Therapeutic Potential and Main Methods of Obtaining Selenium Nanoparticles
Abstract
1. Introduction
2. The Main Methods of Obtaining Selenium Nanoparticles
3. The Role of SeNP in Medicine and Human Health
3.1. Role of SeNP in the Immune System
| Cellular Effects | Ref. | |
|---|---|---|
| Immune cells | ↑ Ca2+-mobilization, ROS, NO-release, IL-2 receptor, lymphocytes proliferation, production of TNFα, IL-1β and IL-6, NKG2D, CD16 ↓ PD-1, | [38,41,48,56] |
| Antibacterial | ↑ ROS, DNA damage, ↓free intracellular thiol, enzyme activity | [45,46,47] |
| Anti-tuberculosis | ↑ PI3K/Akt/mTOR | [57,58] |
| Anti-autoimmune skin diseases | ↑ IL-1β, IL-6, IL-17, IL-22, TGF, MAPK, STAT ↓ formation of plaques, erythema, desquamation, Ki67, PCNA, cyclin-D1, mTOR | [59,60,61,62] |
| Anti-fibrosis | ↑ membrane stabilizing capacity, free radical scavenging activity, antioxidative potential and anti-inflammatory action ↓ TGF-β1, inflammation and alveolitis, infiltration of monocytes, granulocytes, leukocytes | [63,64] |
3.2. Role of SeNP in Neurological Diseases
3.3. The Role of SeNP in Oncology
Molecular Mechanisms of Anticancer Effects of SeNPs
3.4. SeNP and Diabetes Mellitus
3.5. Role of SeNP in Oxidative Stress, Bone Health, and Inflammation
3.6. Future Perspectives
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoffaneller, R.; Morse, N.L. A Review of Dietary Selenium Intake and Selenium Status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef]
- Tutel’ian, V.A. Norms of physiological requirements in energy and nutrients in various groups of population in Russian Federation. Vopr. Pitan. 2009, 78, 4–15. (In Russian) [Google Scholar] [PubMed]
- Tretyak, L.N.; Gerasimov, E.M. Specificity of the Effect of Selenium on the Human Body and Animals (In Relation to the Problem of Creating Selenium-Containing Foods); Bulletin of the Orenburg State University: Orenburg, Russia, 2007; p. 12. [Google Scholar]
- Hart, W.E.; Marczak, S.P.; Kneller, A.R.; French, R.A.; Morris, D.L. The abilities of selenium dioxide and selenite ion to coordinate DNAbound metal ions and decrease oxidative DNA damage. J. Inorg. Biochem. 2013, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ramoutar, R.R.; Brumaghim, J.L. Effects of inorganic selenium compounds on oxidative DNA damage. J. Inorg. Biochem. 2007, 101, 1028–1035. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A. The main cytotoxic effects of methylseleninic acid on various cancer cells. Int. J. Mol. Sci. 2021, 22, 6614. [Google Scholar] [CrossRef]
- Guan, B.; Yan, R.; Li, R.; Zhang, X. Selenium as a pleiotropic agent for medical discovery and drug delivery. Int. J. Nanomed. 2018, 13, 7473–7490. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Ks, S.D.; Santhiya, R.; Rajeshkumar, S.; Venkat Kumar, S. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B Biointerfaces 2018, 170, 280–292. [Google Scholar] [CrossRef]
- Selmani, A.; Ulm, L.; Kasemets, K.; Kurvet, I.; Erceg, I.; Barbir, R.; Pem, B.; Santini, P.; Marion, I.D.; Vinković, T.; et al. Stability and toxicity of differently coated selenium nanoparticles under model environmental exposure settings. Chemosphere 2020, 250, 126265. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Muthukumaran, A. A novel one-pot green synthesis of selenium nanoparticles and evaluation of its toxicity in zebrafish embryos. Artif. Cells Nanomed. Biotechnol. 2016, 44, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wu, Z.; Ma, Y.; Jian, W.; Xiong, H.; Zhou, L. Preparation and growth-promoting effect of selenium nanoparticles capped by polysaccharide-protein complexes on tilapia. J. Sci. Food. Agric. 2021, 101, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Loeschner, K.; Hadrup, N.; Hansen, M.; Pereira, S.A.; Gammelgaard, B.; Møller, L.H.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Absorption, distribution, metabolism and excretion of selenium following oral administration of elemental selenium nanoparticles or selenite in rats. Metallomics 2014, 6, 330–337. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, S.; Liu, Z.; Cheng, C.; Li, H.; Wang, M. Toxicity of selenium nanoparticles in male Sprague-Dawley rats at supranutritional and nonlethal levels. Life Sci. 2014, 115, 44–51. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chauhan, P.; Kumar, R.; Bhasin, K.K. Toxicological responses of surfactant functionalized selenium nanoparticles: A quantitative multi-assay approach. Sci. Total Environ. 2018, 643, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Ramoutar, R.R.; Brumaghim, J.L. Antioxidant and anticancer properties and mechanisms of inorganic selenium, oxo-sulfur, and oxo-selenium compounds. Cell. Biochem. Biophys. 2010, 58, 1–23. [Google Scholar] [CrossRef]
- Sieja, K.; Talerczyk, M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol. Oncol. 2004, 93, 320–327. [Google Scholar] [CrossRef]
- Davy, T.; Castellano, S. The genomics of selenium: Its past, present and future. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2427–2432. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, D. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox (Review). Mol. Med. Rep. 2012, 5, 299–304. [Google Scholar]
- Quintana, M.; Haro-Poniatowski, E.; Morales, J.; Batina, N. Synthesis of nanoparticles by pulsed laser ablation. Appl. Surf. Sci. 2002, 195, 175–186. [Google Scholar] [CrossRef]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Arellano-Jimenez, M.J.; Webster, T.J.; Larese-Casanova, P.; Nash, K.L. Anti-bacterial selenium nanoparticles produced by UV/VIS/NIR pulsed nanosecond laser ablation in liquids. Laser Phys. Lett. 2014, 12, 016003. [Google Scholar] [CrossRef]
- Hou, J.Y.; Ai, S.Y.; Shi, W.J. Preparation and Characterization of nanoSe/silk Fibroin Colloids. Chem. Res. Chin. Univ. 2011, 27, 158–160. [Google Scholar]
- Questera, K.; Avalos-Borjab, M.; Castro-Longoria, E. Biosynthesis and microscopic study of metallic nanoparticles. Micron 2013, 54, 1–27. [Google Scholar] [CrossRef]
