Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview
Abstract
:1. Introduction
2. Chemistry of Melatonin
2.1. N1-Acetyl-N2-Formyl-5-Methoxykynuramine (AFMK)
2.2. N1-Acetyl-5-Methoxykynuramine (AMK)
2.3. 3-Hydroxymelatonin (C3-OHM)
2.4. 6-Hydroxymelatonin (6-OHM)
2.5. 2-Hydroxymelatonin (2-OHM)
3. Biosynthesis of Melatonin
3.1. Biosynthetic Route in Plants
3.2. Biosynthetic Route in Animals
3.3. Focus on the Enzymes Involved in the Biosynthetic Routes
3.3.1. L-Tryptophan Decarboxylase (TDC) (EC 4.1.1.105)
3.3.2. Tryptamine 5-Hydroxylase (T5H) (EC 1.14.-.-)
3.3.3. Serotonin N-Acetyltransferase (SNAT) (EC 2.3.1.87)
3.3.4. Acetylserotonin O-Methyltransferase (ASMT) (EC 2.1.1.4)
3.3.5. Caffeic Acid 3-O-Methyltransferase (COMT) (EC 2.1.1.68)
4. Distribution of Melatonin in Plants
4.1. Phytomelatonin Content within Plant Species
4.2. Phytomelatonin Content within Plant Families
4.3. Phytomelatonin Content in Plant Tissues
5. Role of Phytomelatonin in Plants
5.1. Melatonin as a Promoter or Inhibitor of Plant Growth
5.2. Melatonin Affects Seed Germination and Plant Performances
5.3. Melatonin Affects Photosynthetic Efficiency
5.4. Melatonin Affects Antioxidant Defence System
5.5. Melatonin Interactions with Plant Hormones
5.6. Melatonin Affects Primary and Secondary Metabolism
6. Role of Melatonin in Animals
6.1. Melatonin in Sleep Disorders
6.2. Melatonin as Antioxidant
6.3. Melatonin as Geroprotective Agent
6.4. Melatonin in Other Pathological Conditions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of Melatonin and 5-Methoxyindole-3-acetic Acid from Bovine Pineal Glands. J. Biol. Chem. 1960, 235, 1992–1997. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Pshenichnyuk, S.A.; Modelli, A.; Jones, D.; Lazneva, E.F.; Komolov, A.S. Low-Energy Electron Interaction with Melatonin and Related Compounds. J. Phys. Chem. B 2017, 121, 3965–3974. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar]
- Tilden, A.R.; Becker, M.A.; Amma, L.L.; Arciniega, J.; McGaw, A.K. Melatonin production in an aerobic photosynthetic bacterium: An evolutionarily early association with darkness. J. Pineal Res. 1997, 22, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Majima, A.; Hattori, A.; Wada, M.; Chiba, A.; Aoki, K. Dynamic Release of Melatonin in Cyanobacterium, Spirulina Platensis. Proc. Jpn. Soc. Comp. Endocrinol. 1999, 14, 50. [Google Scholar]
- Hattori, A.; Wada, M.; Majima, A.; Tabata, M.; Itoh, M. Detection of Melatonin and Synthesizing Enzyme Activities in Cyanobacterium, Spirulina Platensis. Proc. Jpn. Soc. Comp. Endocrinol. 1999, 14, 49. [Google Scholar]
- Pöggeler, B.; Balzer, I.; Fischer, J.; Behrmann, G.; Hardeland, R.; Zoologisches Institut. A role of melatonin in dinoflagellates? Eur. J. Endocrinol. 1989, 29, S97. [Google Scholar] [CrossRef]
- Balzer, I.; Hardeland, R. Photoperiodism and effects of indoleamines in a unicellular alga, Gonyaulax polyedra. Science 1991, 253, 795–797. [Google Scholar] [CrossRef]
- Pöggeler, B.; Balzer, I.; Hardeland, R.; Lerchl, A. Pineal hormone melatonin oscillates also in the dinoflagellate Gonyaulax polyedra. Naturwissenschaften 1991, 78, 268–269. [Google Scholar] [CrossRef]
- Fuhrberg, B.; Balzer, I.; Hardeland, R.; Werner, A.; Lüning, K. The vertebrate pineal hormone melatonin is produced by the brown alga Pterygophora californica and mimics dark effects on growth rate in the light. Planta 1996, 200, 125–131. [Google Scholar] [CrossRef]
- Kurland, C.G.; Andersson, S.G.E. Origin and Evolution of the Mitochondrial Proteome. Microbiol. Mol. Biol. Rev. 2000, 64, 786–820. [Google Scholar] [CrossRef] [Green Version]
- Murch, S.J.; Erland, L.A.E. A Systematic Review of Melatonin in Plants: An Example of Evolution of Literature. Front. Plant Sci. 2021, 12, 1060. [Google Scholar] [CrossRef]
- Mannino, G.; Caradonna, F.; Cruciata, I.; Lauria, A.; Perrone, A.; Gentile, C. Melatonin reduces inflammatory response in human intestinal epithelial cells stimulated by interleukin-1β. J. Pineal Res. 2019, 67, e12598. [Google Scholar] [CrossRef]
- Goldman, B.D.; Nelson, R.J. Melatonin and seasonality in mammals. In Melatonin; CRC Press: New York NY, USA, 2020; pp. 225–252. ISBN 100306857X. [Google Scholar]
- Tosches, M.A.; Bucher, D.; Vopalensky, P.; Arendt, D. Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 2014, 159, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Schippers, K.J.; Nichols, S.A. Deep, dark secrets of melatonin in animal evolution. Cell 2014, 159, 9–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Manchanda, R.; Goswami, R.; Chawla, S. Biodiversity and Therapeutic Potential of Medicinal Plants. In Environmental Concerns and Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2020; pp. 27–44. [Google Scholar]
- Pallante, L.A.; Gala, D.N.; Farajzadeh-Asl, S.; Ullal, G. The Potential Applicability of Melatonin as an Immunosuppressive Agent for COVID-19. EC Neurol. 2021, 13, 11–21. [Google Scholar]
- Hardeland, R.; Fuhrberg, B.; Uría, H.; Behrmann, G.; Meyer, T.J.; Burkhardt, S.; Poeggeler, B. Chronobiology of indoleamines in the dinoflagellate Gonyaulax polyedra: Metabolism and effects related to circadian rhythmicity and photoperiodism. Braz. J. Med. Biol. Res. 1996, 29, 119–123. [Google Scholar] [PubMed]
- Kolář, J.; Macháčková, I.; Eder, J.; Prinsen, E.; Van Dongen, W.; Van Onckelen, H.; Illnerová, H. Melatonin: Occurrence and daily rhythm in Chenopodium rubrum. Phytochemistry 1997, 44, 1407–1413. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Roy, J.; Veresoglou, S.D.; Rillig, M.C. Soil biodiversity enhances the persistence of legumes under climate change. New Phytol. 2021, 229, 2945–2956. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Tan, X.; Fan, Z.; Zeng, Z.; Shan, W.; Kuang, J.; Lu, W.; Su, X.; Tao, N.; Lakshmanan, P.; Chen, J. Exogenous melatonin maintains leaf quality of postharvest Chinese flowering cabbage by modulating respiratory metabolism and energy status. Postharvest Biol. Technol. 2021, 177, 111524. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Zhang, Z.-W.; Chen, Y.-E.; Ding, C.-B.; Yuan, S.; Reiter, R.J.; Yuan, M. Melatonin: A potential agent in delaying leaf senescence. CRC Crit. Rev. Plant Sci. 2021, 40, 1–22. [Google Scholar] [CrossRef]
- Mansouri, S.; Sarikhani, H.; Sayyari, M.; Aghdam, M.S. Melatonin accelerates strawberry fruit ripening by triggering GAMYB gene expression and promoting ABA accumulation. Sci. Hortic. 2021, 281, 109919. [Google Scholar] [CrossRef]
- Shi, H.; Chen, K.; Wei, Y.; He, C. Fundamental issues of melatonin-mediated stress signaling in plants. Front. Plant Sci. 2016, 7, 1124. [Google Scholar] [CrossRef] [Green Version]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Zheng, B. Melatonin mediated regulation of drought stress: Physiological and molecular aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, J.; Sharma, A.; Tao, S.; Zheng, B.; Landi, M.; Yuan, H.; Yan, D. Melatonin Stimulates Activities and Expression Level of Antioxidant Enzymes and Preserves Functionality of Photosynthetic Apparatus in Hickory Plants (Carya cathayensis Sarg.) under PEG-Promoted Drought. Agronomy 2019, 9, 702. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- Arnao, M.B. Phytomelatonin: Discovery, Content, and Role in Plants. Adv. Bot. 2014, 2014, 815769. [Google Scholar] [CrossRef]
- Omer, R.A.; Koparir, P.; Ahmed, L.; Koparir, M. Computational and spectroscopy study of melatonin. Indian J. Chem. B 2021, 60, 732–741. [Google Scholar]
- Tan, D.; Reiter, R.; Manchester, L.; Yan, M.; El-Sawi, M.; Sainz, R.; Mayo, J.; Kohen, R.; Allegra, M.; Hardelan, R. Chemical and Physical Properties and Potential Mechanisms: Melatonin as a Broad Spectrum Antioxidant and Free Radical Scavenger. Curr. Top. Med. Chem. 2005, 2, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P.; Tan, D.X.; Manchester, L.C.; Reiter, R.J. Purslane: A plant source of omega-3 fatty acids and melatonin. J. Pineal Res. 2005, 39, 331–332. [Google Scholar] [CrossRef]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Reina, M.; Martínez, A. A new free radical scavenging cascade involving melatonin and three of its metabolites (3OHM, AFMK and AMK). Comput. Theor. Chem. 2018, 1123, 111–118. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Burkhardt, S.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Shohami, E.; Huo, Y.S.; Hardeland, R.; Reiter, R.J. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001, 15, 2294–2296. [Google Scholar] [CrossRef]
