Abstract
Tau plays a central role in a group of neurodegenerative disorders collectively named tauopathies. Despite the wide range of diverse symptoms at the onset and during the progression of the pathology, all tauopathies share two common hallmarks, namely the misfolding and aggregation of Tau protein and progressive synaptic dysfunctions. Tau aggregation correlates with cognitive decline and behavioural impairment. The mechanistic link between Tau misfolding and the synaptic dysfunction is still unknown, but this correlation is well established in the human brain and also in tauopathy mouse models. At the onset of the pathology, Tau undergoes post-translational modifications (PTMs) inducing the detachment from the cytoskeleton and its release in the cytoplasm as a soluble monomer. In this condition, the physiological enrichment in the axon is definitely disrupted, resulting in Tau relocalization in the cell soma and in dendrites. Subsequently, Tau aggregates into toxic oligomers and amyloidogenic forms that disrupt synaptic homeostasis and function, resulting in neuronal degeneration. The involvement of Tau in synaptic transmission alteration in tauopathies has been extensively reviewed. Here, we will focus on non-canonical Tau functions mediating synapse dysfunction.
1. Tau Canonical Localization and Functions
Tau protein is a hydrophilic intrinsically disordered protein with a small content of secondary structures [1,2,3]. In physiological conditions, Tau assumes a loop-like conformation in which the N-terminal and C-terminal ends are close when bound to microtubules [4,5]. Two large protein domains, the projection domain and the microtubule binding domain, provide specific properties and functions. The projection domain contains the amino-terminal region (NTD) enriched in acidic residues and the proline-rich region (PRD). The NTD region includes the KKKK sequence involved in heparin binding and the PPXXP/PXXP motifs in the PRD which mediates the interaction of Tau with tubulin and with proteins containing SH3 domains such as the tyrosine kinase Fyn [6,7,8]. The microtubule-binding domain is subdivided into a true tubulin-binding domain with three or four repeats (MTBD) and the acidic carboxy-terminal region (CTD). The repeats are divided in two small regions, a sequence of 18 residues which contains the minimal region with microtubule binding capacity and a second region of 13/14 residues that is a linker region between the repeats [9]. Tau isoforms are able to bind MTs by the MTBD with different affinity depending on the number of repeats: higher for Tau 4R isoforms and lower Tau 3R [10].
Even if Tau in physiological conditions is intrinsically disordered and does not present tertiary structures, pathological events result in the formation of Tau β-structures typical of amyloidogenic proteins in the repeats of MTBD, in particular in R2 and R3, which can assemble by their own in filaments [11]. The pathological process can involve different Tau isoforms and the 3R/4R ratio generate peculiar aggregate structures in different cell types determining distinct tauopathies [12]. Indeed, while in AD both 3R and 4R contribute to aggregation, 4R isoforms are predominant in PSP, CBD, AGD and GGT and 3R are predominant in Pick disease. FTDP-17 is the most variable showing both 3R and 4R or just one isoform [12].
Self-aggregation is inhibited by the presence of charged intact N-terminal and C-terminal domains; however, when Tau undergoes post-translational modifications (PTMs), in particular hyperphosphorylation, acetylation and truncation, the conformational structure changes and exposes the sticky repeat regions, which result in the formation of aggregates [13]. Tau PTMs modify the protein altering Tau conformations and properties. Among PTMs, phosphorylation is the most characterized and actually pathology relevant. Seventy-nine putative serine and threonine phosphorylation sites have been identified. Phosphorylation in physiological conditions controls the MT dynamics during normal neurite growth and maturation. In addition, phosphorylation can alter Tau subcellular localization. Phosphorylation of the proline-rich region favours Tau localization mainly in the soma and in the dendritic compartment, whereas, both the dephosphorylation of PRD and the phosphorylation in the C-terminal domain favours Tau localization in the distal axonal region [14,15]. In tauopathies, the uncontrolled phosphorylation events cause an aberrant hyperphosphorylated Tau with increased insolubility and aggregation propensity. All six isoforms hyperphosphorylated at 40 different residues have been identified in aggregates [9,16]. Several critical residues have been identified (Figure 1). Phosphorylation at the AT8 epitope (Ser199/Ser202/Thr205) is sufficient to cause MTs’ remodelling and instability, diminished mitochondrial transport, cell death and neurodegeneration [17]. A similar effect is observed by phosphorylation of Thr212/Thr231/Ser262 [18]. In vitro kinetic studies of the interaction of unphosphorylated and hyperphosphorylated Tau with tubulin identified Ser199/Ser202/Thr205, Thr212, Thr231/Ser235, Ser262/Ser356 and Ser422 as key critical phosphorylation sites that convert Tau to a pathological molecule [19,20,21,22,23]. The phosphorylation of these residues depends on the concerted activity of kinases and phosphatases, for which its equilibrium plays a key role in Tau pathology. Several enzymes involved in phosphorylation pathways have been observed to modulate Tau physiological and pathological behaviour. GSK3b, ERK1/2, Cdk5, JNK, PKA, p38mapK and other kinases are commonly found to interact with Tau protein and NFTs [24,25,26,27]. Different kinases seem to target and phosphorylate specific residues of Tau and have been associated with early or late phosphorylation events. Kinases can induce initial destabilization, as described for the phosphorylation of T231 and S235 mediated by GSK3b, or are involved in aggregation events as described for ERK1/2 [26,28,29,30]. The action of these kinases in normal conditions is countered by the activity of phosphatases, in particular PP2A, PP2B and PP5, which modulate Tau and MTs dynamics. These phosphatases can reduce Tau phosphorylation by direct and indirect mechanisms, and the gain or loss of function in vitro and in vivo induces Tau hyperphosphorylation and aggregation. Accordingly, in AD brains, the reduced level of phosphatases is a peculiar mark and correlates with the aberrant phosphorylation detected during the disease progression [31,32].