- Fardsadegh, B.; Jafarizadeh-Malmiri, H. Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their In vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process Synth. 2019, 8, 399–407. [Google Scholar] [CrossRef]
- Ahmadi, O.; Jafarizadeh-Malmiri, H.; Jodeiri, N. Eco-friendly microwave-enhanced green synthesis of silver nanoparticles using Aloe vera leaf extract and their physico-chemical and antibacterial studies. Green Process. Synth. 2018, 7, 231–240. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Sendón, R.; Sanches-Silva, A. Aloe vera: Ancient knowledge with new frontiers. Trends Food Sci. Technol. 2017, 61, 94–102. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Bhavesh, R.; Park, J.; Ganbold, B.; Nam, J.S.; Lee, S.S. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules 2014, 19, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Anu, K.; Singaravelu, G.; Murugan, K.; Benelli, G. Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): Biophysical characterization and cytotoxicity on vero cells. J. Clust. Sci. 2017, 28, 551–563. [Google Scholar] [CrossRef]
- Krishnan, M.; Ranganathan, K.; Maadhu, P.; Thangavelu, P.; Kundan, S.; Arjunan, N. Leaf extract of Dillenia indica as a source of selenium nanoparticles with larvicidal and antimicrobial potential toward vector mosquitoes and pathogenic microbes. Coatings 2020, 10, 626. [Google Scholar] [CrossRef]
- Sawant, V.J.; Sawant, V.J. Biogenic capped selenium nano rods as naked eye and selective hydrogen peroxide spectrometric sensor. Sens. Bio-Sens. Res. 2020, 27, 100314. [Google Scholar] [CrossRef]
- Fan, D.; Li, L.; Li, Z.; Zhang, Y.; Ma, X.; Wu, L.; Zhang, H.; Guo, F. Biosynthesis of selenium nanoparticles and their protective, antioxidative effects in streptozotocin induced diabetic rats. Sci. Technol. Adv. Mater. 2020, 21, 505–514. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Nimbalkar, M.S.; Sharma, K.K.K.; Patil, P.S.; Pawar, K.D. Sustainable approach to almond skin mediated synthesis of tunable selenium microstructures for coating cotton fabric to impart specific antibacterial activity. J. Colloid Interface Sci. 2020, 569, 346–357. [Google Scholar] [CrossRef]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium nanoparticles for biomedical applications: From development and characterization to therapeutics. Adv. Healthc. Mater. 2021, 10, e2100598. [Google Scholar] [CrossRef]
- Aizik, G.; Waiskopf, N.; Agbaria, M.; Levi-Kalisman, Y.; Banin, U.; Golomb, G. Delivery of liposomal quantum dots via monocytes for imaging of inflamed tissue. ACS Nano 2017, 11, 3038–3051. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radic. Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Zhao, G.; Sun, H.; Che, H.; Leng, X. Effect of molecular weight of chitosan and its oligosaccharides on antitumor activities of chitosan-selenium nanoparticles. Carbohydr. Polym. 2020, 231, 115689. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; John, P.; Bhatti, A. Biogenic selenium nanoparticles: Potential solution to oxidative stress mediated inflammation in rheumatoid arthritis and associated complications. Nanomaterials 2021, 11, 2005. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Kiremidjian-Schumacher, L.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Selenium supplementation enhances the expression of interleukin 2 receptor subunits and internalization of interleukin 2. Proc. Soc. Exp. Biol. Med. 1993, 202, 295–301. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Ganther, H.E. Reduction of the selenotrisulfide derivative of glutathione to a persulfide analog by glutathione reductase. Biochemistry 1971, 10, 4089–4098. [Google Scholar] [CrossRef]
- Versantvoort, C.H.; Withoff, S.; Broxterman, H.J.; Kuiper, C.M.; Scheper, R.J.; Mulder, N.H.; de Vries, E.G. Resistance-associated factors in human small-cell lung-carcinoma GLC4 sub-lines with increasing adriamycin resistance. Int. J. Cancer 1995, 61, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Scheper, R.J.; Broxterman, H.J.; Scheffer, G.L.; Kaaijk, P.; Dalton, W.S.; van Heijningen, T.H.; van Kalken, C.K.; Slovak, M.L.; de Vries, E.G.; van der Valk, P.; et al. Overexpression of a M(r) 110,000 vesicular protein in non-P-glycoprotein-mediated multidrug resistance. Cancer Res. 1993, 53, 1475–1479. [Google Scholar]
- Björkhem-Bergman, L.; Jönsson, K.; Eriksson, L.C.; Olsson, J.M.; Lehmann, S.; Paul, C.; Björnstedt, M. Drug-resistant human lung cancer cells are more sensitive to selenium cytotoxicity. Effects on thioredoxin reductase and glutathione reductase. Biochem. Pharmacol. 2002, 63, 1875–1884. [Google Scholar] [CrossRef]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked selenium nanoparticles for antibacterial and anticancer treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Husen, A.; Siddiqi, K.S. Plants and microbes assisted selenium nanoparticles: Characterization and application. J. Nanobiotechnol. 2014, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sonkusre, P.; Singh Cameotra, S. Biogenic selenium nanoparticles inhibit Staphylococcus aureus adherence on different surfaces. Colloids Surf. B Biointerfaces 2015, 136, 1051–1057. [Google Scholar] [CrossRef]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.Y.; Wang, Y.Y.; Wang, M.; Yan, J.K. Construction, stability, and enhanced antioxidant activity of pectin-decorated selenium nanoparticles. Colloids Surf. B Biointerfaces 2018, 170, 692–700. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.U.R. Biomedical potential of plant-based selenium nanoparticles: A comprehensive review on therapeutic and mechanistic sspects. Int. J. Nanomed. 2021, 16, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Holden, J.A.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Engineering highly effective antimicrobial selenium nanoparticles through control of particle size. Nanoscale 2019, 11, 14937–14951. [Google Scholar] [CrossRef]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Mimun, L.C.; Mendoza-Cruz, R.; Larese-Casanova, P.; Webster, T.J.; Nash, K.L. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016, 11, 3731–3736. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Reynolds, E.C.; Pantarat, N.; Biswas, D.P.; O’Connor, A.J. Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology 2016, 27, 045101. [Google Scholar] [CrossRef]