- Rosen, J.; Than, N.N.; Koch, D.; Poeggeler, B.; Laatsch, H.; Hardeland, R. Interactions of melatonin and its metabolites with the ABTS cation radical: Extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res. 2006, 41, 374–381. [Google Scholar] [CrossRef]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Iwashita, H.; Matsumoto, Y.; Maruyama, Y.; Watanabe, K.; Chiba, A.; Hattori, A. The melatonin metabolite N1-acetyl-5-methoxykynuramine facilitates long-term object memory in young and aging mice. J. Pineal Res. 2021, 70, e12703. [Google Scholar] [CrossRef]
- Silva, S.O.; Rodrigues, M.R.; Carvalho, S.R.Q.; Catalani, L.H.; Campa, A.; Ximenes, V.F. Oxidation of melatonin and its catabolites, N1-acetyl-N 2-formyl-5-methoxykynuramine and N1-acetyl-5-methoxykynuramine, by activated leukocytes. J. Pineal Res. 2004, 37, 171–175. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Tan, D.-X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and synergistic antioxidative actions of melatonin: Studies with vitamin E, vitamin C, glutathione and desferrrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 2010, 53, 1393–1401. [Google Scholar] [CrossRef]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef]
- Ressmeyer, A.R.; Mayo, J.C.; Zelosko, V.; Sáinz, R.M.; Tan, D.X.; Poeggeler, B.; Antolín, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef] [Green Version]
- León, J.; Escames, G.; Rodríguez, M.I.; López, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrión, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N 1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006, 98, 2023–2033. [Google Scholar] [CrossRef]
- Hardeland, R.; Backhaus, C.; Fadavi, A.; Hess, M. N1-acetyl-5-methoxykynuramine contrasts with other tryptophan metabolites by a peculiar type of NO scavenging: Cyclization to a cinnolinone prevents formation of unstable nitrosamines. J. Pineal Res. 2007, 43, 104–105. [Google Scholar] [CrossRef]
- Hacışevki, A.; Baba, B. An overview of melatonin as an antioxidant molecule: A biochemical approach. Melatonin Mol. Biol. Clin. Pharm. Approaches 2018, 59–85. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Poeggeler, B.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J. Pineal Res. 2003, 34, 249–259. [Google Scholar] [CrossRef]
- Hara, M.; Iigo, M.; Ohtani-Kaneko, R.; Nakamura, N.; Suzuki, T.; Reiter, R.J.; Hirata, K. Administration of melatonin and related indoles prevents exercise-induced cellular oxidative changes in rats. Neurosignals 1997, 6, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Siu, A.W.; Garcia, J.J. Increased levels of oxidatively damaged DNA induced by chromium(III) and H2O2: Protection by melatonin and related molecules. J. Pineal Res. 2000, 29, 54–61. [Google Scholar] [CrossRef] [PubMed]
- López-Burillo, S.; Tan, D.X.; Rodriguez-Gallego, V.; Manchester, L.C.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin and its derivatives cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine and 6-methoxymelatonin reduce oxidative DNA damage induced by Fenton reagents. J. Pineal Res. 2003, 34, 178–184. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, Z.; Lu, T.; Chen, J.; Wang, X. Comparison of 6-hydroxylmelatonin or melatonin in protecting neurons against ischemia/reperfusion-mediated injury. J. Pineal Res. 2006, 41, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Sakano, K.; Oikawa, S.; Hiraku, Y.; Kawanishi, S. Oxidative DNA damage induced by a melatonin metabolite, 6-hydroxymelatonin, via a unique non-o-quinone type of redox cycle. Biochem. Pharmacol. 2004, 68, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Dellegar, S.M.; Murphy, S.A.; Bourne, A.E.; Dicesare, J.C.; Purser, G.H. Identification of the factors affecting the rate of deactivation of hypochlorous acid by melatonin. Biochem. Biophys. Res. Commun. 1999, 257, 431–439. [Google Scholar] [CrossRef]
- Agozzino, P.; Avellone, G.; Bongiorno, D.; Ceraulo, L.; Filizzola, F.; Natoli, M.C.; Livrea, M.A.; Tesoriere, L. Melatonin: Structural characterization of its non-enzymatic mono-oxygenate metabolite. J. Pineal Res. 2003, 35, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Horstman, J.A.; Wrona, M.Z.; Dryhurst, G. Further insights into the reaction of melatonin with hydroxyl radical. Bioorg. Chem. 2002, 30, 371–382. [Google Scholar] [CrossRef]
- Semak, I.; Naumova, M.; Korik, E.; Terekhovich, V.; Wortsman, J.; Slominski, A. A novel metabolic pathway of melatonin: Oxidation by cytochrome c. Biochemistry 2005, 44, 9300–9307. [Google Scholar] [CrossRef]
- Yu, Y.; Bian, L.; Jiao, Z.; Yu, K.; Wan, Y.; Zhang, G.; Guo, D. Molecular cloning and characterization of a grapevine (Vitis vinifera L.) serotonin N-acetyltransferase (VvSNAT2) gene involved in plant defense. BMC Genom. 2019, 20, 880. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Xie, Q.; Liu, Y.; Lv, H.; Bai, R.; Ma, R.; Li, X.; Zhang, X.; Guo, Y.D.; et al. SlSNAT Interacts with HSP40, a Molecular Chaperone, to Regulate Melatonin Biosynthesis and Promote Thermotolerance in Tomato. Plant Cell Physiol. 2020, 61, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef] [PubMed]
- Vigliante, I.; Mannino, G.; Maffei, M.E. Chemical Characterization and DNA Fingerprinting of Griffonia simplicifolia Baill. Molecules 2019, 24, 1032. [Google Scholar] [CrossRef] [Green Version]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, Distribution, Ecological Role, and Use of Biostimulants to Increase Their Content in Plant Foods—A Review. Agriculture 2021, 11, 212. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Byeon, Y.; Lee, K.; Lee, H.J.; Back, K. Cloning of Arabidopsis serotonin N-acetyltransferase and its role with caffeic acid O-methyltransferase in the biosynthesis of melatonin in vitro despite their different subcellular localizations. J. Pineal Res. 2014, 57, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Byeon, Y.; Back, K. Functional analyses of three ASMT gene family members in rice plants. J. Pineal Res. 2013, 55, 409–415. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Back, K. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J. Pineal Res. 2014, 57, 219–227. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, L.; Liu, X.; Liu, B.; Chen, X.; Guo, Y.; Huang, C.; Zhao, Y.; Zeng, Z. Crystal structure of Oryza sativa TDC reveals the substrate specificity for TDC-mediated melatonin biosynthesis. J. Adv. Res. 2020, 24, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yao, Z.; Zhang, J.; Zhang, R.; Mou, Z.; Zhang, X.; Li, Z.; Feng, X.; Chen, S.; Reiter, R.J. Melatonin synthesis genes N-acetylserotonin methyltransferases evolved into caffeic acid O-methyltransferases and both assisted in plant terrestrialization. J. Pineal Res. 2021, e12737. [Google Scholar] [CrossRef]
- Kang, K.; Park, S.; Kim, Y.S.; Lee, S.; Back, K. Biosynthesis and biotechnological production of serotonin derivatives. Appl. Microbiol. Biotechnol. 2009, 83, 27–34. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa). J. Pineal Res. 2015, 58, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zawadzka, A.; Czarnocki, Z.; Reiter, R.J.; Back, K. Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J. Pineal Res. 2016, 61, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Yin, X.; Yu, L.; Zheng, S.J.; Cai, W.J.; Wu, Y.; Feng, Y.Q. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J. Pineal Res. 2019, 66, e12531. [Google Scholar] [CrossRef] [Green Version]
- Byeon, Y.; Choi, G.H.; Lee, H.Y.; Back, K. Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice. J. Exp. Bot. 2015, 66, 6917–6925. [Google Scholar] [CrossRef] [Green Version]
- Koshiba, T.; Hirose, N.; Mukai, M.; Yamamura, M.; Hattori, T.; Suzuki, S.; Sakamoto, M.; Umezawa, T. Characterization of 5-hydroxyconiferaldehyde O-methyltransferase in Oryza sativa. Plant Biotechnol. 2013, 30, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Byeon, Y.; Park, S.; Kim, Y.S.; Park, D.H.; Lee, S.; Back, K. Light-regulated melatonin biosynthesis in rice during the senescence process in detached leaves. J. Pineal Res. 2012, 53, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in plants and other phototrophs: Advances and gaps concerning the diversity of functions. J. Exp. Bot. 2015, 66, 627–646. [Google Scholar] [CrossRef] [Green Version]
