Mechanism of Phosgene-Induced Acute Lung Injury and Treatment Strategy
Abstract
1. Introduction
2. Toxicology Studies
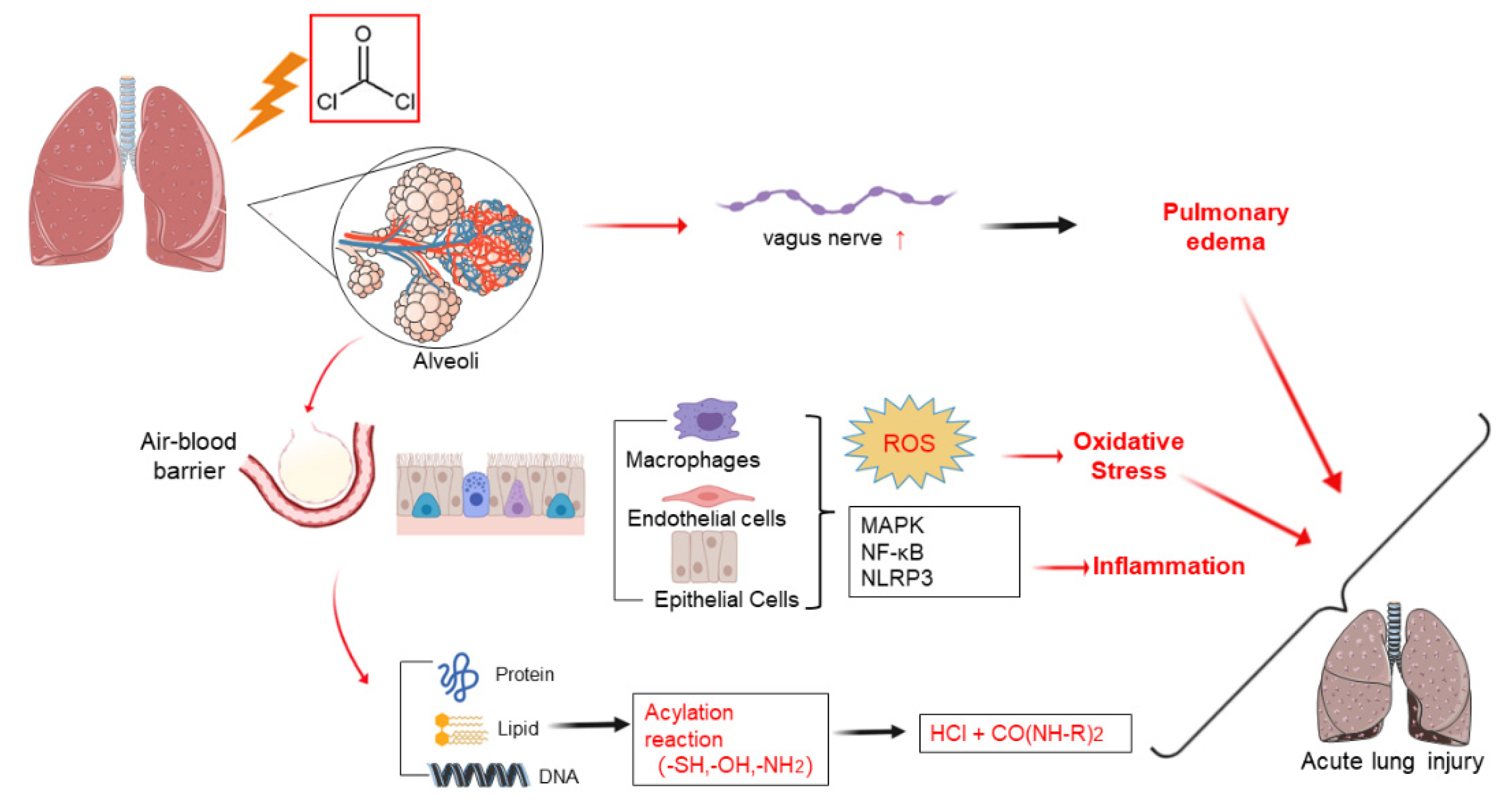
3. Pathophysiology Mechanisms
4. Cellular and Molecular Mechanisms
4.1. Plasma Membrane Impairment
4.2. Inflammation
4.3. Oxidative Stress
5. Medical Treatments
5.1. Anti-Inflammatory Drugs
5.1.1. Glucocorticoids
5.1.2. Ulinastatin
5.1.3. NOS-2 Inhibitors
5.1.4. Melatonin
5.1.5. Angiopoietin-1
5.2. Antioxidant Drugs
5.2.1. N-Acetylcysteine
5.2.2. Caffeic Acid Phenethyl Ester
5.2.3. Ibuprofen
5.2.4. Bio300
5.2.5. 5,8,11,14-Eicosatetraynoic acid
5.3. Others
5.3.1. TRP Channel Inhibitors
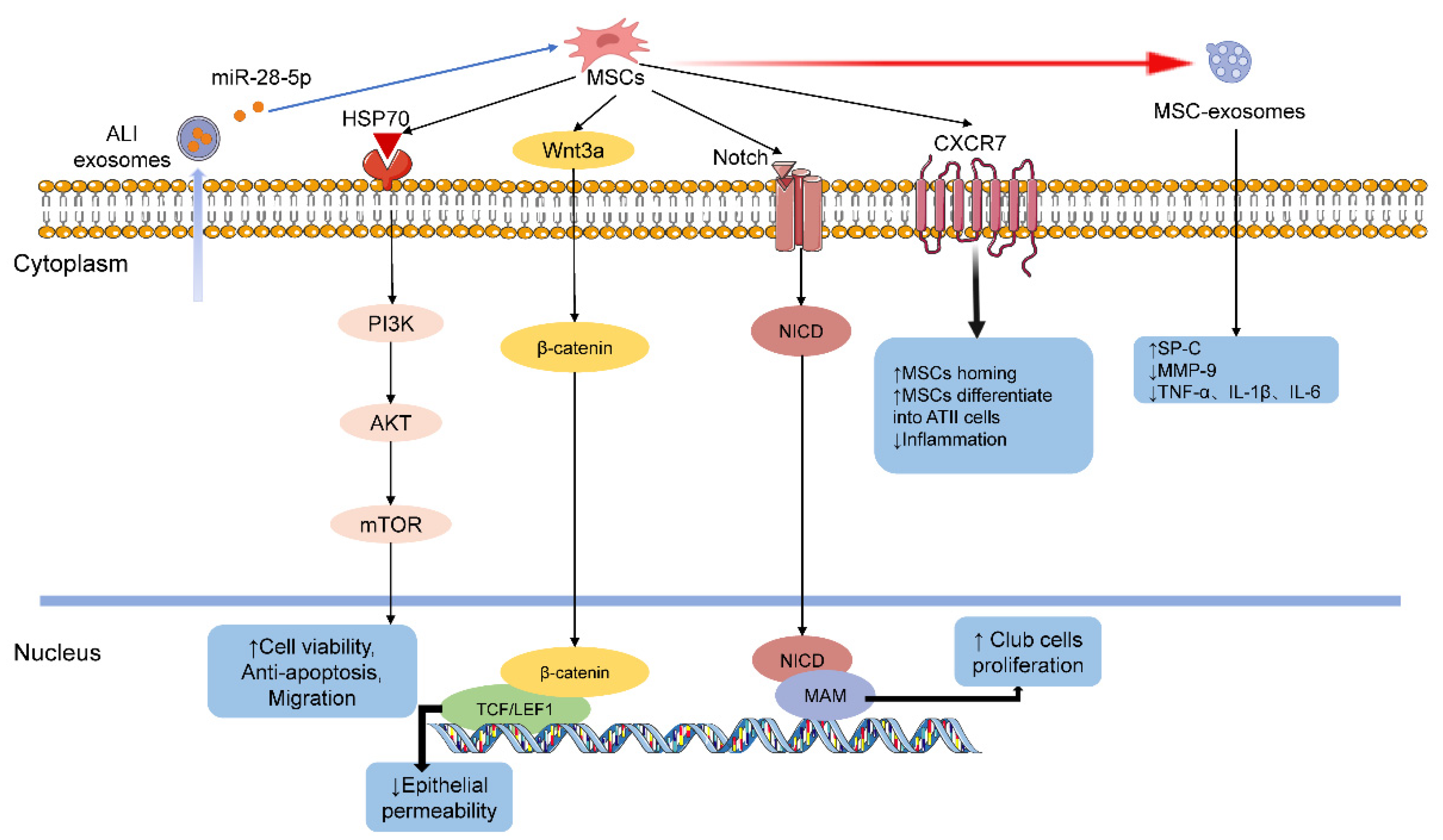
5.3.2. Mesenchymal Stem Cells
5.3.3. FV-HSP72
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholson-Roberts, T.C. Phosgene use in World War 1 and early evaluations of pathophysiology. J. R. Army Med. Corps 2019, 165, 183–187. [Google Scholar] [CrossRef]
- Jones, E. Terror weapons: The British experience of gas and its treatment in the First World War. War Hist. 2014, 21, 355–375. [Google Scholar] [CrossRef]
- Fitzgerald, G.J. Chemical warfare and medical response during World War I. Am. J. Public Health 2008, 98, 611–625. [Google Scholar] [CrossRef]
- Chauhan, S.; Chauhan, S.; D’Cruz, R.; Faruqi, S.; Singh, K.K.; Varma, S.; Singh, M.; Karthik, V. Chemical warfare agents. Environ. Toxicol. Pharmacol. 2008, 26, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, T.; Mori, W.; Ogawa, M.; Fujinaga, M.; Zhang, M.R. [(11)C]phosgene: Synthesis and application for development of PET radiotracers. Nucl. Med. Biol. 2021, 92, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, D.; Yoon, J. Recent advances in the development of chromophore-based chemosensors for nerve agents and phosgene. ACS Sens. 2018, 3, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Bessac, B.F.; Jordt, S.E. Sensory detection and responses to toxic gases: Mechanisms, health effects, and countermeasures. Proc. Am. Thorac. Soc. 2010, 7, 269–277. [Google Scholar] [CrossRef]
- Hardison, L.S., Jr.; Wright, E.; Pizon, A.F. Phosgene exposure: A case of accidental industrial exposure. J. Med. Toxicol. 2014, 10, 51–56. [Google Scholar] [CrossRef]
- Li, W.; Rosenbruch, M.; Pauluhn, J. Effect of PEEP on phosgene-induced lung edema: Pilot study on dogs using protective ventilation strategies. Exp. Toxicol. Pathol. 2015, 67, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Grainge, C.; Jugg, B.J.; Smith, A.J.; Brown, R.F.; Jenner, J.; Parkhouse, D.A.; Rice, P. Delayed low-dose supplemental oxygen improves survival following phosgene-induced acute lung injury. Inhal. Toxicol. 2010, 22, 552–560. [Google Scholar] [CrossRef]
