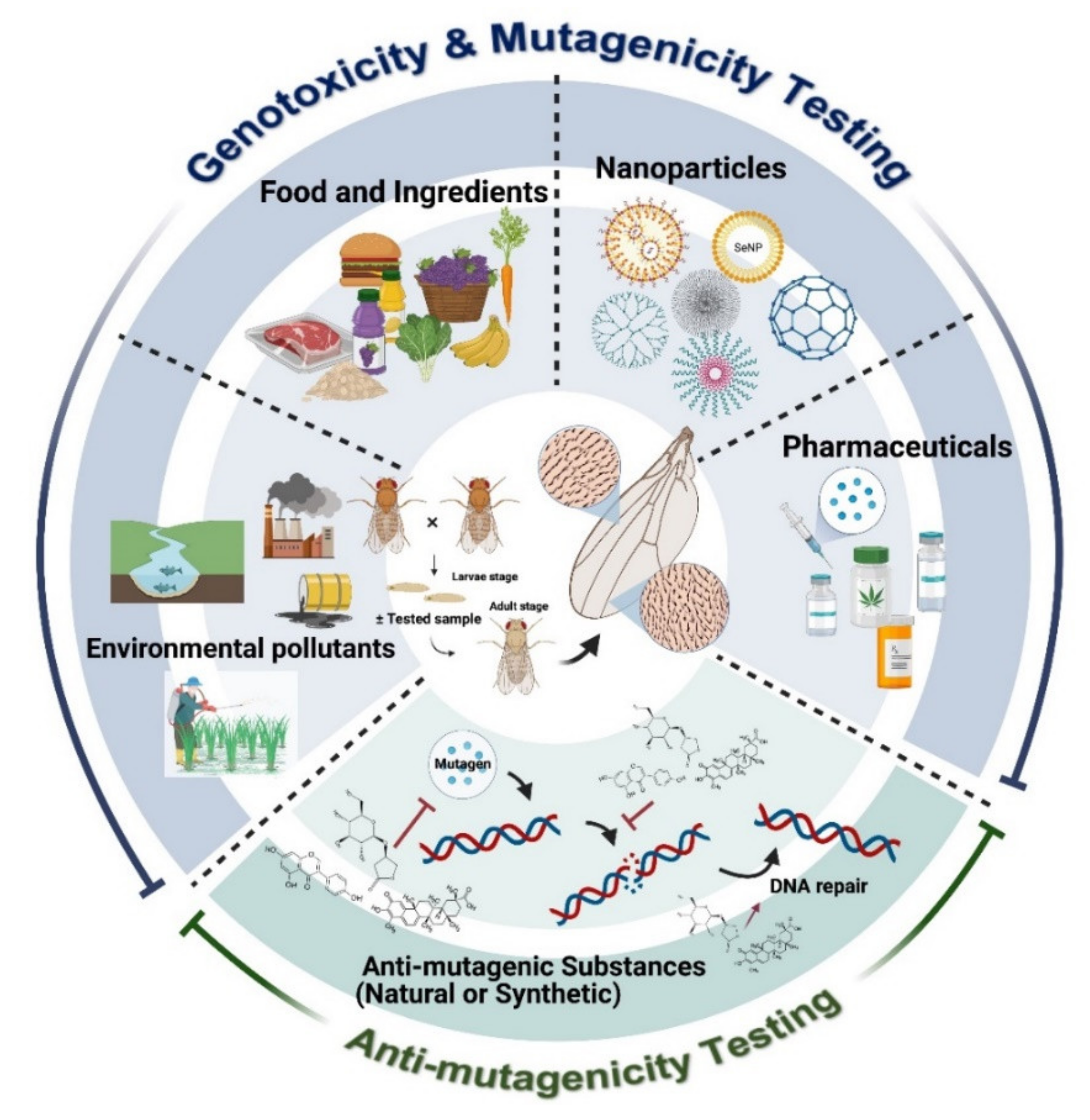
Human Hazard Assessment Using Drosophila Wing Spot Test as an Alternative In Vivo Model for Genotoxicity Testing—A Review
Abstract
1. Introduction
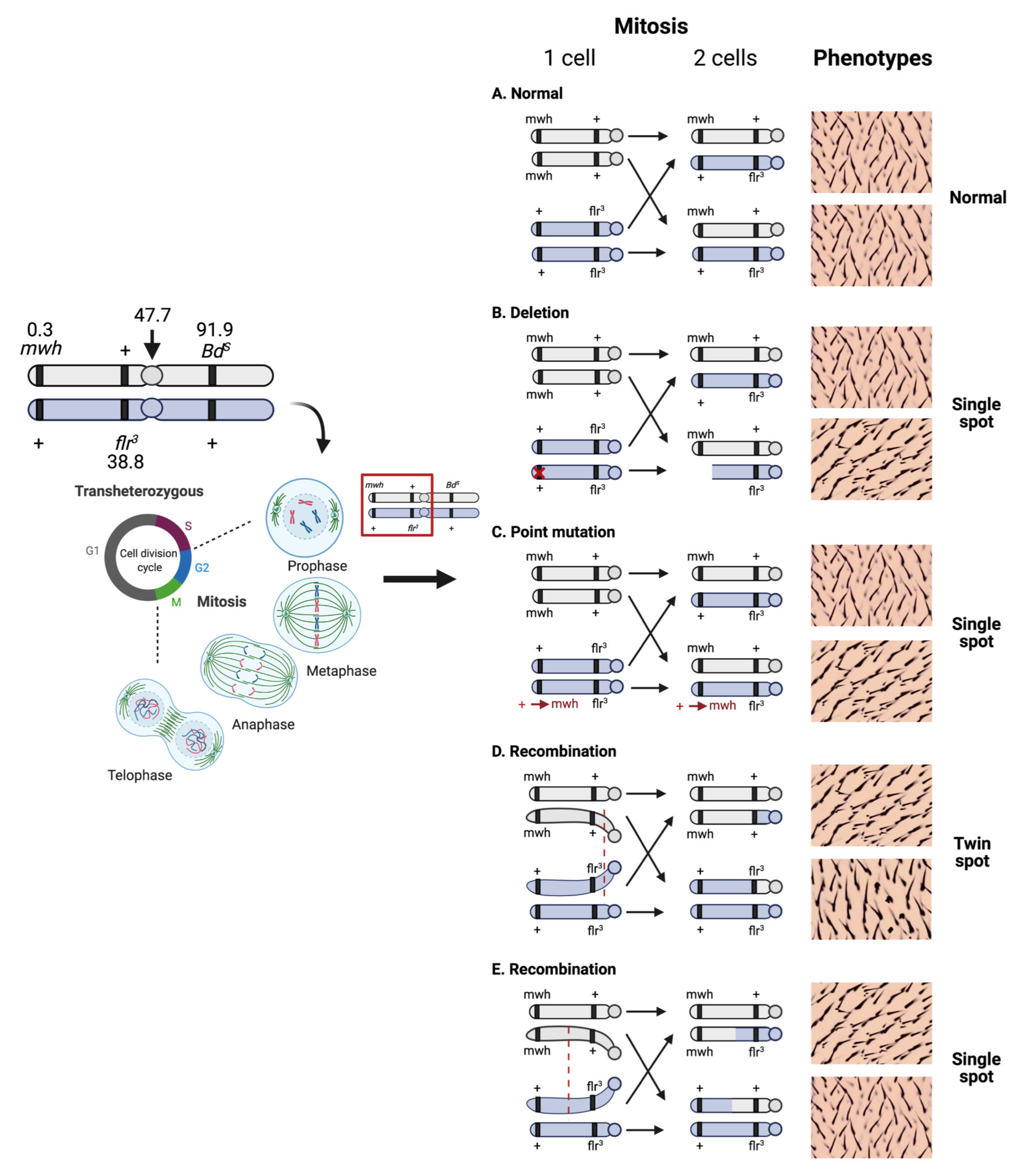
2. Principle of the SMART Assay or Wing Spot Test
3. Applications of SMART for Food Safety
4. Applications of SMART in Drug Safety Assessment
5. Applications of SMART for Genotoxicity Assessment of Environmental Pollutants
5.1. Insecticides and Herbicides
5.2. Chemicals in Daily Life
| Types | Result in ST Cross | Result in HB Cross | Range of Treated Doses and Remarks | Ref. |
|---|---|---|---|---|
| 1,5-Dinitronaphthalene | + | + | 1–100 mM, inconclusive at 20 and 50 mM and positive at 1, 10, and 100 mM positive in ST cross; however, all doses positive in HT cross | [78] |
| 2-Methylisoborneol | - | nd | 125–500 µg/mL, all doses lack genotoxicity | [92] |
| 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) | + | + | 0.6–2.4 mM, all doses positive both ST and HB cross | [93] |
| 1-Nitronaphthalene | + | + | 1–5 mM, lack genotoxicity at 1 mM and positive at 2–5 mM in ST cross, all doses positive in HB cross | [78] |
| 9-Nitroanthracene | + | + | 1–20 mM, inconclusive up to 10 mM, and positive at 20 mM in ST cross; however, lack of genotoxicity at 5 mM, inconclusive at 20 mM and positive at 1, 10, and 50 mM in HB cross | [78] |
| α-Terpineol | - | - | 0.05 and 2.5 µg/mL, lack genotoxicity at 0.05 µg/mL and inconclusive at 2.5 µg/mL in ST cross; however, lack genotoxicity in HB cross | [94] |
| Anthracene | + | + | 1–50 mM, lack genotoxicity at 5, 20, and 50 mM and positive at 1 and 10 mM in ST cross, HB cross shows 50 mM lack genotoxicity, weak positive at 1 and 10 mM, and positive at 5 and 20 mM | [78] |
| Bisphenol A | - | - | 0.1–5 µg/mL, all doses show inconclusive genotoxicity both ST and HB cross | [91] |
| Dimethylarsinic acid | + | nd | 0.05–0.5 mM, lack of genotoxicity at 0.1 mM but 0.05 mM inconclusive and positive at 0.25 and 0.5 mM | [95] |
| Linalool | - | - | 0.025 and 2.5 µg/mL, inconclusive at 0.025 µg/mL and lack genotoxicity at 2.5 µg/mL in ST cross; however, lack genotoxicity HB cross | [94] |
| Methylparaben | - | nd | 100–250 mM, all doses lack genotoxicity | [82] |
| Naphthalene | + | + | 1–10 mM, inconclusive at 1 mM and positive at 5 and 10 mM in ST cross; moreover, weak positive at 1mM and positive at 5 and 10 mM in HB cross | [78] |
| N-Nitrosonornicotine (NNN) | + | + | 7.2–28.8 mM, all doses positive both ST and HB cross | [93] |
| Propylparaben | - | nd | 100–250 mM, lack of genotoxicity up to 150 mM and inconclusive at 200 and 250 mM | [82] |
| Trans-pinocarveol | - | - | 0.025 and 2.5 µg/mL, inconclusive both concentrations in ST cross; however, lack genotoxicity HB cross | [94] |
| Verbenone | - | - | 0.05 and 2.5 µg/mL, inconclusive at 0.05 µg/mL and lack of genotoxicity at 2.5 µg/mL in ST cross; all doses lack genotoxicity in HB cross | [94] |
5.3. Domestic and Industrial Sewage
6. Applications of SMART for Nanoparticle Genotoxicity Assessment
7. Applications of SMART in Anti-Genotoxic Studies
8. Perspective: High-Throughput Screening Using SMART Assay
9. Limitations of SMART
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4,5-T | 2,4,5-Trichlorophenoxyacetic acid |
| 2-MIB | 2-methylisoborneol |
| BaP | Benzo[a]pyrene |
| BPA | bisphenol A |
| BPDE | Benzo(a)pyrene-7,8-diol 9,10-Epoxide |
| Cd | cadmium |
| CNTs | carbon nanotubes |
| Co-NPs | cobalt nanoparticles |
| CuO NPs | copper oxide nanoparticles |
| DWCNTs | double-walled carbon nanotubes |
| DXR | Doxorubicin |
| EFSA | European Food Safety Authority |
| EMS | Ethyl methanesulfonate |
| ENU | N-nitroso N-ethylurea |
| H2O2 | Hydrogen peroxide |
| HB cross | High bioactivation cross |
| LOH | Loss of heterozygosity |
| MMC | Mitomycin C |
| NaNO2 | Sodium nitrite |
| NaNO3 | Sodium nitrate |
| NCDs | non-communicable diseases |
| NiO-NPs | nickel-based nanoparticles |
| NNK | 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone |
| NNN | N-Nitrosonornicotine |
| NOCs | N-nitroso compounds |
| PAHs | polycyclic aromatic hydrocarbons |
| ROS | Reactive oxygen species |
| SMART | Somatic mutation and recombination test |
| SSRIs | selective serotonin reuptake inhibitors |
| ST cross | Standard cross |
| SWNTs | single-walled carbon nanotubes |
| T2DM | type 2 diabetes mellitus |
| TiO2 NCs | Titanium dioxide nanocrystals |
| URE | Urethane |
References
- Yousefzadeh, M.; Henpita, C.; Vyas, R.; Soto-Palma, C.; Robbins, P.; Niedernhofer, L. DNA damage—how and why we age? eLife 2021, 10, e62852. [Google Scholar] [CrossRef] [PubMed]
- Niedernhofer, L.J.; Gurkar, A.U.; Wang, Y.; Vijg, J.; Hoeijmakers, J.H.J.; Robbins, P.D. Nuclear Genomic Instability and Aging. Annu. Rev. Biochem. 2018, 87, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Fakouri, N.B.; Hou, Y.; Demarest, T.G.; Christiansen, L.S.; Okur, M.N.; Mohanty, J.G.; Croteau, D.L.; Bohr, V.A. Toward understanding genomic instability, mitochondrial dysfunction and aging. FEBS J. 2019, 286, 1058–1073. [Google Scholar] [CrossRef]
- Basu, A.K. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Maurici, D.; Aardema, M.; Corvi, R.; Kleber, M.; Krul, C.; Laurent, C.; Loprieno, N.; Pasanen, M.; Pfuhler, S.; Phillips, B.; et al. Genotoxicty and mutagenicity. Altern. Lab. Anim. 2005, 33 (Suppl. 1), 117–130. [Google Scholar] [CrossRef]
- Kanvah, S.; Joseph, J.; Schuster, G.B.; Barnett, R.N.; Cleveland, C.L.; Landman, U. Oxidation of DNA: Damage to nucleobases. Acc. Chem. Res. 2010, 43, 280–287. [Google Scholar] [CrossRef]
- Poetsch, A.R. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Graf, U.; Wurgler, F.E.; Katz, A.J.; Frei, H.; Juon, H.; Hall, C.B.; Kale, P.G. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mutagen. 1984, 6, 153–188. [Google Scholar] [CrossRef]
- Aldaz, S.; Escudero, L.M. Imaginal discs. Curr. Biol. 2010, 20, R429–R431. [Google Scholar] [CrossRef] [PubMed]
- Beira, J.V.; Paro, R. The legacy of Drosophila imaginal discs. Chromosoma 2016, 125, 573–592. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, H.H.R.; Reguly, M.L.; Lehmann, M. Wing Somatic Mutation and Recombination Test. In Drosophila Cytogenetics Protocols; Henderson, D.S., Ed.; Humana Press: Totowa, NJ, USA, 2004; pp. 389–412. [Google Scholar]
- Graf, U.; Alonso Moraga, A.; Castro, R.; Diaz Carrillo, E. Genotoxicity testing of different types of beverages in the Drosophila wing somatic mutation and recombination test. Food Chem. Toxicol. 1994, 32, 423–430. [Google Scholar] [CrossRef]
- Munerato, M.C.; Sinigaglia, M.; Reguly, M.L.; de Andrade, H.H.R. Genotoxic effects of eugenol, isoeugenol and safrole in the wing spot test of Drosophila melanogaster. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 582, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, D.L.; Zimm, G.G. The Genome of Drosophila Melanogaster; Academic Press: San Diego, CA, USA, 1992; 1133p. [Google Scholar]
