Diosmin Inhibits Glioblastoma Growth through Inhibition of Autophagic Flux
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of GBM Lines
2.2. Chemicals
2.3. Colony Formation Assays
2.4. Western Blotting
2.5. Migration and Invasion
2.6. Flow Cytometric Analysis of Cell Viability and Proliferation, Cell Cycle Progression, Autophagy, and Senescence
2.7. Immunofluorescent Staining and Quantification
2.8. Glioma Orthotopic Xenograft in Nude Mice
2.9. Hematoxylin and Eosin (H&E) and Ki67staining
2.10. Global Gene Expression Profile Analysis and Gene Ontology
2.11. Bioinformatics Investigation of the CGGA Dataset
2.12. Sphere Formation Assay
2.13. Statistical Analysis
3. Results
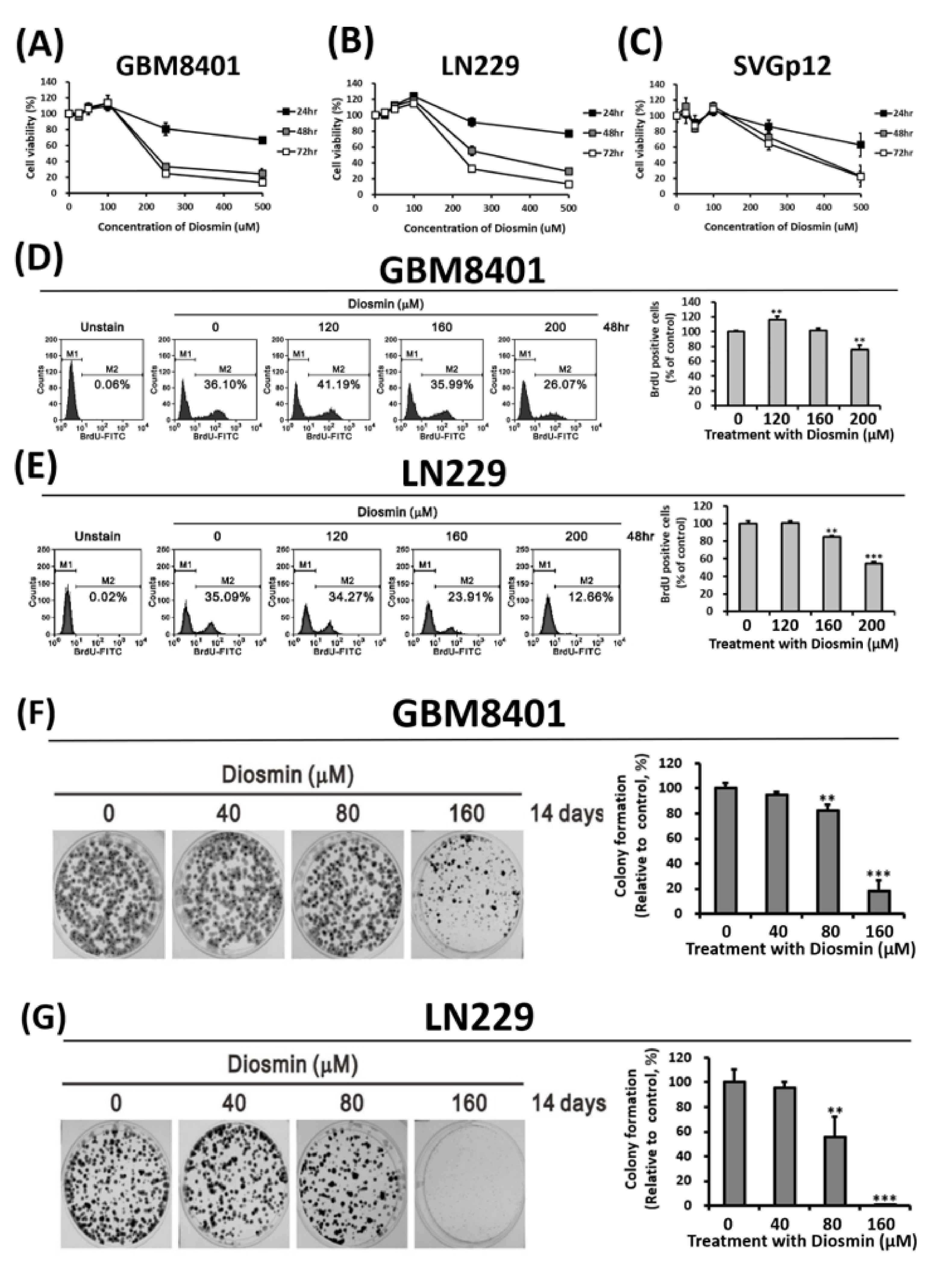
3.1. Diosmin Concentration-Dependently Suppresses GBM Cell Growth and Proliferation
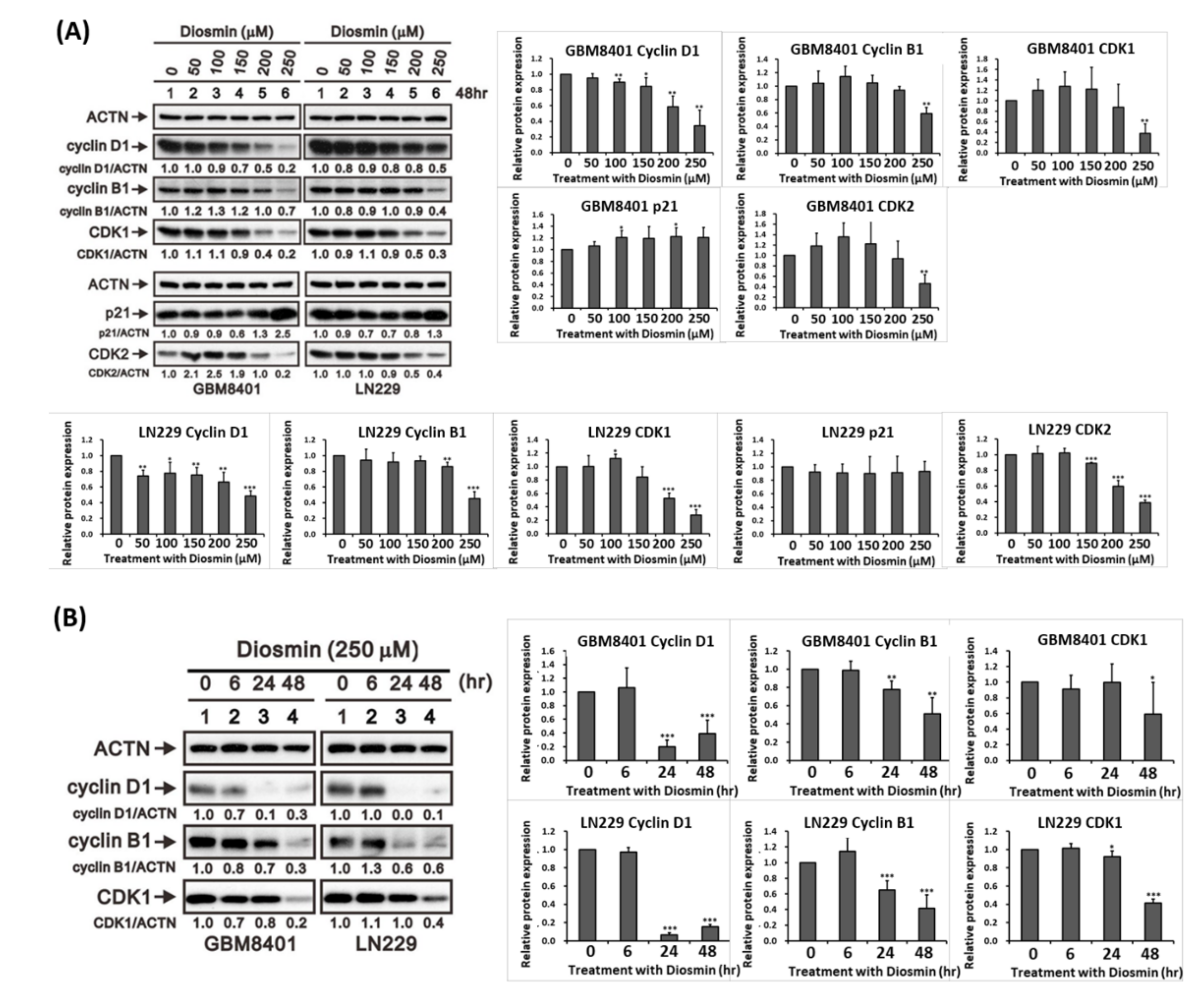
3.2. Diosmin Induces G1 Phase Arrest Associated with Upregulation of p21 and Downregulation of Cyclin B1, Cyclin D1, CDK1, and CDK2
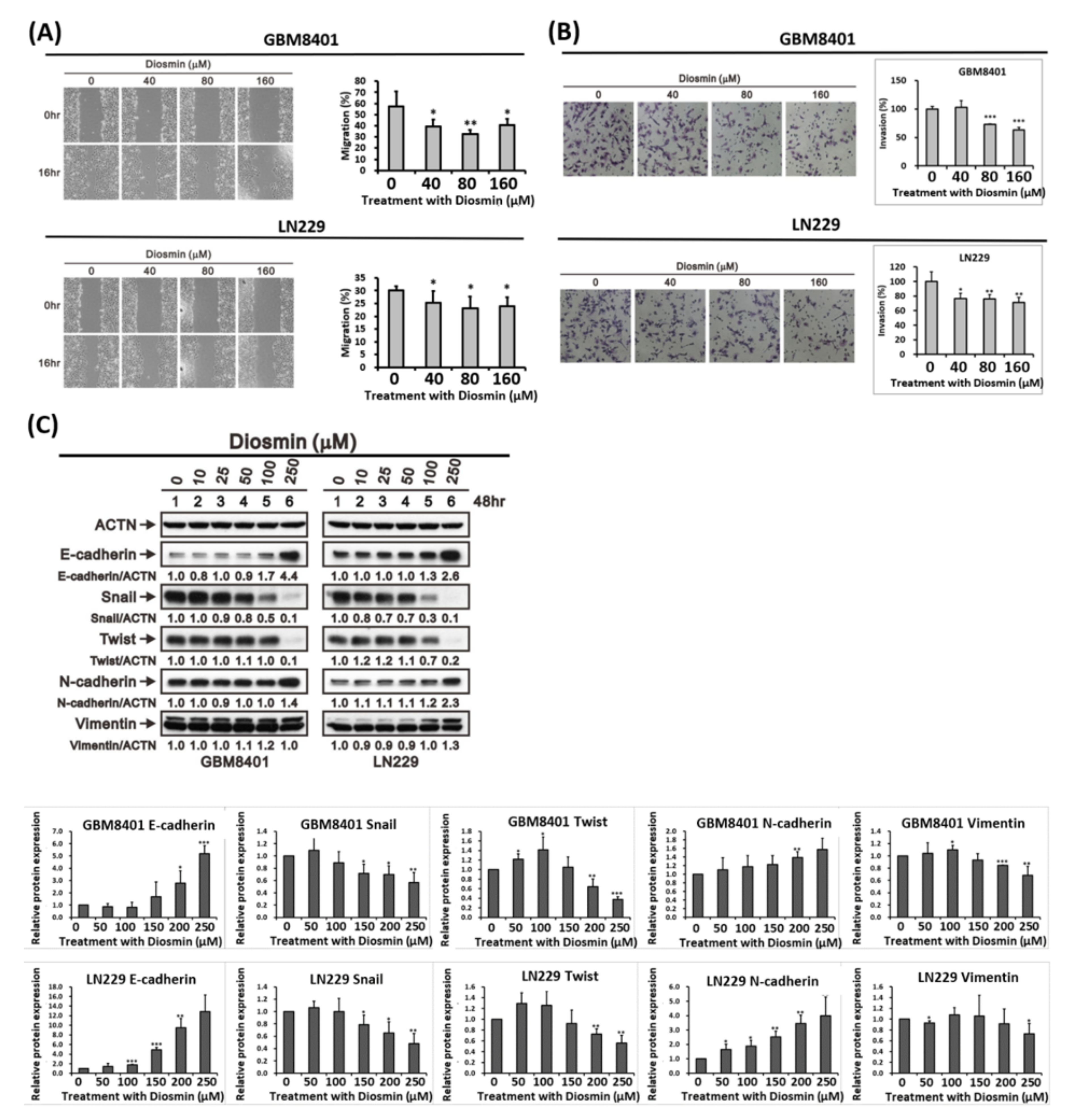
3.3. Diosmin Inhibits Glioma Cells Migration by Decreasing EMT Transcription Factors
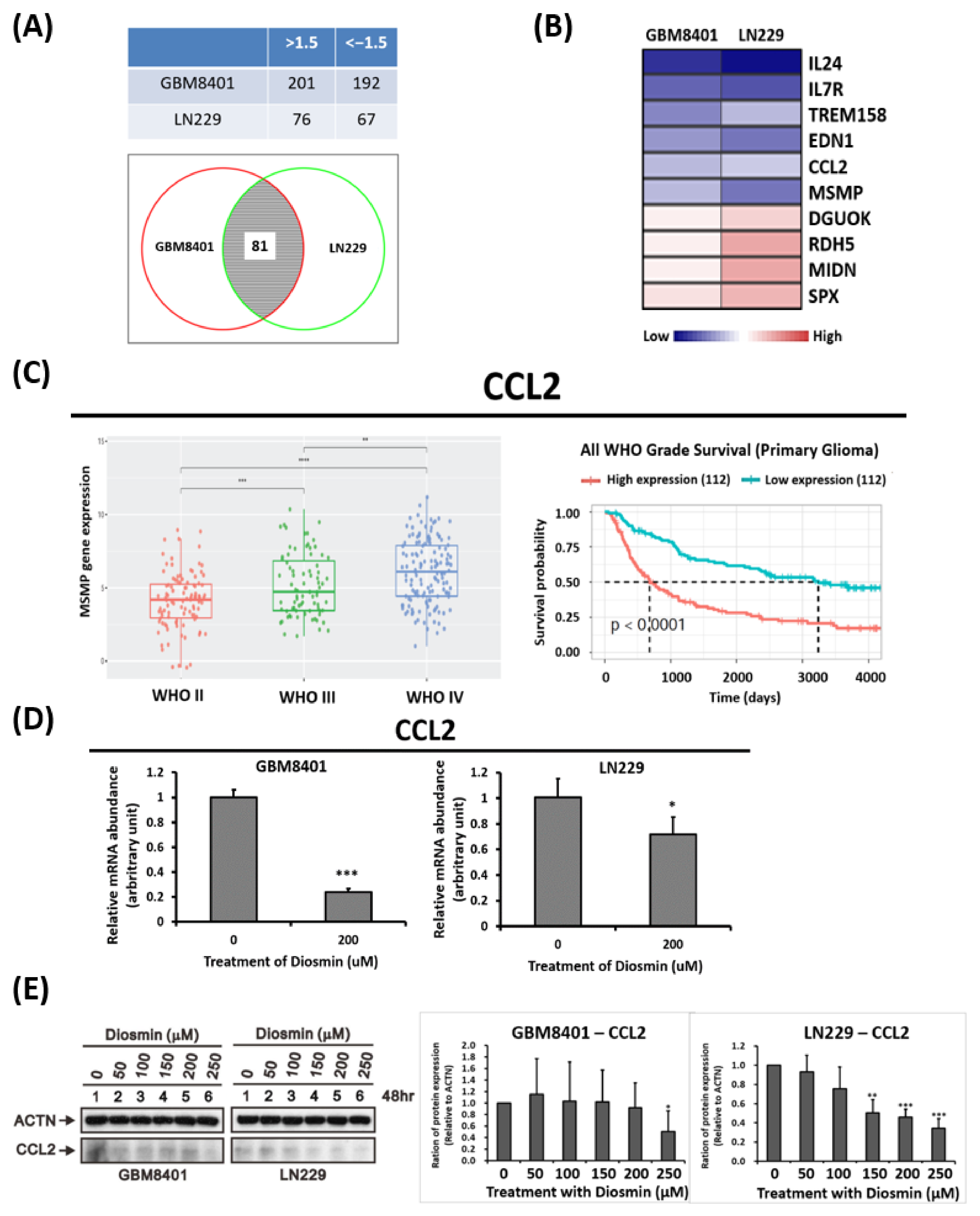
3.4. Gene Expression Profile Analysis Revealed CCL2 Are Downstream Targets Affected by Diosmin
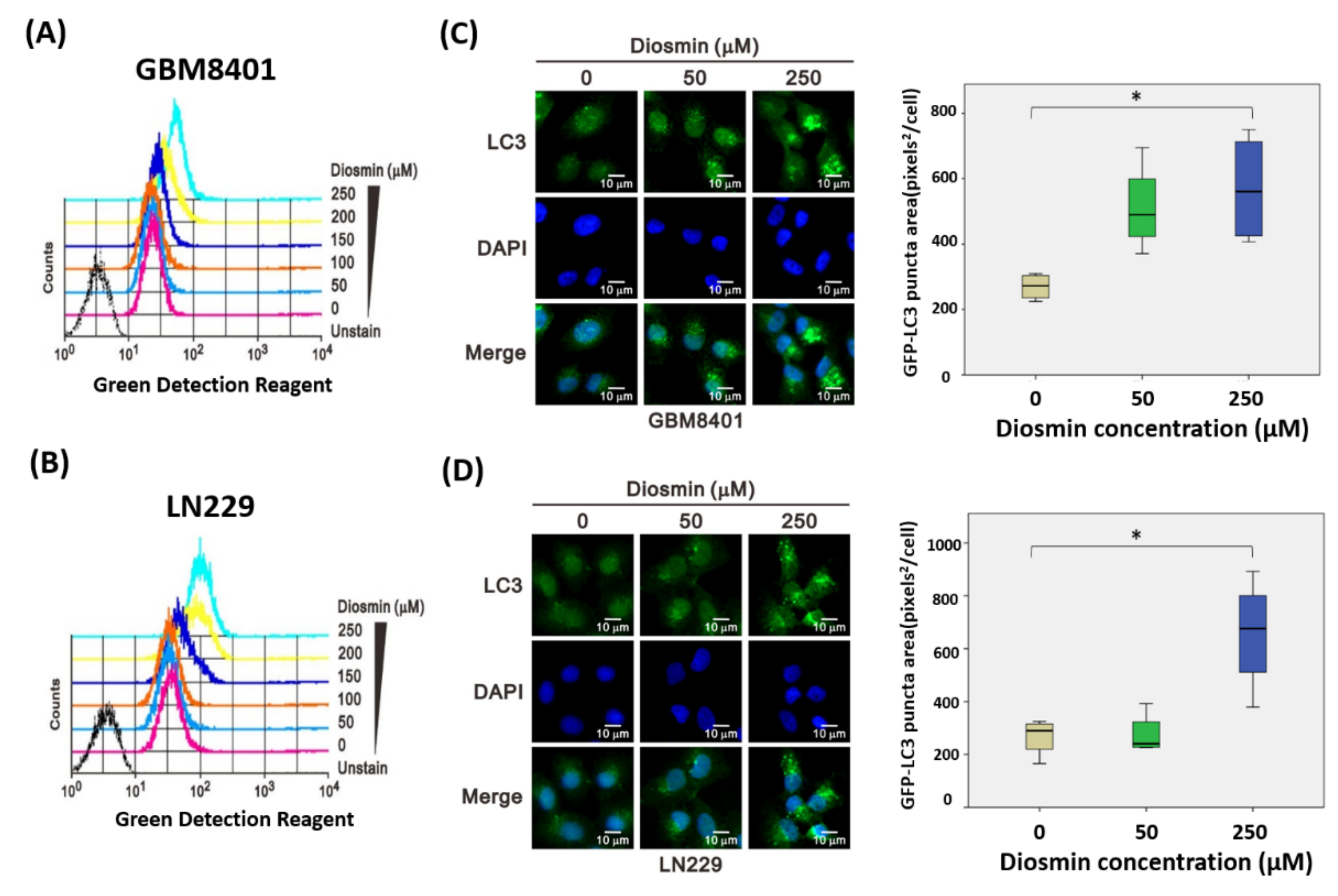