- Byeon, Y.; Lee, H.J.; Lee, H.Y.; Back, K. Cloning and functional characterization of the Arabidopsis N-acetylserotonin O-methyltransferase responsible for melatonin synthesis. J. Pineal Res. 2016, 60, 65–73. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Back, K. Chloroplastic and cytoplasmic overexpression of sheep serotonin N-acetyltransferase in transgenic rice plants is associated with low melatonin production despite high enzyme activity. J. Pineal Res. 2015, 58, 461–469. [Google Scholar] [CrossRef]
- Park, S.; Byeon, Y.; Back, K. Transcriptional suppression of tryptamine 5-hydroxylase, a terminal serotonin biosynthetic gene, induces melatonin biosynthesis in rice (Oryza sativa L.). J. Pineal Res. 2013, 55, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.; Kim, Y.S.; Back, K. Tryptamine 5-hydroxylase-deficient Sekiguchi rice induces synthesis of 5-hydroxytryptophan and N-acetyltryptamine but decreases melatonin biosynthesis during senescence process of detached leaves. J. Pineal Res. 2012, 52, 211–216. [Google Scholar] [CrossRef]
- Balemans, M.G.M.; Ebels, I.; Hendriks, H.G.; van Berlo, M.; de Moreé, A. The influence of some pteridines on pineal 5-methoxyindole synthesis in male Wistar rats periodically exposed to either white or green light. J. Neural Transm. 1983, 58, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ortiz, M.; Sayed, R.K.A.; Fernández-Martínez, J.; Cionfrini, A.; Aranda-Martínez, P.; Escames, G.; de Haro, T.; Acuña-Castroviejo, D. Melatonin/Nrf2/NLRP3 connection in mouse heart mitochondria during aging. Antioxidants 2020, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R.; Poeggeler, B. Non-vertebrate melatonin. J. Pineal Res. 2003, 34, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Weissbach, H.; Redfield, B.G.; Axelrod, J. Biosynthesis of melatonin: Enzymic conversion of serotonin to N-acetylserotonin. BBA-Biochim. Biophys. Acta 1960, 43, 352–353. [Google Scholar] [CrossRef]
- Pevet, P.; Klosen, P.; Felder-Schmittbuhl, M.-P. The hormone melatonin: Animal studies. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin-A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [Green Version]
- Velarde, E.; Cerdá-Reverter, J.M.; Alonso-Gómez, A.L.; Sánchez, E.; Isorna, E.; Delgado, M.J. Melatonin-synthesizing enzymes in pineal, retina, liver, and gut of the goldfish (Carassius): MRNA expression pattern and regulation of daily rhythms by lighting conditions. Chronobiol. Int. 2010, 27, 1178–1201. [Google Scholar] [CrossRef] [Green Version]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Julius Axelrod, J.; Weissbach, H. Purification and Properties of Hydroxyindole-O-meth-vl Transferase. J. Biol. Chem. 1961, 236, 211–213. [Google Scholar] [CrossRef]
- Skene, D.J. Optimization of light and melatonin to phase-shift human circadian rhythms. J. Neuroendocrinol. 2003, 15, 438–441. [Google Scholar] [CrossRef]
- Ganguly, S.; Weller, J.L.; Ho, A.; Chemineau, P.; Malpaux, B.; Klein, D.C. Melatonin synthesis: 14-3-3-Dependent activation and inhibition of arylalkylamine N-acetyltransferase mediated by phosphoserine-205. Proc. Natl. Acad. Sci. USA 2005, 102, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.X.; Manchester, L.C.; Fuentes-Broto, L.; Paredes, S.D.; Reiter, R.J. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: Relation to human obesity. Obes. Rev. 2011, 12, 167–188. [Google Scholar] [CrossRef]
- Noé, W.; Mollenschott, C.; Berlin, J. Tryptophan decarboxylase from Catharanthus roseus cell suspension cultures: Purification, molecular and kinetic data of the homogenous protein. Plant Mol. Biol. 1984, 3, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kang, K.; Lee, K.; Back, K. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 2007, 227, 263–272. [Google Scholar] [CrossRef]
- De Luca, V.; Marineau, C.; Brisson, N. Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: Comparison with animal dopa decarboxylases. Proc. Natl. Acad. Sci. USA 1989, 86, 2582–2586. [Google Scholar] [CrossRef] [Green Version]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J. Pineal Res. 2014, 57, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Maisonneuve, S.; Isshiki, M.; Mizutani, M.; Chen, L.; Ling Wong, H.; Kawasaki, T.; Shimamoto, K. Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice. J. Biol. Chem. 2010, 285, 11308–11313. [Google Scholar] [CrossRef] [Green Version]
- Byeon, Y.; Park, S.; Lee, H.Y.; Kim, Y.S.; Back, K. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J. Pineal Res. 2014, 56, 275–282. [Google Scholar] [CrossRef]
- Nguyen Le, T.N.; Byeon, Y.; Kim, Y.S.; Back, K. Transient induction of melatonin biosynthesis in rice (Oryza sativa L.) during the reproductive stage Sangkyu Park1. J. Pineal Res. 2013, 55, 40–45. [Google Scholar]
- Murch, S.J.; Saxena, P.K. A melatonin-rich germplasm line of St John’s wort (Hypericum perforatum L.). J. Pineal Res. 2006, 41, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kang, K.; Lee, K.; Back, K. Characterization of tryptamine 5-hydroxylase and serotonin synthesis in rice plants. Plant Cell Rep. 2007, 26, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Coon, S.L.; Mazuruk, K.; Bernard, M.; Roseboom, P.H.; Klein, D.C.; Rodriguez, I.R. The human serotonin N-acetyltransferase (EC 2.3.1.87) Gene (AANAT): Structure, chromosomal localization, and tissue expression. Genomics 1996, 34, 76–84. [Google Scholar] [CrossRef]
- Okazaki, M.; Higuchi, K.; Hanawa, Y.; Shiraiwa, Y.; Ezura, H. Cloning and characterization of a Chlamydomonas reinhardtii cDNA arylalkylamine N-acetyltransferase and its use in the genetic engineering of melatonin content in the Micro-Tom tomato. J. Pineal Res. 2009, 46, 373–382. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Choi, D.W.; Back, K. Chloroplast-encoded serotonin N-acetyltransferase in the red alga Pyropia yezoensis: Gene transition to the nucleus from chloroplasts. J. Exp. Bot. 2015, 66, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.; Lee, K.; Park, S.; Byeon, Y.; Back, K. Molecular cloning of rice serotonin N-acetyltransferase, the penultimate gene in plant melatonin biosynthesis. J. Pineal Res. 2013, 55, 7–13. [Google Scholar] [CrossRef]
- Park, S.; Byeon, Y.; Lee, H.Y.; Kim, Y.S.; Ahn, T.; Back, K. Cloning and characterization of a serotonin N-acetyltransferase from a gymnosperm, loblolly pine (Pinus taeda). J. Pineal Res. 2014, 57, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Wang, L.; Tan, D.X.; Zhao, Y.; Zheng, X.D.; Chen, H.; Li, Q.T.; Zuo, B.X.; Kong, J. Identification of genes for melatonin synthetic enzymes in “Red Fuji” apple (Malus domestica Borkh.cv.Red) and their expression and melatonin production during fruit development. J. Pineal Res. 2013, 55, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Sagan, L. On the Origin of Mitosing Cdls. J. Theor. Biol. 1967, 14, 255–274. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin, hormone of darkness and more—Occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef] [Green Version]
- Yet, S.-F.; Chen, C.-H. Hydroxyindole o-methyltransferase in 5-methoxytryptop-han synthesis and function in vascular smooth muscle cells. Atherosclerosis 2020, 315, e61. [Google Scholar] [CrossRef]
- Kang, K.; Kong, K.; Park, S.; Natsagdorj, U.; Kim, Y.S.; Back, K. Molecular cloning of a plant N-acetylserotonin methyltransferase and its expression characteristics in rice. J. Pineal Res. 2011, 50, 304–309. [Google Scholar] [CrossRef]
- Park, S.; Byeon, Y.; Kim, Y.S.; Back, K. Kinetic analysis of purified recombinant rice N-acetylserotonin methyltransferase and peak melatonin production in etiolated rice shoots. J. Pineal Res. 2013, 54, 139–144. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Park, S.; Back, K. Cellular localization and kinetics of the rice melatonin biosynthetic enzymes SNAT and ASMT. J. Pineal Res. 2014, 56, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Back, K. Melatonin synthesis in rice seedlings in vivo is enhanced at high temperatures and under dark conditions due to increased serotonin N-acetyltransferase and N-acetylserotonin methyltransferase activities. J. Pineal Res. 2014, 56, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-H.; Xu, Y. Characterization of a caffeic acid 3-O-methyltransferase from wheat and its function in lignin biosynthesis. Biochimie 2008, 90, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014, 57, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Du, Y.T.; Zhou, Y.B.; Chen, J.; Xu, Z.S.; Ma, Y.Z.; Chen, M.; Min, D.H. Overexpression of TaCOMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 652. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin in plants—Diversity of levels and multiplicity of functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth conditions influence the melatonin content of tomato plants. Food Chem. 2013, 138, 1212–1214. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [Green Version]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Ayuso, M.C.; Bernalte, M.J.; Rodríguez, A.B. Detection and quantification of melatonin and serotonin in eight Sweet Cherry cultivars (Prunus avium L.). Eur. Food Res. Technol. 2009, 229, 223–229. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Arnao, M.B. Distribution of melatonin in different zones of lupin and barley plants at different ages in the presence and absence of light. J. Agric. Food Chem. 2008, 56, 10567–10573. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef]
