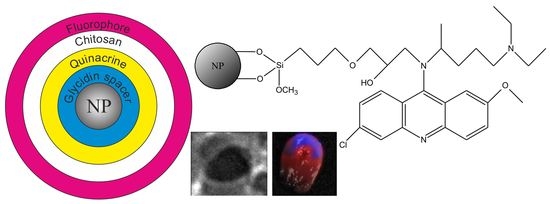
Theranostic Platforms Based on Silica and Magnetic Nanoparticles Containing Quinacrine, Chitosan, Fluorophores, and Quantum Dots
Abstract
:1. Introduction
2. Results and Discussion
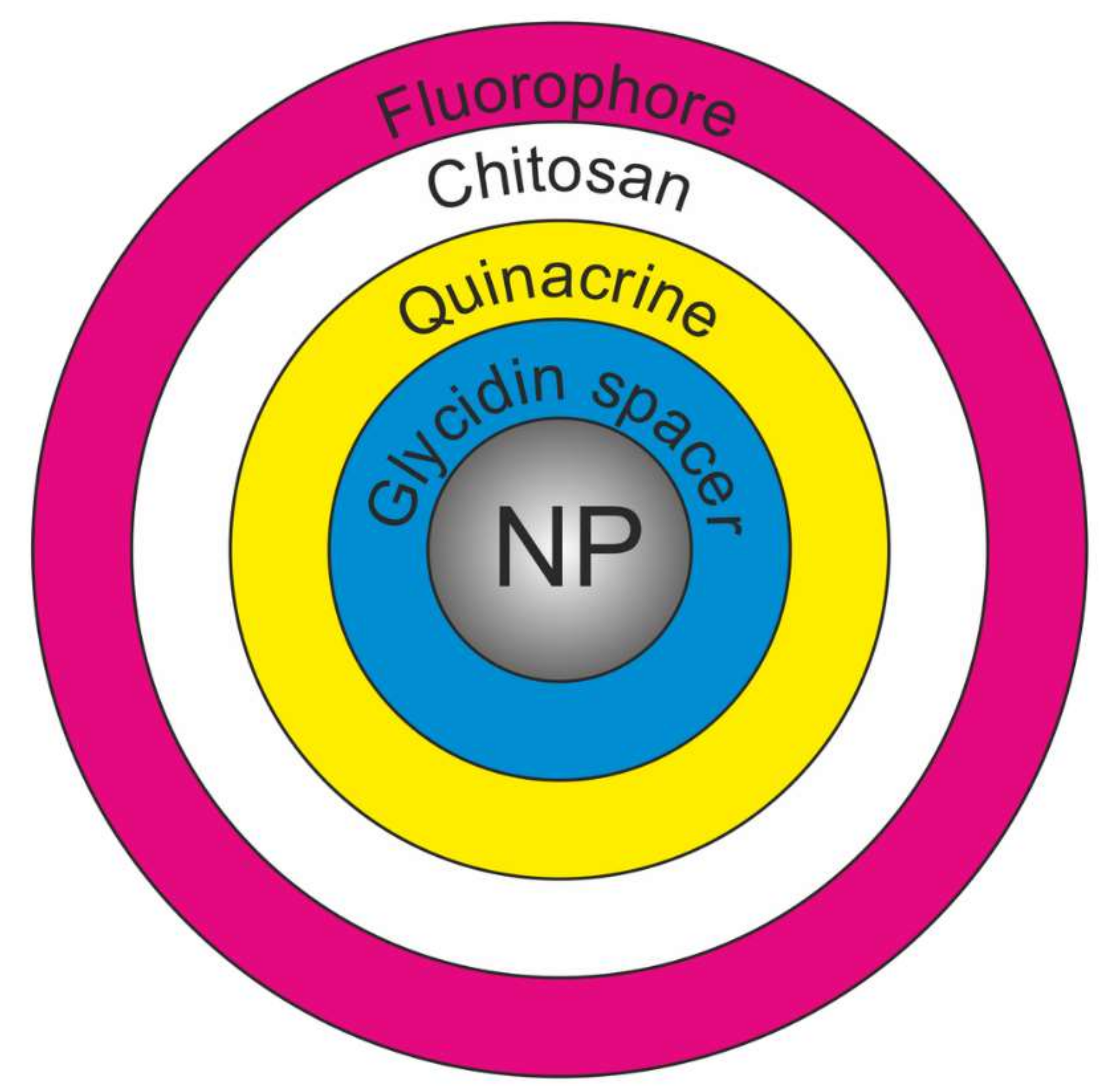
2.1. Synthesis Concept and Development
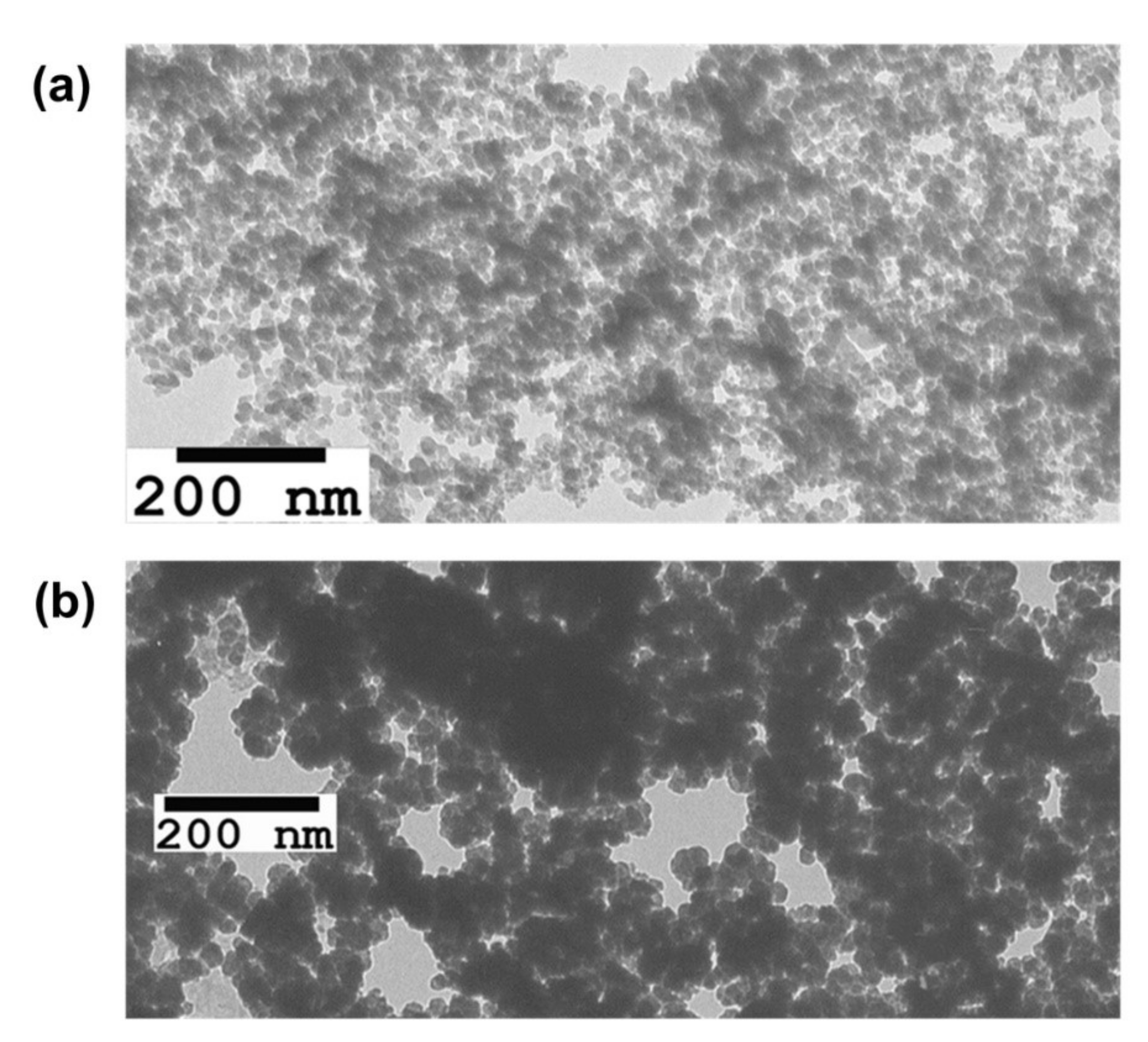
2.2. Investigation of Physical and Chemical Properties
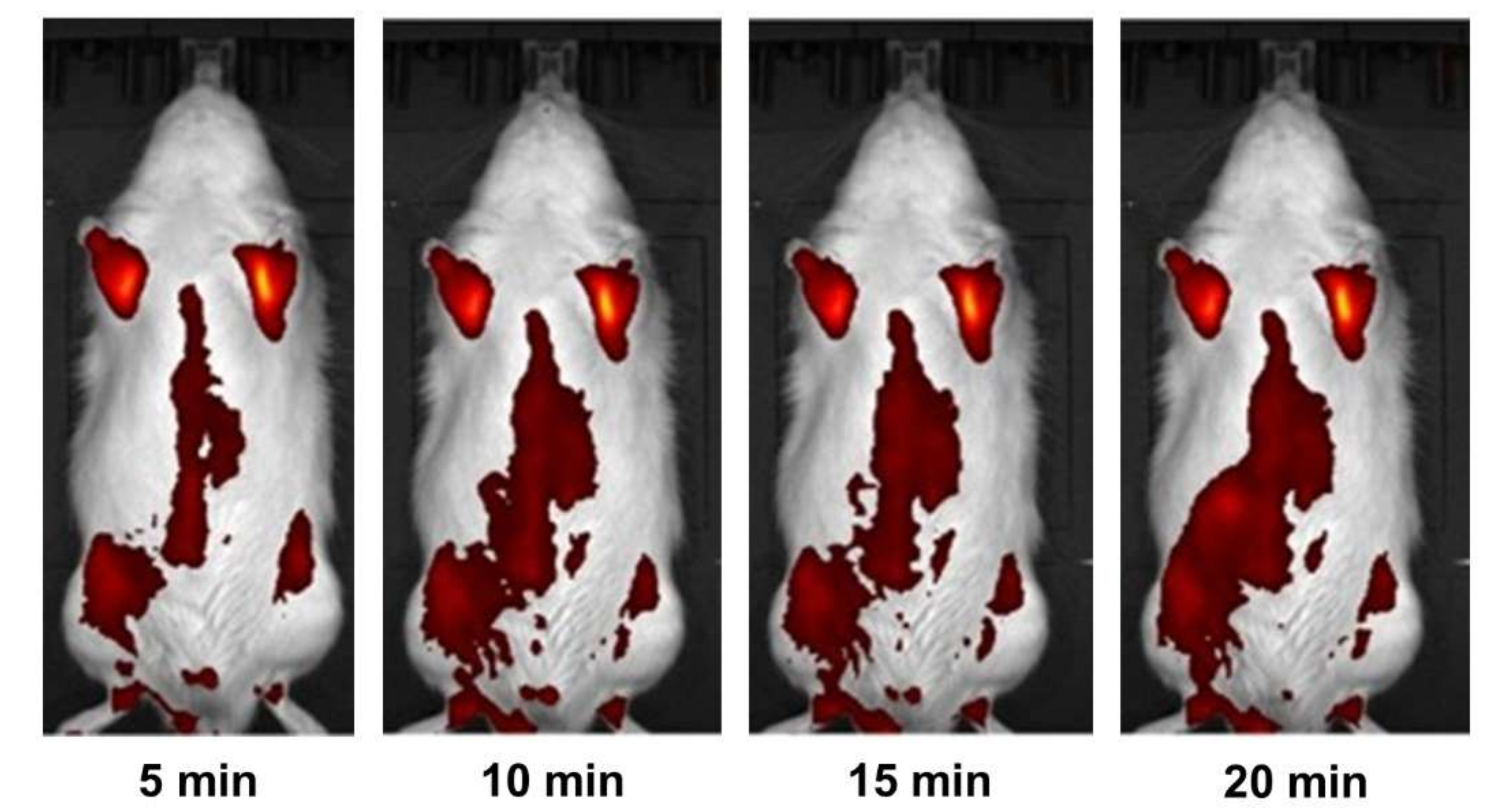
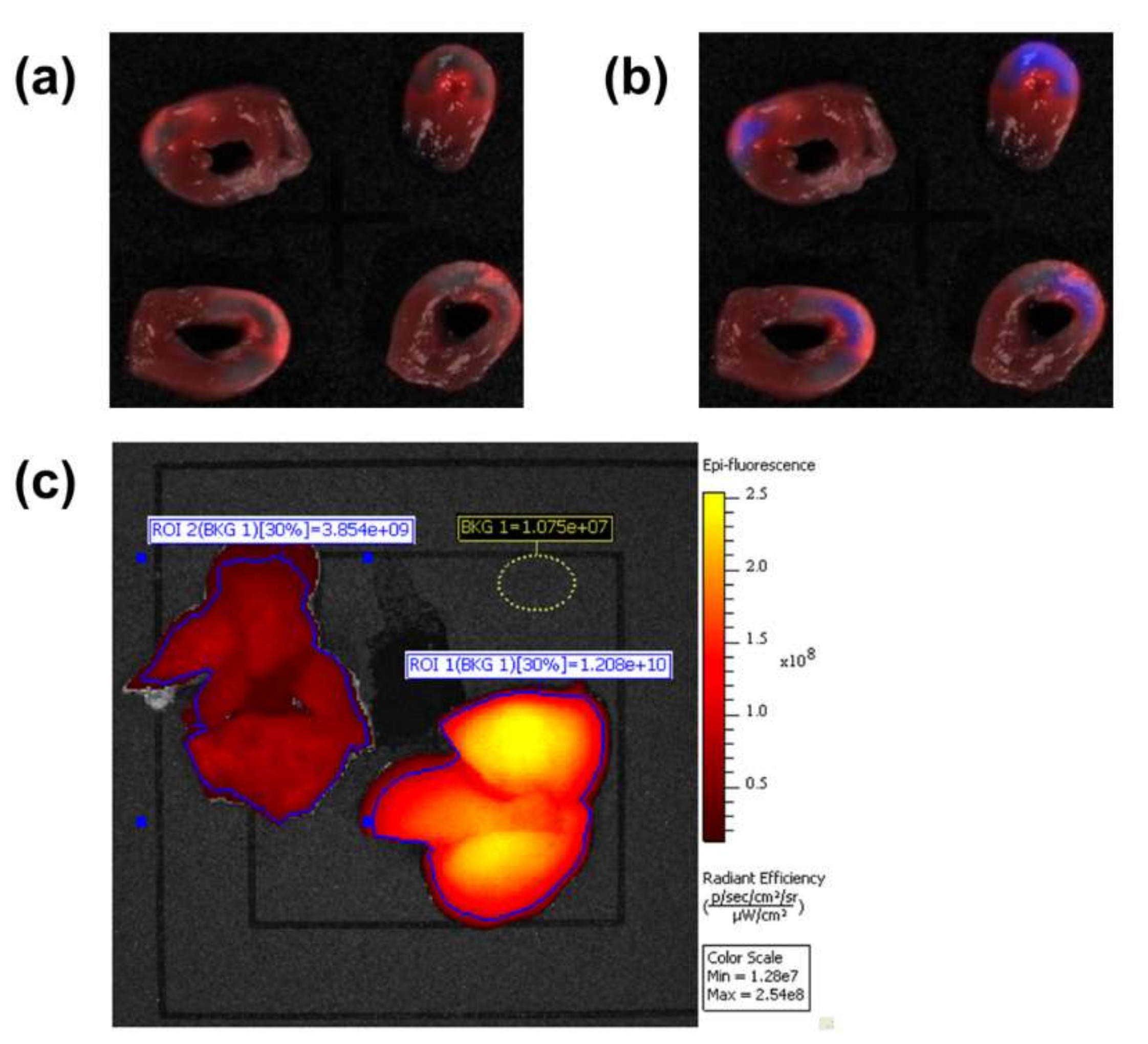
2.3. Research in Laboratory Animals
3. Materials and Methods
3.1. Characteristics of the Used Nanoparticles
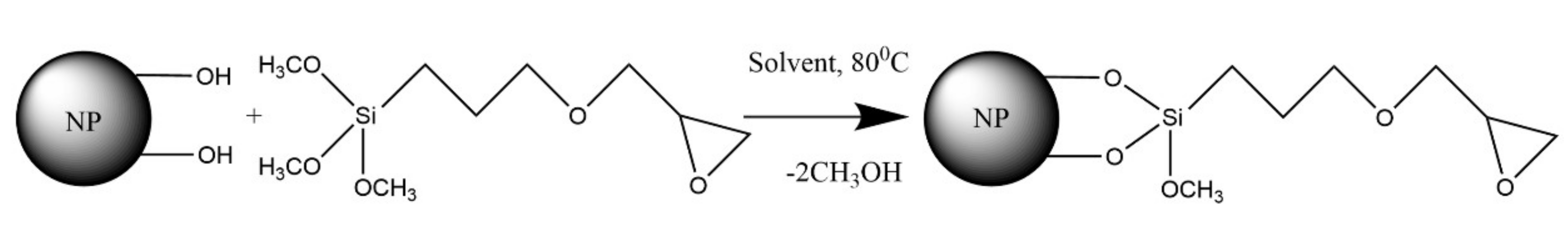
3.2. Glycidine Spacer
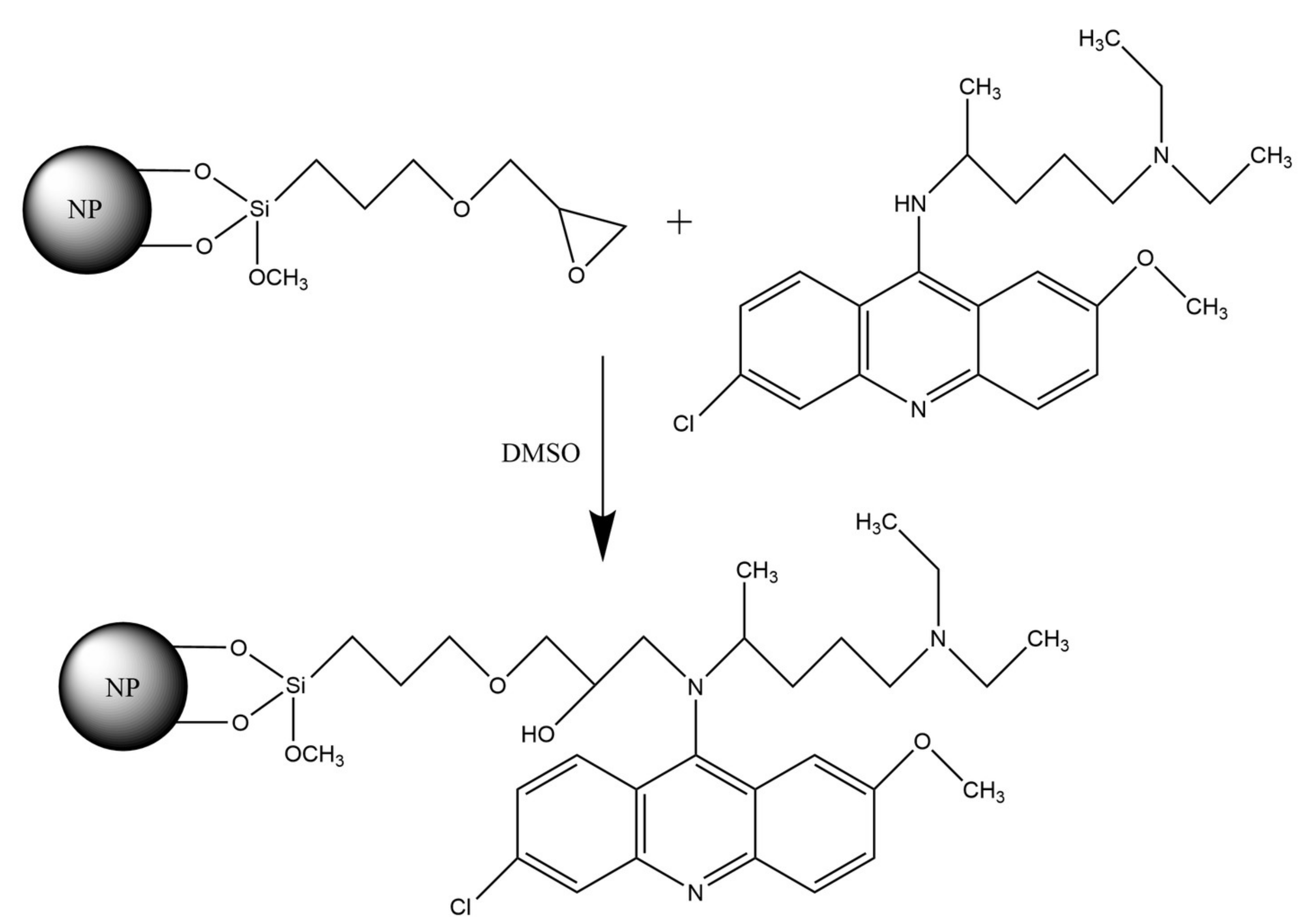
3.3. Immobilization of Quinacrine
3.4. Chitosan Coating
3.5. Immobilization of a Secondary Radiation Source
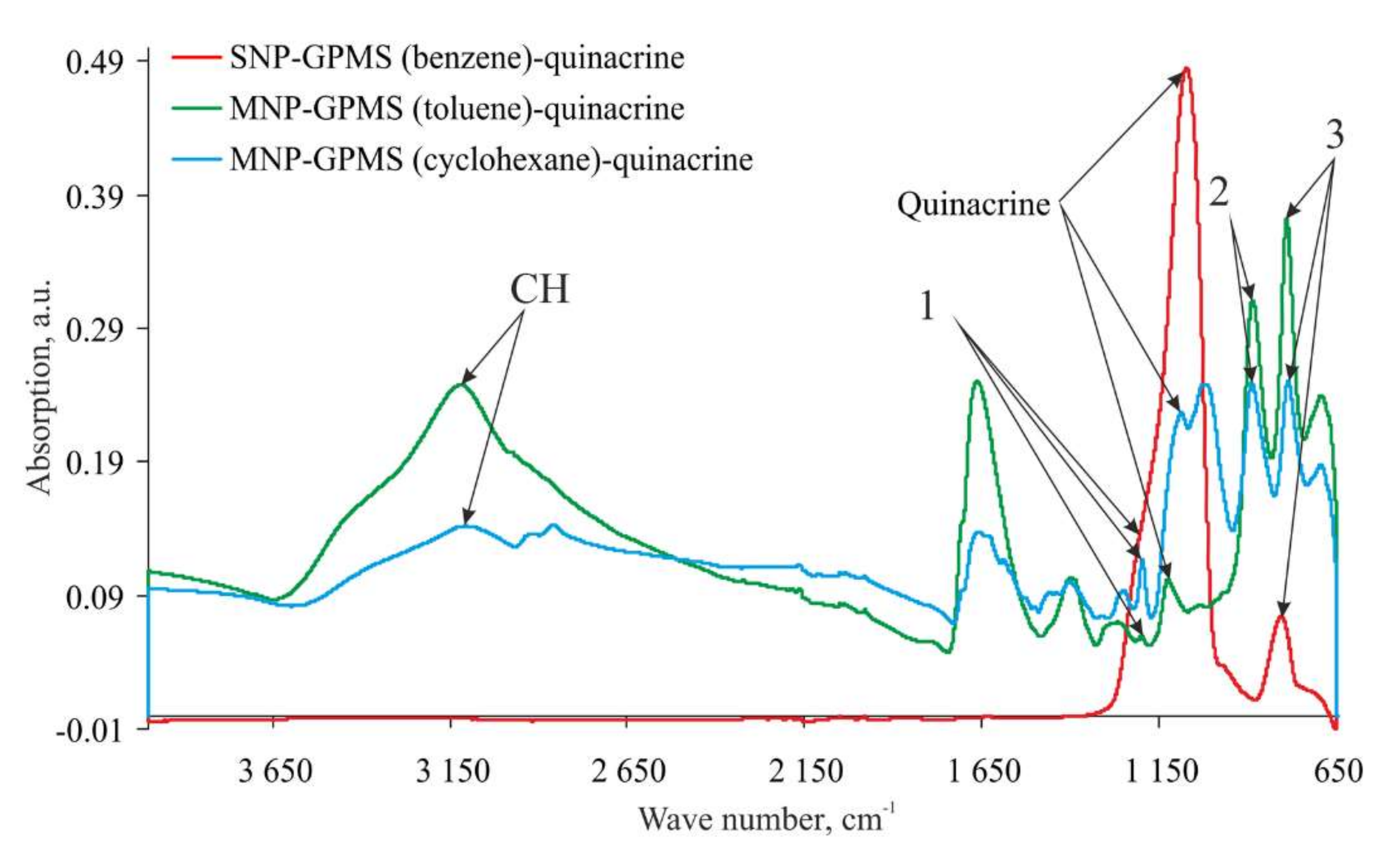
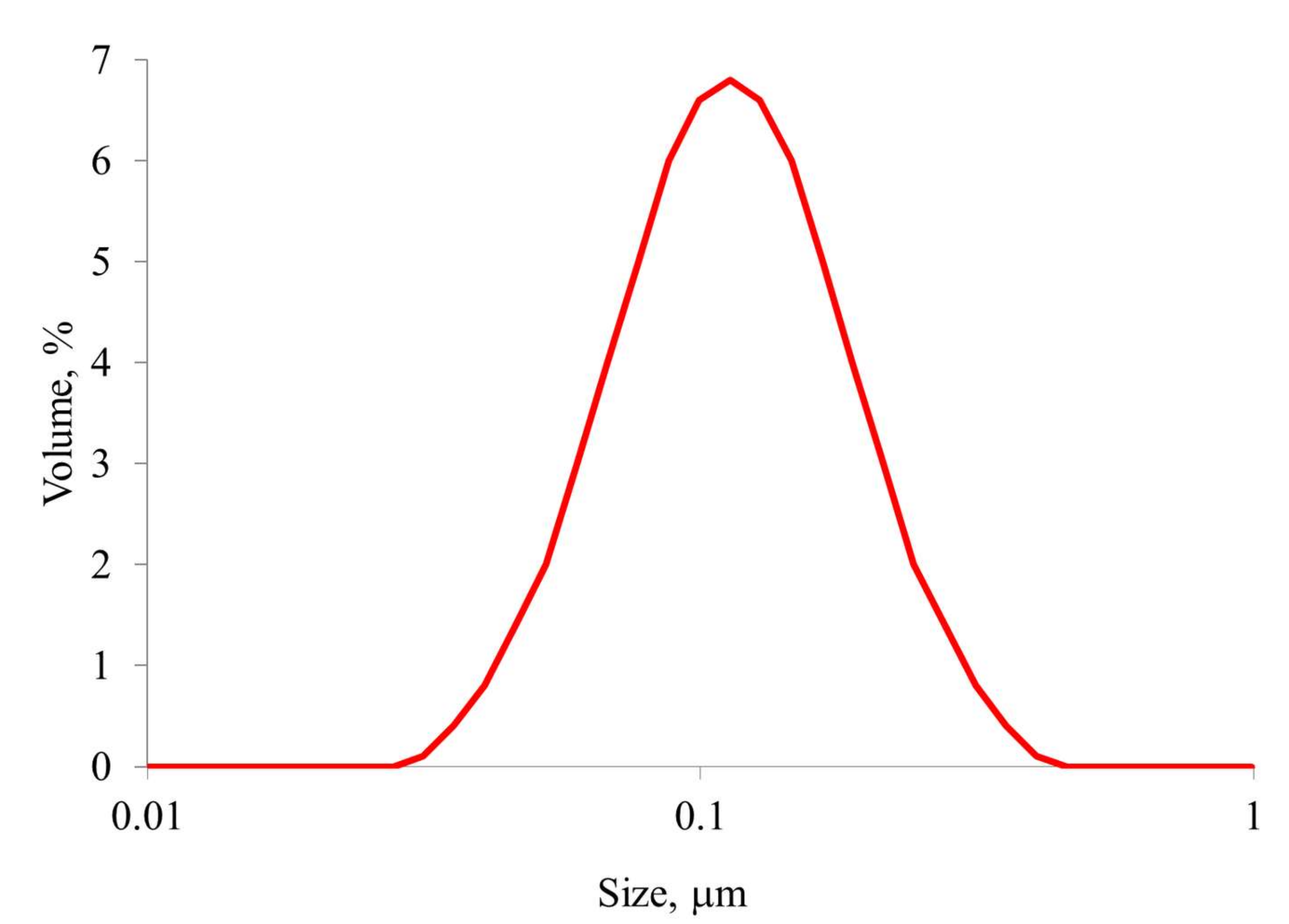
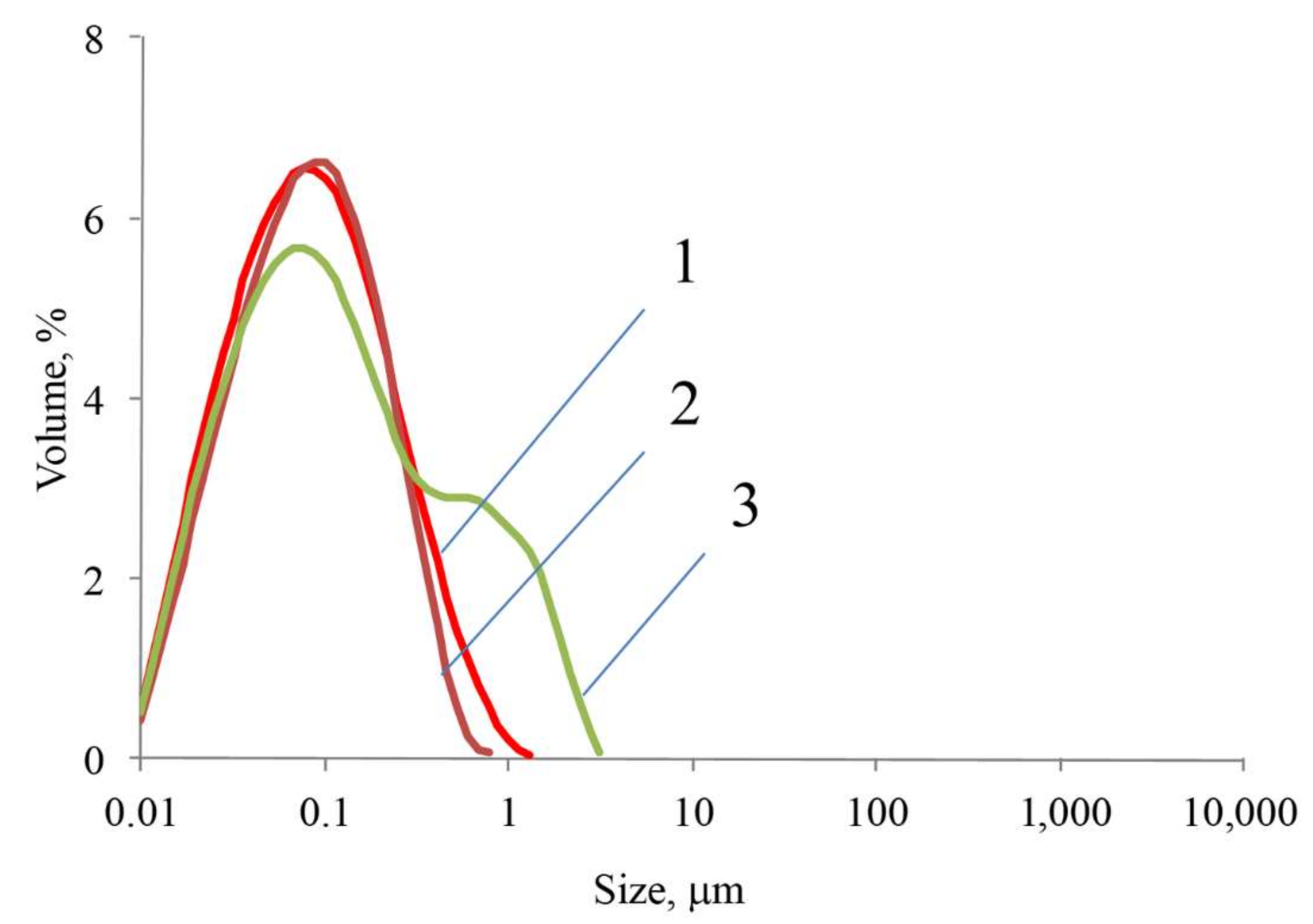
3.6. Investigation of the Physicochemical Properties of the Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Salamanca, A.E.; Diebold, Y.; Calonge, M.; García-Vazquez, C.; Callejo, S.; Vila, A.; Alonso, M.J. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: Toxicity, uptake mechanism and in vivo tolerance. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1416–1425. [Google Scholar] [CrossRef]
- Li, L.; Chen, D.; Zhang, Y.; Deng, Z.; Ren, X.; Meng, X.; Tang, F.; Ren, J.; Zhang, L. Magnetic and fluorescent multifunctional chitosan nanoparticles as a smart drug delivery system. Nanotechnology 2007, 18, 405102. [Google Scholar] [CrossRef]
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, Y.N.; Zhang, J.P.; Wang, A.Q.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar]