- Frölich, A.; Würgler, F.E. New tester strains with improved bioactivation capacity for the Drosophila wing-spot test. Mutat. Res. 1989, 216, 179–187. [Google Scholar] [CrossRef]
- Frölich, A.; Würgler, F.E. Drosophila wing-spot test: Improved detectability of genotoxicity of polycyclic aromatic hydrocarbons. Mutat Res. 1990, 234, 71–80. [Google Scholar] [CrossRef]
- Graf, U.; van Schaik, N. Improved high bioactivation cross for the wing somatic mutation and recombination test in Drosophila melanogaster. Mutat. Res. 1992, 271, 59–67. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of potassium nitrite (E 249) and sodium nitrite (E 250) as food additives. EFSA J. 2017, 15, e04786. [Google Scholar] [PubMed]
- Demir, E.; Kocaoğlu, S.; Kaya, B. Genotoxicity testing of four benzyl derivatives in the Drosophila wing spot test. Food Chem. Toxicol. 2008, 46, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Ayar, A.; Uysal, H. Examination of the Genotoxic Effects of Various Parabens Used as Food Additives with the Drosophila Wing Spot Test (SMART). J. Appl. Biol. 2013, 7, 83–88. [Google Scholar]
- Sarikaya, R.; Cakir, S. Genotoxicity testing of four food preservatives and their combinations in the Drosophila wing spot test. Environ. Toxicol. Pharmacol. 2005, 20, 424–430. [Google Scholar] [CrossRef]
- Sarıkaya, R.; Selvi, M.; Erkoç, F. Evaluation of potential genotoxicity of five food dyes using the somatic mutation and recombination test. Chemosphere 2012, 88, 974–979. [Google Scholar] [CrossRef]
- Mademtzoglou, D.; Akmoutsou, P.; Kounatidis, I.; Franzios, G.; Drosopoulou, E.; Vokou, D.; Mavragani-Tsipidou, P. Applying the Drosophila wing spot test to assess the genotoxic impact of 10 essential oil constituents used as flavouring agents or cosmetic ingredients. Flavour. Fragr. J. 2011, 26, 447–451. [Google Scholar] [CrossRef]
- Srichamnong, W.; Ting, P.; Pitchakarn, P.; Nuchuchua, O.; Temviriyanukul, P. Safety assessment of Plukenetia volubilis (Inca peanut) seeds, leaves, and their products. Food Sci. Nutr. 2018, 6, 962–969. [Google Scholar] [CrossRef]
- Kounatidis, I.; Papoti, V.T.; Nenadis, N.; Franzios, G.; Oikonomou, M.; Partheniou, F.; Tsimidou, M.; Mavragani-Tsipidou, P. Evaluation of Potential Genotoxicity of Virgin Olive Oil (VOO) Using the Drosophila Wing-Spot Test. J. Agric. Food Chem. 2009, 57, 7785–7789. [Google Scholar] [CrossRef]
- da Silva, R.A.; Dihl, R.R.; e Santos, D.N.; de Abreu, B.R.R.; de Lima, A.; de Andrade, H.H.R.; Lehmann, M. Evaluation of antioxidant and mutagenic activities of honey-sweetened cashew apple nectar. Food Chem. Toxicol. 2013, 62, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bedmar, Z.; Anter, J.; Alonso Moraga, Á. Anti/genotoxic, longevity inductive, cytotoxic, and clastogenic-related bioactivities of tomato and lycopene. Environ. Mol. Mutagen. 2018, 59, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Marcos, R.; Kaya, B. Genotoxicity studies in the ST cross of the Drosophila wing spot test of sunflower and soybean oils before and after frying and boiling procedures. Food Chem. Toxicol. 2012, 50, 3619–3624. [Google Scholar] [CrossRef] [PubMed]
- Budluang, P.; Pitchakarn, P.; Ting, P.; Temviriyanukul, P.; Wongnoppawich, A.; Imsumran, A. Anti-inflammatory and anti-insulin resistance activities of aqueous extract from Anoectochilus burmannicus. Food Sci. Nutr. 2017, 5, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Karinchai, J.; Budluang, P.; Temviriyanukul, P.; Ting, P.; Nuchuchua, O.; Wongnoppavich, A.; Imsumran, A.; Pitchakarn, P. Bioassay-guided study of the anti-inflammatory effect of Anoectochilus burmannicus ethanolic extract in RAW 264.7 cells. J. Ethnopharmacol. 2021, 280, 114452. [Google Scholar] [CrossRef]
- Amkiss, S.; Dalouh, A.; Idaomar, M. Chemical composition, genotoxicity and antigenotoxicity study of Artemisia herba-alba using the eye and wing SMART assay of Drosophila melanogaster. Arab. J. Chem. 2021, 14, 102976. [Google Scholar] [CrossRef]
- Carmona, E.R.; Reyes-Díaz, M.; Parodi, J.; Inostroza-Blancheteau, C. Antimutagenic evaluation of traditional medicinal plants from South America Peumus boldus and Cryptocarya alba using Drosophila melanogaster. J. Toxicol. Environ. Health A 2017, 80, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Téllez, M.G.O.; Rodríguez, H.B.; Olivares, G.Q.; Sortibrán, A.N.C.; Cetto, A.A.; Rodríguez-Arnaiz, R. A phytotherapeutic extract of Equisetum myriochaetum is not genotoxic either in the in vivo wing somatic test of Drosophila or in the in vitro human micronucleus test. J. Ethnopharmacol. 2007, 111, 182–189. [Google Scholar] [CrossRef]
- Khantamat, O.; Dukaew, N.; Karinchai, J.; Chewonarin, T.; Pitchakarn, P.; Temviriyanukul, P. Safety and bioactivity assessment of aqueous extract of Thai Henna (Lawsonia inermis Linn.) Leaf. J. Toxicol. Environ. Health A 2021, 84, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Villas Boas, G.R.; Rodrigues Lemos, J.M.; de Oliveira, M.W.; dos Santos, R.C.; Stefanello da Silveira, A.P.; Bacha, F.B.; Aguero Ito, C.N.; Cornelius, E.B.; Lima, F.B.; Sachilarid Rodrigues, A.M.; et al. Preclinical safety evaluation of the aqueous extract from Mangifera indica Linn. (Anacardiaceae): Genotoxic, clastogenic and cytotoxic assessment in experimental models of genotoxicity in rats to predict potential human risks. J. Ethnopharmacol. 2019, 243, 112086. [Google Scholar] [CrossRef]
- Senes-Lopes, T.F.; López, J.A.; do Amaral, V.S.; Brandão-Neto, J.; de Rezende, A.A.; da Luz, J.R.D.; Guterres, Z.d.R.; Almeida, M.d.G. Genotoxicity of Turnera subulata and Spondias mombin × Spondias tuberosa Extracts from Brazilian Caatinga Biome. J. Med. Food 2018, 21, 372–379. [Google Scholar] [CrossRef]
- Anter, J.; Romero-Jiménez, M.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Analla, M.; Alonso-Moraga, Á.; Muñoz-Serrano, A. Antigenotoxicity, Cytotoxicity, and Apoptosis Induction by Apigenin, Bisabolol, and Protocatechuic Acid. J. Med. Food 2010, 14, 276–283. [Google Scholar] [CrossRef]
- Oliveira, V.C.; Naves, M.P.C.; de Morais, C.R.; Constante, S.A.R.; Orsolin, P.C.; Alves, B.S.; Rinaldi Neto, F.; da Silva, L.H.D.; de Oliveira, L.T.S.; Ferreira, N.H.; et al. Betulinic acid modulates urethane-induced genotoxicity and mutagenicity in mice and Drosophila melanogaster. Food Chem. Toxicol. 2020, 138, 111228. [Google Scholar] [CrossRef]
- Costa, W.F.; de Oliveira, A.B.; Nepomuceno, J.C. Genotoxicity of lapachol evaluated by wing spot test of Drosophila melanogaster. Genet. Mol. Biol. 2010, 33, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.M.; da Rosa Guterres, Z.; Almeida, I.V.; Vicentini, V.E.P. Genotoxicity and Antigenotoxicity Assessments of the Flavonoid Vitexin by the Drosophila melanogaster Somatic Mutation and Recombination Test. J. Med. Food 2017, 20, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Brody, T. Chapter 25—Drug Safety. In Clinical Trials, 2nd ed.; Brody, T., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 483–568. [Google Scholar]