- Iriti, M.; Rossoni, M.; Faoro, F. Melatonin content in grape: Myth or panacea? J. Sci. Food Agric. 2006, 86, 1432–1438. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.M.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Kocadaǧli, T.; Yilmaz, C.; Gökmen, V. Determination of melatonin and its isomer in foods by liquid chromatography tandem mass spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Bałabusta, M.; Szewczyk, R.; Posmyk, M.M. The levels of melatonin and its metabolites in conditioned corn (Zea mays L.) and cucumber (Cucumis sativus L.) seeds during storage. Acta Physiol. Plant. 2015, 37, 105. [Google Scholar] [CrossRef]
- Korkmaz, A.; Deǧer, Ö.; Cuci, Y. Profiling the melatonin content in organs of the pepper plant during different growth stages. Sci. Hortic. 2014, 172, 242–247. [Google Scholar] [CrossRef]
- Aguilera, Y.; Herrera, T.; Benítez, V.; Arribas, S.M.; López De Pablo, A.L.; Esteban, R.M.; Martín-Cabrejas, M.A. Estimation of scavenging capacity of melatonin and other antioxidants: Contribution and evaluation in germinated seeds. Food Chem. 2015, 170, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Mena, P.; Gil-Izquierdo, Á.; Moreno, D.A.; Martí, N.; García-Viguera, C. Assessment of the melatonin production in pomegranate wines. LWT-Food Sci. Technol. 2012, 47, 13–18. [Google Scholar] [CrossRef]
- Mercolini, L.; Mandrioli, R.; Raggi, M.A. Content of melatonin and other antioxidants in grape-related foodstuffs: Measurement using a MEPS-HPLC-F method. J. Pineal Res. 2012, 53, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Ezura, H. Profiling of melatonin in the model tomato (Solanum lycopersicum L.) cultivar Micro-Tom. J. Pineal Res. 2009, 46, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Padumanonda, T.; Johns, J.; Sangkasat, A.; Tiyaworanant, S. Determination of melatonin content in traditional Thai herbal remedies used as sleeping aids. DARU J. Pharm. Sci. 2014, 22, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pothinuch, P.; Tongchitpakdee, S. Melatonin contents in mulberry (Morus spp.) leaves: Effects of sample preparation, cultivar, leaf age and tea processing. Food Chem. 2011, 128, 415–419. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in walnuts: Influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef]
- Riga, P.; Medina, S.; García-Flores, L.A.; Gil-Izquierdo, Á. Melatonin content of pepper and tomato fruits: Effects of cultivar and solar radiation. Food Chem. 2014, 156, 347–352. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Barbero, G.F.; Palma, M.; García Barroso, C. Determination of melatonin in rice (Oryza sativa) grains by pressurized liquid extraction. J. Agric. Food Chem. 2015, 63, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Allegrone, G.; Razzano, F.; Pollastro, F.; Grassi, G. Determination of melatonin content of different varieties of hemp (Cannabis sativa L.) by liquid chromatography tandem mass spectrometry. SN Appl. Sci. 2019, 1, 720. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.; Wang, X.; Tu, H.; Zhang, X.; Liu, F.; Zhou, G.H.; Liang, D.; Xia, H. Diurnal variation of melatonin content in sweet cherry leaves. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 145, p. 01022. [Google Scholar]
- Stege, P.W.; Sombra, L.L.; Messina, G.; Martinez, L.D.; Silva, M.F. Determination of melatonin in wine and plant extracts by capillary electrochromatography with immobilized carboxylic multi-walled carbon nanotubes as stationary phase. Electrophoresis 2010, 31, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Stürtz, M.; Cerezo, A.B.; Cantos-Villar, E.; Garcia-Parrilla, M.C. Determination of the melatonin content of different varieties of tomatoes (Lycopersicon esculentum) and strawberries (Fragaria ananassa). Food Chem. 2011, 127, 1329–1334. [Google Scholar] [CrossRef]
- Tan, D.X.; Zanghi, B.M.; Manchester, L.C.; Reiter, R.J. Melatonin identified in meats and other food stuffs: Potentially nutritional impact. J. Pineal Res. 2014, 57, 213–218. [Google Scholar] [CrossRef]
- Tapia, M.I.; Sánchez-Morgado, J.R.; García-Parra, J.; Ramírez, R.; Hernández, T.; González-Gómez, D. Comparative study of the nutritional and bioactive compounds content of four walnut (Juglans regia L.) cultivars. J. Food Compos. Anal. 2013, 31, 232–237. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Simonetti, P.; Faoro, F.; Fico, G.; Iriti, M. The presence of melatonin in grapevine (Vitis vinifera L.) berry tissues. J. Pineal Res. 2011, 51, 331–337. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.Y.; Shi, X.Y.; Xiao, H.; Kang, K.; Liu, X.Y.; Zhan, J.C.; Huang, W.D. Effect of Cultivar, Temperature, and Environmental Conditions on the Dynamic Change of Melatonin in Mulberry Fruit Development and Wine Fermentation. J. Food Sci. 2016, 81, M958–M967. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, C.; Li, S.; Zheng, J. Study on analysis method of melatonin and melatonin content in corn & rice seeds. Chin. Agric. Sci. Bull. 2009, 25, 20–24. [Google Scholar]
- Wang, L.; Zhao, Y.; Reiter, R.J.; He, C.; Liu, G.; Lei, Q.; Zuo, B.; Zheng, X.D.; Li, Q.; Kong, J. Changes in melatonin levels in transgenic “Micro-Tom” tomato overexpressing ovine AANAT and ovine HIOMT genes. J. Pineal Res. 2014, 56, 134–142. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Phytomelatonin, natural melatonin from plants as a novel dietary supplement: Sources, activities and world market. J. Funct. Foods 2018, 48, 37–42. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, D.X.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef]
- Zieliński, H.; Lewczuk, B.; Przybylska-Gornowicz, B.; Kozłowska, H. Melatonin in germinated legume seeds as a potentially significant agent for health. In Biologically-Active Phytochemicals in Food: Analysis, Metabolism, Bioavailability and Function. Proceedings of the EUROFOODCHEM XI Meeting, Norwich, UK, 26–28 September 2001; Royal Society of Chemistry: London, UK, 2001; pp. 110–117. [Google Scholar]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Brown, P.N.; Turi, C.E.; Shipley, P.R.; Murch, S.J. Comparisons of large (Vaccinium macrocarpon Ait) and small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) cranberry in British Columbia by phytochemical determination, antioxidant potential, and metabolomic profiling with chemometric analysis. Planta Med. 2012, 78, 630–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murch, S.J.; Simmons, C.B.; Saxena, P.K. Melatonin in feverfew and other medicinal plants. Lancet 1997, 350, 1598–1599. [Google Scholar] [CrossRef]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. Spectrofluorimetric determination of melatonin in kernels of four different Pistacia varieties after ultrasound-assisted solid-liquid extraction. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2014, 132, 326–329. [Google Scholar] [CrossRef]
- Badria, F.A. Melatonin, serotonin, and tryptamine in some Egyptian food and medicinal plants. J. Med. Food 2002, 5, 153–157. [Google Scholar] [CrossRef]
- Boccalandro, H.E.; González, C.V.; Wunderlin, D.A.; Silva, M.F. Melatonin levels, determined by LC-ESI-MS/MS, fluctuate during the day/night cycle in Vitis vinifera cv Malbec: Evidence of its antioxidant role in fruits. J. Pineal Res. 2011, 51, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Back, K. An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. J. Pineal Res. 2014, 56, 408–414. [Google Scholar] [CrossRef]
- Chen, G.; Huo, Y.; Tan, D.X.; Liang, Z.; Zhang, W.; Zhang, Y. Melatonin in Chinese medicinal herbs. Life Sci. 2003, 73, 19–26. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth conditions determine different melatonin levels in Lupinus albus L. J. Pineal Res. 2013, 55, 149–155. [Google Scholar] [CrossRef]