- Graham, S.; Fairhall, S.; Rutter, S.; Auton, P.; Rendell, R.; Smith, A.; Perrott, R.; Roberts, T.N.; Jugg, B. Continuous positive airway pressure: An early intervention to prevent phosgene-induced acute lung injury. Toxicol. Lett. 2018, 293, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Pauluhn, J. Concentration x time analyses of sensory irritants revisited: Weight of evidence or the toxic load approach. That is the question. Toxicol. Lett. 2019, 316, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pauluhn, J. Phosgene-induced lung edema: Comparison of clinical criteria for increased extravascular lung water content with postmortem lung gravimetry and lavage-protein in rats and dogs. Toxicol. Lett. 2019, 305, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Kennedy, T.P.; Hatch, G.E.; Tepper, J.S. Reduction of neutrophil influx diminishes lung injury and mortality following phosgene inhalation. J. Appl. Physiol. 1991, 71, 657–665. [Google Scholar] [CrossRef]
- Russell, D.; Blain, P.G.; Rice, P. Clinical management of casualties exposed to lung damaging agents: A critical review. Emerg. Med. J. 2006, 23, 421–424. [Google Scholar] [CrossRef]
- Diller, W.F. Pathogenesis of phosgene poisoning. Toxicol. Ind. Health 1985, 1, 7–15. [Google Scholar] [CrossRef]
- Borak, J.; Diller, W.F. Phosgene exposure: Mechanisms of injury and treatment strategies. J. Occup. Environ. Med. 2001, 43, 110–119. [Google Scholar] [CrossRef]
- Lee, L.Y. Respiratory sensations evoked by activation of bronchopulmonary C-fibers. Respir. Physiol. Neurobiol. 2009, 167, 26–35. [Google Scholar] [CrossRef]
- Li, W.; Liu, F.; Wang, C.; Truebel, H.; Pauluhn, J. Novel insights into phosgene-induced acute lung injury in rats: Role of dysregulated cardiopulmonary reflexes and nitric oxide in lung edema pathogenesis. Toxicol. Sci. 2013, 131, 612–628. [Google Scholar] [CrossRef]
- Ivanhoe, F.; Meyers, F.H. Phosgene poisoning as an example of neuroparalytic acute pulmonary edema: The sympathetic vasomotor reflex involved. Dis. Chest 1964, 46, 211–218. [Google Scholar] [CrossRef][Green Version]
- Pauluhn, J. Phosgene inhalation toxicity: Update on mechanisms and mechanism-based treatment strategies. Toxicology 2021, 450, 152682. [Google Scholar] [CrossRef]
- Jugg, B.; Jenner, J.; Rice, P. The effect of perfluoroisobutene and phosgene on rat lavage fluid surfactant phospholipids. Hum. Exp. Toxicol. 1999, 18, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef]
- Sciuto, A.M.; Clapp, D.L.; Hess, Z.A.; Moran, T.S. The temporal profile of cytokines in the bronchoalveolar lavage fluid in mice exposed to the industrial gas phosgene. Inhal. Toxicol. 2003, 15, 687–700. [Google Scholar] [CrossRef] [PubMed]
- He, D.K.; Shao, Y.R.; Shen, J.; Zhang, L.; Zhang, J.; Zhang, F. Significance of the NLRP3 inflammasome expression in rats with acute lung injury induced by phosgene. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2017, 35, 491–496. [Google Scholar]
- Qian, F.; Deng, J.; Wang, G.; Ye, R.D.; Christman, J.W. Pivotal role of mitogen-activated protein kinase-activated protein kinase 2 in inflammatory pulmonary diseases. Curr. Protein Pept. Sci. 2016, 17, 332–342. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- He, D.K.; Shao, Y.R.; Zhang, L.; Shen, J.; Zhong, Z.Y.; Wang, J.; Xu, G. Adenovirus-delivered angiopoietin-1 suppresses NF-kappaB and p38 MAPK and attenuates inflammatory responses in phosgene-induced acute lung injury. Inhal. Toxicol. 2014, 26, 185–192. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Riestra, A.M.; Gao, N.J.; LaRock, C.N.; Gupta, N.; Ali, S.R.; Hoffman, H.M.; Ghosh, P.; Nizet, V. Group A streptococcal M protein activates the NLRP3 inflammasome. Nat. Microbiol. 2017, 2, 1425–1434. [Google Scholar] [CrossRef]