- Cremonini, E.; Boaretti, M.; Vandecandelaere, I.; Zonaro, E.; Coenye, T.; Lleo, M.M.; Lampis, S.; Vallini, G. Biogenic selenium nanoparticles synthesized by Stenotrophomonas maltophilia SeITE02 loose antibacterial and antibiofilm efficacy as a result of the progressive alteration of their organic coating layer. Microb. Biotechnol. 2018, 11, 1037–1047. [Google Scholar] [CrossRef]
- Shakibaie, M.; Forootanfar, H.; Golkari, Y.; Mohammadi-Khorsand, T.; Shakibaie, M.R. Anti-biofilm activity of biogenic selenium nanoparticles and selenium dioxide against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus mirabilis. J. Trace Elem. Med. Biol. 2015, 29, 235–241. [Google Scholar] [CrossRef]
- Strahl, H.; Hamoen, L.W. Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. USA 2010, 107, 12281–12286. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, J.; Xu, J.F.; Pi, J. The advancing of selenium nanoparticles against infectious diseases. Front. Pharmacol. 2021, 12, 682284. [Google Scholar] [CrossRef]
- Estevez, H.; Palacios, A.; Gil, D.; Anguita, J.; Vallet-Regi, M.; González, B.; Prados-Rosales, R.; Luque-Garcia, J.L. Antimycobacterial Effect of Selenium Nanoparticles on Mycobacterium tuberculosis. Front. Microbiol. 2020, 11, 800. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Liao, G.; Tang, J.; Wang, D.; Zuo, H.; Zhang, Q.; Liu, Y.; Xiong, H. Selenium nanoparticles (SeNP) have potent antitumor activity against prostate cancer cells through the upregulation of miR-16. World J. Surg. Oncol. 2020, 18, 81. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The diagnosis and treatment of prostate cancer: A review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Tugarova, A.V.; Mamchenkova, P.V.; Dyatlova, Y.A.; Kamnev, A.A. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 192, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Winkler, H.C.; Suter, M.; Naegeli, H. Critical review of the safety assessment of nano-structured silica additives in food. J. Nanobiotechnology 2016, 14, 44. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X. Protective effects of selenium nanoparticles on oxidative stress and antioxidant enzymes activities induced by microcystins in the liver of mice. Acta Hydrobiol. Sin. 2010, 34, 679–683. [Google Scholar] [CrossRef]
- Mohammed, E.; Safwat, G. Assessment of the ameliorative role of selenium nanoparticles on the oxidative stress of acetaminophen in some tissues of male albino rats. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 80–85. [Google Scholar] [CrossRef]
- Shahabi, R.; Anissian, A.; Javadmoosavi, S.A.; Nasirinezhad, F. Protective and anti-inflammatory effect of selenium nano-particles against bleomycin-induced pulmonary injury in male rats. Drug. Chem. Toxicol. 2021, 44, 92–100. [Google Scholar] [CrossRef]
- Zaafan, M.A.; Zaki, H.F.; El-Brairy, A.I.; Kenawy, S.A. Pyrrolidinedithiocarbamate attenuates bleomycin-induced pulmonary fibrosis in rats: Modulation of oxidative stress, fibrosis, and inflammatory parameters. Exp. Lung Res. 2016, 42, 408–416. [Google Scholar] [CrossRef]
- El-Ghazaly, M.A.; Fadel, N.; Rashed, E.; El-Batal, A.; Kenawy, S.A. Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can. J. Physiol. Pharmacol. 2017, 95, 101–110. [Google Scholar] [CrossRef]
- Yu, W.; Guo, F.; Song, X. Effects and mechanisms of pirfenidone, prednisone and acetylcysteine on pulmonary fibrosis in rat idiopathic pulmonary fibrosis models. Pharm. Biol. 2017, 55, 450–455. [Google Scholar] [CrossRef]
- Miroliaee, A.E.; Esmaily, H.; Vaziri-Bami, A.; Baeeri, M.; Shahverdi, A.R.; Abdollahi, M. Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol. Mech. Methods 2011, 21, 200–208. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Guo, M.; Xia, Y.; Zhao, M.; Wang, C.; Xu, T.; Chen, T.; Zhu, B. Inhibitory activity of selenium nanoparticles functionalized with oseltamivir on H1N1 influenza virus. Int. J. Nanomed. 2017, 12, 5733–5743. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Gong, G.; Xia, Y.; Wang, C.; Chen, Y.; Hua, L.; Zhong, J.; Tang, Y.; Liu, X.; et al. Restriction of H1N1 influenza virus infection by selenium nanoparticles loaded with ribavirin via resisting caspase-3 apoptotic pathway. Int. J. Nanomed. 2018, 13, 5787–5797. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, Y.; Xu, T.; Guo, M.; Wang, C.; Zhao, M.; Chen, H.; Kuang, J.; Li, W.; Zhang, Y.; et al. Inhibition of Enterovirus 71 by Selenium Nanoparticles Loaded with siRNA through Bax Signaling Pathways. ACS Omega 2020, 5, 12495–12500. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.K.; Du, P.; Ignacio, C.; Mehta, S.; Newton, I.G.; Steinmetz, N.F. Virus-like particles as positive controls for COVID-19 RT-LAMP diagnostic assays. Biomacromolecules 2021, 22, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Z.; Hu, H.; Zhou, Q.; Liu, W.; Li, X.; Liu, Z.; Wang, Y.; Ma, Y. A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab Chip 2020, 20, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of metal nanoparticles and their pharmacological applications: Present status and application prospects. J. Nanostruct. Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef]
- Chintamani, R.B.; Salunkhe, K.S.; Chavan, M. Emerging use of green synthesis silver nanoparticle: An updated review. Int. J. Pharm. Sci. Res. 2018, 9, 4029–4055. [Google Scholar]
- Abdraboh, M.E.; Essa, Z.S.; Abdelrazzak, A.B.; El-Far, Y.M.; Elsherbini, Y.; El-Zayat, M.M.; Ali, D.A. Radio-sensitizing effect of a cocktail of phytochemicals on HepG2 cell proliferation, motility and survival. Biomed. Pharmacother. 2020, 131, 110620. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, M.J.; Ha, E.; Chung, J.H. Apoptotic effect of hesperidin through CASP3 activation in human colon cancer cells, SNU-C4. Phytomedicine 2008, 15, 147–151. [Google Scholar] [CrossRef]