- Gürbüzel, M.; Çapoğlu, I.; Kızılet, H.; Halıcı, Z.; Özçiçek, F.; Demirtaş, L. Genotoxic evaluation of two oral antidiabetic agents in the Drosophila wing spot test. Toxicol. Ind. Health 2014, 30, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Koksal, P.M.; Gürbüzel, M. Analysis of genotoxic activity of ketamine and rocuronium bromide using the somatic mutation and recombination test in Drosophila melanogaster. Environ. Toxicol. Pharmacol. 2015, 39, 628–634. [Google Scholar] [CrossRef]
- Leone, S.; Di Cianni, S.; Casati, A.; Fanelli, G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed. 2008, 79, 92–105. [Google Scholar] [PubMed]
- Shaik, N.A.; Shaik, J.P.; Ali, S.; Imran, A.; Rao, D.K. Increased frequency of micronuclei in diabetes mellitus patients using pioglitazone and glimepiride in combination. Food Chem. Toxicol. 2010, 48, 3432–3435. [Google Scholar] [CrossRef]
- Gürbüzel, M.; Oral, E.; Kizilet, H.; Halici, Z.; Gulec, M. Genotoxic evaluation of selective serotonin-reuptake inhibitors by use of the somatic mutation and recombination test in Drosophila melanogaster. Mutat. Res. 2012, 748, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Orsolin, P.C.; Silva-Oliveira, R.G.; Nepomuceno, J.C. Modulating effect of synthetic statins against damage induced by doxorubicin in somatic cells of Drosophila melanogaster. Food Chem. Toxicol. 2015, 81, 111–119. [Google Scholar] [CrossRef]
- Gürbüzel, M.; Karaca, U.; Karayilan, N. Genotoxic evaluation of bupivacaine and levobupivacaine in the Drosophila wing spot test. Cytotechnology 2016, 68, 979–986. [Google Scholar] [CrossRef]
- Naves, M.P.C.; de Morais, C.R.; Spanó, M.A.; de Rezende, A.A.A. Mutagenicity and recombinogenicity evaluation of bupropion hydrochloride and trazodone hydrochloride in somatic cells of Drosophila melanogaster. Food Chem. Toxicol. 2019, 131, 110557. [Google Scholar] [CrossRef]
- Bhalli, J.A.; Ali, T.; Asi, M.R.; Khalid, Z.M.; Ceppi, M.; Khan, Q.M. DNA damage in Pakistani agricultural workers exposed to mixture of pesticides. Environ. Mol. Mutagen. 2009, 50, 37–45. [Google Scholar] [CrossRef]
- Bolognesi, C.; Moretto, A. Genotoxic risk in rubber manufacturing industry: A systematic review. Toxicol. Lett. 2014, 230, 345–355. [Google Scholar] [CrossRef]
- Hernández, A.F.; López, O.; Rodrigo, L.; Gil, F.; Pena, G.; Serrano, J.L.; Parrón, T.; Álvarez, J.C.; Lorente, J.A.; Pla, A. Changes in erythrocyte enzymes in humans long-term exposed to pesticides: Influence of several markers of individual susceptibility. Toxicol. Lett. 2005, 159, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, C. Genotoxicity of pesticides: A review of human biomonitoring studies. Mutat. Res. 2003, 543, 251–272. [Google Scholar] [CrossRef]
- de Morais, C.R.; Carvalho, S.M.; Carvalho Naves, M.P.; Araujo, G.; de Rezende, A.A.A.; Bonetti, A.M.; Spanó, M.A. Mutagenic, recombinogenic and carcinogenic potential of thiamethoxam insecticide and formulated product in somatic cells of Drosophila melanogaster. Chemosphere 2017, 187, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ishiwatari, T.; Sugano, M.; Lucas, J.R.; Shono, Y. Biological Efficacy of Metofluthrin, a New Pyrethroid Insecticide, Highly Effective against Mosquitoes. In Advances in Human Vector Control; American Chemical Society: Washington, DC, USA, 2009; Volume 1014, pp. 161–169. [Google Scholar]
- Ujihara, K.; Mori, T.; Iwasaki, T.; Sugano, M.; Shono, Y.; Matsuo, N. Metofluthrin: A potent new synthetic pyrethroid with high vapor activity against mosquitoes. Biosci. Biotechnol. Biochem. 2004, 68, 170–174. [Google Scholar] [CrossRef][Green Version]
- Grosman, N. Influence of pyrethroids and piperonyl butoxide on histamine release from isolated rat mast cells. Inflamm. Res. 2007, 56, 473–478. [Google Scholar] [CrossRef]
- Cole, L.M.; Nicholson, R.A.; Casida, J.E. Action of Phenylpyrazole Insecticides at the GABA-Gated Chloride Channel. Pestic Biochem. Physiol. 1993, 46, 47–54. [Google Scholar] [CrossRef]
- Lourenço, C.T.; Carvalho, S.M.; Malaspina, O.; Nocelli, R.C.F. Oral Toxicity of Fipronil Insecticide Against the Stingless Bee Melipona scutellaris (Latreille, 1811). Bull. Environ. Contam. Toxicol. 2012, 89, 921–924. [Google Scholar] [CrossRef]
- de Morais, C.R.; Bonetti, A.M.; Carvalho, S.M.; de Rezende, A.A.A.; Araujo, G.R.; Spanó, M.A. Assessment of the mutagenic, recombinogenic and carcinogenic potential of fipronil insecticide in somatic cells of Drosophila melanogaster. Chemosphere 2016, 165, 342–351. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Goulson, D. REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef]
- Karabay, N.U.; Oguz, M.G. Cytogenetic and genotoxic effects of the insecticides, imidacloprid and methamidophos. Genet. Mol. Res. 2005, 4, 653–662. [Google Scholar]
- Abolaji, A.O.; Kamdem, J.P.; Farombi, E.O.; Rocha, J.B.T. Drosophila melanogaster as a promising model organism in toxicological studies: A mini review. Arch. Basic Appl. Med. 2013, 1, 33–38. [Google Scholar]
- Zetterberg, G. Genetic Effects of Phenoxy Acids on Microorganisms. Ecol. Bull. 1978, 27, 193–204. [Google Scholar]
- Mortelmans, K.; Haworth, S.; Speck, W.; Zeiger, E. Mutagenicity testing of agent orange components and related chemicals. Toxicol. Appl. Pharm. 1984, 75, 137–146. [Google Scholar] [CrossRef]
- Sarıkaya, R.; Memmi, B.K. Detection of transfluthrin and metofluthrin genotoxicity in the ST cross of the Drosophila Wing Spot Test. Chemosphere 2013, 93, 238–242. [Google Scholar] [CrossRef]
- Kaya, B.; Creus, A.; Yanikoğlu, A.; Cabré, O.; Marcos, R. Use of the Drosophila wing spot test in the genotoxicity testing of different herbicides. Environ. Mol. Mutagen. 2000, 36, 40–46. [Google Scholar] [CrossRef]
- Kaya, B.; Yanikoǧlu, A.; Creus, A.; Marcos, R. Genotoxicity testing of five herbicides in the Drosophila wing spot test. Mutat Res. Genet. Toxicol. Environ. Mutagen. 2000, 465, 77–84. [Google Scholar] [CrossRef]
- Kaya, B.; Marcos, R.; Yanikoğlu, A.; Creus, A. Evaluation of the genotoxicity of four herbicides in the wing spot test of Drosophila melanogaster using two different strains. Mutat. Res. 2004, 557, 53–62. [Google Scholar] [CrossRef]
- Fragiorge, E.J.; Rezende, A.A.; Graf, U.; Spanó, M.A. Comparative genotoxicity evaluation of imidazolinone herbicides in somatic cells of Drosophila melanogaster. Food Chem. Toxicol. 2008, 46, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- Logan, D.T. Perspective on Ecotoxicology of PAHs to Fish. Hum. Ecol. Risk Assess. 2007, 13, 302–316. [Google Scholar] [CrossRef]
- Tong, H.Y.; Karasek, F.W. Quantitation of polycyclic aromatic hydrocarbons in diesel exhaust particulate matter by high-performance liquid chromatography fractionation and high-resolution gas chromatography. Anal. Chem. 1984, 56, 2129–2134. [Google Scholar] [CrossRef]