- He, D.K.; Chen, J.F.; Shao, Y.R.; Zhou, F.Q.; Shen, J. Adenovirus-delivered angiopoietin-1 ameliorates phosgene-induced acute lung injury via inhibition of NLRP3 inflammasome activation. Inhal. Toxicol. 2018, 30, 187–194. [Google Scholar] [CrossRef]
- He, D.K.; Xu, N.; Shao, Y.R.; Shen, J. NLRP3 gene silencing ameliorates phosgene-induced acute lung injury in rats by inhibiting NLRP3 inflammasome and proinflammatory factors, but not anti-inflammatory factors. J. Toxicol. Sci. 2020, 45, 625–637. [Google Scholar] [CrossRef]
- Wiegman, C.H.; Li, F.; Ryffel, B.; Togbe, D.; Chung, K.F. Oxidative stress in ozone-induced chronic lung inflammation and emphysema: A facet of chronic obstructive pulmonary disease. Front. Immunol. 2020, 11, 1957. [Google Scholar] [CrossRef] [PubMed]
- Rendell, R.; Fairhall, S.; Graham, S.; Rutter, S.; Auton, P.; Smith, A.; Perrott, R.; Jugg, B. Assessment of N-acetylcysteine as a therapy for phosgene-induced acute lung injury. Toxicol. Lett. 2018, 290, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ye, X.L.; Liu, R.; Chen, H.L.; Liang, X.; Li, W.L.; Zhang, X.D.; Qin, X.J.; Bai, H.; Zhang, W.; et al. Mechanism of acute lung injury due to phosgene exposition and its protection by cafeic acid phenethyl ester in the rat. Exp. Toxicol. Pathol. 2013, 65, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, A.M.; Cascio, M.B.; Moran, T.S.; Forster, J.S. The fate of antioxidant enzymes in bronchoalveolar lavage fluid over 7 days in mice with acute lung injury. Inhal. Toxicol. 2003, 15, 675–685. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Khazaei, M. Oxidative stress and cancer: The role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef]
- Jaskot, R.H.; Grose, E.C.; Richards, J.H.; Doerfler, D.L. Effects of inhaled phosgene on rat lung antioxidant systems. Fundam. Appl. Toxicol. 1991, 17, 666–674. [Google Scholar] [CrossRef]
- He, Z.; Yang, X.; Yang, C. Extracorporeal membrane oxygenation for acute respiratory distress syndrome caused by acute phosgene poisoning: A report of 4 cases. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019, 31, 232–235. [Google Scholar]
- Qiu, S.B.; Hu, Y.T.; He, Q.; Zhu, R.K. Investigation and analysis of 7 cases of acute lung injury caused by a welding operation. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2019, 37, 60–62. [Google Scholar]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Desmet, S.J.; De Bosscher, K. Glucocorticoid receptors: Finding the middle ground. J. Clin. Investig. 2017, 127, 1136–1145. [Google Scholar] [CrossRef]
- He, D.K.; Shen, J.; Zhang, L.; Huang, W.B. Effects of dexamethasone pretreatment on expression of matrix metalloproteinase-9 in rats with acute lung injury induced by phosgene. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2011, 29, 289–293. [Google Scholar]
- Zhou, F.Q.; He, D.K.; Shao, Y.R.; Shen, J. The effects of methylprednisolone on NLRP3 inflammasome in rats with acute lung injury Induced by Phosgene. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2018, 36, 573–579. [Google Scholar]