- El-Zayat, M.M.; Eraqi, M.M.; Alrefai, H.; El-Khateeb, A.Y.; Ibrahim, M.A.; Aljohani, H.M.; Aljohani, M.M.; Elshaer, M.M. The Antimicrobial, antioxidant, and anticancer activity of greenly synthesized selenium and zinc composite nanoparticles using Ephedra aphylla extract. Biomolecules 2021, 11, 470. [Google Scholar] [CrossRef]
- Zhuang, C.; Yao, D.; Li, F.; Zhang, K.; Feng, Q.; Gan, Z. Study of micron-thick MgB2 films on niobium substrates. Supercond. Sci. Technol. 2007, 20, 287–291. [Google Scholar] [CrossRef]
- Yang, L.; Wang, W.; Chen, J.; Wang, N.; Zheng, G. A comparative study of resveratrol and resveratrol-functional selenium nanoparticles: Inhibiting amyloid β aggregation and reactive oxygen species formation properties. J. Biomed. Mater. Res. A 2018, 106, 3034–3041. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, J.; Liu, P.; Ji, D.; Zhang, L.; Zhang, M.; Li, Y.; Xiao, Y. Preparation and in vitro evaluation of multi-target-directed selenium-chondroitin sulfate nanoparticles in protecting against the Alzheimer’s disease. Int. J. Biol. Macromol. 2020, 142, 265–276. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Liu, C.; Song, C.; Li, P.; Yin, F.; Xiao, Y.; Jiang, W.; Zong, A. Protective effects of low molecular weight chondroitin sulfate on amyloid beta (Aβ)-induced damage in vitro and in vivo. Neuroscience 2015, 305, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Bai, R.; Li, Y.F.; Wang, L.; Chen, C. Selenium nanoparticles as an efficient nanomedicine for the therapy of Huntington’s disease. ACS Appl. Mater. Interfaces 2019, 11, 34725–34735. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatr. 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Tatton, W.G.; Eastman, M.J.; Bedingham, W.; Verrier, M.C.; Bruce, I.C. Defective utilization of sensory input as the basis for bradykinesia, rigidity and decreased movement repertoire in Parkinson’s disease: A hypothesis. Can. J. Neurol. Sci. 1984, 11, 136–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fedorova, T.N.; Logvinenko, A.A.; Poleshchuk, V.V.; Illarioshkin, S.N. The state of systemic oxidative stress during Parkinson’s disease. Neurochem. J. 2017, 11, 340–345. [Google Scholar] [CrossRef]
- Exner, N.; Lutz, A.K.; Haass, C.; Winklhofer, K.F. Mitochondrial dysfunction in Parkinson’s disease: Molecular mechanisms and pathophysiological consequences. EMBO J. 2012, 31, 3038–3062. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Zeng, C.; Okyere, S.K.; Chen, Z.; Hu, Y. Glycine nano-selenium prevents brain oxidative stress and neurobehavioral abnormalities caused by MPTP in rats. J. Trace Elem. Med. Biol. 2021, 64, 126680. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, B.Y.; Kho, A.R.; Jeong, J.H.; Hong, D.K.; Lee, S.H.; Lee, S.Y.; Lee, M.W.; Song, H.K.; Choi, H.C.; et al. Protective effects of protocatechuic acid on seizure-induced neuronal death. Int. J. Mol. Sci. 2018, 19, 187. [Google Scholar] [CrossRef]
- Demirci, K.; Naziroglu, M.; Ovey, I.S.; Balaban, H. Selenium attenuates apoptosis, inflammation and oxidative stress in the blood and brain of aged rats with scopolamine-induced dementia. Metab. Brain Dis. 2017, 32, 321–329. [Google Scholar] [CrossRef]
- Yuan, X.; Fu, Z.; Ji, P.; Guo, L.; Al-Ghamdy, A.O.; Alkandiri, A.; Habotta, O.A.; Abdel Moneim, A.E.; Kassab, R.B. Selenium nanoparticles pre-treatment reverse behavioral, oxidative damage, neuronal loss and neurochemical alterations in pentylenetetrazole-induced epileptic seizures in mice. Int. J. Nanomed. 2020, 15, 6339–6353. [Google Scholar] [CrossRef]
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239. [Google Scholar] [CrossRef]
- Mehta, S.L.; Kumari, S.; Mendelev, N.; Li, P.A. Selenium preserves mitochondrial function, stimulates mitochondrial biogenesis, and reduces infarct volume after focal cerebral ischemia. BMC Neurosci. 2012, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.R.; Wright, D.K.; Zheng, P.; Stuchbery, R.; Liu, S.J.; Sashindranath, M.; Medcalf, R.L.; Johnston, L.A.; Hovens, C.M.; Jones, N.C.; et al. Sodium selenate reduces hyper-phosphorylated tau and improves outcomes after traumatic brain injury. Brain 2015, 138, 1297–1313. [Google Scholar] [CrossRef]
- Li, C.; Sun, T.; Jiang, C. Recent advances in nanomedicines for the treatment of ischemic stroke. Acta. Pharm. Sin. B 2021, 11, 1767–1788. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Abou Zaid, O.A.R.; El-Sonbaty, S.M.; Barakat, W.M. Ameliorative effect of selenium nanoparticles and ferulic acid on acrylamide-induced neurotoxicity in rats. Ann. Med. Biomed. Sci. 2017, 3, 35–45. [Google Scholar]
- Mitchell, J.H.; Nicol, F.; Beckett, G.J.; Arthur, J.R. Selenoprotein expression and brain development in preweanling selenium-and iodine deficient rats. J. Mol. Endocrinol. 1998, 20, 203–210. [Google Scholar] [CrossRef]
- Gaidin, S.G.; Turovskaya, M.V.; Gavrish, M.S.; Babaev, A.A.; Mal’tseva, V.N.; Blinova, E.V.; Turovsky, E.A. The selective BDNF overexpression in neurons protects neuroglial networks against OGD and glutamate-induced excitotoxicity. Int. J. Neurosci. 2020, 130, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G.; Goltyaev, M.V.; Mal’tseva, V.N.; Turovsky, E.A.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V. Mechanisms of the cytotoxic effect of selenium nanoparticles in different human cancer cell lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef] [PubMed]
- Rocca, C.; Pasqua, T.; Boukhzar, L.; Anouar, Y.; Angelone, T. Progress in the emerging role of selenoproteins in cardiovascular disease: Focus on endoplasmic reticulum–resident selenoproteins. Cell Mol. Life Sci. 2019, 76, 3969–3985. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Liu, L.; Tao, W.; Wang, G.; Sun, W.; Pei, X.; Xiao, Z.; Jin, Y.; Wang, M. Prooxidation and cytotoxicity of selenium nanoparticles at nonlethal level in Sprague-Dawley rats and buffalo rat liver cells. Oxid. Med. Cell. Longev. 2020, 14, 7680276. [Google Scholar] [CrossRef] [PubMed]
- Varlamova, E.G. Protein-protein interactions of ER-resident selenoproteins with their physiological partners. Biochimie 2020, 171–172, 197–204. [Google Scholar] [CrossRef]