- Delgado-Rodriguez, A.; Ortíz-Marttelo, R.; Graf, U.; Villalobos-Pietrini, R.; Gómez-Arroyo, S. Genotoxic activity of environmentally important polycyclic aromatic hydrocarbons and their nitro derivatives in the wing spot test of Drosophila melanogaster. Mutat. Res. 1995, 341, 235–247. [Google Scholar] [CrossRef]
- Guzmán Rincón, J.; Espinosa, J.; Graf, U. Analysis of the in vivo nitrosation capacity of the larvae used in the wing somatic mutation and recombination test of Drosophila melanogaster. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1998, 412, 69–81. [Google Scholar] [CrossRef]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (Parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 2004, 24, 5–13. [Google Scholar] [CrossRef]
- Ayar, A.; Uysal, H. Genotoxic and safety assessment of 2 parabens in somatic cells of in vivo Drosophila melanogaster. Turk. J. Biol. 2013, 37, 683–688. [Google Scholar] [CrossRef]
- Mikołajewska, K.; Stragierowicz, J.; Gromadzińska, J. Bisphenol A—Application, sources of exposure and potential risks in infants, children and pregnant women. Int. J. Occup. Med. Environ. Health 2015, 28, 209–241. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Fabbri, E. Environmental Effects of BPA: Focus on Aquatic Species. Dose-Response 2015, 13, 1559325815598304. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.R.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef]
- Huang, R.P.; Liu, Z.H.; Yuan, S.F.; Yin, H.; Dang, Z.; Wu, P.X. Worldwide human daily intakes of bisphenol A (BPA) estimated from global urinary concentration data (2000–2016) and its risk analysis. Environ. Pollut. 2017, 230, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Valentino, R.; D’Esposito, V.; Ariemma, F.; Cimmino, I.; Beguinot, F.; Formisano, P. Bisphenol A environmental exposure and the detrimental effects on human metabolic health: Is it necessary to revise the risk assessment in vulnerable population? J. Endocrinol. Investig. 2016, 39, 259–263. [Google Scholar] [CrossRef]
- Mouneimne, Y.; Nasrallah, M.; Khoueiry-Zgheib, N.; Nasreddine, L.; Nakhoul, N.; Ismail, H.; Abiad, M.; Koleilat, L.; Tamim, H. Bisphenol A urinary level, its correlates, and association with cardiometabolic risks in Lebanese urban adults. Environ. Monit. Assess. 2017, 189, 517. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Jiang, L.; Liu, X.; Geng, C.; Wang, W.; Zhong, L.; Yang, G.; Chen, M. Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutat Res. Genet. Toxicol. Environ. Mutagen. 2014, 769, 29–33. [Google Scholar] [CrossRef]
- Naik, P.; Vijayalaxmi, K.K. Cytogenetic evaluation for genotoxicity of Bisphenol-A in bone marrow cells of Swiss albino mice. Mutat Res. Genet. Toxicol. Environ. Mutagen. 2009, 676, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.; Olakkaran, S.; Purayil, A.K.; Puttaswamygowda, G.H. Bisphenol A induced oxidative stress mediated genotoxicity in Drosophila melanogaster. J. Hazard. Mater. 2019, 370, 42–53. [Google Scholar]
- Burgos, L.; Lehmann, M.; de Andrade, H.H.; de Abreu, B.R.; de Souza, A.P.; Juliano, V.B.; Dihl, R.R. In vivo and in vitro genotoxicity assessment of 2-methylisoborneol, causal agent of earthy-musty taste and odor in water. Ecotoxicol. Environ. Saf. 2014, 100, 282–286. [Google Scholar] [CrossRef]
- Guzmán-Rincόn, J.; Ramίrez-Victoria, P.; Benitez, L. Somatic Mutation and Recombination Test in Drosophila Used for Biomonitoring of Environmental Pollutants. In Biomonitors and Biomarkers as Indicators of Environmental Change 2: A Handbook; Butterworth, F.M., Gunatilaka, A., Gonsebatt, M.E., Eds.; Springer: Boston, MA, USA, 2001; pp. 221–237. [Google Scholar]
- Drosopoulou, E.; Vlastos, D.; Efthimiou, I.; Kyrizaki, P.; Tsamadou, S.; Anagnostopoulou, M.; Kofidou, D.; Gavriilidis, M.; Mademtzoglou, D.; Mavragani-Tsipidou, P. In vitro and in vivo evaluation of the genotoxic and antigenotoxic potential of the major Chios mastic water constituents. Sci. Rep. 2018, 8, 12200. [Google Scholar] [CrossRef]
- Rizki, M.; Kossatz, E.; Velázquez, A.; Creus, A.; Farina, M.; Fortaner, S.; Sabbioni, E.; Marcos, R. Metabolism of arsenic in Drosophila melanogaster and the genotoxicity of dimethylarsinic acid in the Drosophila wing spot test. Environ. Mol. Mutagen. 2006, 47, 162–168. [Google Scholar] [CrossRef]
- Bishop, A.J.R.; Schiestl, R.H. Homologous Recombination and Its Role in Carcinogenesis. J. Biomed. Biotechnol. 2002, 2, 75–85. [Google Scholar] [CrossRef]
- Olegário de Campos Júnior, E.; da Silva Oliveira, R.G.; Pereira, B.B.; Souto, H.N.; Campos, C.F.; Nepomuceno, J.C.; Morelli, S. Assessment of genotoxic, mutagenic, and recombinogenic potential of water resources in the Paranaíba River basin of Brazil: A case study. J. Toxicol. Environ. Health A 2016, 79, 1190–1200. [Google Scholar] [CrossRef]
- Porta, C.S.; dos Santos, D.L.; Bernardes, H.V.; Bellagamba, B.C.; Duarte, A.; Dias, J.F.; da Silva, F.R.; Lehmann, M.; da Silva, J.; Dihl, R.R. Cytotoxic, genotoxic and mutagenic evaluation of surface waters from a coal exploration region. Chemosphere 2017, 172, 440–448. [Google Scholar] [CrossRef]
- Resende de Morais, C.; Bonetti, A.M.; Mota, A.A.; Campos, C.F.; Souto, H.N.; Carvalho Naves, M.P.; Vieira Santos, V.S.; Olegário de Campos Júnior, E.; Pavanin, L.A.; Alves de Rezende, A.A.; et al. Evaluation of toxicity, mutagenicity and carcinogenicity of samples from domestic and industrial sewage. Chemosphere 2018, 201, 342–350. [Google Scholar] [CrossRef]
- do Amaral, V.S.; da Silva, R.M.; Reguly, M.L.; de Andrade, H.H. Drosophila wing-spot test for genotoxic assessment of pollutants in water samples from urban and industrial origin. Mutat. Res. 2005, 583, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Jacociunas, L.V.; Dihl, R.R.; Lehmann, M.; Reguly, M.L.; de Andrade, H.H.R. Recombinagenic activity of water and sediment from Sinos River and Araçá and Garças Streams (Canoas, Brazil), in the Drosophila wing spot test. Sci. Total Environ. 2010, 408, 571–577. [Google Scholar] [CrossRef]
- Gorth, D.J.; Rand, D.M.; Webster, T.J. Silver nanoparticle toxicity in Drosophila: Size does matter. Int. J. Nanomed. 2011, 6, 343–350. [Google Scholar]
- Carmona, E.R.; Inostroza-Blancheteau, C.; Rubio, L.; Marcos, R. Genotoxic and oxidative stress potential of nanosized and bulk zinc oxide particles in Drosophila melanogaster. Toxicol. Ind. Health 2016, 32, 1987–2001. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.R.; Escobar, B.; Vales, G.; Marcos, R. Genotoxic testing of titanium dioxide anatase nanoparticles using the wing-spot test and the comet assay in Drosophila. Mutat Res. Genet. Toxicol. Environ. Mutagen. 2015, 778, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.R.; Inostroza-Blancheteau, C.; Obando, V.; Rubio, L.; Marcos, R. Genotoxicity of copper oxide nanoparticles in Drosophila melanogaster. Mutat Res. Genet. Toxicol. Environ. Mutagen. 2015, 791, 1–11. [Google Scholar] [CrossRef]
- Vales, G.; Demir, E.; Kaya, B.; Creus, A.; Marcos, R. Genotoxicity of cobalt nanoparticles and ions in Drosophila. Nanotoxicology 2013, 7, 462–468. [Google Scholar] [CrossRef]