- Smith, A.; Brown, R.; Jugg, B.; Platt, J.; Mann, T.; Masey, C.; Jenner, J.; Rice, P. The effect of steroid treatment with inhaled budesonide or intravenous methylprednisolone on phosgene-induced acute lung injury in a porcine model. Mil. Med. 2009, 174, 1287–1294. [Google Scholar] [CrossRef]
- Liu, F.; Pauluhn, J.; Trubel, H.; Wang, C. Single high-dose dexamethasone and sodium salicylate failed to attenuate phosgene-induced acute lung injury in rats. Toxicology 2014, 315, 17–23. [Google Scholar] [CrossRef]
- Luo, S.; Pauluhn, J.; Trubel, H.; Wang, C. Corticosteroids found ineffective for phosgene-induced acute lung injury in rats. Toxicol. Lett. 2014, 229, 85–92. [Google Scholar] [CrossRef]
- Liu, B.Z.; Hai, C.X.; Li, W.L. Clinical diagnosis and treatment of acute phosgene poisoning and its research progress. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2020, 38, 66–70. [Google Scholar] [PubMed]
- Li, S.T.; Dai, Q.; Zhang, S.X.; Liu, Y.J.; Yu, Q.Q.; Tan, F.; Lu, S.H.; Wang, Q.; Chen, J.W.; Huang, H.Q.; et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-kappaB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol. Sin. 2018, 39, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liu, S.; Luo, L.; Gu, N.; Zeng, Y.; Chen, X.; Xu, S.; Zhang, D. Anti-inflammatory mechanism of ulinastatin: Inhibiting the hyperpermeability of vascular endothelial cells induced by TNF-alpha via the RhoA/ROCK signal pathway. Int. Immunopharmacol. 2017, 46, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Gan, Z.; Zhao, J.; Zhang, L.; Xu, G. Ulinastatin reduces pathogenesis of phosgene-induced acute lung injury in rats. Toxicol. Ind. Health 2014, 30, 785–793. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Zhong, Z.; He, D.; Zhang, J.; Shen, J. Dynamic changes of a group of cytokines in phosgene-induced lung injury and the function of ulinastatin. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2014, 32, 813–818. [Google Scholar] [PubMed]
- Liu, F.; Li, W.; Pauluhn, J.; Trubel, H.; Wang, C. Rat models of acute lung injury: Exhaled nitric oxide as a sensitive, noninvasive real-time biomarker of prognosis and efficacy of intervention. Toxicology 2013, 310, 104–114. [Google Scholar] [CrossRef]
- Zheng, H.; Liang, W.; He, W.; Huang, C.; Chen, Q.; Yi, H.; Long, L.; Deng, Y.; Zeng, M. Ghrelin attenuates sepsis-induced acute lung injury by inhibiting the NF-kappaB, iNOS, and Akt signaling in alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 317, L381–L391. [Google Scholar] [CrossRef]
- Ma, H.; Mo, S.; Yi, Q.; Lai, J.; Liu, H.; Shi, Z. Role and mechanism of maresin-1 in acute lung injury induced by trauma-hemorrhagic shock. Med. Sci. Monit. 2020, 26, e923518. [Google Scholar] [CrossRef]
- Luo, S.; Trubel, H.; Wang, C.; Pauluhn, J. Phosgene- and chlorine-induced acute lung injury in rats: Comparison of cardiopulmonary function and biomarkers in exhaled breath. Toxicology 2014, 326, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Bai, H.; Xi, M.M.; Liu, R.; Qin, X.J.; Liang, X.; Zhang, W.; Zhang, X.D.; Li, W.L.; Hai, C.X. Ethyl pyruvate protects rats from phosgene-induced pulmonary edema by inhibiting cyclooxygenase2 and inducible nitric oxide synthase expression. J. Appl. Toxicol. 2013, 33, 71–77. [Google Scholar] [CrossRef]