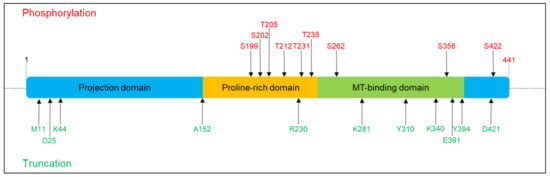
Figure 1.
Phosphorylation and truncation sites on Tau protein described in this review. The scheme refers to 2N4R Tau (441 aa). Red colour denotes the phosphorylation sites, and green colour denotes the truncation sites.
In addition to phosphorylation, truncation has been demonstrated to significantly affect Tau stability, resulting in the formation of short Tau species with strong aggregation predisposition [33,34,35,36]. Truncation seems to work synergically with phosphorylation since deprivation of GSK3β prevents the formation of Tau short species [37] (Figure 1). Several enzymes are involved in Tau truncation, and early and late cleavage events have been identified in tauopathies which are associated with Tau species prone to premature nucleation or mature aggregation. Indeed, Tau can be the substrate of caspase3, and the C-terminal truncated Tau becomes prone to forming aggregates [38]. The relevance of Tau fragmentation in neurotoxicity is evident in mice that coexpress truncated and full-length human Tau and showing axonal transport failure, clumping of mitochondria, disruption of the Golgi apparatus and missorting of synaptic proteins. However, halting the expression of truncated Tau determined a functional rescue [39]. Moreover, it has been shown that N-terminally truncated Tau-derived peptides can be extracellularly released and exert neurotoxic actions [40,41].
Another relevant PTM involved in Tau pathology is lysine acetylation, which neutralizes charges in the MTBD thus interfering with Tau interaction with MTs. Hyperacetylation has been detected mostly in intracellular NFTs rather than in pretangles or in extracellular aggregates and anticipates Tau truncation [42,43]. Interestingly, a recent work demonstrated that the overexpression of the hyperacetylated Tau in mice was able to induce a time-dependent progression of neurodegeneration from the entorhinal cortex to the hippocampus that was associated with glial activation and memory deficit [44].
Among PTMs’ glycosylation, glycation, ubiquitination and oxidation are strongly associated with Tau aggregation, in particular with mature NFTs, but their effects on Tau physiological and pathological behaviour are still under investigation.
The downstream effect of these PTMs is Tau aggregation, the hallmark of all tauopathies, although fibrils composition and structure can differ [45,46,47,48,49,50,51,52]. In addition to PTMs, other factors including site-specific mutations or Hsp90 can mediate Tau aggregation [53]. The aggregation process involves several steps, beginning with Tau PTMs, which cause the conformational alteration of the protein, followed by small Tau inclusions with low content in β-sheet structures formed in the process called nucleation; after that, other Tau molecules associate to this core to form neurofibrillary tangles (NFTs). The administration of Tau oligomers in the extracellular medium can induce prion-like homotypic-seeding both in vivo and in vitro, skipping the nucleation process [54,55]. The smallest assembly having these seeding properties is the Tau trimer [56], even if it has been recently demonstrated that monomers isolated from tauopathy brains induce a tauopathy-specific Tau aggregation. This indicates that monomers may possess peculiar pathological characteristics independent from oligomerization and specific for each tauopathy [50,57], possibly related to Tau PTMs. However, the impact that different pathological Tau monomers can have on neuronal homeostasis and pathways is still not completely elucidated and needs further investigation. Moreover, Tau mutated species have proved to be prone to self-aggregation in vivo [53,58,59,60]. It has been extensively reported that Tau aggregation causes cellular stress and damage to neuronal and non-neuronal cells [61]. However, the molecular mechanisms of Tau aggregates toxicity are still debated.
In neuronal cells, Tau is mainly enriched in axons, where it interacts and stabilizes MTs. Each Tau domain mediates tubulin interaction with different mechanisms. The PRD is positively charged and binds the negatively charged MT surface, while the MTBD binds specific pockets in β-tubulin at the inner surface of the MTs. Different repeats of the same MT-binding domain can occupy the β-tubulin pockets of adjacent filaments, thus causing the crosslink of three or four dimers [62,63]. The projection domain outdistances MTs in the axon and may increase the axonal diameter. The acidic Tau N-terminal domain branches away from the MT-surface probably for electrostatic repulsion. By interaction with other cytoskeletal components such as spectrin and actin filaments, the projection domain mediates the interconnection of Tau-stabilized MTs with neurofilaments, thus, restricting the flexibility of MTs lattices [64]. In addition, with tubulin polymerization and stabilization, several pieces of evidence associate Tau with the regulation of axonal transport. Tau does not alter the speed of kinesin along MTs, but it can induce the detachment of cargoes anchored to kinesin [65]. In vitro, Tau knockdown increases the transport velocity in iPSC-derived dopaminergic neurons, suggesting that the presence of the protein may interfere with motor proteins dynamics [66,67]. Moreover, in SHSY5y cells, Tau is also able to bind the p150 subunit of dynactin and is able to stabilize it on MTs, thus, promoting dynein transport [67,68]. Tau is also localized at the axon terminal upon stimulation with NGF and can potentiate NGF and EGF signalling, enhancing the activation of the downstream pathway and modulating neurites extension [69,70].