- Reis Éde, M.; de Rezende, A.A.; Santos, D.V.; de Oliveria, P.F.; Nicolella, H.D.; Tavares, D.C.; Silva, A.C.; Dantas, N.O.; Spanó, M.A. Assessment of the genotoxic potential of two zinc oxide sources (amorphous and nanoparticles) using the in vitro micronucleus test and the in vivo wing somatic mutation and recombination test. Food Chem. Toxicol. 2015, 84, 55–63. [Google Scholar] [CrossRef] [PubMed]
- De Carli, R.F.; Chaves, D.d.S.; Cardozo, T.R.; de Souza, A.P.; Seeber, A.; Flores, W.H.; Honatel, K.F.; Lehmann, M.; Dihl, R.R. Evaluation of the genotoxic properties of nickel oxide nanoparticles in vitro and in vivo. Mutat Res. Genet. Toxicol. Environ. Mutagen. 2018, 836, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.M.; Lopes, J.C.; Saturnino, R.S.; Fagan, E.B.; Nepomuceno, J.C. Lack of mutagenic effect by multi-walled functionalized carbon nanotubes in the somatic cells of Drosophila melanogaster. Food Chem. Toxicol. 2013, 62, 355–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Andrade, L.R.; Sandin Brito, A.; de Souza Melero, A.M.G.; Zanin, H.; José Ceragioli, H.; Baranauskas, V.; Silva Cunha, K.; Pierre Irazusta, S. Absence of mutagenic and recombinagenic activity of multi-walled carbon nanotubes in the Drosophila wing-spot test and Allium cepa test. Ecotoxicol. Environ. Saf. 2014, 99, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Reis Éde, M.; Rezende, A.A.; Oliveira, P.F.; Nicolella, H.D.; Tavares, D.C.; Silva, A.C.; Dantas, N.O.; Spanó, M.A. Evaluation of titanium dioxide nanocrystal-induced genotoxicity by the cytokinesis-block micronucleus assay and the Drosophila wing spot test. Food Chem. Toxicol. 2016, 96, 309–319. [Google Scholar] [CrossRef]
- Ávalos, A.; Haza, A.I.; Mateo, D.; Morales, P. In vitro and in vivo genotoxicity assessment of gold nanoparticles of different sizes by comet and SMART assays. Food Chem. Toxicol. 2018, 120, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Kaygisiz, Ş.Y.; Ciğerci, İ.H. Genotoxic evaluation of different sizes of iron oxide nanoparticles and ionic form by SMART, Allium and comet assay. Toxicol. Ind. Health 2017, 33, 802–809. [Google Scholar] [CrossRef]
- Parvathi, V.D.; Rajagopal, K.; Sumitha, R. Standardization of Alternative Methods for Nanogenotoxicity Testing in Drosophila melanogaster Using Iron Nanoparticles: A Promising Link to Nanodosimetry. J. Nanotechnol. 2016, 2016, 2547467. [Google Scholar] [CrossRef]
- Cardozo, T.R.; De Carli, R.F.; Seeber, A.; Flores, W.H.; da Rosa, J.A.N.; Kotzal, Q.S.G.; Lehmann, M.; da Silva, F.R.; Dihl, R.R. Genotoxicity of zinc oxide nanoparticles: An in vivo and in silico study. Toxicol. Res. 2019, 8, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R. Antimutagens as cancer chemopreventive agents in the diet. Mutat. Res. 1994, 307, 395–410. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Philpott, M.; Karunasinghe, N. Dietary cancer and prevention using antimutagens. Toxicology 2004, 198, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Hrdinaa, R.; Geršlb, V.; Klimtováa, I.; Šimůneka, T.; Macháčkováb, J.; Adamcovác, M. Anthracycline-Induced Cardiotoxicity. Acta Med. 2000, 43, 75–82. [Google Scholar] [CrossRef]
- Pawlus, A.D.; Su, B.-N.; Keller, W.J.; Kinghorn, A.D. An Anthraquinone with Potent Quinone Reductase-Inducing Activity and Other Constituents of the Fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2005, 68, 1720–1722. [Google Scholar] [CrossRef]
- Carmona, E.R.; Escobar, B.; Obando, V. Antimutagenic activity of Buddleja globosa extracts in the Drosophila wing-spot test. Fresenius Environ. Bull. 2016, 25, 5758–5764. [Google Scholar]
- Vale, C.R.; Silva, C.R.; Oliveira, C.M.; Silva, A.L.; Carvalho, S.; Chen-Chen, L. Assessment of toxic, genotoxic, antigenotoxic, and recombinogenic activities of Hymenaea courbaril (Fabaceae) in Drosophila melanogaster and mice. Genet. Mol. Res. 2013, 12, 2712–2724. [Google Scholar] [CrossRef]
- Franchi, L.P.; Guimaraes, N.N.; De Andrade, L.R.; De Andrade, H.H.; Lehmann, M.; Dihl, R.R.; Cunha, K.S. Antimutagenic and antirecombinagenic activities of noni fruit juice in somatic cells of Drosophila melanogaster. An. Acad. Bras. Cienc. 2013, 85, 585–594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Valadares, B.L.B.; Graf, U.; Spanó, M.A. Inhibitory effects of water extract of propolis on doxorubicin-induced somatic mutation and recombination in Drosophila melanogaster. Food Chem. Toxicol. 2008, 46, 1103–1110. [Google Scholar] [CrossRef]
- Sarikaya, R.; Erciyas, K.; Kara, M.I.; Sezer, U.; Erciyas, A.F.; Ay, S. Evaluation of genotoxic and antigenotoxic effects of boron by the somatic mutation and recombination test (SMART) on Drosophila. Drug Chem. Toxicol. 2016, 39, 400–406. [Google Scholar] [CrossRef]
- Demir, E.; Kocaoğlu, S.; Cetin, H.; Kaya, B. Antigenotoxic effects of Citrus aurentium L. fruit peel oil on mutagenicity of two alkylating agents and two metals in the Drosophila wing spot test. Environ. Mol. Mutagen. 2009, 50, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Baena, M.-D.; Tasset, I.; Obregón-Cano, S.; De Haro-Bailon, A.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Antigenotoxicity and Tumor Growing Inhibition by Leafy Brassica carinata and Sinigrin. Molecules 2015, 20, 15748–15765. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bedmar, Z.; Anter, J.; de La Cruz-Ares, S.; Muñoz-Serrano, A.; Alonso-Moraga, Á.; Pérez-Guisado, J. Role of Citrus Juices and Distinctive Components in the Modulation of Degenerative Processes: Genotoxicity, Antigenotoxicity, Cytotoxicity, and Longevity in Drosophila. J. Toxicol. Environ. Health A 2011, 74, 1052–1066. [Google Scholar] [CrossRef]
- Véras, J.H.; do Vale, C.R.; da Silva Lima, D.C.; dos Anjos, M.M.; Bernardes, A.; de Moraes Filho, A.V.; e Silva, C.R.; de Oliveira, G.R.; Pérez, C.N.; Chen-Chen, L. Modulating effect of a hydroxychalcone and a novel coumarin–chalcone hybrid against mitomycin-induced genotoxicity in somatic cells of Drosophila melanogaster. Drug Chem. Toxicol. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B. Anti-genotoxic effect of Ascorbic acid on mutagenic dose of three alkylating agents. Turk. J. Biol. 2003, 27, 241–246. [Google Scholar]
- de Freitas, P.L.; Dias, A.C.; Moreira, V.R.; Monteiro, S.G.; Pereira, S.R. Antimutagenic action of the triterpene betulinic acid isolated from Scoparia dulcis (Scrophulariaceae). Genet. Mol. Res. 2015, 14, 9745–9752. [Google Scholar] [CrossRef] [PubMed]
- Mezzoug, N.; Elhadri, A.; Dallouh, A.; Amkiss, S.; Skali, N.S.; Abrini, J.; Zhiri, A.; Baudoux, D.; Diallo, B.; El Jaziri, M.; et al. Investigation of the mutagenic and antimutagenic effects of Origanum compactum essential oil and some of its constituents. Mutat Res. Genet. Toxicol. Environ. Mutagen. 2007, 629, 100–110. [Google Scholar] [CrossRef]