- Filipczak, P.T.; Senft, A.P.; Seagrave, J.; Weber, W.; Kuehl, P.J.; Fredenburgh, L.E.; McDonald, J.D.; Baron, R.M. NOS-2 inhibition in phosgene-induced acute lung injury. Toxicol. Sci. 2015, 146, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Grailer, J.J.; Wang, N.; Wang, M.; Yao, J.; Zhong, R.; Gao, G.F.; Ward, P.A.; Tan, D.X.; et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J. Pineal Res. 2016, 60, 405–414. [Google Scholar] [CrossRef]
- Aliasgharzadeh, A.; Farhood, B.; Amini, P.; Saffar, H.; Motevaseli, E.; Rezapoor, S.; Nouruzi, F.; Shabeeb, D.H.; Eleojo Musa, A.; Mohseni, M.; et al. Melatonin attenuates upregulation of Duox1 and Duox2 and protects against lung injury following chest irradiation in rats. Cell J. 2019, 21, 236–242. [Google Scholar]
- Zhang, L.; Zhang, F.; He, D.; Xu, D.; Zhong, Z.; Shen, J. Melatonin attenuates phosgene-induced acute lung injury via the upregulation Wnt/beta-catenin pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 11281–11287. [Google Scholar] [PubMed]
- Zhang, L.; He, D.; Shao, Y.; Xu, D.; Shen, J. Effect of melatonin on p38MAPKsignaling pathway in rats with phosgene-induced lung injury. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2014, 32, 648–652. [Google Scholar] [PubMed]
- Zhang, L.; Shen, J.; Gan, Z.Y.; He, D.K.; Zhong, Z.Y. Protective effect of melatonin in rats with phosgene-induced lung injury. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2012, 30, 834–838. [Google Scholar]
- Gutbier, B.; Neuhauss, A.K.; Reppe, K.; Ehrler, C.; Santel, A.; Kaufmann, J.; Scholz, M.; Weissmann, N.; Morawietz, L.; Mitchell, T.J.; et al. Prognostic and pathogenic role of angiopoietin-1 and -2 in pneumonia. Am. J. Respir. Crit. Care Med. 2018, 198, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ryczko, M.; Xie, X.; Baardsnes, J.; Lord-Dufour, S.; Duroche, Y.; Hicks, E.A.; Taiyab, A.; Sheardown, H.; Quaggin, S.E.; et al. New soluble angiopoietin analog of Hepta-ANG1 prevents pathological vascular leakage. Biotechnol. Bioeng. 2021, 118, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, K.S.; Park, S.J.; Min, K.H.; Lee, K.Y.; Choe, Y.H.; Hong, S.H.; Koh, G.Y.; Lee, Y.C. Angiopoietin-1 variant, COMP-Ang1 attenuates hydrogen peroxide-induced acute lung injury. Exp. Mol. Med. 2008, 40, 320–331. [Google Scholar] [CrossRef]
- Mei, S.H.; McCarter, S.D.; Deng, Y.; Parker, C.H.; Liles, W.C.; Stewart, D.J. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007, 4, e269. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, J.; Shen, J. The changes of the ratio of angiopoietin-2 to angiopoietin-1 in the acute lung injury induced by phosgene in rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2011, 29, 608–610. [Google Scholar]
- Shen, J.; Wang, J.; Shao, Y.R.; He, D.K.; Zhang, L.; Nadeem, L.; Xu, G. Adenovirus-delivered angiopoietin-1 treatment for phosgene-induced acute lung injury. Inhal. Toxicol. 2013, 25, 272–279. [Google Scholar] [CrossRef]