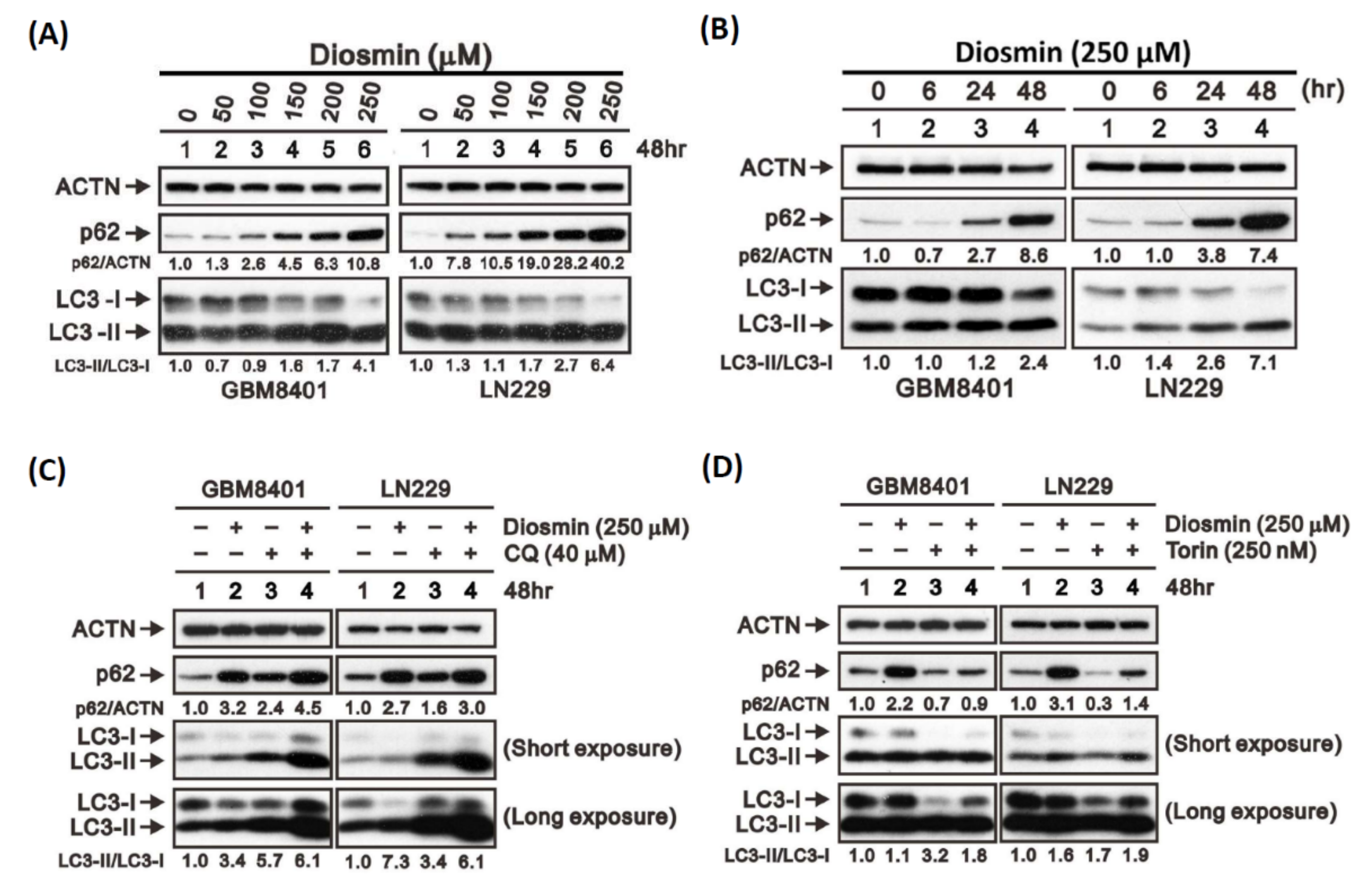
3.5. Diosmin Is a Potent Inhibitor of Autophagic Flux
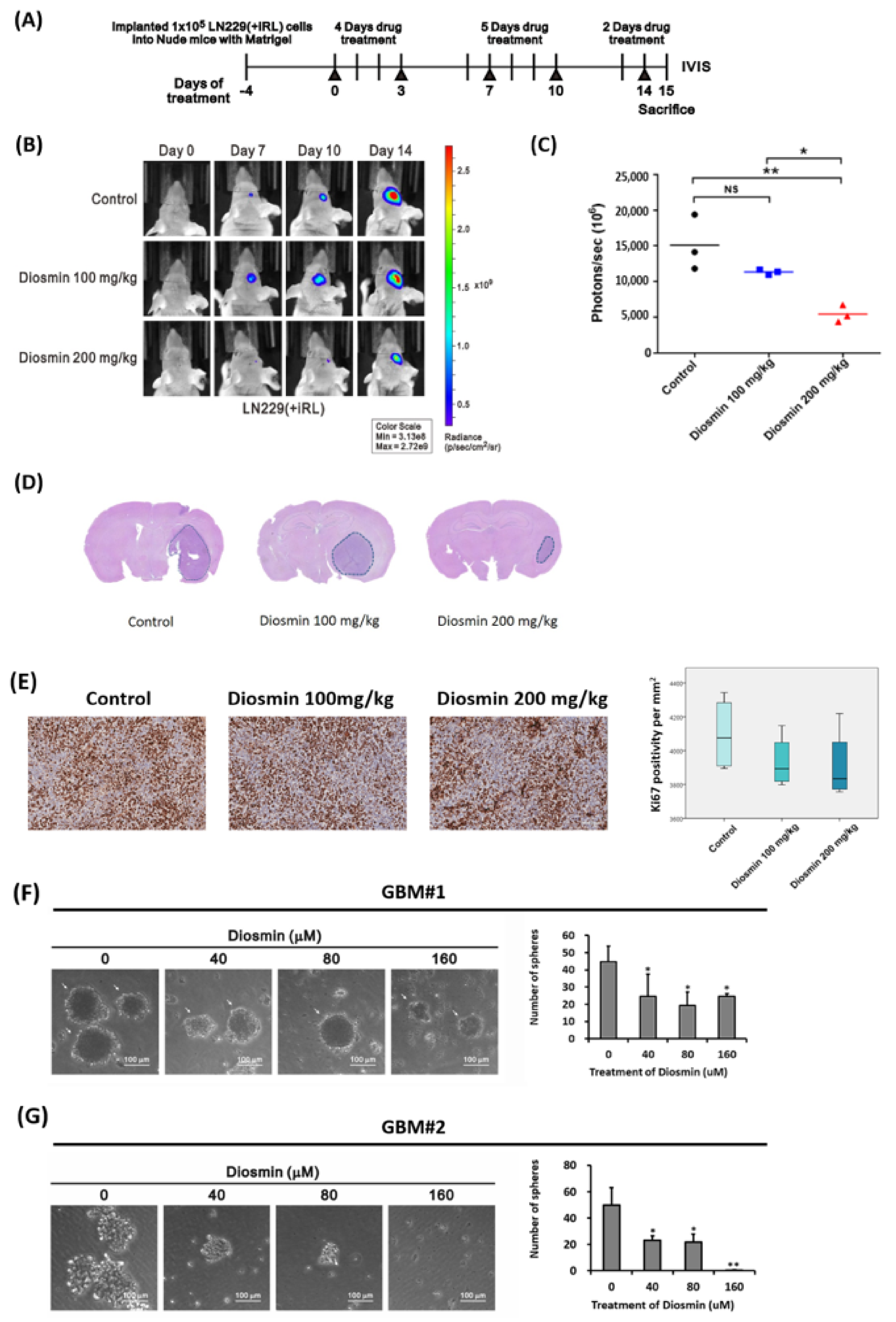
3.6. Diosmin Diminishes Tumor Bulk in a Xenograft Mouse Model and Tumor Sphere Formation in Clinical Specimens
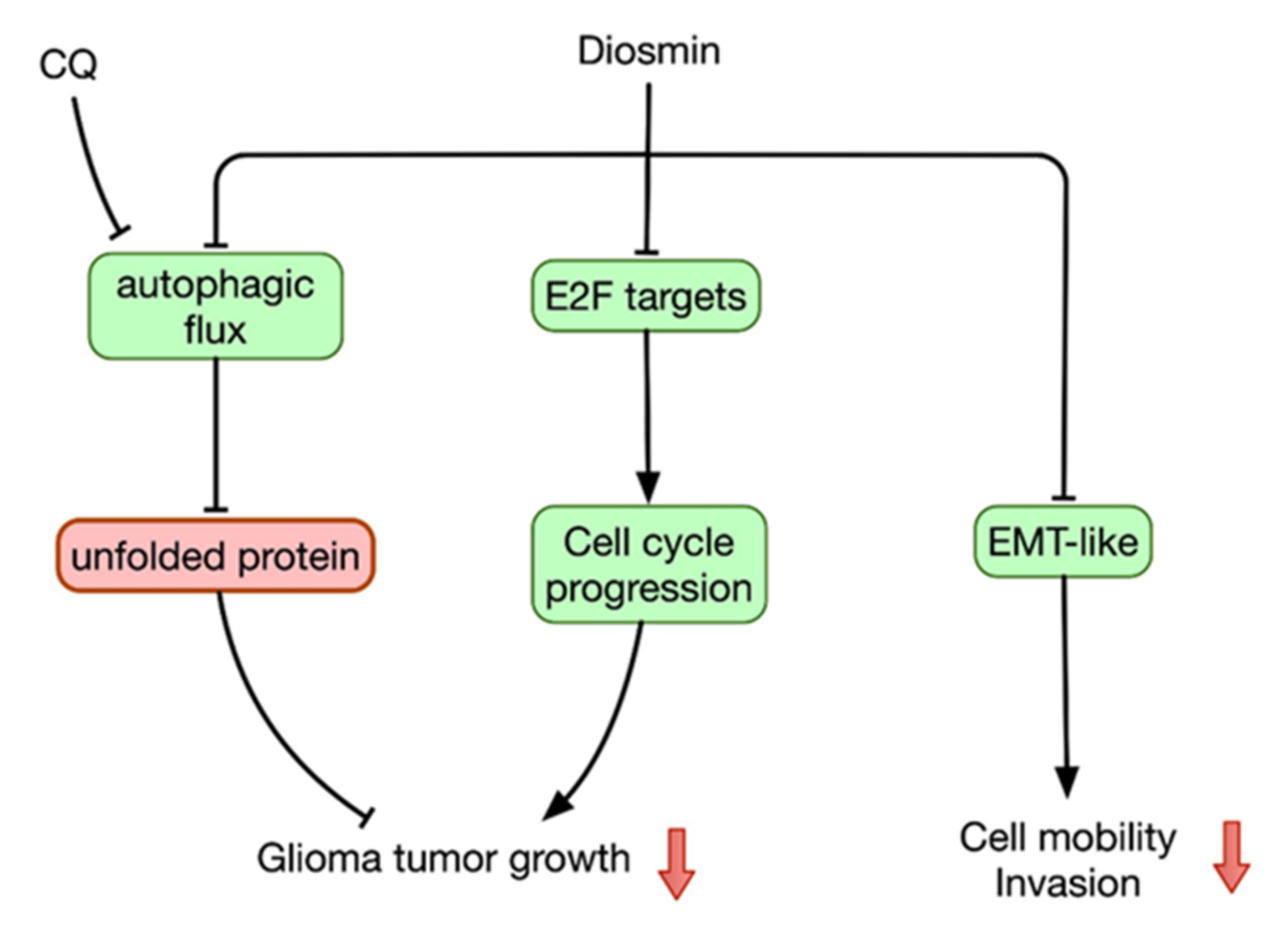
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Dolecek, T.A.; Propp, J.M.; Stroup, N.E.; Kruchko, C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-Oncology 2012, 14, v1–v49. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Selmin, O.I. Flavonoids and cancer prevention: A review of the evidence. J. Nutr. Gerontol. Geriatr. 2012, 31, 206–238. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, X.; Zhang, J.; Kang, N.; Zhang, N.; Wang, H.; Xue, J.; Yu, J.; Yang, Y.; Cui, H.; et al. Diosmin protects against cerebral ischemia/reperfusion injury through activating JAK2/STAT3 signal pathway in mice. Neuroscience 2014, 268, 318–327. [Google Scholar] [CrossRef]
- Lewinska, A.; Siwak, J.; Rzeszutek, I.; Wnuk, M. Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol. In Vitro 2015, 29, 417–425. [Google Scholar] [CrossRef]