- Kitts, D.; Wijewickreme, A.; Hu, C. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000, 203, 1–10. [Google Scholar] [CrossRef]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evalution of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar]
- Egorova, E.M.; Revina, A.A. Synthesis of metallic nanoparticles in reverse micelles in the presence of quercetin. Colloids Surf. A Physicochem. Eng. Asp. 2000, 168, 87–96. [Google Scholar] [CrossRef]
- El-Refai, A.A.; Ghoniem, G.A.; El-Khateeb, A.Y.; Hasaan, M.M. Eco-friendly synthesis of metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. J. Nanostruct. Chem. 2018, 8, 71–81. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; De Capriles, C.H.; Perez, C.; Colella, M.; Olaizola, C.; Ontiveros, Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Yu, Q.; Yang, L.; Sun, D.; Zhou, Y.; Liu, J. Epigallocatechin-3-gallate (EGCG)-stabilized selenium nanoparticles coated with Tet-1 peptide to reduce amyloid-β aggregation and cytotoxicity. ACS Appl. Mater. Interfaces 2014, 6, 8475–8487. [Google Scholar] [CrossRef] [PubMed]
- Sathiyamoorthy, P.; Lugasi-Evgi, H.; Van-Damme, P.; Abu-Rabia, A.; Gopas, J.; Golan-Goldhirsh, A. Larvicidal activity in desert plants of the Negev and Bedouin market plant products. Int. J. Pharm. 1997, 35, 265–273. [Google Scholar] [CrossRef]
- Al-Hussaini, R.; Mahasneh, A.M. Microbial growth and quorum sensing antagonist activities of herbal plants extracts. Molecules 2009, 14, 3425–3435. [Google Scholar] [CrossRef]
- EL-Shahaby, O.A.; Reicha, F.M.; Nabil Aboushadi, M.M.; El-Zayat, M.M. Green synthesis and biological assessments of silver nanoparticles using the plant extract of Crataegus sinaica Boiss. Fruits. Prog. Chem. Biochem. Res. 2020, 3, 105–113. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Fagét, K.Y.; Huang, X.; Tanzi, R.E.; Bush, A.I. Metal chelation as a potential therapy for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000, 920, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, U.; Kamran, Z.; Raza, I.; Ahmad, S.; Babar, W.; Riaz, M.H.; Iqbal, Z. Role of selenium in male reproduction—A review. Anim. Reprod. Sci. 2014, 146, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. Dietary selenium and human health. Nutrients 2017, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Sorribas, A.; Howes, M.J. Natural products as a source of Alzheimer’s drug leads. Chem. Inform. 2011, 42, 48–77. [Google Scholar] [CrossRef]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Dreseswerringloer, U.; Zhao, H.; Davies, P.; Marambaud, P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008, 9, 1–5. [Google Scholar] [CrossRef]
- Kwok, J.C.F.; Carulli, D.; Fawcett, J.W. In vitro modeling of perineuronal nets: Hyaluronan synthase and link protein are necessary for their formation and integrity. J. Neurochem. 2010, 114, 1447–1459. [Google Scholar] [CrossRef]
- Kwok, J.C.F.; Warren, P.; Fawcett, J.W. Chondroitin sulfate: A key molecule in the brain matrix. Int. J. Biochem. Cell. Biol. 2012, 44, 582–586. [Google Scholar] [CrossRef]
- Mclaughlin, R.W.; De Stigter, J.K.; Sikkink, L.A.; Baden, E.M.; Ramirez-Alvarado, M. The effects of sodium sulfate, glycosaminoglycans, and Congo red on the structure, stability, and amyloid formation of an immunoglobulin lightchain protein. Protein Sci. 2006, 15, 1710–1722. [Google Scholar] [CrossRef]
- Solovyev, N.; Drobyshev, E.; Bjorklund, G.; Dubrovskii, Y.; Lysiuk, R.; Rayman, M.P. Selenium, selenoprotein P, and Alzheimer’s disease: Is there a link? Free Radic. Biol. Med. 2018, 127, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Hald, A.; Lotharius, J. Oxidative stress and inflammation in Parkinson’s disease: Is there a causal link? Exp. Neurol. 2005, 193, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, W.; Lu, Y. The causes of new-onset epilepsy and seizures in the elderly. Neuropsychiatr. Dis. Treat. 2016, 12, 1425–1434. [Google Scholar] [CrossRef]
- de Kinderen, R.J.; Evers, S.M.; Rinkens, R.; Postulart, D.; Vader, C.I.; Majoie, M.H.; Aldenkamp, A.P. Side-effects of antiepileptic drugs: The economic burden. Seizure 2014, 23, 184–190. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, J.; Han, B.; Huang, R.; Zhang, A.; Xia, Z.; Chang, H.; Chao, J.; Yao, H. Neuronal nitric oxide synthase contributes to PTZ kindling epilepsy-induced hippocampal endoplasmic reticulum stress and oxidative damage. Front. Cell Neurosci. 2017, 11, 377. [Google Scholar] [CrossRef]
- Gaidin, S.G.; Turovskaya, M.V.; Mal’tseva, V.N.; Zinchenko, V.P.; Blinova, E.V.; Turovsky, E.A. A complex neuroprotective effect of alpha-2-adrenergic receptor agonists in a model of cerebral ischemia–reoxygenation in vitro. Biochem. (Mosc.) Suppl. Ser. A: Membr. Cell Biol. 2019, 4, 319–333. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium anticancer properties and impact on cellular redox status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Goltyaev, M.V.; Mal’tseva, V.N.; Varlamova, E.G. Expression of ER-resident selenoproteins and activation of cancer cells apoptosis mechanisms under ER-stress conditions caused by methylseleninic acid. Gene 2020, 755, 144884. [Google Scholar] [CrossRef]
- Varlamova, E.G. Participation of selenoproteins localized in the ER in the processes occurring in this organelle and in the regulation of carcinogenesis-associated processes. J. Trace Elem. Med. Biol. 2018, 48, 172–180. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The epidemiology of selenium and human cancer. Adv. Cancer Res. 2017, 136, 1–48. [Google Scholar]
- Kipp, A.P. Selenium in colorectal and differentiated thyroid cancer. Hormones 2020, 19, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium exposure and cancer risk: An updated meta-analysis and meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef] [PubMed]
- Murdolo, G.; Bartolini, D.; Tortoioli, C.; Piroddi, M.; Torquato, P.; Galli, F. Selenium and cancer stem cells. Adv. Cancer Res. 2017, 136, 235–257. [Google Scholar]