- Zhang, R.H.; Li, C.H.; Wang, C.L.; Xu, M.J.; Xu, T.; Wei, D.; Liu, B.J.; Wang, G.H.; Tian, S.F. N-acetyl-l-cystine (NAC) protects against H9N2 swine influenza virus-induced acute lung injury. Int. Immunopharmacol. 2014, 22, 1–8. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, H.; Zhou, W.G.; Guo, X.C.; Wu, M.J.; Xu, Z.Y.; Jiang, J.F.; Shen, C.; Liu, H.Q. N-acetylcysteine-pretreated human embryonic mesenchymal stem cell administration protects against bleomycin-induced lung injury. Am. J. Med. Sci. 2013, 346, 113–122. [Google Scholar] [CrossRef]
- Ji, L.; Liu, R.; Zhang, X.D.; Chen, H.L.; Bai, H.; Wang, X.; Zhao, H.L.; Liang, X.; Hai, C.X. N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal. Toxicol. 2010, 22, 535–542. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, Y.; Li, N.G.; Zhu, Y.; Duan, J.A. Bioactivity and chemical synthesis of caffeic acid phenethyl ester and its derivatives. Molecules 2014, 19, 16458–16476. [Google Scholar] [CrossRef]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef]
- Kantor, T.G. Ibuprofen. Ann. Intern. Med. 1979, 91, 877–882. [Google Scholar] [CrossRef]
- Sciuto, A.M.; Stotts, R.R.; Hurt, H.H. Efficacy of ibuprofen and pentoxifylline in the treatment of phosgene-induced acute lung injury. J. Appl. Toxicol. 1996, 16, 381–384. [Google Scholar] [CrossRef]
- Kennedy, T.P.; Rao, N.V.; Noah, W.; Michael, J.R.; Jafri, M.H., Jr.; Gurtner, G.H.; Hoidal, J.R. Ibuprofen prevents oxidant lung injury and in vitro lipid peroxidation by chelating iron. J. Clin. Investig. 1990, 86, 1565–1573. [Google Scholar] [CrossRef]
- Holmes, W.W.; Keyser, B.M.; Paradiso, D.C.; Ray, R.; Andres, D.K.; Benton, B.J.; Rothwell, C.C.; Hoard-Fruchey, H.M.; Dillman, J.F.; Sciuto, A.M.; et al. Conceptual approaches for treatment of phosgene inhalation-induced lung injury. Toxicol. Lett. 2016, 244, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Tobias, L.D.; Hamilton, J.G. The effect of 5,8,11,14-eicosatetraynoic acid on lipid metabolism. Lipids 1979, 14, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, A.M. Posttreatment with ETYA protects against phosgene-induced lung injury by amplifying the glutathione to lipid peroxidation ratio. Inhal. Toxicol. 2000, 12, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Banner, K.H.; Igney, F.; Poll, C. TRP channels: Emerging targets for respiratory disease. Pharmacol. Ther. 2011, 130, 371–384. [Google Scholar] [CrossRef]
- Prandini, P.; De Logu, F.; Fusi, C.; Provezza, L.; Nassini, R.; Montagner, G.; Materazzi, S.; Munari, S.; Gilioli, E.; Bezzerri, V.; et al. Transient receptor potential ankyrin 1 channels modulate inflammatory response in respiratory cells from patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2016, 55, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Brayden, J.E. Transient receptor potential channels in the vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef]