- Tan, D.; Manchester, L.C.; Di Mascio, P.; Martinez, G.R.; Prado, F.M.; Reiter, R.J. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: Importance for phytoremediation. FASEB J. 2007, 21, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernàndez-Ruiz, J. Melatonin possible role as light-protector in plants. In UV Radiation: Properties, Effects, and Applications; Nova Science Publishing: Hauppauge, NY, USA, 2014. [Google Scholar]
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Jia, J.F.; Xu, Y.; Wang, Y.; Hao, J.G.; Li, T.K. Production of transgenic Nicotiana sylvestris plants expressing melatonin synthetase genes and their effect on UV-B-induced DNA damage. Vitr. Cell. Dev. Biol.-Plant 2012, 48, 275–282. [Google Scholar] [CrossRef]
- Kang, K.; Lee, K.; Park, S.; Kim, Y.S.; Back, K. Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J. Pineal Res. 2010, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The Application of a Plant Biostimulant Based on Seaweed and Yeast Extract Improved Tomato Fruit Development and Quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A Biostimulant Based on Seaweed (Ascophyllum nodosum and Laminaria digitata) and Yeast Extracts Mitigates Water Stress Effects on Tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.; Sainz, R.M.; Mayo, J.C.; Lopez-Burillo, S. Melatonin: Reducing the toxicity and increasing the efficacy of drugs. J. Pharm. Pharmacol. 2002, 54, 1299–1321. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ruiz, J.; Arnao, M.B. Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant Growth Regul. 2008, 55, 29–34. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A growth-stimulating compound present in lupin tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, W.B.; Reiter, R.J.; Wei, W.; Wang, B.M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sarrou, E.; Therios, I.; Dimassi-Theriou, K. Melatonin and other factors that promote rooting and sprouting of shoot cuttings in Punica granatum cv. Wonderful. Turk. J. Bot. 2014, 38, 293–301. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Muñoz-Parra, E.; Ortiz-Castro, R.; López-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal Res. 2012, 53, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Koyama, F.C.; Carvalho, T.L.G.; Alves, E.; Da Silva, H.B.; De Azevedo, M.F.; Hemerly, A.S.; Garcia, C.R.S. The structurally related auxin and melatonin tryptophan-derivatives and their roles in Arabidopsis thaliana and in the human malaria parasite Plasmodium falciparum. J. Eukaryot. Microbiol. 2013, 60, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Back, K.; Park, S. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J. Pineal Res. 2012, 53, 385–389. [Google Scholar]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S.; et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Growth activity, rooting capacity, and tropism: Three auxinic precepts fulfilled by melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiol. Biochem. 2019, 139, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. By regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; An, B.; Wei, Y.; Reiter, R.J.; Shi, H.; Luo, H.; He, C. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in arabidopsis. Front. Plant Sci. 2016, 7, 1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.; Rutto, L.; Katuuramu, D. Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS ONE 2019, 14, e0221687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Li, A.; Yu, H.; Li, W.; Liang, C.; Guo, S.; Zhang, R.; Chu, C. Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant Sci. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Zhang, P.; Wang, R.; Sun, L.; Ju, Q.; Xu, J. Comparative physiological responses and transcriptome analysis reveal the roles of melatonin and serotonin in regulating growth and metabolism in Arabidopsis. BMC Plant Biol. 2018, 18, 362. [Google Scholar] [CrossRef] [Green Version]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci. Rep. 2021, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Campobenedetto, C.; Grange, E.; Mannino, G.; Van Arkel, J.; Beekwilder, J.; Karlova, R.; Garabello, C.; Contartese, V.; Bertea, C.M. A Biostimulant Seed Treatment Improved Heat Stress Tolerance during Cucumber Seed Germination by Acting on the Antioxidant System and Glyoxylate Cycle. Front. Plant Sci. 2020, 11, 836. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Mannino, G.; Agliassa, C.; Acquadro, A.; Contartese, V.; Garabello, C.; Bertea, C.M. Transcriptome Analyses and Antioxidant Activity Profiling Reveal the Role of a Lignin-Derived Biostimulant Seed Treatment in Enhancing Heat Stress Tolerance in Soybean. Plants 2020, 9, 1308. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Janas, K.M.; Malgorzata, P.M.; Marta, B.; Krystyna, J.M. Melatonin Applied by Osmopriming as a Biostimulator Improving Cucumber (Cucumis sativus L.) Seedling Growth at Abiotic Stress Conditions. In Proceedings of the International Symposium on Environmental Science and Technology (ISEST), Shanghai, China, 2–5 June 2009. [Google Scholar]
- Posmyk, M.M.; Bałabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Popovich, I.G.; Zabezhinski, M.A.; Anisimov, S.V.; Vesnushkin, G.M.; Vinogradova, I.A. Melatonin as antioxidant, geroprotector and anticarcinogen. Biochim. Biophys. Acta-Bioenerg. 2006, 1757, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Szafrańska, K.; Glińska, S.; Janas, K.M. Ameliorative effect of melatonin on meristematic cells of chilled and re-warmed Vigna radiata roots. Biol. Plant. 2013, 57, 91–96. [Google Scholar] [CrossRef]
- Janas, K.M.; Posmyk, M.M. Melatonin, an underestimated natural substance with great potential for agricultural application. Acta Physiol. Plant. 2013, 35, 3285–3292. [Google Scholar] [CrossRef] [Green Version]
- Posmyk, M.M.; Janas, K.M. Melatonin in plants. Acta Physiol. Plant. 2009, 31, 1. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Zhang, N.; Wang, J.; Cao, Y.; Li, X.; Zhang, H.; Zhang, L.; Tan, D.X.; Guo, Y.D. A label-free differential proteomics analysis reveals the effect of melatonin on promoting fruit ripening and anthocyanin accumulation upon postharvest in tomato. J. Pineal Res. 2016, 61, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-Q.; Li, Z.-L.; Zhu, Y.-Y.; Chen, F.; Liang, B.; Nan, J.; Wang, A.-J. Palladium/iron nanoparticles stimulate tetrabromobisphenol a microbial reductive debromination and further mineralization in sediment. Environ. Int. 2020, 135, 105353. [Google Scholar] [CrossRef]
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X.; et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Lazár, D.; Murch, S.J.; Beilby, M.J.; Al Khazaaly, S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal. Behav. 2013, 8, e23279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, J.; Peng, Z.; Ma, W.; Yang, Q.; Song, Z.; Sun, W.; Yang, W.; Yuan, L.; Xu, X. Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in Arabidopsis. J. Pineal Res. 2020, 68, e12640. [Google Scholar] [CrossRef]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; He, Y.-J.; Zhou, L.; Liu, Y.; Jiang, M.; Ren, L.; Chen, H. Transcriptome profiling of genes related to light-induced anthocyanin biosynthesis in eggplant (Solanum melongena L.) before purple color becomes evident. BMC Genom. 2018, 19, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, B.; Hussain, M.; Irshad, M.; Mitra, S.; Li, M.; Liu, S.; Qiu, D. Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules 2018, 23, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Chang, C.; Feng, F.; Liang, D.; Cheng, L.; Ma, F. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 2013, 55, 424–434. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Gong, B.; Yan, Y.; Wen, D.; Shi, Q. Hydrogen peroxide produced by NADPH oxidase: A novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum. Physiol. Plant. 2017, 160, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, R.; Meng, J.; Li, Z.; Wu, Y.; Tao, J. Ameliorative effects of melatonin on dark-induced leaf senescence in gardenia (Gardenia jasminoides Ellis): Leaf morphology, anatomy, physiology and transcriptome. Sci. Rep. 2017, 7, 10423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.N.; Zhang, L.; Yang, L.; Islam, M.R.; Liu, Y.; Luo, H.; Yang, P.; Wang, Q.; Chan, Z. Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24-epibrassinolide. BMC Genom. 2018, 19, 224. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. Melatonin plays a pivotal role in conferring tolerance against endoplasmic reticulum stress via mitogen-activated protein kinases and bZIP60 in Arabidopsis thaliana. Melatonin Res. 2018, 1, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 2009, 46, 295–299. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Helton, P.; Reiter, R.J. Phytoremediative capacity of plants enriched with melatonin. Plant Signal. Behav. 2007, 2, 514–516. [Google Scholar] [CrossRef] [Green Version]