- Suttisansanee, U.; Pitchakarn, P.; Ting, P.; Inthachat, W.; Thiyajai, P.; Rodthayoy, D.; Karinchai, J.; Chanatarasuwan, B.; Nuchuchua, O.; Temviriyanukul, P. Health-promoting bioactivity and in vivo genotoxicity evaluation of a hemiepiphyte fig, Ficus dubia. Food Sci. Nutr. 2021, 9, 2269–2279. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Meijers, M.; van Hees-Stuivenberg, S.; Boei, J.J.; Delbos, F.; Ohmori, H.; de Wind, N.; Jansen, J.G. Different sets of translesion synthesis DNA polymerases protect from genome instability induced by distinct food-derived genotoxins. Toxicol. Sci. 2012, 127, 130–138. [Google Scholar] [CrossRef][Green Version]
- Shimada, T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab. Pharm. 2006, 21, 257–276. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Gocke, E.; Burgin, H.; Muller, L.; Pfister, T. Literature review on the genotoxicity, reproductive toxicity, and carcinogenicity of ethyl methanesulfonate. Toxicol. Lett. 2009, 190, 254–265. [Google Scholar] [CrossRef]
- Jansen, J.G.; Vrieling, H.; van Teijlingen, C.M.; Mohn, G.R.; Tates, A.D.; van Zeeland, A.A. Marked differences in the role of O6-alkylguanine in hprt mutagenesis in T-lymphocytes of rats exposed in vivo to ethylmethanesulfonate, N-(2-hydroxyethyl)-N-nitrosourea, or N-ethyl-N-nitrosourea. Cancer Res. 1995, 55, 1875–1882. [Google Scholar] [PubMed]
- López, A.; Xamena, N.; Marcos, R.; Velázquez, A. Germ cells microsatellite instability: The effect of different mutagens in a mismatch repair mutant of Drosophila (spel1). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002, 514, 87–94. [Google Scholar] [CrossRef]
- Paz, M.M.; Das, T.A.; Tomasz, M. Mitomycin C linked to DNA minor groove binding agents: Synthesis, reductive activation, DNA binding and cross-linking properties and in vitro antitumor activity. Bioorg. Med. Chem. 1999, 7, 2713–2726. [Google Scholar] [CrossRef]
- Shibuya, T.; Morimoto, K. A review of the genotoxicity of 1-ethyl-1-nitrosourea. Mutat. Res. 1993, 297, 3–38. [Google Scholar] [CrossRef]
- Ryu, D.; Choi, B.; Kim, E.; Park, S.; Paeng, H.; Kim, C.I.; Lee, J.Y.; Yoon, H.J.; Koh, E. Determination of Ethyl Carbamate in Alcoholic Beverages and Fermented Foods Sold in Korea. Toxicol. Res. 2015, 31, 289–297. [Google Scholar] [CrossRef]
- Rodrigues, M.A. Automation of the in vitro micronucleus assay using the Imagestream((R)) imaging flow cytometer. Cytom. A 2018, 93, 706–726. [Google Scholar] [CrossRef]
- Braafladt, S.; Reipa, V.; Atha, D.H. The Comet Assay: Automated Imaging Methods for Improved Analysis and Reproducibility. Sci. Rep. 2016, 6, 32162. [Google Scholar] [CrossRef]
- Lombardot, B.; Oh, C.T.; Kwak, J.; Genovesio, A.; Kang, M.; Hansen, M.A.; Han, S.J. High-throughput in vivo genotoxicity testing: An automated readout system for the somatic mutation and recombination test (SMART). PLoS ONE 2015, 10, e0121287. [Google Scholar]
- OECD. Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- OECD. Test No. 489: In Vivo Mammalian Alkaline Comet Assay, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, K.; Almasan, A. Histone H2AX phosphorylation: A marker for DNA damage. Methods Mol. Biol. 2012, 920, 613–626. [Google Scholar] [PubMed]
- Madigan, J.P.; Chotkowski, H.L.; Glaser, R.L. DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 2002, 30, 3698–3705. [Google Scholar] [CrossRef] [PubMed]

| Types | Result in ST Cross | Result in HB Cross | Range of Treated Doses and Remarks | Ref. |
|---|---|---|---|---|
| Food preservatives | ||||
| Benzaldehyde (C6H5CHO) | + | nd | 0.1–50 mM, inconclusive up to 0.5 mM and positive at 1–50 mM | [21] |
| Benzyl acetate (CH3COOCH2C6H5) | + | nd | 0.1–50 mM, lack of genotoxicity or inconclusive up to 1 mM and positive at 10–50 mM | [21] |
| Benzyl alcohol (C6H5CH2OH) | + | nd | 0.1–50 mM, lack of genotoxicity or inconclusive up to 25 mM and positive at 50 mM | [21] |
| Benzoic acid (C6H5COOH) | + | nd | 0.1–50 mM, lack of genotoxicity or inconclusive up to 25 mM and positive at 50 mM | [21] |
| Butylparaben (C11H14O3) | - | nd | 100–250 mM, lack genotoxicity up to 150 mM and inconclusive up to 250 mM | [22] |
| Ethylparaben (C9H10O3) | - | nd | 100–250 mM, lack genotoxicity up to 200 mM and inconclusive at 250 mM | [22] |
| Potassium nitrite (KNO2) | + | nd | 25–100 mM, KNO2 lacks genotoxicity at 25 mM | [23] |
| Potassium nitrate (KNO3) | + | nd | 25–100 mM, KNO3 lacks genotoxicity at 25 mM | [23] |
| Sodium nitrite (NaNO2) | + | nd | 25–100 mM, NaNO2 lacks genotoxicity at 25 mM | [23] |
| Sodium nitrate (NaNO3) | + | nd | 25–100 mM, NaNO3 lacks genotoxicity at 25 mM | [23] |
| Food dyes | ||||
| Amaranth (C20H11N2Na3O10S3) | + | nd | 1–50 mg/mL, inconclusive at 1 mg/mL and positive at 12.5–50 mg/mL | [24] |
| Carminic acid (C22H20O13) | - | nd | 1–20 mg/mL, lack of genotoxicity up to 10 mg/mL and inconclusive at 20 mg/mL | [24] |
| Erythrosine (C20H6I4Na2O5) | - | nd | 1–6 mg/mL, lack of genotoxicity up to 3 mg/mL and inconclusive at 6 mg/mL | [24] |
| Indigotine (C16H8N2Na2O8S2) | - | nd | 0.25–1 mg/mL, lack of genotoxicity up to 1 mg/mL | [24] |
| Patent Blue (C27H31N2O7S2 · 0.5Ca) | + | nd | 6.25–25 mg/mL, inconclusive up to 12.5 mg/mL and positive at 25 mg/mL | [24] |
| Food flavors | ||||
| L-Carveol (C10H16O) | - | nd | 1.5–5 µL/mL, all doses lack genotoxicity | [25] |
| (−)-Carvyl acetate (C12H18O2) | - | nd | 1.5–5 µL/mL, all doses lack genotoxicity | [25] |
| (+)-Dihydrocarvone (C10H16O) | - | nd | 1.5–5 µL/mL, all doses lack genotoxicity | [25] |
| Dihydrocarveol (C10H18O) | - | nd | 1.5 and 2.5 µL/mL, all doses lack genotoxicity | [25] |
| (−)-Fenchone (C10H16O) | - | nd | 1.5–5 µL/mL, all doses lack genotoxicity | [25] |
| S-(−)-Limonene (C10H16) | - | nd | 1.5–5 µL/mL, 1.5, and 2.5 µL/mL lack genotoxicity and inconclusive at 5 µL/mL | [25] |
| (±)-Linalool | - | nd | 1.5–10 µL/mL, lack of genotoxicity up to 2.5 µL/mL and inconclusive at 10 µL/mL | [25] |
| α-Phellandrene (C10H16) | + | nd | 1.5–10 µL/mL, all doses show genotoxicity | [25] |
| Food products | ||||
| Fresh Inca peanut seed | nd | - | 145 mg/mL, lack of genotoxicity | [26] |
| Heated virgin olive oil | - | nd | 6 and 12% v/v, all doses lack genotoxicity | [27] |
| Honey-sweetened cashew- apple nectar | - | - | 12.5–100% v/v, all doses lack genotoxicity | [28] |
| Red pear tomato | - | nd | 0.625 and 5 mg/mL, all doses lack genotoxicity | [29] |
| Soybean oil | + | nd | 6, 12, and 24% v/v, all doses show genotoxicity | [30] |
| Sunflower oil | - | nd | 6, 12, and 24% v/v, only 12% v/v shows genotoxicity | [30] |
| Unheated virgin olive oil | - | nd | 6 and 12% v/v, all doses lack genotoxicity | [27] |
| Plant/herbal extracts | ||||
| Anoectochilus burmannicus, (hot water extract) | nd | - | 500 µg/mL, lack of genotoxicity | [31] |
| Anoectochilus burmannicus (ethanolic extract) | nd | - | 2 and 4 mg/mL, all doses lack genotoxicity | [32] |
| Artemisia herba-alba (ethanolic extract) | - | nd | 0.5, 1, and 1.5% v/v, all doses lack genotoxicity | [33] |