- Carballo-Villalobos, A.I.; Gonzalez-Trujano, M.E.; Pellicer, F.; Lopez-Munoz, F.J. Antihyperalgesic Effect of Hesperidin Improves with Diosmin in Experimental Neuropathic Pain. Biomed. Res. Int. 2016, 2016, 8263463. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.K.; Huang, L.C.; Tsai, W.C.; Huang, S.M.; Lee, J.T.; Hueng, D.Y. Overexpression of PLOD3 promotes tumor progression and poor prognosis in gliomas. Oncotarget 2018, 9, 15705–15720. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.K.; Huang, L.C.; Wu, Y.P.; Kan, I.Y.; Hueng, D.Y. SNAP reverses temozolomide resistance in human glioblastoma multiforme cells through down-regulation of MGMT. FASEB J. 2019, 33, 14171–14184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.; Zhang, W.; You, G.; Zhang, J.; Han, L.; Bao, Z.; Wang, Y.; Liu, Y.; Jiang, C.; Kang, C.; et al. Molecular classification of gliomas based on whole genome gene expression: A systematic report of 225 samples from the Chinese Glioma Cooperative Group. Neuro-Oncology 2012, 14, 1432–1440. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahlert, U.D.; Nikkhah, G.; Maciaczyk, J. Epithelial-to-mesenchymal(-like) transition as a relevant molecular event in malignant gliomas. Cancer Lett. 2013, 331, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Rusten, T.E.; Stenmark, H. p62, an autophagy hero or culprit? Nat. Cell Biol. 2010, 12, 207–209. [Google Scholar] [CrossRef]