- Fischer, T.W.; Kleszczyński, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Behnam, B.; Iuchi, S.; Fujita, M.; Fujita, Y.; Takasaki, H.; Osakabe, Y.; Yamaguchi-Shinozaki, K.; Kobayashi, M.; Shinozaki, K. Characterization of the promoter region of an arabidopsis gene for 9-cis-epoxycarotenoid dioxygenase involved in dehydration-inducible transcription. DNA Res. 2013, 20, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, O.J.; Back, K. Melatonin is involved in skotomorphogenesis by regulating brassinosteroid biosynthesis in rice plants. J. Pineal Res. 2018, 65, e12495. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; ur Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Kumar, R.; Shahzad, B.; Thukral, A.K.; Bhardwaj, R. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food Agric. 2018, 4, 1436212. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Khan, I.; Xue, L. Methyl Jasmonate-Induced Alteration in Lipid Peroxidation, Antioxidative Defence System and Yield in Soybean Under Drought. J. Agron. Crop Sci. 2011, 197, 296–301. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Tung, S.A.; Samad, R.A.; Wang, L.; Khan, I.; ur Rehman, N.; Shah, A.N.; Shahzad, B. Exogenously applied methyl jasmonate improves the drought tolerance in wheat imposed at early and late developmental stages. Acta Physiol. Plant. 2016, 38, 25. [Google Scholar] [CrossRef]
- Shan, C.; Zhou, Y.; Liu, M. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 2015, 252, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Yuan, H.; Kanwar, M.K.; Bhardwaj, R.; Thukral, A.K.; Zheng, B. Jasmonic acid seed treatment stimulates insecticide detoxification in Brassica juncea L. Front. Plant Sci. 2018, 871, 1609. [Google Scholar] [CrossRef]
- Debnath, B.; Hussain, M.; Li, M.; Lu, X.; Sun, Y.; Qiu, D. Exogenous melatonin improves fruit quality features, health promoting antioxidant compounds and yield traits in tomato fruits under acid rain stress. Molecules 2018, 23, 1868. [Google Scholar] [CrossRef] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; Li, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 2016, 7, 197. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Bian, F.; Sun, H.; Zhai, H.; Yao, Y. Melatonin enhances phenolics accumulation partially via ethylene signaling and resulted in high antioxidant capacity in grape berries. Front. Plant Sci. 2017, 18, 1426. [Google Scholar] [CrossRef] [Green Version]
- Hardeland, R. Melatonin in aging and disease—Multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012, 3, 194–225. [Google Scholar]
- Srinivasan, V.; Pandi-Perumal, S.R.; Maestroni, G.J.M.; Esquifino, A.I.; Hardeland, R.; Cardinali, D.P. Role of melatonin in neurodegenerative diseases. Neurotox. Res. 2005, 7, 293–318. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Ahmed, J.; Ak, P.S.; Robertson, N.J.; More, K. Melatonin for neuroprotection in neonatal encephalopathy: A systematic review & meta-analysis of clinical trials. Eur. J. Paediatr. Neurol. 2021, 31, 38–45. [Google Scholar]
- Witt-Enderby, P.A.; Radio, N.M.; Doctor, J.S.; Davis, V.L. Therapeutic treatments potentially mediated by melatonin receptors: Potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J. Pineal Res. 2006, 41, 297–305. [Google Scholar] [CrossRef]
- Kubatka, P.; Bojková, B. Mammary Carcinogenesis Induced in Wistar: Han Rats by the Combination of Ionizing Radiation and Dimethylbenz(a)anthracene: Prevention with melatonin. Neoplasma 2000, 47, 227–229. [Google Scholar]
- Koyun, D.; Tetik, Ş.; Ozsavci, D.; Yiginer, O.; Çetinel, Ş.; Tok, O.E.; Kaya, Z.; Akkiprik, M.; Kiliç, E.; Şener, G. Melatonin protects against ischemic heart failure in rats Ahmet Özer Şehirli1. J. Pineal Res. 2013, 55, 138–148. [Google Scholar]
- Solís-Muñoz, P.; Solís-Herruzo, J.A.; Fernández-Moreira, D.; Gõmez-Izquierdo, E.; García-Consuegra, I.; Muñoz-Yagüe, T.; García Ruiz, I. Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. J. Pineal Res. 2011, 51, 113–123. [Google Scholar] [CrossRef]
- Sartori, C.; Dessen, P.; Mathieu, C.; Monney, A.; Bloch, J.; Nicod, P.; Scherrer, U.; Duplain, H. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology 2009, 150, 5311–5317. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Llamas, F.; Hernández-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a phytomelatonin-rich extract from cultured plants with excellent biochemical and functional properties as an alternative to synthetic melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Seabra, M.L.V.; Bignotto, M.; Pinto, L.R.; Tufik, S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J. Pineal Res. 2000, 29, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordlund, J.J.; Lerner, A.B. The effects of oral melatonin on skin color and on the release of pituitary hormones. J. Clin. Endocrinol. Metab. 1977, 45, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.E.; Hamilton, D.; Seward, N.; Fast, D.K.; Freeman, R.D.; Laudon, M. Clinical trials of controlled-release melatonin in children with sleep-wake cycle disorders. J. Pineal Res. 2000, 29, 34–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Rebollo-Hernanz, M.; Herrera, T.; Cayuelas, L.T.; Rodríguez-Rodríguez, P.; De Pablo, Á.L.L.; Arribas, S.M.; Martin-Cabrejas, M.A. Intake of bean sprouts influences melatonin and antioxidant capacity biomarker levels in rats. Food Funct. 2016, 7, 1438–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido, M.; Espino, J.; González-Gómez, D.; Lozano, M.; Cubero, J.; Toribio-Delgado, A.F.; Maynar-Mariño, J.I.; Terrón, M.P.; Muñoz, J.L.; Pariente, J.A.; et al. A nutraceutical product based on Jerte Valley cherries improves sleep and augments the antioxidant status in humans. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2009, 4, e321–e323. [Google Scholar] [CrossRef] [Green Version]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodríguez, A.B. Jerte valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2010, 65, 909–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Flores, D.; Belén, V.; Garrido, M.; Gonzalez-Gomez, D. Ingestion of Japanese plums Prunus salicina Lindl. cv. Crimson Globe increases the urinary 6 sulfatoxymelatonin and total antioxidant capacity levels in young middleaged and elderly humans. J. Food Nutr. Res. 2011, 50, 229–236. [Google Scholar]
- González-Flores, D.; Gamero, E.; Garrido, M.; Ramírez, R.; Moreno, D.; Delgado, J.; Valdés, E.; Barriga, C.; Rodríguez, A.B.; Paredes, S.D. Urinary 6-sulfatoxymelatonin and total antioxidant capacity increase after the intake of a grape juice cv. Tempranillo stabilized with HHP. Food Funct. 2012, 3, 34–39. [Google Scholar] [CrossRef]
- Johns, N.P.; Johns, J.; Porasuphatana, S.; Plaimee, P.; Sae-Teaw, M. Dietary intake of melatonin from tropical fruit altered urinary excretion of 6-sulfatoxymelatonin in healthy volunteers. J. Agric. Food Chem. 2013, 61, 913–919. [Google Scholar] [CrossRef]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef]
- Chase, J.E.; Gidal, B.E. Melatonin: Therapeutic use in sleep disorders. Ann. Pharmacother. 1997, 31, 1218–1226. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Wurtman, R.J. Efficacy of Melatonin as a Sleep-Promoting Agent. J. Biol. Rhythms 1997, 12, 644–650. [Google Scholar] [CrossRef]
- Leger, D.; Laudon, M.; Zisapel, N. Nocturnal 6-sulfatoxymelatonin excretion in insomnia and its relation to the response to melatonin replacement therapy. Am. J. Med. 2004, 116, 91–95. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Wurtman, R.J.; Regan, M.M.; Taylor, J.A.; Shi, J.P.; Leclair, O.U. Melatonin treatment for age-related insomnia. J. Clin. Endocrinol. Metab. 2001, 86, 4727–4730. [Google Scholar] [CrossRef]
- Waterhouse, J.; Reilly, T.; Atkinson, G. Jet-lag. Lancet 1997, 350, 1611–1616. [Google Scholar] [CrossRef]