- Gareev, K.G.; Babikova, K.Y.; Postnov, V.N.; Naumisheva, E.B.; Korolev, D.V. Fluorescence imaging of the nanoparticles modified with indocyanine green. J. Phys. Conf. Ser. 2017, 917, 042008. [Google Scholar] [CrossRef]
- Korolev, D.; Istomina, M.; Belorus, A.; Brovko, A.; Sonin, D.; Shulmeyster, G.; Evreinova, N.; Moshnikov, V. Fluorescent Nanoagents for Biomedical Applications. In Fluorescence Methods for Investigation of Living Cells and Microorganisms; Grigoryeva, N., Ed.; IntechOpen: London, UK, 2020; p. 72635. ISBN 978-1-83968-040-3. [Google Scholar] [CrossRef]
- Sun, X.; Shen, J.; Yu, D.; Ouyang, X.K. Preparation of pH-sensitive Fe3O4@C/carboxymethyl cellulose/chitosan composite beads for diclofenac sodium delivery. Int. J. Biol. Macromol. 2019, 127, 594–605. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.M.; Lu, W.; Zhang, S.; Jiang, X.; Wu, H.; Yu, D.; Ouyang, X.K. pH-sensitive ZnO/carboxymethyl cellulose/chitosan bio-nanocomposite beads for colon-specific release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 128, 468–479. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Sillanpää, M. Magnetic xanthate modified chitosan as an emerging adsorbent for cationic azo dyes removal: Kinetic, thermodynamic and isothermal studies. Int. J. Biol. Macromol. 2019, 121, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Shi, L.; Lou, S.; Liu, B.; Zhang, W.; Zhang, L. Synthesis of spherical magnetic calcium modified chitosan micro-particles with excellent adsorption performance for anionic-cationic dyes. Int. J. Biol. Macromol. 