- Gohel, M.; Davies, A. Pharmacological Agents in the Treatment of Venous Disease: An Update of the Available Evidence. Curr. Vasc. Pharmacol. 2009, 7, 303–308. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Cao, J.D. The impact of micronized purified flavonoid fraction on the treatment of acute haemorrhoidal episodes. Curr. Med. Res. Opin. 2006, 22, 1141–1147. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Kandel, J.J.; Yamashiro, D.J.; Canoll, P.; Anastassiou, D. A multi-cancer mesenchymal transition gene expression signature is associated with prolonged time to recurrence in glioblastoma. PLoS ONE 2012, 7, e34705. [Google Scholar] [CrossRef]
- Yan, Y.R.; Xie, Q.; Li, F.; Zhang, Y.; Ma, J.W.; Xie, S.M.; Li, H.Y.; Zhong, X.Y. Epithelial-to-mesenchymal transition is involved in BCNU resistance in human glioma cells. Neuropathology 2014, 34, 128–134. [Google Scholar] [CrossRef]
- Mahabir, R.; Tanino, M.; Elmansuri, A.; Wang, L.; Kimura, T.; Itoh, T.; Ohba, Y.; Nishihara, H.; Shirato, H.; Tsuda, M.; et al. Sustained elevation of Snail promotes glial-mesenchymal transition after irradiation in malignant glioma. Neuro-Oncology 2014, 16, 