- Arendt, J.; Skene, D.J.; Middleton, B.; Lockley, S.W.; Deacon, S. Efficacy of Melatonin Treatment in Jet Lag, Shift Work, and Blindness. J. Biol. Rhythm. 1997, 12, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Trakht, I.; Spence, D.W.; Srinivasan, V.; Dagan, Y.; Cardinali, D.P. The roles of melatonin and light in the pathophysiology and treatment of circadian rhythm sleep disorders. Nat. Clin. Pract. Neurol. 2008, 4, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Humbert, W.; Pevet, P. The Decrease of Pineal Melatonin Production with Age: Causes and Consequences. Ann. N. Y. Acad. Sci. 1994, 719, 43–63. [Google Scholar] [CrossRef]
- Poeggeler, B. Melatonin, aging, and age-related diseases: Perspectives for prevention, intervention, and therapy. Endocrine 2005, 27, 201–212. [Google Scholar] [CrossRef]
- Turek, F.W.; Gillette, M.U. Melatonin, sleep, and circadian rhythms: Rationale for development of specific melatonin agonists. Sleep Med. 2004, 5, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.M.; Turner, C.; Mills, S.L.; Nicholson, A.N. Hypnotic Activity of Melatonin. Sleep 2000, 23, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavie, P. Melatonin: Role in Gating Nocturnal Rise in Sleep Propensity. J. Biol. Rhythms 1997, 12, 657–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Chao, H.; Li, Z.; Xu, X.; Liu, Y.; Hou, L.; Liu, N.; Ji, J. Melatonin attenuates traumatic brain injury-induced inflammation: A possible role for mitophagy. J. Pineal Res. 2016, 61, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Macías, M.; León, J.; Escames, G.; Khaldy, H.; Acuña-Castroviejo, D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell Biol. 2002, 34, 348–357. [Google Scholar] [CrossRef]
- López, A.; García, J.A.; Escames, G.; Venegas, C.; Ortiz, F.; López, L.C.; Acuña-Castroviejo, D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 2009, 46, 188–198. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Reiter, R.J.; Ruggiero, F.M. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J. Pineal Res. 2010, 48, 297–310. [Google Scholar] [CrossRef]
- Acuna Castroviejo, D.; Lopez, L.C.; Escames, G.; Lopez, A.; Garcia, J.A.; Reiter, R.J. Melatonin-mitochondria Interplay in Health and Disease. Curr. Top. Med. Chem. 2012, 11, 221–240. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; Lõpez-Pingarrõn, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Marino, A. Melatonin protects band 3 protein in human erythrocytes against H2O2-induced oxidative stress. Molecules 2019, 24, 2741. [Google Scholar] [CrossRef] [Green Version]
- Allegra, M.; Gentile, C.; Tesoriere, L.; Livrea, M.A. Protective effect of melatonin against cytotoxic actions of malondialdehyde: An in vitro study on human erythrocytes. J. Pineal Res. 2002, 32, 187–193. [Google Scholar] [CrossRef]
- Morreale, M.; Livrea, M.A. Synergistic effect of glycolic acid on the antioxidant activity of α-tocopherol and melatonin in lipid bilayers and in human skin homogenates. Biochem. Mol. Biol. Int. 1997, 42, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.; Sauer, L.; Dauchy, R. Melatonin as a Chronobiotic/Anticancer Agent: Cellular, Biochemical, and Molecular Mechanisms of Action and their Implications for Circadian-Based Cancer Therapy. Curr. Top. Med. Chem. 2005, 2, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Benot, S.; Goberna, R.; Reiter, R.J.; Garcia-Mauriño, S.; Osuna, C.; Guerrero, J.M. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J. Pineal Res. 1999, 27, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Pandi-Perumal, S.R. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2005, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvoa, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. NeuroSignals 2000, 9, 137–159. [Google Scholar] [CrossRef]
- Sarti, P.; Magnifico, M.C.; Altieri, F.; Mastronicola, D.; Arese, M. New Evidence for Cross Talk between Melatonin and Mitochondria Mediated by a Circadian-Compatible Interaction with Nitric Oxide. Int. J. Mol. Sci. 2013, 14, 11259–11276. [Google Scholar] [CrossRef] [Green Version]
- Martinis, P.; Zago, L.; Maritati, M.; Battaglia, V.; Grancara, S.; Rizzoli, V.; Agostinelli, E.; Bragadin, M.; Toninello, A. Interactions of melatonin with mammalian mitochondria. Reducer of energy capacity and amplifier of permeability transition. Amino Acids 2012, 42, 1827–1837. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y.; Zhang, B.X. The role of mitochondrial complex III in melatonin-induced ROS production in cultured mesangial cells. J. Pineal Res. 2011, 50, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, H.-M.; Wu, L.-P.; Tan, D.-X.; Kamat, A.; Li, Y.-Q.; Katz, M.S.; Abboud, H.E.; Reiter, R.J.; Zhang, B.-X. Impaired mitochondrial complex III and melatonin responsive reactive oxygen species generation in kidney mitochondria of db/db mice. J. Pineal Res. 2011, 51, 338. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Tomás-Zapico, C.; Coto-Montes, A. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes. J. Pineal Res. 2005, 39, 99–104. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.-Y.; Zhou, J.-N.; van Heerikhuize, J.; Hofman, M.A.; Swaab, D.F. Decreased Melatonin Levels in Postmortem Cerebrospinal Fluid in Relation to Aging, Alzheimer’s Disease, and Apolipoprotein E-ε4/4 Genotype 1. J. Clin. Endocrinol. Metab. 1999, 84, 323–327. [Google Scholar] [PubMed]
- Brown, G.M.; Young, S.N.; Gauthier, S.; Tsui, H.; Grota, L.J. Melatonin in human cerebrospinal fluid in daytime; Its origin and variation with age. Life Sci. 1979, 25, 929–936. [Google Scholar] [CrossRef]
- Kripke, D.F.; Youngstedt, S.D.; Elliott, J.A.; Tuunainen, A.; Rex, K.M.; Hauger, R.L.; Marler, M.R. Circadian phase in adults of contrasting ages. Chronobiol. Int. 2005, 22, 695–709. [Google Scholar] [CrossRef]
- Skene, D.J.; Vivien-Roels, B.; Sparks, D.L.; Hunsaker, J.C.; Pévet, P.; Ravid, D.; Swaab, D.F. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: Effect of age and Alzheimer’s disease. Brain Res. 1990, 528, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Mahlberg, R.; Tilmann, A.; Salewski, L.; Kunz, D. Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroendocrinology 2006, 31, 634–641. [Google Scholar] [CrossRef]
- Youngstedt, S.D.; Kripke, D.F.; Elliott, J.A.; Klauber, M.R. Circadian abnormalities in older adults. J. Pineal Res. 2001, 31, 264–272. [Google Scholar] [CrossRef]
- Skene, D.J.; Swaab, D.F. Melatonin rhythmicity: Effect of age and Alzheimer’s disease. In Proceedings of the Experimental Gerontology. Exp. Gerontol. 2003, 38, 199–206. [Google Scholar] [CrossRef]
- Tan, D.X.; Xu, B.; Zhou, X.; Reiter, R.J. Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef] [Green Version]
- Kunz, D.; Schmitz, S.; Mahlberg, R.; Mohr, A.; Stöter, C.; Wolf, K.J.; Herrmann, W.M. A new concept for melatonin deficit: On pineal calcification and melatonin excretion. Neuropsychopharmacology 1999, 21, 765–772. [Google Scholar] [CrossRef]
- Schmid, H.A. Decreased melatonin biosynthesis, calcium flux, pineal gland calcification and aging: A hypothetical framework. Gerontology 1993, 39, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. The antiapoptotic activity of melatonin in neurodegenerative diseases. CNS Neurosci. Ther. 2009, 15, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, E.; Bryant-Thomas, T.; Quinto, J.P.; Henry, T.L.; Poeggeler, B.; Herbert, D.; Cruz-Sanchez, F.; Chyan, Y.J.; Smith, M.A.; Perry, G.; et al. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J. Neurochem. 2003, 85, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chang, Y.; Cheng, Y.; Zhang, B.L.; Qu, Z.W.; Qin, C.; Zhang, J.T. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. J. Pineal Res. 2004, 37, 129–136. [Google Scholar] [CrossRef]
- Brusco, L.I.; Márquez, M.; Cardinali, D.P. Monozygotic twins with Alzheimer’s disease treated with melatonin: Case report. J. Pineal Res. 1998, 25, 260–263. [Google Scholar] [CrossRef]
- Quinn, J.; Kulhanek, D.; Nowlin, J.; Jones, R.; Praticò, D.; Rokach, J.; Stackman, R. Chronic melatonin therapy fails to alter amyloid burden or oxidative damage in old Tg2576 mice: Implications for clinical trials. Brain Res. 2005, 1037, 209–213. [Google Scholar] [CrossRef]
- Hardeland, R. Cognitive enhancers in moderate to severe Alzheimer’s disease. Clin. Med. Insights Ther. 2011, 3, 459–476. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, J.T. Protective effect of melatonin on β-amyloid-induced apoptosis in rat astroglioma c6 cells and its mechanism. Free Radic. Biol. Med. 2004, 37, 1790–1801. [Google Scholar] [CrossRef]