- Andres, D.; Keyser, B.; Benton, B.; Melber, A.; Olivera, D.; Holmes, W.; Paradiso, D.; Anderson, D.; Ray, R. Transient receptor potential (TRP) channels as a therapeutic target for intervention of respiratory effects and lethality from phosgene. Toxicol. Lett. 2016, 244, 21–27. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Lee, J.W.; Fang, X.; Krasnodembskaya, A.; Howard, J.P.; Matthay, M.A. Concise review: Mesenchymal stem cells for acute lung injury: Role of paracrine soluble factors. Stem Cells 2011, 29, 913–919. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z.; et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef]
- Chen, J.; Shao, Y.; Xu, G.; Lim, C.; Li, J.; Xu, D.; Shen, J. Bone marrow-derived mesenchymal stem cells attenuate phosgene-induced acute lung injury in rats. Inhal. Toxicol. 2015, 27, 254–261. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, Y.; He, D.; Zhang, L.; Xu, G.; Shen, J. Evidence that bone marrow-derived mesenchymal stem cells reduce epithelial permeability following phosgene-induced acute lung injury via activation of wnt3a protein-induced canonical wnt/beta-catenin signaling. Inhal. Toxicol. 2016, 28, 572–579. [Google Scholar] [CrossRef]
- Zepp, J.A.; Zacharias, W.J.; Frank, D.B.; Cavanaugh, C.A.; Zhou, S.; Morley, M.P.; Morrisey, E.E. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell 2017, 170, 1134–1148.e10. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; He, D.; Shao, Y.; Xu, N.; Jin, C.; Zhang, L.; Shen, J. Exogenous mesenchymal stem cells affect the function of endogenous lung stem cells (club cells) in phosgene-induced lung injury. Biochem. Biophys. Res. Commun. 2019, 514, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Szydlak, R. Mesenchymal stem cells’ homing and cardiac tissue repair. Acta Biochim. Pol. 2019, 66, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhou, F.; Zhang, L.; Shen, J. Overexpression of heat shock protein 70 enhanced mesenchymal stem cell treatment efficacy in phosgene-induced acute lung injury. J. Biochem. Mol. Toxicol. 2020, 34, e22515. [Google Scholar] [CrossRef]
- Naumann, U.; Cameroni, E.; Pruenster, M.; Mahabaleshwar, H.; Raz, E.; Zerwes, H.G.; Rot, A.; Thelen, M. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS ONE 2010, 5, e9175. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, F.; He, D.; Zhang, L.; Shen, J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed. Pharmacother. 2019, 109, 1233–1239. [Google Scholar] [CrossRef]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Lee, J.W. Therapeutic use of mesenchymal stem cell-derived extracellular vesicles in acute lung injury. Transfusion 2019, 59, 876–883. [Google Scholar] [CrossRef]
- Xu, N.; Shao, Y.; Ye, K.; Qu, Y.; Memet, O.; He, D.; Shen, J. Mesenchymal stem cell-derived exosomes attenuate phosgene-induced acute lung injury in rats. Inhal. Toxicol. 2019, 31, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; He, D.; Shao, Y.; Qu, Y.; Ye, K.; Memet, O.; Zhang, L.; Shen, J. Lung-derived exosomes in phosgene-induced acute lung injury regulate the functions of mesenchymal stem cells partially via miR-28–5p. Biomed. Pharmacother. 2020, 121, 109603. [Google Scholar] [CrossRef] [PubMed]