671–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.A.; Srinivasan, S.; Patric, I.R.; Hegde, A.S.; Chandramouli, B.A.; Arimappamagan, A.; Santosh, V.; Kondaiah, P.; Rao, M.R.; Somasundaram, K. A 16-gene signature distinguishes anaplastic astrocytoma from glioblastoma. PLoS ONE 2014, 9, e85200. [Google Scholar] [CrossRef] [PubMed]
- Velpula, K.K.; Dasari, V.R.; Tsung, A.J.; Dinh, D.H.; Rao, J.S. Cord blood stem cells revert glioma stem cell EMT by down regulating transcriptional activation of Sox2 and Twist1. Oncotarget 2011, 2, 1028–1042. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Xiao, L.; Liu, Y.; Wang, H.; Li, H.; Zhou, Q.; Pan, J.; Lei, B.; Huang, A.; Qi, S. MIR517C inhibits autophagy and the epithelial-to-mesenchymal (-like) transition phenotype in human glioblastoma through KPNA2-dependent disruption of TP53 nuclear translocation. Autophagy 2015, 11, 2213–2232. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.L.; Wustenberg, R.; Rubsam, A.; Schmitz-Salue, C.; Warnecke, G.; Bucker, E.M.; Pettkus, N.; Speidel, D.; Rohde, V.; Schulz-Schaeffer, W.; et al. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro-Oncology 2010, 12, 389–400. [Google Scholar] [CrossRef]