- Sun, F.Y.; Lin, X.; Mao, L.Z.; Ge, W.H.; Zhang, L.M.; Huang, Y.L.; Gu, J. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. J. Pineal Res. 2002, 33, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Wang, J.H.; Kang, J.K.; Son, C.G. Experimental evidence for the protective effects of coffee against liver fibrosis in SD rats. J. Sci. Food Agric. 2010, 90, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Morisco, F.; Mazzone, G.; Amoruso, D.C.; Ribecco, M.T.; Romano, A.; Fogliano, V.; Caporaso, N.; D’Argenio, G. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 2010, 52, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.G.; Chavez, E.; Aldaba-Muruato, L.R.; Segovia, J.; Vergara, P.; Tsutsumi, V.; Shibayama, M.; Rivera-Espinoza, Y.; Muriel, P. Coffee prevents CCl4-induced liver cirrhosis in the rat. Hepatol. Int. 2011, 5, 857–863. [Google Scholar] [CrossRef]
- Prieto-Domínguez, N.; Ordóñez, R.; Fernández, A.; Méndez-Blanco, C.; Baulies, A.; Garcia-Ruiz, C.; Fernández-Checa, J.C.; Mauriz, J.L.; González-Gallego, J. Melatonin-induced increase in sensitivity of human hepatocellular carcinoma cells to sorafenib is associated with reactive oxygen species production and mitophagy. J. Pineal Res. 2016, 61, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Crespo, I.; Fernández-Palanca, P.; San-Miguel, B.; Álvarez, M.; González-Gallego, J.; Tuñón, M.J. Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral-induced fulminant hepatic failure. J. Cell. Mol. Med. 2020, 24, 7625–7636. [Google Scholar] [CrossRef]
- Cruz, A.; Padillo, F.J.; Torres, E.; Navarrete, C.M.; Munoz-Castañeda, J.R.; Caballero, F.J.; Briceño, J.; Marchal, T.; Túnez, I.; Montilla, P.; et al. Melatonin prevents experimental liver cirrhosis induced by thioacetamide in rats. J. Pineal Res. 2005, 39, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, W.; Wang, N.P.; Gui, S.Y.; Wu, L.; Sun, W.Y.; Xu, S.Y. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005, 77, 1902–1915. [Google Scholar] [CrossRef] [PubMed]
- Tahan, V.; Atug, O.; Akin, H.; Eren, F.; Tahan, G.; Tarcin, O.; Uzun, H.; Ozdogan, O.; Tarcin, O.; Imeryuz, N.; et al. Melatonin ameliorates methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis in rats. J. Pineal Res. 2009, 46, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Tahan, G.; Akin, H.; Aydogan, F.; Ramadan, S.S.; Yapicier, O.; Tarcin, O.; Uzun, H.; Tahan, V.; Zengin, K. Melatonin ameliorates liver fibrosis induced by bile-duct ligation in rats. Can. J. Surg. 2010, 53, 313–318. [Google Scholar]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Borsani, E.; Favero, G.; Rodella, L.F.; Rezzani, R. Dietary melatonin supplementation could be a promising preventing/therapeutic approach for a variety of liver diseases. Nutrients 2018, 10, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, K.; Nduhirabandi, F.; Adam, T.; Thomas, D.P.; Opie, L.H.; Lecour, S. Role of melatonin, melatonin receptors and STAT3 in the cardioprotective effect of chronic and moderate consumption of red wine. Biochem. Biophys. Res. Commun. 2015, 465, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Jiki, Z.; Lecour, S.; Nduhirabandi, F. Cardiovascular benefits of dietary melatonin: A myth or a reality? Front. Physiol. 2018, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Maarman, G.; Blackhurst, D.; Thienemann, F.; Blauwet, L.; Butrous, G.; Davies, N.; Sliwa, K.; Lecour, S. Melatonin as a preventive and curative therapy against pulmonary hypertension. J. Pineal Res. 2015, 59, 343–353. [Google Scholar] [CrossRef]
- Srinivasan, V.; Spence, D.W.; Moscovitch, A.; Pandi-Perumal, S.R.; Trakht, I.; Brown, G.M.; Cardinali, D.P. Malaria: Therapeutic implications of melatonin. J. Pineal Res. 2010, 48, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.M.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Miller, S.C.; Pandi, P.S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J.M. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef]
- Jung-Hynes, B.; Reiter, R.J.; Ahmad, N. Sirtuins, melatonin and circadian rhythms: Building a bridge between aging and cancer. J. Pineal Res. 2010, 48, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiter, R.J. Mechanisms of cancer inhibition by melatonin. J. Pineal Res. 2004, 37, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Martín, V.; Herrera, F.; García-Santos, G.; Rodriguez-Blanco, J.; Casado-Zapico, S.; Sánchez-Sánchez, A.M.; Suárez, S.; Puente-Moncada, N.; Anítua, M.J.; et al. Mechanisms involved in the pro-apoptotic effect of melatonin in cancer cells. Int. J. Mol. Sci. 2013, 14, 6597–6613. [Google Scholar] [CrossRef]
- Kamdar, B.B.; Tergas, A.I.; Mateen, F.J.; Bhayani, N.H.; Oh, J. Night-shift work and risk of breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 138, 291–301. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhou, Y.; Meng, X.; Zhang, J.J.; Xu, D.P.; Li, H. Bin Melatonin for the prevention and treatment of cancer. Oncotarget 2017, 8, 39896–39921. [Google Scholar] [CrossRef] [Green Version]
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A.; Krause, J.A. Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: Melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential rol. Carcinogenesis 2004, 25, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, K.; Castaño-Vinyals, G.; Espinosa, A.; Aragonés, N.; Pérez-Gõmez, B.; Burgos, J.; Gõmez-Acebo, I.; Llorca, J.; Peirõ, R.; Jimenez-Moleõn, J.J.; et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int. J. Cancer 2015, 137, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Åkerstedt, T.; Knutsson, A.; Narusyte, J.; Svedberg, P.; Kecklund, G.; Alexanderson, K. Night work and breast cancer in women: A Swedish cohort study. BMJ Open 2015, 5, e008127. [Google Scholar] [CrossRef]
- Wang, F.; Yeung, K.L.; Chan, W.C.; Kwok, C.C.H.; Leung, S.L.; Wu, C.; Chan, E.Y.Y.; Yu, I.T.S.; Yang, X.R.; Tse, L.A. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann. Oncol. 2013, 24, 2724–2732. [Google Scholar] [CrossRef]
- Mirick, D.K.; Bhatti, P.; Chen, C.; Nordt, F.; Stanczyk, F.Z.; Davis, S. Night shift work and levels of 6-sulfatoxymelatonin and cortisol in men. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1079–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, L.; Dauchy, R.T.; Blask, D.E.; Slakey, L.M.; Xiang, S.; Yuan, L.; Dauchy, E.M.; Shan, B.; Brainard, G.C.; Hanifin, J.P.; et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin-regulation of GSK3β. Mol. Endocrinol. 2012, 26, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G.; Blask, D.E.; Brainard, G.C.; Hansen, J.; Lockley, S.W.; Provencio, I.; Rea, M.S.; Reinlib, L. Meeting report: The role of environmental lighting and circadian disruption in cancer and other diseases. Environ. Health Perspect. 2007, 115, 1357–1362. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.P.; Lamarque, A.L.; Comba, A.; Berra, M.A.; Silva, R.A.; Labuckas, D.O.; Das, U.N.; Eynard, A.R.; Pasqualini, M.E. Synergistic anti-tumor effects of melatonin and PUFAs from walnuts in a murine mammary adenocarcinoma model. Nutrition 2015, 31, 570–577. [Google Scholar] [CrossRef] [PubMed]
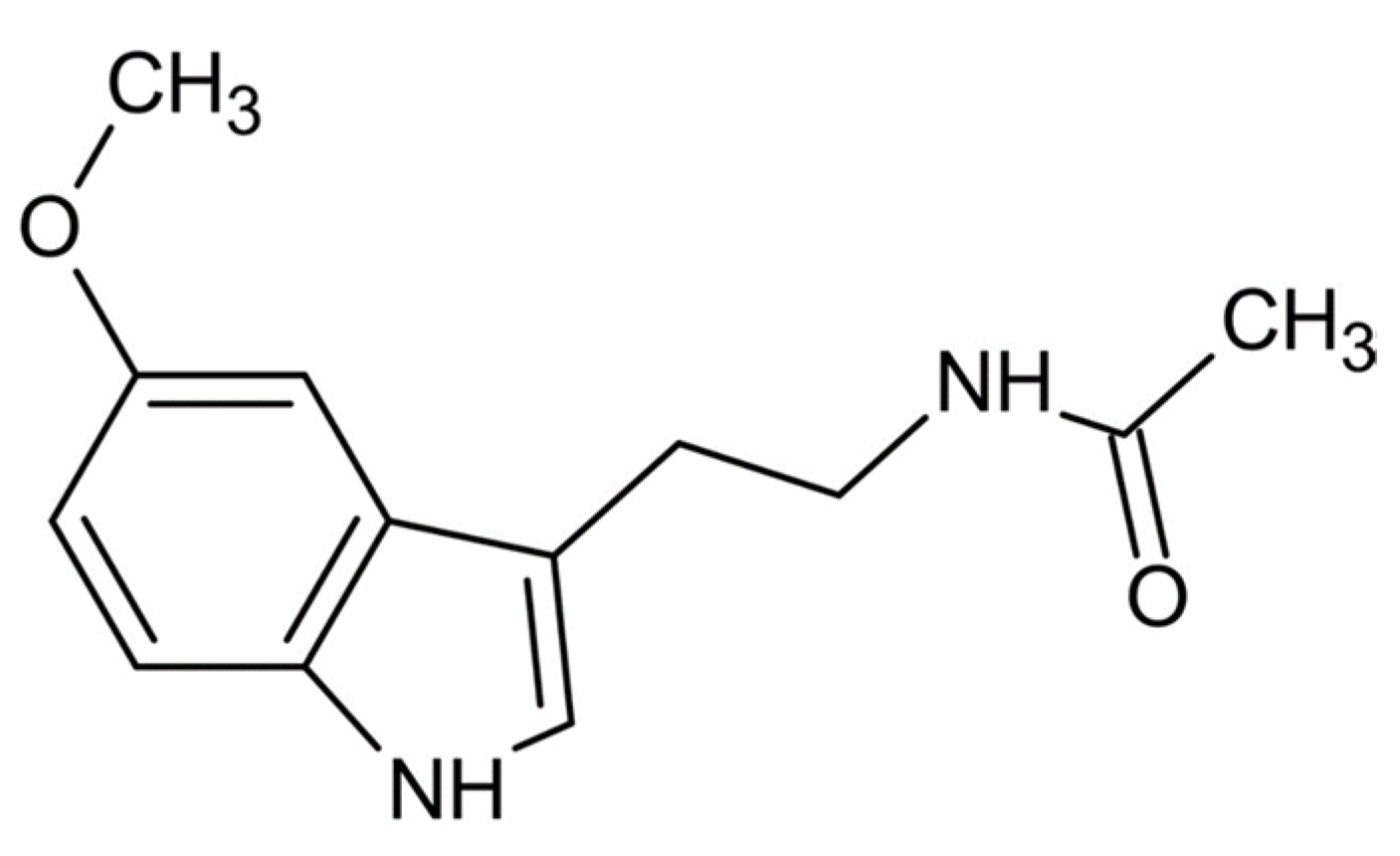
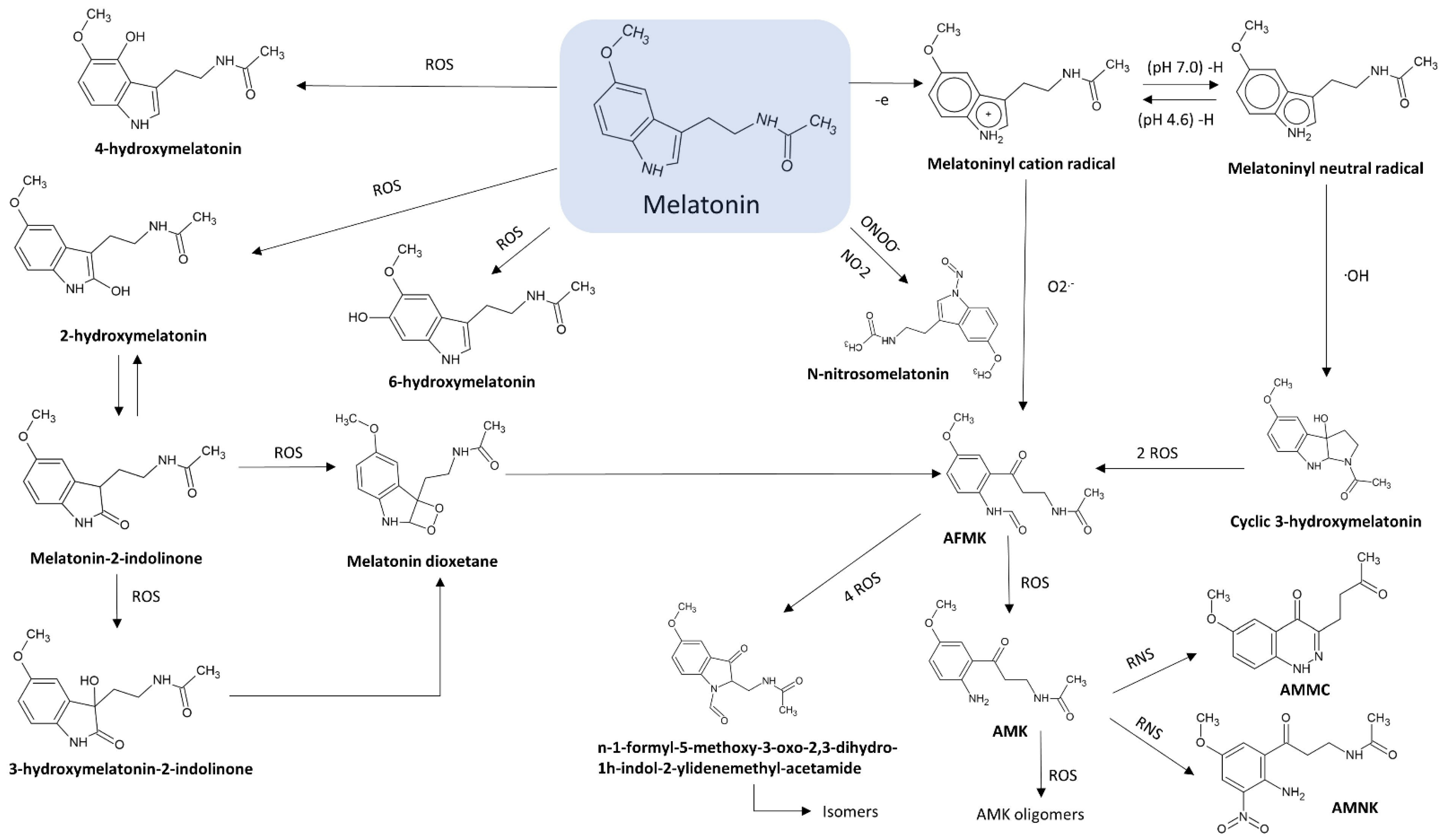
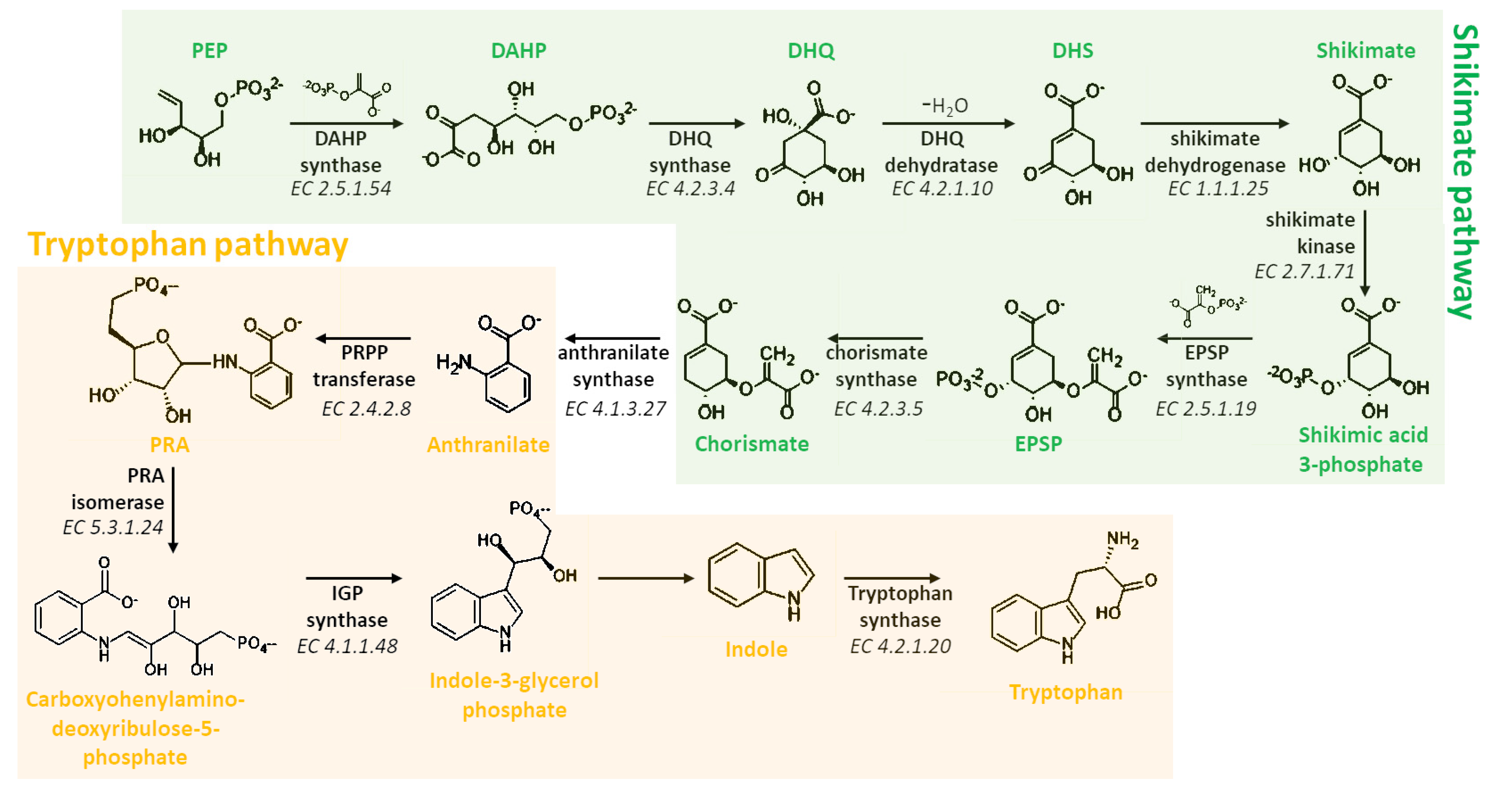
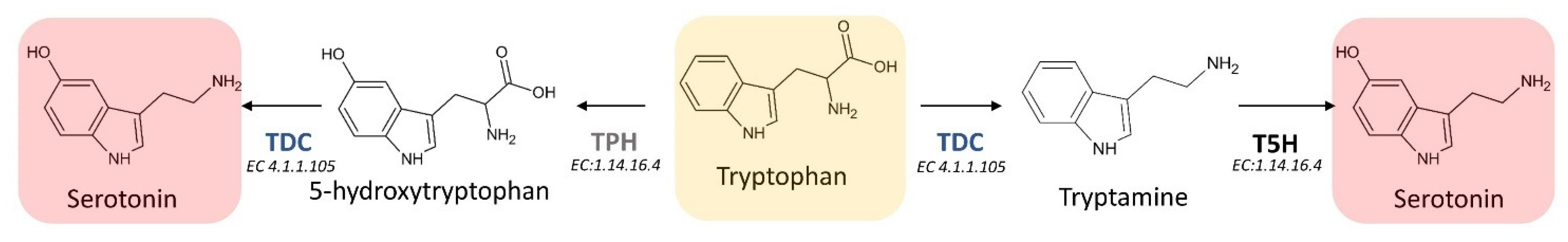
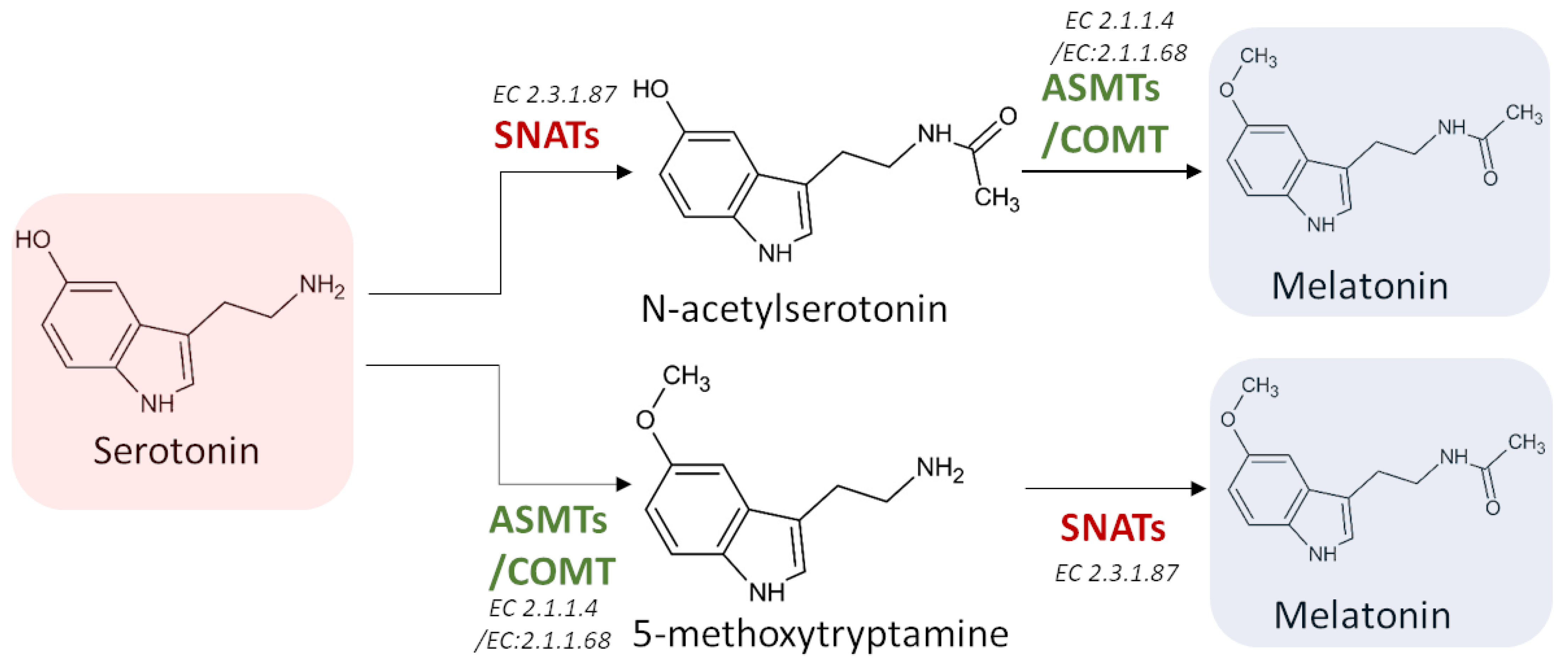
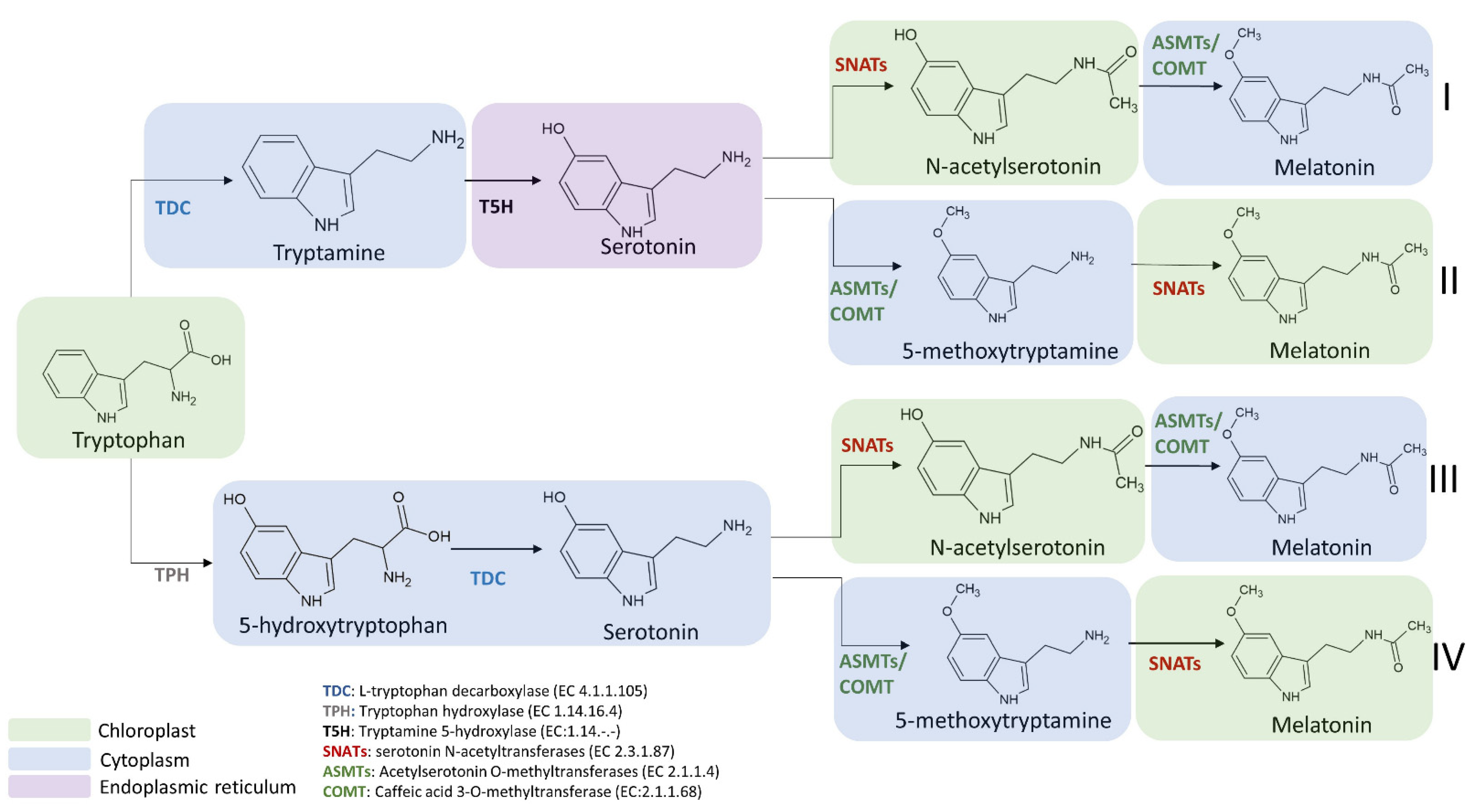

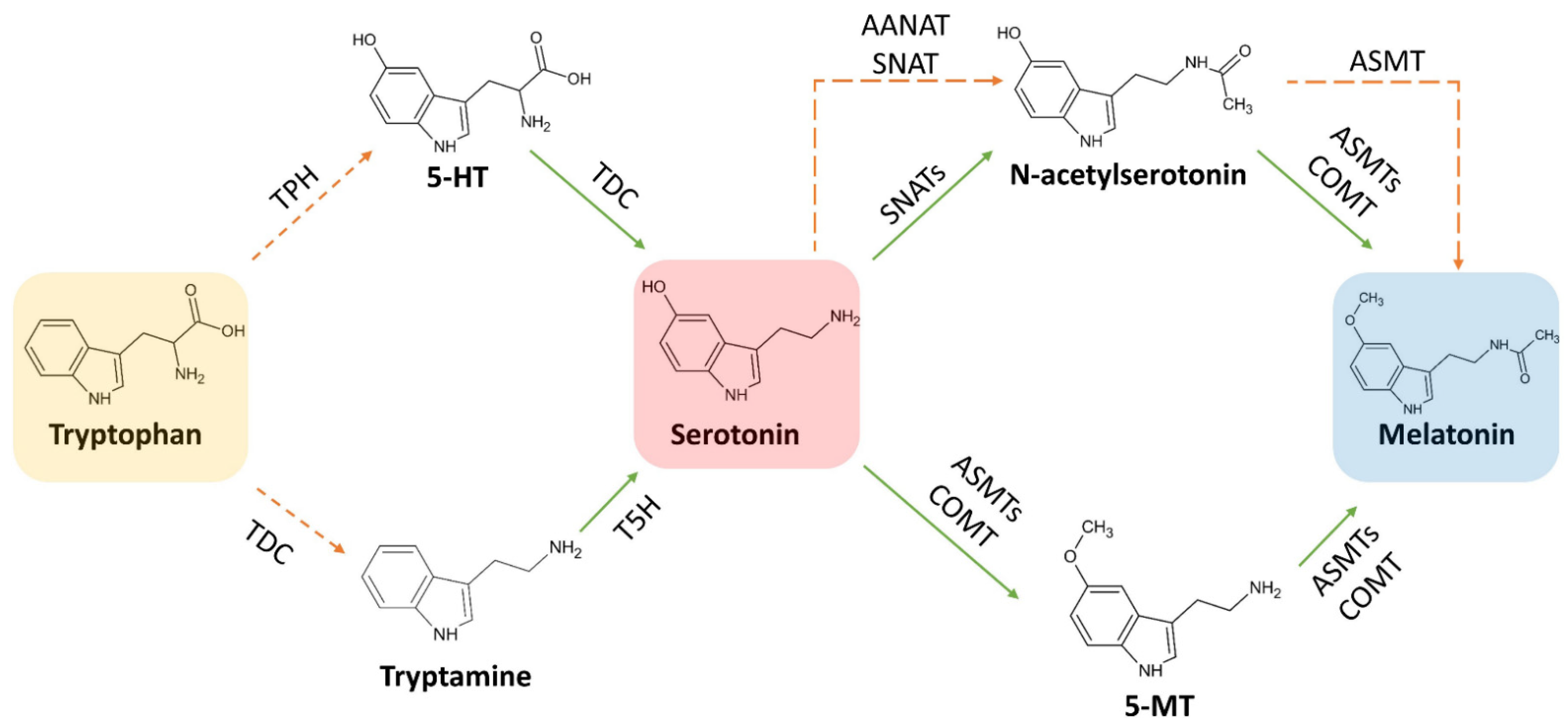
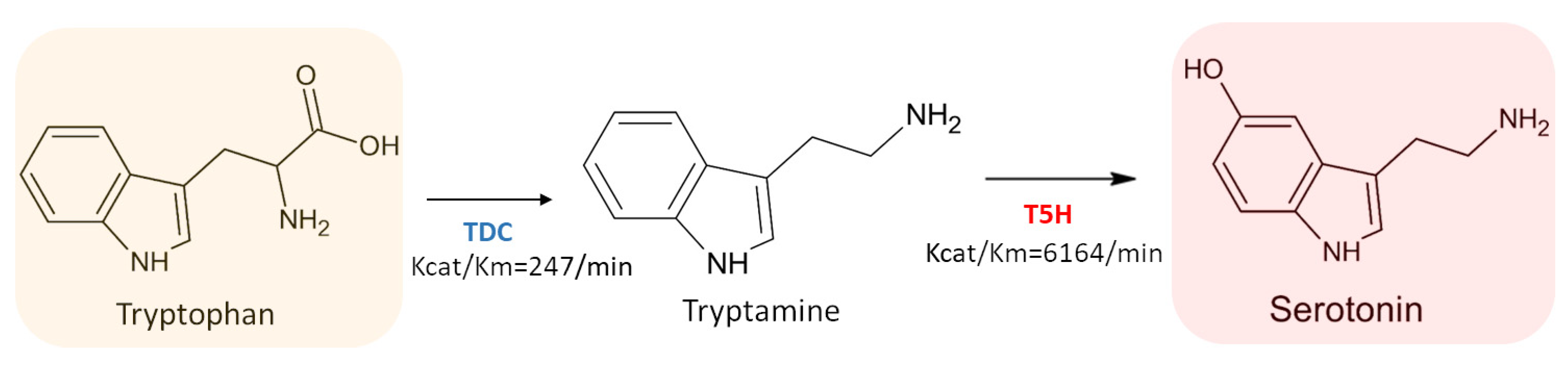
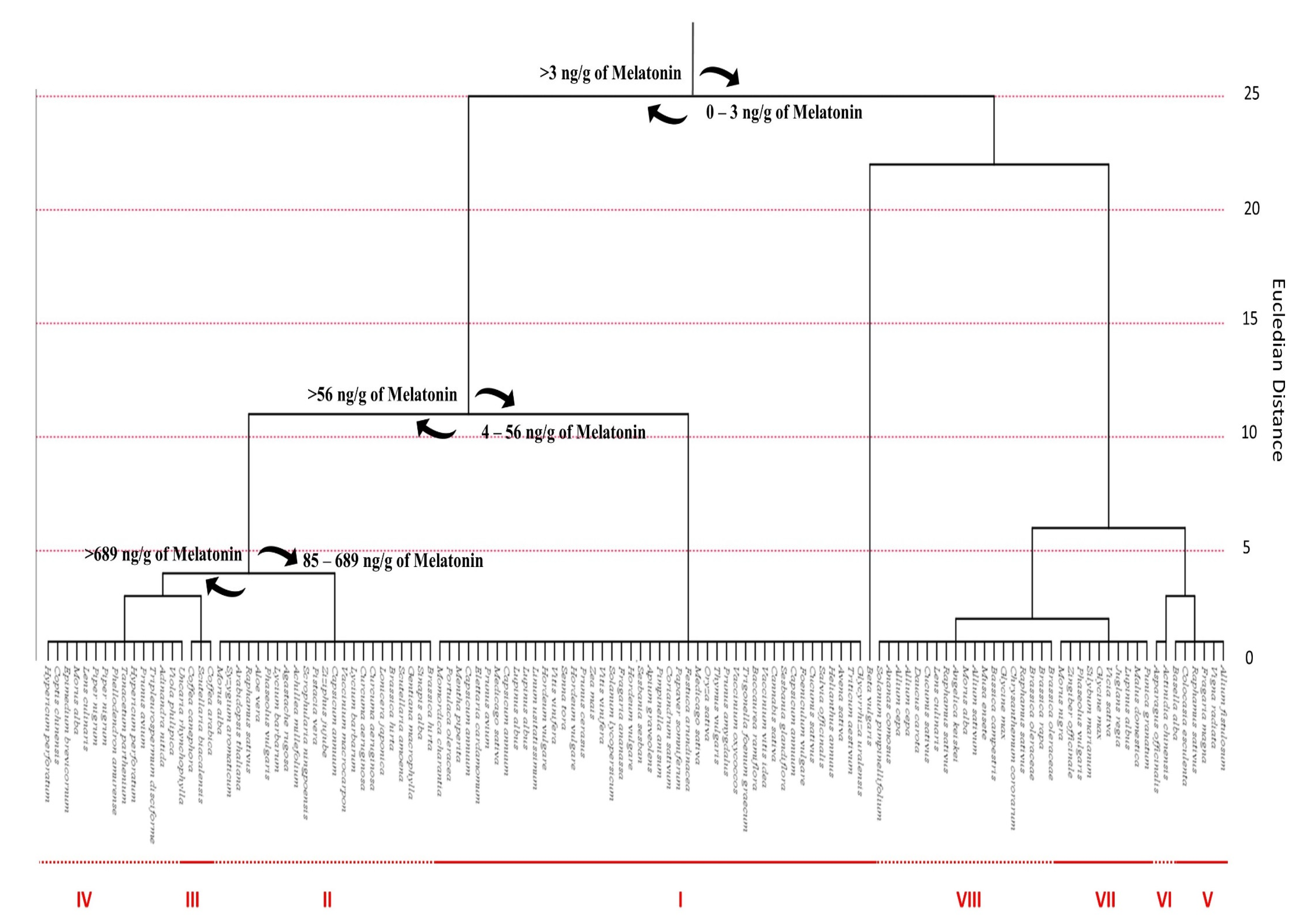

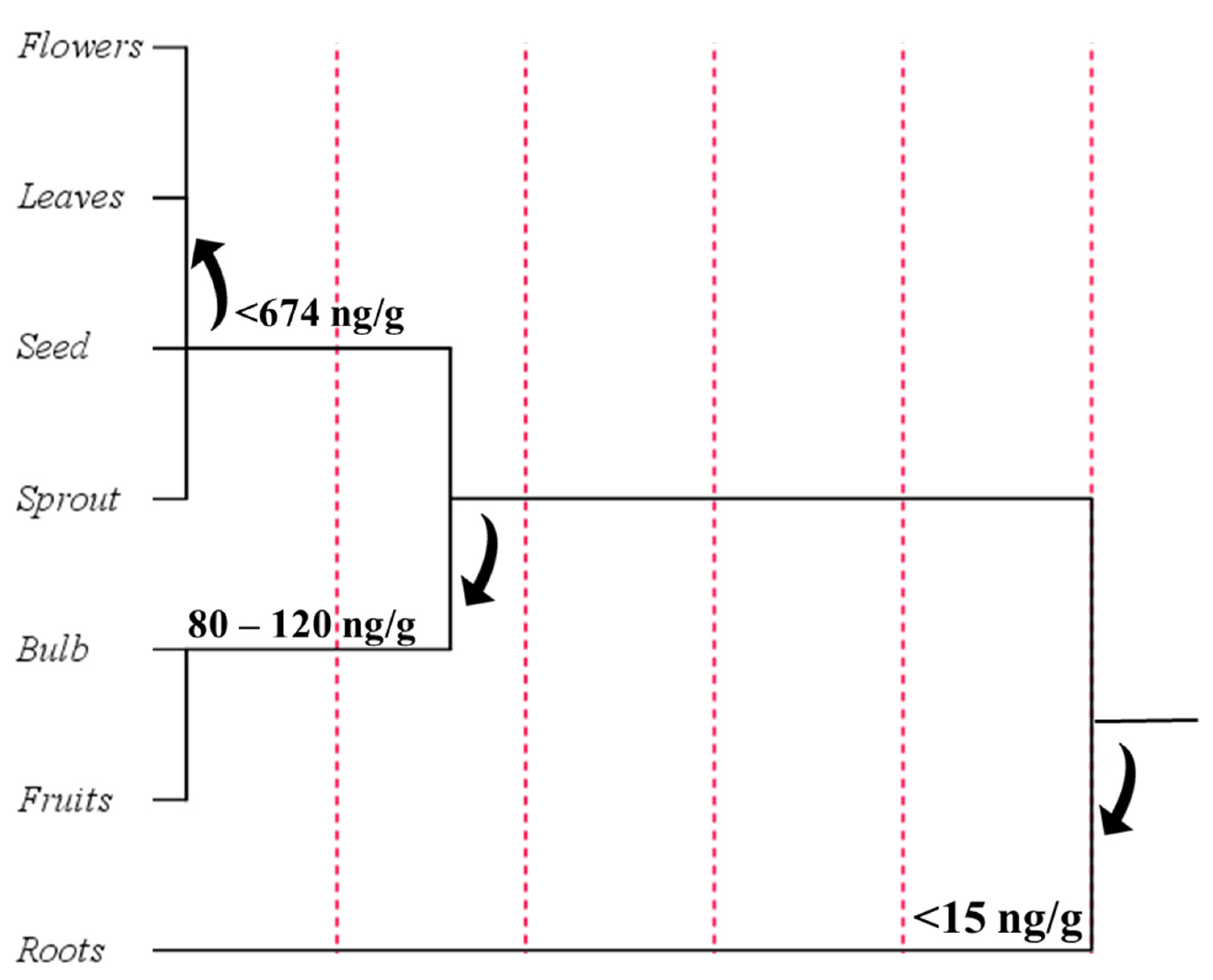
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996. https://doi.org/10.3390/ijms22189996
Mannino G, Pernici C, Serio G, Gentile C, Bertea CM. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. International Journal of Molecular Sciences. 2021; 22(18):9996. https://doi.org/10.3390/ijms22189996
Chicago/Turabian StyleMannino, Giuseppe, Carlo Pernici, Graziella Serio, Carla Gentile, and Cinzia M. Bertea. 2021. "Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview" International Journal of Molecular Sciences 22, no. 18: 9996. https://doi.org/10.3390/ijms22189996
APA StyleMannino, G., Pernici, C., Serio, G., Gentile, C., & Bertea, C. M. (2021). Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. International Journal of Molecular Sciences, 22(18), 9996. https://doi.org/10.3390/ijms22189996