2019, 128, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yang, J.; Zhu, L.; Yang, Y.; Yuan, H.; Yang, Y.; Liu, X. Removal of Pb2+, Hg2+, and Cu2+ by Chain-Like Fe3O4@SiO2@Chitosan Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, W.; Luo, X.; Zhao, F.; Li, Y.; Li, R.; Li, Z. Efficient removal and environmentally benign detoxification of Cr(VI) in aqueous solutions by Zr(IV) cross-linking chitosan magnetic microspheres. Chemosphere 2017, 185, 991–1000. [Google Scholar] [CrossRef]
- Jiang, X.; Cheng, J.; Zhou, H.; Li, F.; Wu, W.; Ding, K. Polyaniline-coated chitosan-functionalized magnetic nanoparticles: Preparation for the extraction and analysis of endocrine-disrupting phenols in environmental water and juice samples. Talanta 2015, 141, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qian, Y.; Zhang, H.; Ullah, A.; He, X.; Zhou, Z.; Fenniri, H.; Shen, J. Advances in cancer theranostics using organic-inorganic hybrid nanotechnology. Appl. Mater. Today 2021, 23, 101003. [Google Scholar] [CrossRef]
- Sedyakina, N.E.; Silaeva, A.O.; Krivoshchepov, A.F.; Avramenko, G.V. Preparation and properties of chitosan microspheres based on polyglycerol polyricinoleate stabilized emulsions. Mendeleev. Commun. 2018, 28, 70–72. [Google Scholar] [CrossRef]
- Sedyakina, N.E.; Feldman, N.B.; Lutsenko, S.V.; Avramenko, G.V. Fabrication and drug release behavior of ionically cross-linked chitosan microspheres. Mendeleev. Commun. 2018, 28, 598–600. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Efimova, A.A.; Mulashkin, F.D.; Rudenskaya, G.N.; Krivtsov, G.G. Biodegradable liposome–chitosan complexes: Enzyme-mediated release of encapsulated substances. Mendeleev. Commun. 2018, 28, 140–142. [Google Scholar] [CrossRef]