- Sotelo, J.; Briceno, E.; Lopez-Gonzalez, M.A. Adding chloroquine to conventional treatment for glioblastoma multiforme: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2006, 144, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Buccarelli, M.; Marconi, M.; Pacioni, S.; De Pascalis, I.; D’Alessandris, Q.G.; Martini, M.; Ascione, B.; Malorni, W.; Larocca, L.M.; Pallini, R.; et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Tsai, S.Y.; Leone, G. Emerging roles of E2Fs in cancer: An exit from cell cycle control. Nat. Rev. Cancer 2009, 9, 785–797. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef]
- Jiang, H.; Martin, V.; Gomez-Manzano, C.; Johnson, D.G.; Alonso, M.; White, E.; Xu, J.; McDonnell, T.J.; Shinojima, N.; Fueyo, J. The RB-E2F1 pathway regulates autophagy. Cancer Res. 2010, 70, 7882–7893. [Google Scholar] [CrossRef] [Green Version]
- Polager, S.; Ofir, M.; Ginsberg, D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene 2008, 27, 4860–4864. [Google Scholar] [CrossRef] [Green Version]
- Madden, E.; Logue, S.E.; Healy, S.J.; Manie, S.; Samali, A. The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance. Biol. Cell 2019, 111, 1–17. [Google Scholar] [CrossRef]
- Corazzari, M.; Gagliardi, M.; Fimia, G.M.; Piacentini, M. Endoplasmic Reticulum Stress, Unfolded Protein Response, and Cancer Cell Fate. Front. Oncol. 