- Raganová, A.; Gažová, A.; Tomo, I.; Kristová, V. Selenium in the prevention and subsidiary therapy of cancer of soft tissues. Ceska Slov. Farm. 2018, 67, 66–70. [Google Scholar]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Whanger, P.D. Selenium and its relationship to cancer: An update dagger. Br. J. Nutr. 2004, 91, 11–28. [Google Scholar] [CrossRef]
- Drutel, A.; Archambeaud, F.; Caron, P. Selenium and the thyroid gland: More good news for clinicians. Clin. Endocrinol. 2013, 78, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Prabhu, K.S.; Mastro, A.M. Is selenium a potential treatment for cancer metastasis? Nutrients 2013, 5, 1149–1168. [Google Scholar] [CrossRef]
- Olm, E.; Fernandes, A.P.; Hebert, C.; Rundlöf, A.K.; Larsen, E.H.; Danielsson, O.; Björnstedt, M. Extracellular thiol-assisted selenium uptake dependent on the x(c)- cystine transporter explains the cancer-specific cytotoxicity of selenite. Proc. Natl. Acad. Sci. USA 2009, 106, 11400–11405. [Google Scholar] [CrossRef]
- Banjac, A.; Perisic, T.; Sato, H.; Seiler, A.; Bannai, S.; Weiss, N.; Kölle, P.; Tschoep, K.; Issels, R.D.; Daniel, P.T.; et al. The cystine/cysteine cycle: A redox cycle regulating susceptibility versus resistance to cell death. Oncogene 2008, 27, 1618–1628. [Google Scholar] [CrossRef]
- Mandal, P.K.; Seiler, A.; Perisic, T.; Kölle, P.; Banjac Canak, A.; Förster, H.; Weiss, N.; Kremmer, E.; Lieberman, M.W.; Bannai, S.; et al. System x(c)- and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J. Biol. Chem. 2010, 285, 22244–22253. [Google Scholar] [CrossRef]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Sharifiaghdam, M.; Shaabani, E.; Sharifiaghdam, Z.; De Keersmaecker, H.; De Rycke, R.; De Smedt, S.; Faridi-Majidi, R.; Braeckmans, K.; Fraire, J.C. Enhanced siRNA delivery and selective apoptosis induction in H1299 cancer cells by layer-by-layer-assembled Se nanocomplexes: Toward more efficient cancer therapy. Front. Mol. Biosci. 2021, 8, 639184. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wei, X.; Zhang, W.; Xu, A. Effects of selenium nanoparticles combined with radiotherapy on lung cancer cells. Front. Bioeng. Biotechnol. 2020, 8, 598997. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Wong, Y.S.; Chen, T.; Zhang, H.; Liu, C. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials 2011, 32, 9068–9076. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, H.; Niu, Y.; Wu, Y.; Sun, W.; Li, H.; Kong, J.; Ding, K.; Shen, H.M.; Wu, H.; et al. Erratum to: Long non-coding RNA linc00673 regulated non-small cell lung cancer proliferation, migration, invasion and epithelial mesenchymal transition by sponging miR-150-5p. Mol. Cancer 2017, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, L.; Liu, W.; Fan, C.; Zheng, W.; Wong, Y.S.; Chen, T. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials 2013, 34, 7106–7116. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-small cell lung cancer: Epidemiology. Screen. Diagn. Treat. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Cai, W.; Mastrandrea, N.; Tham, K.; Monks, T.; Lau, S. Pentoxifylline induces GSK-3β−independent proteasomal degradation of cyclin D1 and arrests renal cancer cells in the G1 phase (616.5). Int. J. Biochem. Cell Biol. 2014, 54, 223–235. [Google Scholar]
- Zhu, Y.; Xia, Y.; Niu, H.; Chen, Y. MiR-16 induced the suppression of cell apoptosis while promote proliferation in esophageal squamous cell carcinoma. Cell Physiol. Biochem. 2014, 33, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.K.; Zhao, G.Y.; Tian, L.Y.; Liu, L.; Yan, K.; Ma, Y.L.; Ji, Z.W.; Li, X.X.; Han, K.; Gao, J.; et al. miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncol. Rep. 2012, 28, 1764–1770. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, G.; Guo, M.; Xu, T.; Chen, H.; Lin, Z.; Li, Y.; Chen, Y.; Zhu, B.; Liu, H.; et al. Silencing KLK12 expression via RGDfC-decorated selenium nanoparticles for the treatment of colorectal cancer in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110594. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Varlamova, E.G. Mechanism of Ca2+-dependent pro-apoptotic action of selenium nanoparticles, mediated by activation of Cx43 hemichannels. Biology 2021, 10, 743. [Google Scholar] [CrossRef]
- Sonkusre, P.; Cameotra, S.S. Biogenic selenium nanoparticles induce ROS mediated necroptosis in PC-3 cancer cells through TNF activation. J. Nanobiotechnol. 2017, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Yuan, Q.; Zhu, H.; Li, Y.; Guo, Q.; Wang, Q.; Bi, X.; Gao, X. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials 2011, 32, 6515–6522. [Google Scholar] [CrossRef]
- Gao, F.; Yuan, Q.; Gao, L.; Cai, P.; Zhu, H.; Liu, R.; Wang, Y.; Wei, Y.; Huang, G.; Liang, J.; et al. Cytotoxicity and therapeutic effect of irinotecan combined with selenium nanoparticles. Biomaterials 2014, 35, 8854–8866. [Google Scholar] [CrossRef] [PubMed]
- Barbanente, A.; Nadar, R.A.; Esposti, L.D.; Palazzo, B.; Iafisco, M.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Margiotta, N. Platinum-loaded, selenium-doped hydroxyapatite nanoparticles selectively reduce proliferation of prostate and breast cancer cells co-cultured in the presence of stem cells. J. Mater. Chem. B 2020, 8, 2792–2804. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tang, G.; Wang, C.; Zhong, J.; Chen, Y.; Hua, L.; Li, Y.; Liu, H.; Zhu, B. Functionalized selenium nanoparticles for targeted siRNA delivery silence Derlin1 and promote antitumor efficacy against cervical cancer. Drug Deliv. 2020, 27, 15–25. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, G.; Chen, Y.; Wang, C.; Guo, M.; Xu, T.; Zhao, M.; Zhou, Y. Tumor-targeted delivery of siRNA to silence Sox2 gene expression enhances therapeutic response in hepatocellular carcinoma. Bioact. Mater. 2020, 6, 1330–1340. [Google Scholar] [CrossRef]