Tau protein has been detected also in the postsynaptic compartment, where it interacts with PSD-95 in dendritic spines [6]. Upon synaptic activation, Tau delocalizes from the dendritic shaft to spines in cultured neurons and hippocampal slices, suggesting a potential role in the modulation of postsynaptic response [71]. With the PRD region, Tau interacts with the SH3 domains of Fyn, a tyrosine kinase from the Src-family involved in protein trafficking, and this interaction is necessary for Fyn localization at the postsynaptic compartment. Fyn phosphorylates NMDA subunit NR2B, thereby stabilizing its interaction with PSD-95 [7,8].
Moreover, Tau has also been localized close to, or associated to, many organelles, such as ribosomes and mitochondria, or to subcellular compartments such as endoplasmic reticulum and the nucleus. Evidence connecting nuclear Tau with the regulation of gene expression and synaptic functions is significantly growing, as described in the following paragraphs [72,73].
2. Impact of Pathogenic Tau Localization
Tau Mislocalization in the Somato-Dendritic Compartment and Consequent Synaptic Dysfunction
In tauopathies, Tau affinity to tubulin is reduced, resulting in destabilization and disorganization of the axonal cytoskeleton. Moreover, it has been observed that Tau aggregates can sequester not only normal Tau but also the two other major neuronal MAPs, MAP1 and 2 [74]. This loss of function is due to conformational change and misfolding caused by PTMs resulting in aggregation in intracellular fibrillary toxic structures. Remarkably, the number of NFTs correlates with the level of cognitive impairment in tauopathy patients.
The destabilization of axonal MTs affects the plus end-directed transport mediated by kinesin [75]. Altered transport slows down exocytosis and the organelles’ localization; the damage of these mechanisms causes a decrease in lipid and glucose metabolism, ATP synthesis and a loss of Ca2+ homeostasis, which result in a distal degeneration process [76,77]. Altogether, these findings raise the hypothesis that the alterations associated with Tau conformational changes may contribute to either pre-synaptic or post-synaptic deficits as early pathological signs of AD. These aspects of the pathological protein behaviour are currently the most studied and characterized by the scientific community. However, recently, preclinical and clinical studies focused on the prevention of cytoskeletal destabilization or on the reduction in Tau aggregates with promising but still unripe results, probably due to the lack of knowledge on unexplored Tau pathological roles [78,79,80,81,82,83]. Indeed, novel non-canonical functions and locations have been discovered, supporting their involvement in neuronal physiology and pathology. Under pathological conditions, Tau is relocalized in the somato-dendritic compartment and in isolated processes of affected neurons [64]. Remarkably, the relocalization of Tau into the somato-dendritic compartment causes the increase in Fyn in dendrites, resulting in toxic hyperexcitability mediated by NMDA receptors. On the contrary, Tau loss prevents deregulated postsynaptic Fyn and, as a consequence, NMDA-dependent excitotoxicity and memory impairment [6,7,8]. The finely tuned interplay between Tau, Fyn and NMDA is relevant for the regulation of the glutamatergic signalling, and it may mediate the process of excitotoxicity in tauopathies. The association between Tau misfolding and synaptic transmission is further supported by the fact that Tau reduction protects from pathological network hyperexcitability in several in vitro and in vivo models, and Tau KO shows impaired LTP [84,85,86]. In addition, the involvement of dendritic Tau in pathology is further underlined by the fact that it is necessary to induce the Aβ-mediated LTP impairment typical of AD conditions, thus supporting that Tau is necessary for and mediates Aβ-induced neurotoxicity in AD [87]. Although abnormal Tau accumulation has an abnormal effect on synapsis, it has also been observed that abnormal signaling at the synapsis can facilitate local tau phosphorylation and translocation [71,88].
To date, thousands of studies have identified Tau protein as a key factor in the pathophysiology of Alzheimer’s disease (AD). Under normal conditions, Tau has been detected in small concentrations at dendrites, but Tau becomes largely missorted into the somato-dendritic compartment and causes synaptic dysfunction in tauopathy conditions [89]. Tau can undergo post-translational changes that result in the characteristic formation of neurofibrillary tangles (NFTs), which are self-assembled paired helical filaments that form inside cell bodies. An early sign of AD neurodegeneration is the presence of NFTs in the entorhinal cortex, which then spread to the hippocampus, amygdala and basal magnocellular complex [90]. Tau modifications that result in NFTs may include hyperphosphorylation, truncation or even acetylation [91]. However, Tau can form not only insoluble deposits but it is also present as non-fibrillar soluble monomeric and oligomeric species that have been found to increase in the brain of AD patients [92,93]. It is worth noting that recent literature pointed at soluble Tau forms (including soluble oligomeric aggregates), but not NFTs or insoluble Tau, as responsible for mediating synaptic dysfunction and toxicity [94]. Therefore, it has been suggested that Tau can be directly involved in the regulation of synaptic function. This was first demonstrated to occur by presynaptic modulation. In particular, presynaptic microinjection of recombinant human Tau protein in the squid giant synapse model was capable of altering synaptic transmission by an increase in transmitter release [95]. This event was mediated by calcium release from intracellular stores and was followed by a reduction in evoked transmitter release. Moreover, this study demonstrated that exogenously injected Tau requires IP3 receptors, GSK3 and Cdk5 activities to block synaptic transmission [95]. This is in agreement with the well-known role of the many kinases that can modulate Tau function contributing to neuronal impairment. On the other hand, the in vivo administration of a calpain inhibitor was capable of decreasing cdk5 activation, thereby diminishing the hyperphosphorylation of Tau, which ameliorated synaptic function and cognition in the 3xTgAD mouse model [96]. Moreover, in the work by Roy et al., the age-related cognitive impairment occurred in the mouse model overexpressing a human mutant Tau form (P301L) known to result in dementia (rTg4510) could be prevented by the administration of a selective p38αMAPK inhibitor, further strengthening the role of this specific kinase in Tau-related pathology [97].