2017, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Dastghaib, S.; Shojaei, S.; Mostafavi-Pour, Z.; Sharma, P.; Patterson, J.B.; Samali, A.; Mokarram, P.; Ghavami, S. Simvastatin Induces Unfolded Protein Response and Enhances Temozolomide-Induced Cell Death in Glioblastoma Cells. Cells 2020, 9, 2339. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; Day, L.L.; Harwood, J.; Ying, C.; St John, L.N.; Giles, R.; Neeley, C.K.; Pienta, K.J. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia 2006, 8, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Zhou, Y.; Su, Z.; Yan, A.; Ding, P. Effect of CCL2 siRNA on proliferation and apoptosis in the U251 human glioma cell line. Mol. Med. Rep. 2017, 16, 3387–3394. [Google Scholar] [CrossRef]
- Shono, K.; Yamaguchi, I.; Mizobuchi, Y.; Kagusa, H.; Sumi, A.; Fujihara, T.; Nakajima, K.; Kitazato, K.T.; Matsuzaki, K.; Saya, H.; et al. Downregulation of the CCL2/CCR2 and CXCL10/CXCR3 axes contributes to antitumor effects in a mouse model of malignant glioma. Sci. Rep. 2020, 10, 15286. [Google Scholar] [CrossRef] [PubMed]
- Roca, H.; Varsos, Z.S.; Mizutani, K.; Pienta, K.J. CCL2, survivin and autophagy: New links with implications in human cancer. Autophagy 2008, 4, 969–971. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.M.; Faria, B.M.; Ascari, L.M.; Souza, J.M.; Soares, A.G.; Cordeiro, Y.; Romao, L.F. Diosmin induces caspase-dependent apoptosis in human glioblastoma cells. An. Acad. Bras. Cienc. 2019, 91, e20191031. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-L.; Li, Y.-F.; Chou, C.-H.; Huang, L.-C.; Wu, Y.-P.; Kao, Y.; Tsai, C.-K. Diosmin Inhibits Glioblastoma Growth through Inhibition of Autophagic Flux. Int. J. Mol. Sci. 2021, 22, 10453. https://doi.org/10.3390/ijms221910453
Chang Y-L, Li Y-F, Chou C-H, Huang L-C, Wu Y-P, Kao Y, Tsai C-K. Diosmin Inhibits Glioblastoma Growth through Inhibition of Autophagic Flux. International Journal of Molecular Sciences. 2021; 22(19):10453. https://doi.org/10.3390/ijms221910453
Chicago/Turabian StyleChang, Yung-Lung, Yao-Feng Li, Chung-Hsing Chou, Li-Chun Huang, Yi-Ping Wu, Ying Kao, and Chia-Kuang Tsai. 2021. "Diosmin Inhibits Glioblastoma Growth through Inhibition of Autophagic Flux" International Journal of Molecular Sciences 22, no. 19: 10453. https://doi.org/10.3390/ijms221910453
APA StyleChang, Y.-L., Li, Y.-F., Chou, C.-H., Huang, L.-C., Wu, Y.-P., Kao, Y., & Tsai, C.-K. (2021). Diosmin Inhibits Glioblastoma Growth through Inhibition of Autophagic Flux. International Journal of Molecular Sciences, 22(19), 10453. https://doi.org/10.3390/ijms221910453