- Parseghian, M.H.; Hobson, S.T.; Richieri, R.A. Targeted heat shock protein 72 for pulmonary cytoprotection. Ann. N. Y. Acad. Sci. 2016, 1374, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Hobson, S.T.; Richieri, R.A.; Parseghian, M.H. Phosgene: Toxicology, animal models, and medical countermeasures. Toxicol. Mech. Methods 2021, 31, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef]
- Zheng, Z.; Kim, J.Y.; Ma, H.; Lee, J.E.; Yenari, M.A. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J. Cereb. Blood Flow Metab. 2008, 28, 53–63. [Google Scholar] [CrossRef]
- Tanimoto, T.; Parseghian, M.H.; Nakahara, T.; Kawai, H.; Narula, N.; Kim, D.; Nishimura, R.; Weisbart, R.H.; Chan, G.; Richieri, R.A.; et al. Cardioprotective Effects of HSP72 administration on ischemia-reperfusion Injury. J. Am. Coll. Cardiol. 2017, 70, 1479–1492. [Google Scholar] [CrossRef]
- Lei, J.; Wei, Y.; Song, P.; Li, Y.; Zhang, T.; Feng, Q.; Xu, G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2018, 818, 110–114. [Google Scholar] [CrossRef]
- Beltran-Garcia, J.; Osca-Verdegal, R.; Pallardo, F.V.; Ferreres, J.; Rodriguez, M.; Mulet, S.; Sanchis-Gomar, F.; Carbonell, N.; Garcia-Gimenez, J.L. Oxidative stress and inflammation in COVID-19-associated sepsis: The potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants 2020, 9, 936. [Google Scholar] [CrossRef]
- De Carvalho, F.O.; Felipe, F.A.; de Melo Costa, A.C.; Teixeira, L.G.; Silva, E.R.; Nunes, P.S.; Shanmugam, S.; de Lucca Junior, W.; Quintans, J.S.; de Souza Araujo, A.A. Inflammatory mediators and oxidative stress in animals subjected to smoke inhalation: A systematic review. Lung 2016, 194, 487–499. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Liu, J.; Gao, J.; Fang, L.E.; Liu, Z.; Xia, B.; Fan, X.; Li, C.; Lu, Q.; et al. NF-kappaB and FosB mediate inflammation and oxidative stress in the blast lung injury of rats exposed to shock waves. Acta Biochim. Biophys. Sin. 2021, 53, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, Y.; Shen, J.; Mehra, R.; Kallianpur, A.; Culver, D.A.; Gack, M.U.; Farha, S.; Zein, J.; Comhair, S.; et al. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biol. 2020, 18, e3000970. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Huang, S.; Meng, X.; Zhang, J.; Yu, S.; Li, J.; Shi, M.; Fan, H.; Zhao, Y. Mechanism of Phosgene-Induced Acute Lung Injury and Treatment Strategy. Int. J. Mol. Sci. 2021, 22, 10933. https://doi.org/10.3390/ijms222010933
Lu Q, Huang S, Meng X, Zhang J, Yu S, Li J, Shi M, Fan H, Zhao Y. Mechanism of Phosgene-Induced Acute Lung Injury and Treatment Strategy. International Journal of Molecular Sciences. 2021; 22(20):10933. https://doi.org/10.3390/ijms222010933
Chicago/Turabian StyleLu, Qianying, Siyu Huang, Xiangyan Meng, Jianfeng Zhang, Sifan Yu, Junfeng Li, Mingyu Shi, Haojun Fan, and Yanmei Zhao. 2021. "Mechanism of Phosgene-Induced Acute Lung Injury and Treatment Strategy" International Journal of Molecular Sciences 22, no. 20: 10933. https://doi.org/10.3390/ijms222010933
APA StyleLu, Q., Huang, S., Meng, X., Zhang, J., Yu, S., Li, J., Shi, M., Fan, H., & Zhao, Y. (2021). Mechanism of Phosgene-Induced Acute Lung Injury and Treatment Strategy. International Journal of Molecular Sciences, 22(20), 10933. https://doi.org/10.3390/ijms222010933