Regarding the abnormal acetylation of Tau protein at two specific lysine residues in transgenic mice, it promotes memory loss and induces an impairment of hippocampal Long-Term Potentiation (LTP) [98].
Studies based on transgenic Tau models contributed to clarifying the relationship between Tau protein and synaptic function either regarding the wild-type or mutated forms. As reported by Dickstein et al., overexpression of the wild-type human Tau in the mouse correlated with a decrease in spines density and altered morphology in cortical neurons [99]. Moreover the expression of human non-mutated Tau results in a decrease in LTP and learning and memory deficits [100].
As reported above and similarly to what has been demonstrated for the role of β-amyloid in AD, soluble small aggregates of Tau protein have gained attention as the pathogenic form inducing synaptic dysfunction. Indeed, soluble Tau was found to be acutely toxic in animal models of tauopathy [101,102,103]. Researchers demonstrated that acute exposure to the extracellular oligomeric form of Tau affects memory and its cellular correlate, LTP [92,104]. It is important to note that these toxic effects of Tau were observed by different preparations of the protein, either Tau derived from transgenic human Tau mice or Tau derived from AD patients [92]. Other studies confirmed an increased amount of oligomeric Tau in the brain of AD patients compared to control, suggesting the possible role of oligomeric Tau as an early biomarker of the disease and the importance of further investigating the biological significance of this particular aggregation state [93].
3. Tau Functions in the Nuclear Compartment and Pathological Impact on Synaptic Functions
The presence of Tau in the nuclear compartment was first described in the 1990s in neuroblastoma cells and in human brains [73,105]. It is still unclear how Tau is transported to the nucleus; however, it interacts directly with proteins of the Nuclear Pore Complex (NPC) and has been recently demonstrated to interact with TRIM28, a protein involved in transcriptional regulation and chromatin remodelling, which is able to shuttle Tau to the nucleus [106,107,108]. Concomitant Tau and TRIM28 increased levels have been measured in neuronal nuclei of AD human brains supporting their close dependence in nuclear transport and suggesting a pathological involvement of Tau in chromatin remodelling [108]. Moreover, pathological Tau in primary neurons, in tauopathy models and in AD brains determines a depletion of nuclear Ran and an impairment of the nuclear translocation [109]. Tau contributes in regulating the nuclear homeostasis directly inside the nuclear compartment and indirectly by altering nuclear transport, gene expression and genome structure. In pathological conditions, alterations of the nuclear envelope have been described. Indeed, Tau-dependent aberrant invaginations have been observed in post-mortem patient brains, and this evidence is supported by the fact that pathological Tau induces rearrangement and dysfunction of nuclear lamins, thus causing chromatin modifications, DNA damage and apoptosis as a consequence [106,107].
Tau is involved also in DNA protection, and the interaction between Tau oligomers and p53 in AD mouse models and human brains result in the pathological delocalization of p53 outside the nucleus and to increased susceptibility to DNA damage and neuronal cell death [110]. In a similar manner, a Tau-dependent translocation into the cytoplasm of RNA Polymerase II Subunit RPB1 has been observed in the tauopathy mouse model Tg4510 and in AD human brains, suggesting a pathological role of Tau in transcription alterations [111]. Moreover, pathogenic cytoplasmic Tau induces a reduction in nuclear Ca2+ in tauopathy Drosophila model and iPSC-derived hippocampal neurons that causes a CREB depletion from the nucleus and a consequent gene expression alteration which results in neuronal cell death [112]. Recently, Tau involvement in miRNA activity has been reported. Tau interacts with the DEAD box RNA helicase DDX6 involved in translation repression and mRNA decay as well as in the miRNA pathway. The complex Tau/DDX6 increases the silencing activity of the miRNA let-7a, miR-21 and miR-124, affecting their target expression. Indeed, aberrant Tau isoforms are less efficient in modulating the activity of miRNA let-7a, thus impairing miRNA and mRNA homeostasis [113].