- Zemskova, L.A.; Shlyk, D.K.; Voit, A.V.; Barinov, N.N. Chitosan based composite sorbents for arsenic removal. Russ. Chem. Bull. 2019, 68, 9–16. [Google Scholar] [CrossRef]
- Balakina, A.A.; Mumyatova, V.A.; Pliss, E.M.; Terent’ev, A.A.; Sen, V.D. Antioxidant properties of chitosan-(poly)nitroxides under induced oxidative stress. Russ. Chem. Bull. 2018, 67, 2135–2140. [Google Scholar] [CrossRef]
- Filatova, L.Y.; Klyachko, N.L.; Kudryashova, E.V. Targeted delivery of anti-tuberculosis drugs to macrophages: Targeting mannose receptors. Russ. Chem. Rev. 2018, 87, 374–391. [Google Scholar] [CrossRef]
- Otani, H.; Engelman, R.M.; Breyer, R.H.; Rousou, J.A.; Lemeshow, S.; Das, D.K. Mepacrine, a phospholipase inhibitor. A potential tool for modifying myocardial reperfusion injury. J. Thorac. Cardiovasc. Surg. 1986, 92, 247–254. [Google Scholar] [CrossRef]
- Van Bilsen, M.; van der Vusse, G.J.; Willemsen, P.H.; Coumans, W.A.; Roemen, T.H.; Reneman, R.S. Effects of nicotinic acid and mepacrine on fatty acid accumulation and myocardial damage during ischemia and reperfusion. J. Mol. Cell. Cardiol. 1990, 22, 155–163. [Google Scholar] [CrossRef]
- Korolev, D.; Postnov, V.; Aleksandrov, I.; Murin, I. The Combination of Solid-State Chemistry and Medicinal Chemistry as the Basis for the Synthesis of Theranostics Platforms. Biomolecules 2021, 11, 1544. [Google Scholar] [CrossRef]
- Kvitek, R.J.; Watson, M.W.; Evans, J.F.; Carr, P.W. The effect of ethylenediamine vs. glycidoxy side-chains on the reactivity of organosilanes toward silica surfaces. Anal. Chim. Acta 1981, 129, 269–272. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; Wiley: Hoboken, NJ, USA, 1979; p. 896. ISBN 978-0-471-02404-0. [Google Scholar]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds. Tables of Spectral Data, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; p. 433. ISBN 978-3-540-93809-5. [Google Scholar] [CrossRef]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins–Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. In Infrared Spectroscopy. Materials Science, Engineering and Technology; Theophanides, T., Ed.; IntechOpen: London, UK, 2012; p. 13. ISBN 978-953-51-0537-4. [Google Scholar] [CrossRef] [Green Version]
- Gonsalves, K.; Halberstadt, C.; Laurencin, C.T.; Nair, L. Biomedical Nanostructures; Wiley: Hoboken, NJ, USA, 2007; p. 544. ISBN 978-0-471-92552-1. [Google Scholar]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.S. Nanotheranostics–application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef]
- Sun, I.C.; Jo, S.; Dumani, D.; Yun, W.S.; Yoon, H.Y.; Lim, D.K.; Ahn, C.H.; Emelianov, S.; Kim, K. Theragnostic Glycol Chitosan-Conjugated Gold Nanoparticles for Photoacoustic Imaging of Regional Lymph Nodes and Delivering Tumor Antigen to Lymph Nodes. Nanomaterials 2021, 11, 1700. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, X.; Yao, Y.; Luo, J.; Tang, Q.; Wu, H.; Lin, S.; Han, C.; Wei, Q.; Chen, L. Electrophoretic deposition of chitosan/gelatin coatings with controlled porous surface topography to enhance initial osteoblast adhesive responses. J. Mater. Chem. B 2016, 4, 7584–7595. [Google Scholar] [CrossRef] [PubMed]
- Toropova, Y.G.; Golovkin, A.S.; Malashicheva, A.B.; Korolev, D.V.; Gorshkov, A.N.; Gareev, K.G.; Afonin, M.V.; Galagudza, M.M. In vitro toxicity of FemOn, FemOn-SiO2 composite, and SiO2-FemOn core-shell magnetic nanoparticles. Int. J. Nanomed. 2017, 12, 593–603. [Google Scholar] [CrossRef] [Green Version]