- Li, X.S.; He, X.L. Kallikrein 12 downregulation reduces AGS gastric cancer cell proliferation and migration. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Wang, C.; Xia, Y.; Huo, S.; Shou, D.; Mei, Q.; Tang, W.; Li, Y.; Liu, H.; Zhou, Y.; Zhu, B. Silencing of MEF2D by siRNA loaded selenium nanoparticles for ovarian cancer therapy. Int. J. Nanomed. 2020, 15, 9759–9770. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, Y.; Hua, L.; Zhao, M.; Xu, T.; Wang, C.; Li, Y.; Zhu, B. Functionalized selenium nanoparticles for targeted delivery of doxorubicin to improve non-small-cell lung cancer therapy. Int. J. Nanomed. 2018, 13, 6929–6939. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, M.; Xu, T.; Li, Y.; Wang, C.; Lin, Z.; Zhao, M.; Zhu, B. siRNA-loaded selenium nanoparticle modified with hyaluronic acid for enhanced hepatocellular carcinoma therapy. Int. J. Nanomed. 2018, 13, 1539–1552. [Google Scholar] [CrossRef]
- Sekerdag, E.; Solaroglu, I.; Gursoy-Ozdemir, Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr. Neuropharmacol. 2018, 16, 1396–1415. [Google Scholar] [CrossRef]
- Lindholm, D.; Mercer, E.A.; Yu, L.Y.; Chen, Y.; Kukkonen, J.; Korhonen, L.; Arumäe, U. Neuronal apoptosis inhibitory protein: Structural requirements for hippocalcin binding andeffects on survival of NGF-dependent sympathetic neurons. Biochim. Biophys. Acta 2002, 1600, 138–147. [Google Scholar] [CrossRef]
- Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001, 15, 2922–2933. [Google Scholar] [PubMed]
- Liu, L.; Liu, C.; Lu, Y.; Liu, L.; Jiang, Y. ER stress related factor ATF6 and caspase-12 trigger apoptosis in neonatal hypoxic–ischemic encephalopathy. Int. J. Clin. Exp. Pathol. 2015, 8, 6960–6966. [Google Scholar]
- Murata, T.; Shinozuka, Y.; Obata, Y.; Yokoyama, K.K. Phosphorylation of two eukaryotic transcription factors, Jun dimerization protein 2 and activation transcription factor 2, in Escherichia coli by Jun N–terminal kinase 1. Anal. Biochem. 2008, 376, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Najafian, B.; Mauer, M. Progression of diabetic nephropathy in type 1 diabetic patients. Diabetes Res. Clin. Pract. 2009, 83, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, Y.S.; Sun, L.; Xie, P.; Liu, F.Y.; Chen, S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 2011, 6, 395–423. [Google Scholar] [CrossRef]
- Ichinose, K.; Maeshima, Y.; Yamamoto, Y.; Kitayama, H.; Takazawa, Y.; Hirokoshi, K.; Sugiyama, H.; Yamasaki, Y.; Eguchi, K.; Makino, H. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes 2005, 54, 2891–2903. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, Y.S.; Wada, J.; Sun, L.; Xie, P.; Wallner, E.I.; Chen, S.; Chugh, S.; Danesh, F.R. Diabetic nephropathy: Mechanisms of renal disease progression. Exp. Biol. Med. 2008, 233, 4–11. [Google Scholar] [CrossRef]
- Jiang, T.; Huang, Z.; Lin, Y.; Zhang, Z.; Fang, D.; Zhang, D.D. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 2010, 59, 850–860. [Google Scholar] [CrossRef]
- Kumar, G.S.; Kulkarni, A.; Khurana, A.; Kaur, J.; Tikoo, K. Selenium nanoparticles involve HSP-70 and SIRT1 in preventing the progression of type 1 diabetic nephropathy. Chem. Biol. Interact. 2014, 223, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Gao, X.Y.; Zhang, L.D.; Bao, Y.P. Biological effects of a nano red elemental selenium. Biofactors 2001, 15, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, J.; Rao, S.; Su, Y.; Yang, Y. Antihyperglycemic, antihyperlipidemic and antioxidant activities of polysaccharides from Catathelasma ventricosum in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2013, 57, 39–45. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, S.; Liu, Y.; Wu, W.; Shen, Y.; Zhang, L.; Li, C.; Chen, H.; Liu, A.; Shen, L.; et al. Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol. 2018, 114, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalek, S.A.; Balbaa, M. Synergistic effect of nano-selenium and metformin on type 2 diabetic rat model: Diabetic complications alleviation through insulin sensitivity, oxidative mediators and inflammatory markers. PLoS ONE 2019, 14, 0220779. [Google Scholar] [CrossRef]
- Hwang, D.; Seo, S.; Kim, Y.; Kim, C.; Shim, S.; Jee, S.; Lee, S.; Jang, M.; Kim, M.; Yim, S.; et al. Selenium acts as an insulin-like molecule for the down-regulation of diabetic symptoms via endoplasmic reticulum stress and insulin signalling proteins in diabetes-induced non-obese diabetic mice. J. Biosci. 2007, 32, 723–735. [Google Scholar] [CrossRef]
- Ezaki, O. The insulin-like effects of selenate in rat adipocytes. J. Biol. Chem. 1990, 265, 1124–1128. [Google Scholar] [CrossRef]
- Uyoyo Ukperoro, J.; Offiah, N.; Idris, T.; Awogoke, D. Antioxidant effect of zinc, selenium and their combination on the liver and kidney of alloxan-induced diabetes in rats. Mediterr. J. Nutr. Metab. 2010, 3, 25–30. [Google Scholar] [CrossRef]
- El-Borady, O.M.; Othman, M.S.; Atallah, H.H.; Abdel Moneim, A.E. Hypoglycemic potential of selenium nanoparticles capped with polyvinyl-pyrrolidone in streptozotocin-induced experimental diabetes in rats. Heliyon 2020, 6, 04045. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Khater, S.I.; Hamed Arisha, A.; Metwally, M.M.M.; Mostafa-Hedeab, G.; El-Shetry, E.S. Chitosan-stabilized selenium nanoparticles alleviate cardio-hepatic damage in type 2 diabetes mellitus model via regulation of caspase, Bax/Bcl-2, and Fas/FasL-pathway. Gene 2021, 768, 145288. [Google Scholar] [CrossRef] [PubMed]
- Ebokaiwe, A.P.; Okori, S.; Nwankwo, J.O.; Ejike, C.E.C.C.; Osawe, S.O. Selenium nanoparticles and metformin ameliorate streptozotocin-instigated brain oxidative-inflammatory stress and neurobehavioral alterations in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 591–602. [Google Scholar] [CrossRef]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musilek, K. Redox- and non- redoxmetal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Park, D.J.; Koh, P.O. Diabetes aggravates decreases in hippocalcin and parvalbumin expression in focal cerebral ischemia. Neurosci. Lett. 2017, 662, 189–194. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Biofunctionalization of selenium nanoparticles with a polysaccharide from Rosa roxburghii fruit and their protective effect against H2O2-induced apoptosis in INS-1 cells. Food Funct. 2019, 10, 539–553. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food. Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Zrieq, R.; Al-Quraishy, S.; Abdel Moneim, A.E. Selenium Nanoparticles Attenuate Oxidative Stress and Testicular Damage in Streptozotocin-Induced Diabetic Rats. Molecules 2016, 21, 1517. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Bhattacharjee, S.; Daware, A.; Tribedi, P.; Krishnani, K.K.; Minhas, P.S. Selenium Nanoparticles for Stress-Resilient Fish and Livestock. Nanoscale Res. Lett. 2015, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Kondaparthi, P. Selenium nanoparticles: An insight on its Pro-oxidant and antioxidant properties. Front. Nanosci. Nanotechnol. 2019, 6, 1–5. [Google Scholar]