The distribution of Tau in the nuclear compartment may depend on the isoform and on its phosphorylation state. In adult mice brains, 2N Tau isoforms are enriched in the chromatin-bound fraction, while 1N Tau isoforms are over-represented in the soluble nuclear fraction. The functional impact of this asymmetric distribution is unclear; it is probable that the presence of 1 or 2 N-terminal sequences alters the affinity for Tau to nuclear cofactors or chromatin even if this point still needs further investigation [114]. Phosphorylation events may impact Tau nuclear distribution. Tau can be found either in a phosphorylated and non-phosphorylated state in the nucleus. Tau localized in the nucleolus is mainly non-phosphorylated, but it can also be phosphorylated in the nucleoplasm in physiological conditions [73,115]. Low levels of phosphorylation increase the affinity of Tau for DNA, while hyperphosphorylation reduces Tau-DNA interaction [116]. The phosphorylation profile of nuclear Tau by mass spectrometry indicates a specific enrichment of Tau phosphorylated at residues T181, S231, S235 and pAT8 in the nuclei of normal cells. Remarkably, the treatment with molecules that cause DNA damage results in an enrichment of non-phosphorylated Tau in the nucleus in order to protect DNA [117]. This evidence suggests a protective role for Tau in the non-phosphorylated form that is supported by observations in mouse models where non-phosphorylated Tau levels are increased in neuronal nuclei after oxidative stress and hyperthermic conditions [115,118]. In addition, since Tau is hyperphosphorylated in pathological conditions, this event might prevent its interaction with chromatin, thus promoting DNA damage that is a typical hallmark of AD and other tauopathies. Moreover, a differential phosphorylation profile is observed for nuclear Tau with aging such as significant phosphorylation of the AT100 epitope, which localizes Tau to heterochromatin, suggesting an epigenetic role for Tau [119]. In the nuclear compartment, Tau is able to directly bind the DNA with the second half of the PRD and the R2 of the MTBD [116,120]. Biophysical studies identified a preference for GC-rich sequences and demonstrated that Tau binding stabilizes the DNA structure when altered by physical stress [121]. Nuclear Tau binds AT-rich α-satellites and colocalizes with nucleolin at the internal periphery of nucleoli [122]. In addition, in primary neurons, Tau also occupies intergenic chromatin regions and promoter regions with a high specificity for GAGA motifs [123]. The mechanisms involved in Tau-mediated chromatin structure, stability or gene expression still need further clarification; however, several functions have been described for nuclear Tau.
Tau emerged as a key player in DNA protection and stability in neuronal cells. It interacts with the minor groove of the double strand DNA helix, stabilizes DNA structure and, as a matter of fact, it increases the dsDNA melting temperature and protects DNA from oxidative stress induced by hydroxyl radicals [120,124]. This protective function was confirmed in mouse models for tauopathies and patients. In tauopathy models, the translocation of Tau in the nuclei is observed under oxidative stress [115,118]. Intriguingly, the presence of pathology-associated Tau mutants results in remarkable chromosome aberrant recombination and aneuploidy in mouse and human samples, suggesting a loss of protective function and supporting the key role of nuclear Tau in genome stability [125,126,127]. Tau also has a key role in chromatin remodelling and gene expression. In Tau knock-out models, several genes show significant gene expression alteration, and some of them regulate themselves [128,129]. Remarkably, in Tau overexpressing drosophila and mice, higher nuclear Tau levels induce a global chromatin relaxation, suggesting that Tau is a factor involved in the loss of heterochromatin observed in AD human brains. This event results in an increase in the transcription of genes physiologically silenced in heterochromatin regions. The impact of this evidence is relevant for the pathology since heterochromatin recovery in the Drosophila restores locomotor damage [130]. Recently, experiments in Drosophila and in human AD and PSP brains demonstrated that altered Tau levels induced dysregulation of transposable elements that cause genomic damage. This effect is due to the loss of heterochromatin described above and the reduction in piwi elements that prevent transposon expression and translocation [119,131,132]. As described above, Tau directly interacts with TRIM28, which mediates its nuclear translocation in physiological and pathological conditions. TRIM28’s crucial role in chromatin remodelling and its relationship with Tau support the hypothesis that TRIM28 may be a relevant factor that mediates Tau-dependent chromatin alterations in tauopathies [108]. Tau might also have a repressive role in gene expression. Indeed, it has been observed that Tau binds promoter regions and represses specific genes [123]. Moreover, Tau interacts with TIP5 in the nucleolus, and Tau depletion results in the increase in 45S-prerRNA synthesis, suggesting that Tau regulates rRNA synthesis in normal conditions [133,134].
The impact of pathological conditions on these nuclear functions are still unclear, but since hyperphosphorylation prevents Tau translocation in the nucleus, this could cause a nuclear Tau depletion and an alteration in ribogenesis, translation and transcription processes as a consequence. In AD brains, a deregulation of SIRT6 and DNA damage results in a pathological increase in Tau acetylation on residue K174, which favours its nuclear translocation. Remarkably, this pathological nuclear Tau species induces an alteration of the expression of genes related to protein synthesis, translation and energy production typically associated with neurodegeneration [135].
4. Tau-Mediated Gene Expression and Synaptic Dysfunction
The role of nuclear Tau on gene expression suggests that alterations of Tau physiological state might induce gene deregulation leading downstream to dysfunctions of neuronal processes. Due to the relevant differences in Tau biophysical properties in early and late stages of disease, it is conceivable that alterations in its nuclear functions are distinct during the pathology progression. At the onset of the pathology, Tau nuclear translocation increases, and a significantly increased expression of genes related to the glutamatergic pathway is concomitantly observed, in particular, VGluT1 for the presynaptic terminal and NMDA receptor subunits for the post synapse. Moreover, Tau depletion by RNA interference is followed by the reduction in VGluT1 levels, accounting for a bidirectional relationship between Tau and the VGluT1 gene expression. Remarkably, experiments of Tau nuclear enrichment employing nuclear localization and export signals demonstrate that the glutamatergic gene expression alteration mainly depends on the nuclear pool of Tau, with no relevant contribution of cytoplasmic Tau [136,137]. Indeed, the Tau-dependent gene alteration results in higher frequency and amplitude of mEPSCs in primary neurons, suggesting a concomitant presynaptic and postsynaptic contribution for the hyperexcitability observed by electrophysiology experiments [136,137]. Neuronal hyperexcitability, due to altered glutamate release, is a typical event at early stages of tauopathies. A higher release of glutamate neurotransmitter is observed in mouse and patients’ brains in concomitance with increased neuronal activity, and this event results in hyperexcitability associated with a higher probability of seizures, synaptic dysfunction and apoptosis, triggering the pathological cascade [138,139,140,141,142]. The close connection between Tau and hyperexcitability is further reinforced by observations in epilepsy models, where the reduction in Tau results in a reduction in seizures [85,142].