- Istomina, M.S.; Pechnikova, N.A.; Korolev, D.V.; Pochkayeva, E.I.; Mazing, D.S.; Galagudza, M.M.; Moshnikov, V.A.; Shlyakhto, E.V. ZAIS-based colloidal QDs as fluorescent labels for theranostics: Physical properties, biodistribution and biocompatibility. Bull. Russ. State Med. Univ. 2018, 6, 94–101. [Google Scholar] [CrossRef]
- Kondo, T.; Ishizu, A.; Nakano, J. Preparation of glycidyl celluloses from completely allylated methylcellulose and tri-O-allylcellulose. J. Appl. Polym. Sci. 1989, 37, 3003–3009. [Google Scholar] [CrossRef]
- Williams, W.J. Handbook of Anion Determination, 1st ed.; Butterworth-Heinemann: Oxford, UK, 1979; p. 640. ISBN 9781483192550. [Google Scholar]
- Pregl, F. Die Quantitative Organische Mikroanalyse; Springer: Berlin, Germany, 1917; p. 192. ISBN 978-3-662-42226-7. [Google Scholar]
- Agotegaray, M.A.; Campelo, A.E.; Zysler, R.D.; Gumilar, F.; Bras, C.; Gandini, A.; Minetti, A.; Massheimer, V.L.; Lassalle, V.L. Magnetic nanoparticles for drug targeting: From design to insights into systemic toxicity. Preclinical evaluation of hematological, vascular and neurobehavioral toxicology. Biomater. Sci. 2017, 5, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Montero, N.; Pérez, E.; Benito, M.; Teijón, C.; Teijón, J.M.; Olmo, R.; Blanco, M.D. Biocompatibility studies of intravenously administered ionic-crosslinked chitosan-BSA nanoparticles as vehicles for antitumour drugs. Int. J. Pharm. 2019, 554, 337–351. [Google Scholar] [CrossRef] [PubMed]



| Sample | Specific Surface Area, m2/g | Concentration of Glycidine Spacer, mmol/g (μmol/m2) | ||
