bollag, W.B.; Filosa, J.A.; Belin de Chantemele, E.J. Adipocyte-Derived Hormone Leptin Is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation 2015, 132, 2134–2145. [Google Scholar] [CrossRef]
- Bruder-Nascimento, T.; Faulkner, J.L.; Haigh, S.; Kennard, S.; Antonova, G.; Patel, V.S.; Fulton, D.J.R.; Chen, W.; de Chantemele, E.J.B. Leptin Restores Endothelial Function via Endothelial PPARgamma-Nox1-Mediated Mechanisms in a Mouse Model of Congenital Generalized Lipodystrophy. Hypertension 2019, 74, 1399–1408. [Google Scholar] [CrossRef]
- Schoof, E.; Stuppy, A.; Harig, F.; Carbon, R.; Horbach, T.; Stohr, W.; Rascher, W.; Dotsch, J. Comparison of leptin gene expression in different adipose tissues in children and adults. Eur. J. Endocrinol. 2004, 150, 579–584. [Google Scholar] [CrossRef][Green Version]
- Despres, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef]
- Obry-Roguet, V.; Bregigeon, S.; Cano, C.E.; Lions, C.; Zaegel-Faucher, O.; Laroche, H.; Galie, S.; De Lamarliere, P.G.; Orticoni, M.; Soavi, M.J.; et al. Risk factors associated with overweight and obesity in HIV-infected people: Aging, behavioral factors but not cART in a cross-sectional study. Medicine 2018, 97, e10956. [Google Scholar] [CrossRef]
- Koethe, J.R.; Grome, H.; Jenkins, C.A.; Kalams, S.A.; Sterling, T.R. The metabolic and cardiovascular consequences of obesity in persons with HIV on long-term antiretroviral therapy. AIDS 2016, 30, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Petoumenos, K.; Kuwanda, L.; Ryom, L.; Mocroft, A.; Reiss, P.; De Wit, S.; Pradier, C.; Bonnet, F.; Phillips, A.; Hatleberg, C.I.; et al. Effect of Changes in Body Mass Index on the Risk of Cardiovascular Disease and Diabetes Mellitus in HIV-Positive Individuals: Results From the D:A:D Study. J. Acquir. Immune Defic. Syndr. 2021, 86, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Longenecker, C.T.; Dunn, W.; Jiang, Y.; Debanne, S.M.; McComsey, G.A. Adipokines and vascular health in treated HIV infection: An obesity paradox? AIDS 2013, 27, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.L.; Kennard, S.; Huby, A.C.; Antonova, G.; Lu, Q.; Jaffe, I.Z.; Patel, V.S.; Fulton, D.J.R.; Belin de Chantemele, E.J. Progesterone Predisposes Females to Obesity-Associated Leptin-Mediated Endothelial Dysfunction via Upregulating Endothelial MR (Mineralocorticoid Receptor) Expression. Hypertension 2019, 74, 678–686. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacs, L.; Bruder-Nascimento, T.; Greene, L.; Kennard, S.; Belin de Chantemèle, E.J. Chronic Exposure to HIV-Derived Protein Tat Impairs Endothelial Function via Indirect Alteration in Fat Mass and Nox1-Mediated Mechanisms in Mice. Int. J. Mol. Sci. 2021, 22, 10977. https://doi.org/10.3390/ijms222010977
Kovacs L, Bruder-Nascimento T, Greene L, Kennard S, Belin de Chantemèle EJ. Chronic Exposure to HIV-Derived Protein Tat Impairs Endothelial Function via Indirect Alteration in Fat Mass and Nox1-Mediated Mechanisms in Mice. International Journal of Molecular Sciences. 2021; 22(20):10977. https://doi.org/10.3390/ijms222010977
Chicago/Turabian StyleKovacs, Laszlo, Thiago Bruder-Nascimento, Lindsey Greene, Simone Kennard, and Eric J. Belin de Chantemèle. 2021. "Chronic Exposure to HIV-Derived Protein Tat Impairs Endothelial Function via Indirect Alteration in Fat Mass and Nox1-Mediated Mechanisms in Mice" International Journal of Molecular Sciences 22, no. 20: 10977. https://doi.org/10.3390/ijms222010977
APA StyleKovacs, L., Bruder-Nascimento, T., Greene, L., Kennard, S., & Belin de Chantemèle, E. J. (2021). Chronic Exposure to HIV-Derived Protein Tat Impairs Endothelial Function via Indirect Alteration in Fat Mass and Nox1-Mediated Mechanisms in Mice. International Journal of Molecular Sciences, 22(20), 10977. https://doi.org/10.3390/ijms222010977