As stated above, synaptic dysfunction is partially mediated by cytoplasmic pathological Tau, but these data add a significant contribution to nuclear Tau. Remarkably, a RNAseq analysis comparing differentiated SHSY-5y cells with or without Tau overexpression reveals Tau-dependent global gene alteration (4000 differentially expressed genes), and the GO analyses identifies the glutamatergic pathway as significantly modulated by Tau. In addition, the transcriptome profile of the cellular model has been compared with microarray data of human AD hippocampus at different AD stages. This analysis indicates that the Tau-dependent gene alteration specifically resembles the LMCI profile with no correlation with the EMCI and AD stages (unpublished observations).
In the terminal stages of tauopathies, a reduction in glutamate release and lower excitability can be observed. In these stages, Tau forms amyloidogenic aggregates that represent the typical lesion of Tau pathology [143,144,145]. Experiments on a cellular model mimicking late AD condition showed that aggregation causes a significant reduction in VGluT1 levels, suggesting that nuclear inclusions results in a Tau loss of function [146]. Remarkably, this evidence is further supported by studies in the tauopathy mouse model Tau22, where Tau oligomers in the nuclear compartment have a repressive role on gene expression [123].
Altogether, this evidence supports the hypothesis that synaptic alteration might be caused by the synergistic effect of pathological Tau localized at the synapse and the increase in nuclear Tau that modulates the expression of glutamatergic genes. On the contrary, in late tauopathy conditions, when Tau is mostly aggregated, the glutamate release is lower and synaptic hypoexcitability is observed. This event is related to the formation of repressive Tau amyloidogenic inclusions impairing Tau nuclear function, thus resulting in a reduction in glutamatergic gene levels [123,146]. These stage-specific and pathological Tau-dependent glutamatergic pathway changes correlate genetically and functionally with the synaptic alterations observed in tauopathy human brains. Indeed, the prefrontal cortex (PFC) of AD patients shows higher levels of VGluT1 expression at Braak stages 3 and 4 when Tau is displaced from MTs. On the contrary, low levels of VGluT1 can be observed at Braak stage 6 when Tau is aggregated. A GO analysis reveals that not only VGluT1 is present, but a more general synaptic gene alteration is present specifically at Braak stage 3 and 4, and a significant reduction in gene modulation follows at Braak stage 6 in the PFC of AD patients [146].
Glutamatergic gene alterations in intermediate AD phases might explain the functional synaptic damage that results in hyperexcitability and glutamate release deregulation commonly described in AD. Indeed, higher glutamate release and higher susceptibility to seizures are observed in MCI stages in patients. Late AD stages show synaptic transmission impairment and overall synapse reduction at glutamatergic pathways that could be explained by the gene expression changes in the opposite direction to previous phases [138,139,140,145,147,148,149,150,151].
Altogether, these observations strongly suggest that different tauopathy stages result in peculiar alterations of nuclear Tau properties and functions. Indeed, the different Tau pathological species, destabilized and soluble or aggregated, affect the nuclear Tau function and the synaptic pathway as a consequence. These observations correlate with the stage-specific synaptic alterations that can be observed in tauopathy mouse models and in AD brains. The mechanisms that mediate nuclear Tau gene expression alterations are still unclear, but the repressive role of nuclear Tau oligomers on gene expression has been proposed in tauopathy conditions [123]. Moreover, pathological Tau species alter the chromatin structure, reducing heterochromatin formation in AD brain nuclei. This event causes genomic instability, but it could also induce global gene alteration affecting synaptic functions [132]. Tau is able to bind chromatin by direct (or indirect) interactions, and it is plausible that differences in Tau conformation or PTMs could alter its DNA affinity and its structure. Remarkably, nuclear Tau can interact with key proteins involved in chromatin remodelling, such as TRIM28, and their interaction seems to change during AD progression, suggesting a possible impact on heterochromatin formation and gene expression [108].
The elucidation of these aspects is a central future objective that could result in the discovery of new therapeutic targets preventing tauopathy progression and synaptic damage. However, despite the strong correlation between Tau and AD pathology [58,152], the mechanisms by which this protein is involved in synaptic dysfunction and induces memory impairment still remain elusive [104]. Due to the fact that soluble toxic aggregates can self-propagate and spread throughout the brain, successful therapeutic intervention for AD would benefit from detecting these species before aggregated tangle production and before cognitive impairment becomes evident, with the aim of interfering with the destructive biochemical pathways that this protein initiates. In this view, it is crucial to investigate non-canonical actions of Tau that could contribute to the development and progression of synaptic dysfunction.