|---|---|---|---|---|
| Titration | Silicon | Carbon | ||
| SNP-GPMS from benzene | 197 | 0.34 (1.7) | – | 0.42 (2.2) |
| MNP-GPMS from benzene | 70 | 0.27 (3.9) | 0.35 (5.0) | 0.30 (4.3) |
| MNP-GPMS from toluene | 77 | 0.14 (1.8) | 0.40 (5.2) | |
| MNP-GPMS from cyclohexane | 42 | 1.26 (30.0) | 1.10 (26.0) | |
| Sample | Quinacrine Content, mmol /g |
|---|---|
| SNP-GPMS from benzene | 0.007 |
| MNP-GPMS from benzene | 0.014 |
| MNP-GPMS from toluene | 0.006 |
| MNP-GPMS from cyclohexane | 0.009 |
| No. of Sample | Sample Composition | Total Luminous Efficiency, (p/s)/(µW/cm2) × 10−8 |
|---|---|---|
| 1 | SNP-GPMS-quinacrine | 99,870.0 |
| 2 | MNP-GPMS-quinacrine | 142.0 |
| No. | Acid | The Amount of ICG per Unit Mass of the Carrier, mmol/g |
|---|---|---|
| 1 | Acetic acid | 0.0006 |
| 2 | Succinic acid | 0.0017 |
| 3 | Tartaric acid | 0.0026 |
| 4 | Citric acid | 0.0159 |
| No. of Sample | NP Type | Secondary Radiation Source | Acid | Total Luminous Efficiency,(p/s)/(µW/cm2) × 10−8 |
|---|---|---|---|---|
| 1 | SNP | ICG | Acetic | 22.8 |
| 2 | SNP | ICG | Succinic | 4.2 |
| 3 | SNP | ICG | Tartaric | 5.6 |
| 4 | SNP | ICG | Citric | 13.1 |
| 5 | SNP | CQD | Acetic | 1024 |
| 6 | SNP | CQD | Succinic | 1647 |
| 7 | SNP | CQD | Tartaric | 1524 |
| 8 | SNP | CQD | Citric | 931 |
| 9 | MNP | ICG | Acetic | 0 |
| 10 | MNP | ICG | Citric | 55.3 |
| 11 | MNP | CQD | Acetic | 23.1 |
| No. of Sample | Primary Shell Fluorophore | Secondary Shell Fluorophore | Light Filter for Fluorophore | Total Luminous Efficiency, (p/s)/(µW/cm2) × 10−9 |
|---|---|---|---|---|
| 1 | Quinacrine | ICG | Quinacrine | 9.1 |
| Quinacrine | ICG | ICG | 0.24 | |