| Cryptocarya alba Mol. (aqueous extract) | - | nd | 4.74, 9.49, and 18.99 mg/mL, all doses lack genotoxicity | [34] |
| Equisetum myriochaetum | - | - | 0.78–500 µg/mL, all doses lack genotoxicity in both crosses | [35] |
| Lawsonia inermis Linn. | nd | - | 500 and 1000 µg/mL, all doses lack genotoxicity | [36] |
| Mangifera indica Linn. (aqueous extract) | - | - | 1.25, 2.5, and 5 mg/mL, all doses lack genotoxicity in both crosses | [37] |
| Peumus boldus Mol. (aqueous extract) | - | nd | 4.56, 9.12, and 18.25 mg/mL, all doses lack genotoxicity | [34] |
| Turnera subulate (aqueous extract) | + | - | 5, 10, and 20 mg/mL, all doses show genotoxicity in ST cross; however, HB cross shows inconclusive results | [38] |
| Phytochemicals | ||||
| Apigenin | - | nd | 0.46 and 1.85 mM, all doses lack genotoxicity | [39] |
| Betulinic acid | - | - | 0.0312–0.5 mM, all doses lack genotoxicity in both crosses | [40] |
| Bisabolol | - | nd | 0.56 and 2.24 mM, all doses lack genotoxicity | [39] |
| Isoeugenol | - | - | 1–15 mM, all doses lack genotoxicity in both crosses | [15] |
| Lapachol | - | + | 20, 40, and 60 µg/mL, all doses lack genotoxicity in ST cross; however, all doses show genotoxicity in HB cross | [41] |
| Lycopene | - | nd | 7 and 56 µM, all doses lack genotoxicity | [29] |
| Protocatechuic acid | - | nd | 0.25 and 1 mM, all doses lack genotoxicity | [39] |
| Safrole | - | + | 0.125–0.75 mM, all doses lack genotoxicity in ST cross; however, all doses show genotoxicity in HB cross | [15] |
| Vitexin | - | - | 0.15, 0.3, and 0.6 mM, all doses lack genotoxicity | [42] |
| Types | Result in ST Cross | Result in HB Cross | Range of Treated Doses and Remarks | Ref. |
|---|---|---|---|---|
| Atorvastatin | - | - | 0–340 µM, all doses lack genotoxicity both ST and HB cross | [49] |
| Bupivacaine | - | - | 10–500 µg/mL, inconclusive up to 500 µg/mL in ST cross and HB cross, inconclusive at 10 and 100 but lack genotoxicity at 250 and 500 µg/mL | [50] |
| Bupropion hydrochloride | + | + | 0.937–7.5 mg/mL, inconclusive at 0.937 mg/mL and positive at 1.875–7.5 mg/mL in ST cross, whereby all of doses positive in HB cross | [51] |
| Doxorubicin | + | + | 0.125 µM a dose show positive both ST and HB cross | [49] |
| Glimepiride | + | nd | 10–100 µg, inconclusive at 10 µg and positive at 25–100 µg | [44] |
| Glipizide | + | nd | 10–100 µg, inconclusive at up to 10 µg and positive at 25–100 µg | [44] |
| Levobupivacaine | - | + | 100–1000 µg/mL, inconclusive up to 1000 µg/mL in ST cross; however, inconclusive up to 500 and positive at 1000 µg/mL in HB cross | [50] |
| Rosuvastatin | - | - | 0–300 µM, all doses lack genotoxicity both ST and HB cross | [49] |
| Trazodone hydrochloride | + | + | 0.937–7.5 mg/mL, all doses positive both ST and HB cross | [51] |
| Types | Result in ST Cross | Result in HB Cross | Range of Treated Doses and Remarks | Ref. |
|---|---|---|---|---|
| Insecticides | ||||
| Fipronil | + | + | 0.3 × 10−5–3.0 × 10−5 mM, all of doses positive in ST and HB cross; however, inconclusive at 0.7 × 10−5 mM in ST cross | [62] |
| Metofluthrin | + | nd | 6–60 ug/mL, lack of genotoxicity at 6 µg/mL and positive at 60 µg/mL | [70] |
| Thiamethoxam | - | + | 2.4 × 10−4–1.9 × 10−3 mM, all doses lack genotoxicity; however, 9.7 × 10−4–1.9 × 10−3 mM were positive in HB cross. | [56] |
| Transfluthrin | + | nd | 0.0103–0.103 mg/mL, positive at all tested doses | [70] |
| Herbicides | ||||
| 2,4,5-Trichlorophenoxyacetic acid (2,4,5-T) | - | - | 0.05–10 mM, lack of genotoxicity up to 0.1 mM and inconclusive at 0.5–10 mM in ST cross; however, lack of genotoxicity at 0.05, inconclusive at 0.1–5 mM in HB cross | [71] |
| Amitrole | + | nd | 0.05–1 mM, inconclusive at 0.1 mM and positive at 0.5–1 mM | [72] |
| Bentazone | - | + | 0.05–10 mM, all doses inconclusive in ST cross; however, all doses positive in HB cross | [73] |
| Diquat dibromide | - | nd | 1–10 mM, all doses inconclusive | [72] |
| Glyphosate | + | - | 0.1–10 mM, inconclusive up to 1 mM and positive at 2–10 mM in ST cross, whereby lack of genotoxicity at 0.1–0.5 and 2–5 mM, inconclusive at 1 and 10 mM in HB cross | [71] |
| Hydrazide | + | + | 0.1–10 mM, inconclusive up to 0.5 mM and positive 1–10 mM in ST cross; HB cross shows lack of genotoxicity up to 0.5 mM, inconclusive at 1–5 mM and positive at 10 mM in HB cross | [71] |
| Imazamox | + | - | 2.5–20 mM, lack of genotoxicity at 2.5–5 mM, and weak positive at 10–20 mM in ST cross; however, all doses inconclusive both HB cross | [74] |
| Imazapic | - | - | 2.5–20 mM, all doses lack genotoxicity in ST and HB cross | [74] |
| Imazethapyr | - | - | 2.5–20 mM, all doses lack genotoxicity in ST and HB cross | [74] |
| Metribuzin | - | nd | 1–10 mM, lack of genotoxicity at 5 mM and inconclusive at 1, 2, and 10 mM | [72] |
| Molinate | + | + | 0.1–10 mM, inconclusive up to 1 mM and positive at 2–10 mM in ST cross; moreover, inconclusive up to 2 mM and positive at 5–10 mM in HB cross | [72] |
| Prometryn | - | nd | 1–10 mM, all doses inconclusive | [72] |
| Propanil | + | + | 0.1–10 mM, lack of genotoxicity at 0.1 mM, inconclusive at 0.5–5 mM, and positive 10 mM in ST cross; however, inconclusive at 0.1 mM and positive at 0.5–10 mM in HB cross | [71] |
| Terbutryn | + | nd | 1–10 mM, inconclusive at 1, 2, and 10 mM and positive at 5 mM | [72] |
| Thiobencarb | + | - | 0.1–10 mM, inconclusive up to 5 mM and positive at 10 mM in ST cross; all doses lack genotoxicity in HB cross | [73] |
| Trifluralin | + | + | 0.1–10 mM, ST cross shows inconclusive up to 5 mM and positive at 10 mM; however, inconclusive at 0.1 mM and positive at 0.5–10 mM in HB cross | [73] |
| Types | Result in ST Cross | Result in HB Cross | Range of Treated Doses and Remarks | Ref. |
|---|---|---|---|---|
| Water samples from Candiota Stream (Candiota, Brazil) in summer | - | - | Collected from four locations, all locations lack genotoxicity both ST and HB cross | [98] |
| Water samples from Candiota Stream (Candiota, Brazil) in winter | - | - | Collected from four locations, all locations lack genotoxicity both ST and HB cross | [98] |
| Water samples from Mumbuca stream and Perdizes river, Brazil | - | - | Collected from five locations, all locations lack genotoxicity both ST and HB cross | [99] |
| Water samples from Caı’ river, Brazil, in March | + | - | Collected from three locations, all locations positive genotoxicity in ST; however, collected from two locations, all locations lack mutagenicity in HB cross | [100] |
| Water samples from Caı´ river, Brazil, in June | - | - | Collected from three locations, most samples exhibited lack of mutagenicity or inconclusive | [100] |
| Water samples from Caı´ river, Brazil, in September | - | - | Collected from three locations, most samples exhibited lack of mutagenicity or inconclusive | [100] |
| Water samples from Córrego do Óleo and Córrego Liso | + | + | All samples of water showed genotoxicity in ST and HB cross | [97] |