Author Contributions
Conceptualization, investigation, writing—original draft preparation, writing—review and editing, G.S., C.F., N.O., A.C. and C.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Scuola Normale Superiore Institutional fund to Bio@SNS laboratory.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cleveland, D.W.; Hwo, S.Y.; Kirschner, M.W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J. Mol. Biol. 1977, 116, 207–225. [Google Scholar] [CrossRef]
- Hirokawa, N.; Shiomura, Y.; Okabe, S. Tau proteins: The molecular structure and mode of binding on microtubules. J. Cell Biol. 1988, 107, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Mukrasch, M.D.; Bibow, S.; Korukottu, J.; Jeganathan, S.; Biernat, J.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol. 2009, 7, e34. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, S.; Von Bergen, M.; Brutlach, H. Global Hairpin Folding of Tau in Solution †. Biochemistry 2006, 2283–2293. [Google Scholar] [CrossRef]
- Di Primio, C.; Quercioli, V.; Siano, G.; Rovere, M.; Kovacech, B.; Novak, M.; Cattaneo, A. The Distance between N and C Termini of Tau and of FTDP-17 Mutants Is Modulated by Microtubule Interactions in Living Cells. Front. Mol. Neurosci. 2017, 10. [Google Scholar] [CrossRef]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.H.W.; Hogseth, M.; Phillips, E.C.; O’Neill, M.J.; Pooler, A.M.; Noble, W.; Hanger, D.P. Critical residues involved in tau binding to fyn: Implications for tau phosphorylation in Alzheimer’s disease. Acta Neuropathol. Commun. 2016, 4, 49. [Google Scholar] [CrossRef]
- Mondragón-Rodríguez, S.; Trillaud-Doppia, E.; Dudilot, A.; Bourgeois, C.; Lauzon, M.; Leclerc, N.; Boehm, J. Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-D-aspartate receptor-dependent tau phosphorylation. J. Biol. Chem. 2012, 287, 32040–32053. [Google Scholar] [CrossRef]
- Avila, J.; Lucas, J.J.; Perez, M.; Hernandez, F. Role of tau protein in both physiological and pathological conditions. Physiol. Rev. 2004, 84, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Kosik, K.S. Competition for microtubule-binding with dual expression of tau missense and splice isoforms. Mol. Biol. Cell 2001, 12, 171–184. [Google Scholar] [CrossRef]
- Von Bergen, M.; Friedhoff, P.; Biernat, J.; Heberle, J.; Mandelkow, E.M.; Mandelkow, E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif ((306)VQIVYK(311)) forming beta structure. Proc. Natl. Acad. Sci. USA 2000, 97, 5129–5134. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef]
- Alonso, A.; Zaidi, T.; Novak, M.; Grundke-Iqbal, I.; Iqbal, K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. USA 2001, 98, 6923–6928. [Google Scholar] [CrossRef]
- Dotti, C.G.; Banker, G.A.; Binder, L.I. The expression and distribution of the microtubule-associated proteins tau and microtubule-associated protein 2 in hippocampal neurons in the rat in situ and in cell culture. Neuroscience 1987, 23, 121–130. [Google Scholar] [CrossRef]
- Mandell, J.W.; Banker, G.A. A spatial gradient of tau protein phosphorylation in nascent axons. J. Neurosci. 1996, 16, 5727–5740. [Google Scholar] [CrossRef]
- Pevalova, M.; Filipcik, P.; Novak, M.; Avila, J.; Iqbal, K. Post-translational modifications of tau protein. Bratisl. Lek. Listy 2006, 107, 346–353. [Google Scholar]
- Shahpasand, K.; Uemura, I.; Saito, T.; Asano, T.; Hata, K.; Shibata, K.; Toyoshima, Y.; Hasegawa, M.; Hisanaga, S.-I. Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer’s disease. J. Neurosci. 2012, 32, 2430–2441. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.D.; Di Clerico, J.; Li, B.; Corbo, C.P.; Alaniz, M.E.; Grundke-Iqbal, I.; Iqbal, K. Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration. J. Biol. Chem. 2010, 285, 30851–30860. [Google Scholar] [CrossRef] [PubMed]
- Abraha, A.; Ghoshal, N.; Gamblin, T.C.; Cryns, V.; Berry, R.W.; Kuret, J.; Binder, L.I. C-terminal inhibition of tau assembly in vitro and in Alzheimer’s disease. J. Cell Sci. 2000, 113 Pt 21, 3737–3745. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.d.C.; Mederlyova, A.; Novak, M.; Grundke-Iqbal, I.; Iqbal, K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J. Biol. Chem. 2004, 279, 34873–34881. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-X.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K. Post-translational modifications of tau protein in Alzheimer’s disease. J. Neural Transm. 2005, 112, 813–838. [Google Scholar] [CrossRef]
- Haase, C.; Stieler, J.T.; Arendt, T.; Holzer, M. Pseudophosphorylation of tau protein alters its ability for self-aggregation. J. Neurochem. 2004, 88, 1509–1520. [Google Scholar] [CrossRef]
- Sengupta, A.; Kabat, J.; Novak, M.; Wu, Q.; Grundke-Iqbal, I.; Iqbal, K. Phosphorylation of tau at both Thr 231 and Ser 262 is required for maximal inhibition of its binding to microtubules. Arch. Biochem. Biophys. 1998, 357, 299–309. [Google Scholar] [CrossRef]