| 2 | Quinacrine | CQD | Quinacrine | 300 |
| Quinacrine | CQD | CQD | 167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korolev, D.V.; Shulmeyster, G.A.; Evreinova, N.V.; Syrovatkina, M.S.; Istomina, M.S.; Postnov, V.N.; Aleksandrov, I.V.; Krasichkov, A.S.; Galagudza, M.M. Theranostic Platforms Based on Silica and Magnetic Nanoparticles Containing Quinacrine, Chitosan, Fluorophores, and Quantum Dots. Int. J. Mol. Sci. 2022, 23, 932. https://doi.org/10.3390/ijms23020932
Korolev DV, Shulmeyster GA, Evreinova NV, Syrovatkina MS, Istomina MS, Postnov VN, Aleksandrov IV, Krasichkov AS, Galagudza MM. Theranostic Platforms Based on Silica and Magnetic Nanoparticles Containing Quinacrine, Chitosan, Fluorophores, and Quantum Dots. International Journal of Molecular Sciences. 2022; 23(2):932. https://doi.org/10.3390/ijms23020932
Chicago/Turabian StyleKorolev, Dmitry V., Galina A. Shulmeyster, Natalia V. Evreinova, Maria S. Syrovatkina, Maria S. Istomina, Victor N. Postnov, Ilia V. Aleksandrov, Aleksandr S. Krasichkov, and Michael M. Galagudza. 2022. "Theranostic Platforms Based on Silica and Magnetic Nanoparticles Containing Quinacrine, Chitosan, Fluorophores, and Quantum Dots" International Journal of Molecular Sciences 23, no. 2: 932. https://doi.org/10.3390/ijms23020932
APA StyleKorolev, D. V., Shulmeyster, G. A., Evreinova, N. V., Syrovatkina, M. S., Istomina, M. S., Postnov, V. N., Aleksandrov, I. V., Krasichkov, A. S., & Galagudza, M. M. (2022). Theranostic Platforms Based on Silica and Magnetic Nanoparticles Containing Quinacrine, Chitosan, Fluorophores, and Quantum Dots. International Journal of Molecular Sciences, 23(2), 932. https://doi.org/10.3390/ijms23020932