| Water samples of Sinos river and Araçá and Garças Streams (Canoas, Brazil) | - | - | All samples lack genotoxicity both ST and HB cross | [101] |
| Types | Result in ST Cross | Result in HB Cross | Range of Treated Doses and Remarks | Ref. |
|---|---|---|---|---|
| Carbon nanotubes | - | nd | 64–1000 µg/mL, all doses lack genotoxicity | [110] |
| Carbon nanotubes | - | - | 50–250 µg/mL, lack genotoxicity at 50, 100, and 200 µg/mL and inconclusive at 150 and 250 µg/mL in both ST and HB cross | [111] |
| Cobalt nanoparticles | - | nd | 0.1–10 mM, lack genotoxicity up to 5 mM and inconclusive at 10 mM | [112] |
| Copper oxide nanoparticles (CuONPs) | + | nd | 0.24–0.95 mg/mL, lack genotoxicity at 0.24 mg/mL and inconclusive at 0.48 mg/mL but positive at 0.95 mg/mL in ST cross | [105] |
| Gold nanoparticles | - | - | 20–30 µg/mL, all of doses inconclusive in ST cross, whereby all of concentrations lack genotoxicity in HB cross | [113] |
| Iron oxide nanoparticle (<50 nm) | + | nd | 1–10 mM, inconclusive at 2 and 5 mM, positive at 1 and 10 mM | [114] |
| Iron oxide nanoparticle (<100 nm) | - | nd | 1–10 mM, inconclusive at 1 mM and lack genotoxicity 2–10 mM | [114] |
| Iron nanoparticles | - | nd | 0.1–10 mM, all concentrations lack genotoxicity | [115] |
| Nickel oxide nanoparticles | + | + | 1.31–21 mg/mL, all of doses positive in ST cross but lack genotoxicity up to 10.50 mg/mL and positive at 21 mg/mL in HB cross | [108] |
| Titanium dioxide nanocrystal (A3.4 TiO2 NCs) | + | + | 1.5625–12.5 mM, lack genotoxicity up to 6.25 and positive at 12.5 mM in ST cross; moreover, at 12.5 mM lack genotoxicity, 6.25 mM inconclusive, and positive at 1.5625 and 3.125 mM in HB cross | [112] |
| Titanium dioxide nanocrystal (A6.2 TiO2 NCs) | - | - | 1.5625–12.5 mM, lack genotoxicity up to 12.5 and inconclusive at 1.5625 in both ST and HB cross | [112] |
| Titanium dioxide nanoparticles | - | nd | 0.08–1.60 mg/mL, all concentrations lack genotoxicity | [106] |
| Zinc oxide nanoparticles | + | - | 0.075–1.2 mg/mL, lack genotoxicity up to 0.15 mg/mL and positive at 0.3–1.2 mg/mL in ST cross; however, all of doses lack genotoxicity in HB cross | [116] |
| Genotoxins | Tested Compounds/Extracts | Anti-Genotoxic Properties | Results and Remarks |
|---|---|---|---|
| Benzo[a]pyrene (BaP) | |||
| Vitexin | Yes | 0.15, 0.3, and 0.6 mM vitexin inhibit wing spot formation induced by 1 mM BaP [42] | |
| Doxorubicin (DXR) | |||
| Hymenaea courbaril extract | Yes, | 0.3–3 mL of Hymenaea courbaril inhibits wing spot formation induced by 0.125 mg/mL DXR [122] | |
| Noni fruit juice | Yes | 25–75% v/v of noni fruit juice inhibits wing spot formation induced by 0.2 mM DXR [123] | |
| Propolis (aqueous extract) | Yes | 12.5–50 mg/mL of propolis(aqueous extract) inhibits wing spot formation induced by 0.125 mg/mL DXR [124] | |
| Ethyl methanesulfonate (EMS) | |||
| Boron | Yes | 0.1–40 mg/mL of boron inhibits wing spot formation induced by 0.1 mM EMS [125] | |
| Buddleja globosa leaf extract | Yes | 1.91, 3.83, and 7.66 mg/mL of Buddleja globosa inhibit wing spot formation induced by 0.12 mg/mL EMS [121] | |
| Citrus aurentium peel oil | Yes | 0.1–0.5% v/v of Citrus aurentium fruit peel oil inhibits wing spot formation induced by 0.5 mM EMS [126] | |
| Cryptocarya alba leaf extract | Yes | 4.74–9.79 mg/mL of Cryptocarya alba inhibits wing spot formation induced by 0.12 mg/mL EMS [34] | |
| Peumus boldus leaf extract | Yes | 2.28–9.12 mg/mL of Peumus boldus extract inhibits wing spot formation induced by 0.12 mg/mL EMS [34] | |
| Hydrogen peroxide (H2O2) | |||
| Brassica carinata leaf extract | Yes | 1.25–5.0 mg/mL of Brassica carinata extract inhibits wing spot formation induced by 0.12 M H2O2 [127] | |
| Sinigrin | Yes | 0.6–4.81 mM sinigrin, a glucosinolate compound, inhibits wing spot formation induced by 0.12 M H2O2 [127] | |
| Red pear tomato | Yes | 0.625 and 5 mg/mL of red pear tomato extract inhibit wing spot formation induced by 0.12 M H2O2 [29] | |
| Lycopene | Yes | 7 and 56 µM lycopene inhibit wing spot formation induced by 0.12 M H2O2 [29] | |
| Lemon juice | Inconclusive | 0.75 and 50% v/v lemon juice showed inconclusive inhibitory effect induced by 0.15 M H2O2 [128] | |
| Orange juice | Inconclusive | 0.75 and 50% v/v lemon juice showed inconclusive inhibitory effect induced by 0.15 M H2O2 [128] | |
| Hesperidin | Inconclusive | 0.0038 and 0.24 mM of hesperidin showed inconclusive inhibitory effect induced by 0.15 M H2O2 [128] | |
| Limonene | Inconclusive | 0.011 and 0.73 mM of limonene showed inconclusive inhibitory effect induced by 0.15 M H2O2 [128] | |
| Mitomycin C (MMC) | |||
| Chalcone | Yes | 10–400 µg/mL of chalcone inhibits wing spot formation induced by 0.05 mM MMC [129] | |
| Coumarin–chalcone hybrid | Yes | 5–400 µg/mL of coumarin–chalcone hybrid inhibits wing spot formation induced by 0.05 mM MMC [129] | |
| N-nitroso N-ethylurea (ENU) | |||
| Ascorbic acid | Yes | 17 mM Ascorbic acid inhibits wing spot formation induced by 0.01 mM ENU [130] | |
| Citrus aurentium peel oil | Yes | 0.1–0.5% v/v of Citrus aurentium fruit peel oil inhibits wing spot formation induced by 0.01 mM ENU [126] | |
| Urethane (URE) | |||
| Betulinic acid | Yes | 1.64, 3.28, and 6.57 mM of betulinic acid inhibit wing spot formation induced by 10 mM URE [131] | |
| Origanum Compactum essential oil | Yes | 0.05 and 0.1% v/v of Origanum Compactum essential oil inhibits wing spot formation induced by 10 mM URE [132] | |
| Ficus dubia latex | No | 0.25, 1, and 2 mg/mL of Ficus dubia latex did not inhibit wing spot formation induced by 20 mM URE [133] | |
| Ficus dubia root extract | Weak | 0.25, 1, and 2 mg/mL of Ficus dubia root extract weakly inhibits wing spot formation induced by 20 mM URE [133] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitchakarn, P.; Inthachat, W.; Karinchai, J.; Temviriyanukul, P. Human Hazard Assessment Using Drosophila Wing Spot Test as an Alternative In Vivo Model for Genotoxicity Testing—A Review. Int. J. Mol. Sci. 2021, 22, 9932. https://doi.org/10.3390/ijms22189932
Pitchakarn P, Inthachat W, Karinchai J, Temviriyanukul P. Human Hazard Assessment Using Drosophila Wing Spot Test as an Alternative In Vivo Model for Genotoxicity Testing—A Review. International Journal of Molecular Sciences. 2021; 22(18):9932. https://doi.org/10.3390/ijms22189932
Chicago/Turabian StylePitchakarn, Pornsiri, Woorawee Inthachat, Jirarat Karinchai, and Piya Temviriyanukul. 2021. "Human Hazard Assessment Using Drosophila Wing Spot Test as an Alternative In Vivo Model for Genotoxicity Testing—A Review" International Journal of Molecular Sciences 22, no. 18: 9932. https://doi.org/10.3390/ijms22189932
APA StylePitchakarn, P., Inthachat, W., Karinchai, J., & Temviriyanukul, P. (2021). Human Hazard Assessment Using Drosophila Wing Spot Test as an Alternative In Vivo Model for Genotoxicity Testing—A Review. International Journal of Molecular Sciences, 22(18), 9932. https://doi.org/10.3390/ijms22189932