- Kumar, A.; Kumar, S.P. Biogenic selenium nanoparticles for their therapeutic application. Asian J. Pharm. Clin. Res. 2020, 13, 4–9. [Google Scholar]
- Jolly, J.; Rauf, M.A.; Ahmad, Z. Selenium nanoparticles: Small is the new big: Mini review. Open J. Chem. 2020, 6, 013–016. [Google Scholar]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 4. [Google Scholar] [CrossRef]
- Ren, S.-X.; Zhang, B.; Lin, Y.; Ma, D.-S.; Yan, H. Selenium nanoparticles dispersed in phytochemical exert anti-inflammatory activity by modulating catalase, GPx1, and COX-2 gene expression in a rheumatoid arthritis rat model. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 991. [Google Scholar] [CrossRef]
- Lu, K.Y.; Lin, P.Y.; Chuang, E.Y.; Shih, C.M.; Cheng, T.M.; Lin, T.Y.; Sung, H.W.; Mi, F.L. H2O2-depleting and O2-generating selenium nanoparticles for fluorescence imaging and photodynamic treatment of proinflammatory-activated macrophages. ACS Appl. Mater. Interfaces 2017, 9, 5158–5172. [Google Scholar] [CrossRef]
- Chenab, K.K.; Eivazzadeh-Keihan, R.; Maleki, A.; Pashazadeh-Panahi, P.; Hamblin, M.R.; Mokhtarzadeh, A. Biomedical applications of nanoflares: Targeted intracellular fluorescence probes. Nanomedicine 2019, 17, 342–358. [Google Scholar] [CrossRef]
- Rümenapp, C.; Gleich, B.; Haase, A. Magnetic nanoparticles in magnetic resonance imaging and diagnostics. Pharm. Res. 2012, 29, 1165–1179. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, X.; Chen, P.; Zhang, L.; Yang, C.S.; Zhang, J. Selenium nanoparticles are more efficient than sodium selenite in producing reactive oxygen species and hyper-accumulation of selenium nanoparticles in cancer cells generates potent therapeutic effects. Free Radic. Biol. Med. 2018, 126, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, X.; Wong, Y.S.; Zheng, W.; Zhang, Y.; Cao, W.; Chen, T. Selenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergism. ACS Nano 2012, 6, 6578–6591. [Google Scholar] [CrossRef]
- Abd El-Moneim, O.M.; Abd El-Rahim, A.H.; Hafiz, N.A. Evaluation of selenium nanoparticles and doxorubicin effect against hepatocellular carcinoma rat model cytogenetic toxicity and DNA damage. Toxicol. Rep. 2018, 5, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yan, C.; Miao, J.; Zhang, X.; Chen, J.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Synthesis, characterization and antitumor properties of selenium nanoparticles coupling with ferulic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Luesakul, U.; Puthong, S.; Neamati, N.; Muangsin, N. pH-responsive selenium nanoparticles stabilized by folate-chitosan delivering doxorubicin for overcoming drug-resistant cancer cells. Carbohydr. Polym. 2018, 181, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Habibey, R.; Shokri, F.; Hajmiresmail, S.J.; Akhavan, O.; Mashaghi, A.; Pazoki-Toroudi, H. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci. Rep. 2019, 9, 6044. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and use of selenium nanoparticles as fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef] [PubMed]
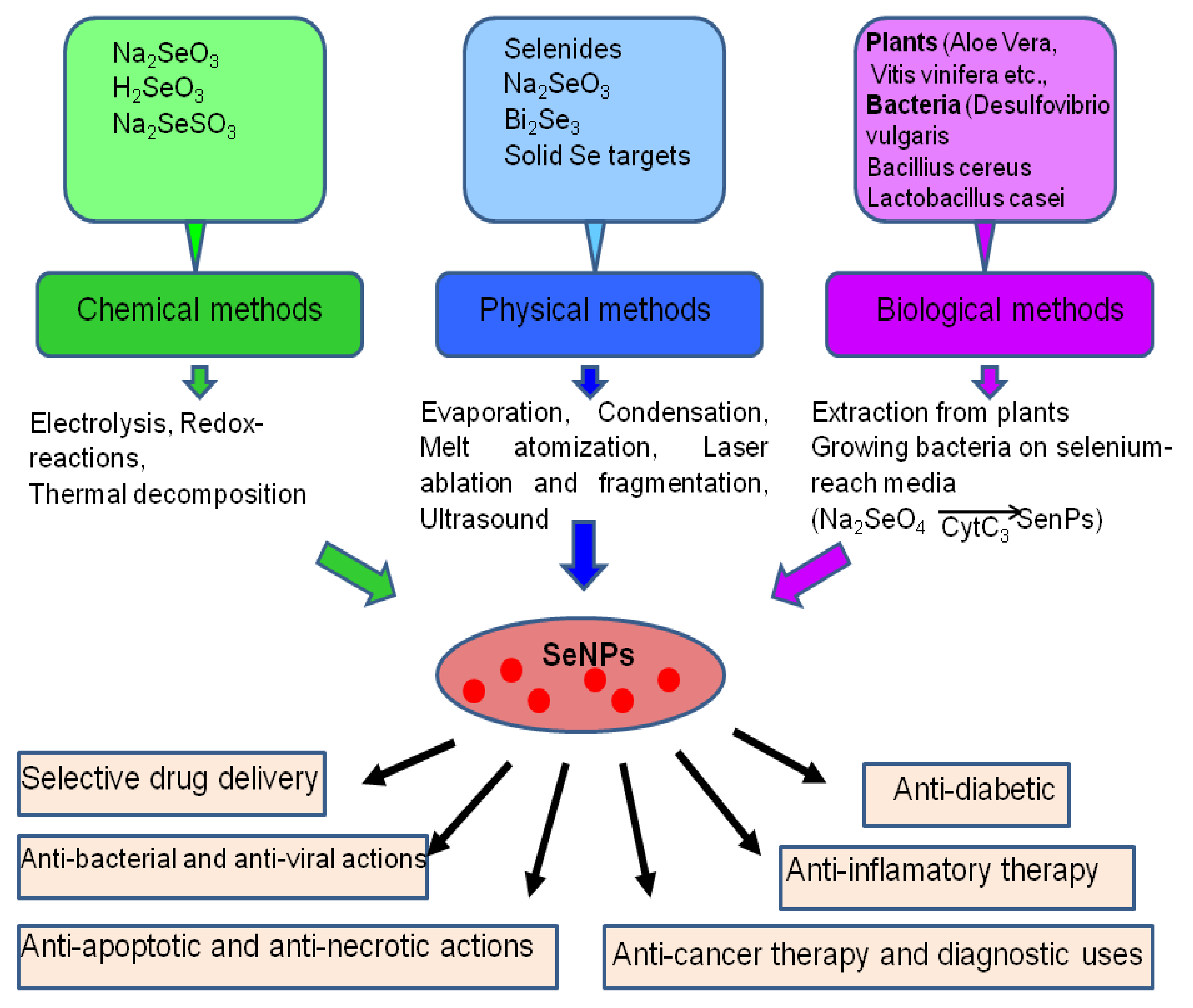
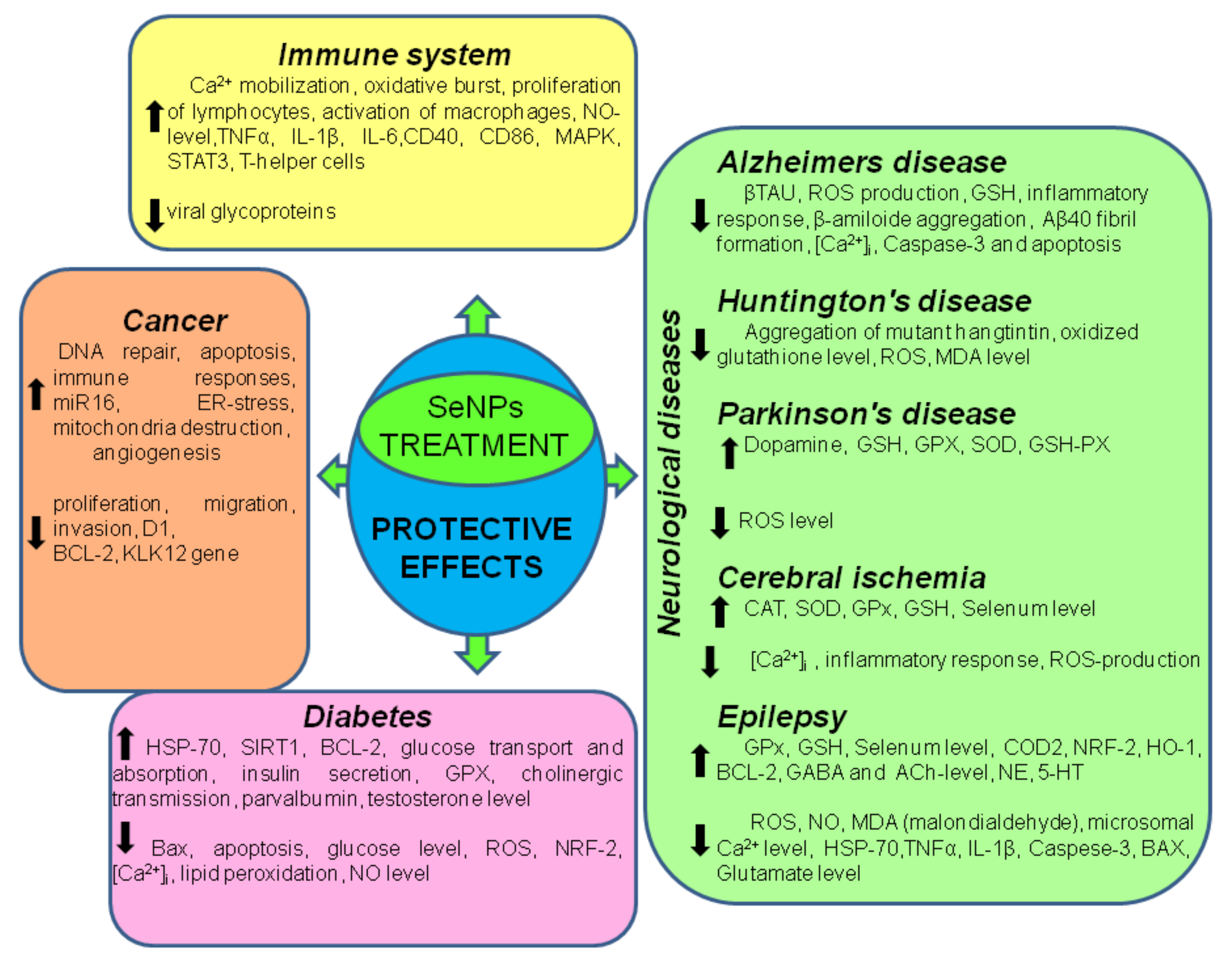
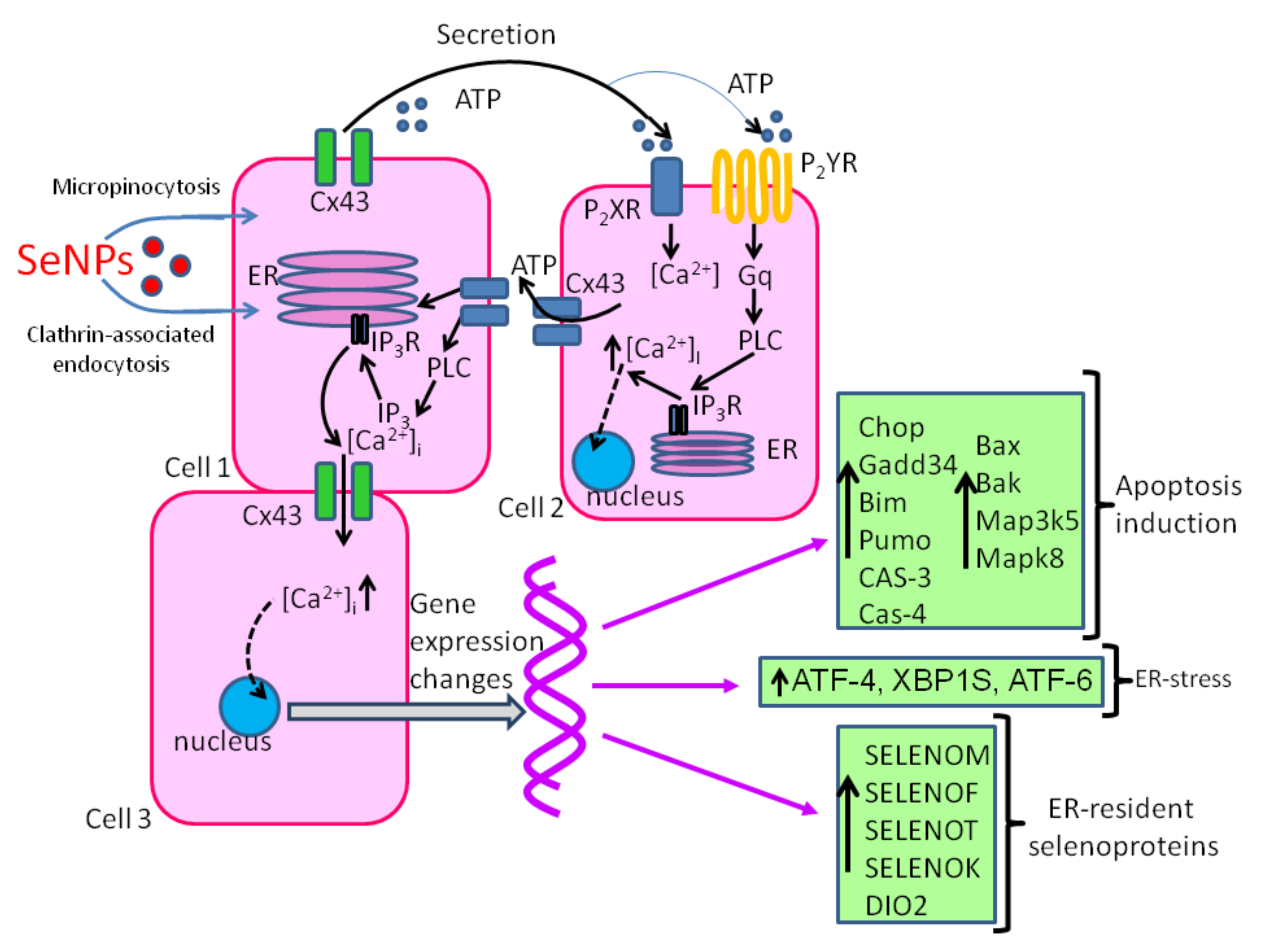
| Cellular Effects | Ref. | |
|---|---|---|
| Alzheimer’s disease | ↑ glutathione peroxidase ↓ ROS, [Ca2+]i, caspase-3, malondialdehyde, Zn2+-induced formation of Aβ40 fibrils, binding of Cu2+ to Aβ42 monomers, tau-hyperphosphorylation, Aβ1-42-induced cytotoxicity | [80,81,82,83] |
| Huntington’s disease | ↑ antioxidant capacity, protective roles from behavioral dysfunction, the sensory and mechanical responses of ASH neurons ↓ ROS, neuronal death, alleviate behavioral dysfunction, aggregation of mutant huntingtin protein | [84], |
| Parkinson’s disease | ↑ SOD, GSH-PX, Dopamine, ↓ ROS, MDA, | [85,86,87,88,89] |
| Epilepsy | ↑ GPx, GSH-PX, COD2, NRF-2, HO-1, BCL-2, GABA, Ach, NE, 5-HT, ↓ ROS, NO, Hsp7, MDA, [Ca2+]i, IL-1β, TNFα, BAX, Glutamate, Caspase-3 | [90,91,92,93] |
| Stroke | ↑ CAT, SOD, GPx, GSH-PX, Se, ER-selenoproteins, BDNF ↓ [Ca2+]i, ROS, inflammation | [94,95,96,97,98,99,100,101,102,103,104] |
| Cellular Effects | Ref. | |
|---|---|---|
| Cancer | ↑ ROS, NO, miR16, mitochondrial destruction, [Ca2+]i, CHOP, GADD34 BIM, PUMA, BAX, caspase-4, MAP3K5, MAPK-8, ATP-release ↓ cell proliferation, migration, invasion, CCND1, zinc-dependent metalloproteinases MMP2 and MMP9, cyclin-D1, Bcl-2 | [147,151,152,153,154,155,156] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varlamova, E.G.; Turovsky, E.A.; Blinova, E.V. Therapeutic Potential and Main Methods of Obtaining Selenium Nanoparticles. Int. J. Mol. Sci. 2021, 22, 10808. https://doi.org/10.3390/ijms221910808
Varlamova EG, Turovsky EA, Blinova EV. Therapeutic Potential and Main Methods of Obtaining Selenium Nanoparticles. International Journal of Molecular Sciences. 2021; 22(19):10808. https://doi.org/10.3390/ijms221910808
Chicago/Turabian StyleVarlamova, Elena G., Egor A. Turovsky, and Ekaterina V. Blinova. 2021. "Therapeutic Potential and Main Methods of Obtaining Selenium Nanoparticles" International Journal of Molecular Sciences 22, no. 19: 10808. https://doi.org/10.3390/ijms221910808
APA StyleVarlamova, E. G., Turovsky, E. A., & Blinova, E. V. (2021). Therapeutic Potential and Main Methods of Obtaining Selenium Nanoparticles. International Journal of Molecular Sciences, 22(19), 10808. https://doi.org/10.3390/ijms221910808