- Pei, J.J.; Tanaka, T.; Tung, Y.C.; Braak, E.; Iqbal, K.; Grundke-Iqbal, I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J. Neuropathol. Exp. Neurol. 1997, 56, 70–78. [Google Scholar] [CrossRef]
- Piedrahita, D.; Hernández, I.; López-Tobón, A.; Fedorov, D.; Obara, B.; Manjunath, B.S.; Boudreau, R.L.; Davidson, B.; Laferla, F.; Gallego-Gómez, J.C.; et al. Silencing of CDK5 reduces neurofibrillary tangles in transgenic alzheimer’s mice. J. Neurosci. 2010, 30, 13966–13976. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.; Roder, H.; Nunomura, A.; Takeda, A.; Friedlich, A.L.; Zhu, X.; Raina, A.K.; Holbrook, N.; Siedlak, S.L.; Harris, P.L.; et al. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. Neuroreport 1999, 10, 2411–2415. [Google Scholar] [CrossRef]
- Zhu, X.; Raina, A.K.; Rottkamp, C.A.; Aliev, G.; Perry, G.; Boux, H.; Smith, M.A. Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J. Neurochem. 2001, 76, 435–441. [Google Scholar] [CrossRef]
- Cho, J.-H.; Johnson, G.V.W. Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J. Biol. Chem. 2003, 278, 187–193. [Google Scholar] [CrossRef]
- Daly, N.L.; Hoffmann, R.; Otvos, L.; Craik, D.J. Role of phosphorylation in the conformation of tau peptides implicated in Alzheimer’s disease. Biochemistry 2000, 39, 9039–9046. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, S.; Klafki, H.W.; Plesnila, N.; Hübinger, G.; Obermeier, A.; Sahagún, H.; Monse, B.; Seneci, P.; Lewis, J.; Eriksen, J.; et al. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9673–9678. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.-X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005, 22, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Singh, T.J.; Grundke-Iqbal, I.; Iqbal, K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J. Neurochem. 1993, 61, 921–927. [Google Scholar] [CrossRef]
- Fasulo, L.; Ugolini, G.; Visintin, M.; Bradbury, A.; Brancolini, C.; Verzillo, V.; Novak, M.; Cattaneo, A. The neuronal microtubule-associated protein tau is a substrate for caspase-3 and an effector of apoptosis. J. Neurochem. 2000, 75, 624–633. [Google Scholar] [CrossRef]
- Jakes, R.; Novak, M.; Davison, M.; Wischik, C.M. Identification of 3- and 4-repeat tau isoforms within the PHF in Alzheimer’s disease. EMBO J. 1991, 10, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Kabat, J.; Wischik, C.M. Molecular characterization of the minimal protease resistant tau unit of the Alzheimer’s disease paired helical filament. EMBO J. 1993, 12, 365–370. [Google Scholar] [CrossRef]
- Novák, M. Truncated tau protein as a new marker for Alzheimer’s disease. Acta Virol. 1994, 38, 173–189. [Google Scholar]
- Cho, J.-H.; Johnson, G.V.W. Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J. Biol. Chem. 2004, 279, 54716–54723. [Google Scholar] [CrossRef] [PubMed]
- Gamblin, T.C.; Chen, F.; Zambrano, A.; Abraha, A.; Lagalwar, S.; Guillozet, A.L.; Lu, M.; Fu, Y.; Garcia-Sierra, F.; LaPointe, N.; et al. Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 10032–10037. [Google Scholar] [CrossRef]
- Ozcelik, S.; Sprenger, F.; Skachokova, Z.; Fraser, G.; Abramowski, D.; Clavaguera, F.; Probst, A.; Frank, S.; Müller, M.; Staufenbiel, M.; et al. Co-expression of truncated and full-length tau induces severe neurotoxicity. Mol. Psychiatry 2016, 21, 1790–1798. [Google Scholar] [CrossRef]
- Florenzano, F.; Veronica, C.; Ciasca, G.; Ciotti, M.T.; Pittaluga, A.; Olivero, G.; Feligioni, M.; Iannuzzi, F.; Latina, V.; Maria Sciacca, M.F.; et al. Extracellular truncated tau causes early presynaptic dysfunction associated with Alzheimer’s disease and other tauopathies. Oncotarget 2017, 8, 64745–64778. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Corsetti, V.; Stringaro, A.; Colone, M.; D’Aguanno, S.; Meli, G.; Ciotti, M.; Sancesario, G.; Cattaneo, A.; Bussani, R.; et al. A NH2 tau fragment targets neuronal mitochondria at AD synapses: Possible implications for neurodegeneration. J. Alzheimers. Dis. 2010, 21, 445–470. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.J.; Guo, J.L.; Hurtado, D.E.; Kwong, L.K.; Mills, I.P.; Trojanowski, J.Q.; Lee, V.M.Y. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat. Commun. 2011, 2, 252. [Google Scholar] [CrossRef]
- Irwin, D.J.; Cohen, T.J.; Grossman, M.; Arnold, S.E.; Xie, S.X.; Lee, V.M.-Y.; Trojanowski, J.Q. Acetylated tau, a novel pathological signature in Alzheimer’s disease and other tauopathies. Brain 2012, 135, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, E.-J.; Liu, Q.; Li, S.-H.; Li, T.; Zhou, Q.-Z.; Liu, Y.-C.; Zhang, H.; Wang, J.-Z. Tau Acetylation in Entorhinal Cortex Induces its Chronic Hippocampal Propagation and Cognitive Deficits in Mice. J. Alzheimers. Dis. 2020, 77, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, N.; David, J.P.; Lefranc, D.; Vermersch, P.; Wattez, A.; Delacourte, A. Different distribution of phosphorylated tau protein isoforms in Alzheimer’s and Pick’s diseases. FEBS Lett. 1997, 412, 578–582. [Google Scholar] [CrossRef]
- Delacourte, A.; Robitaille, Y.; Sergeant, N.; Buée, L.; Hof, P.R.; Wattez, A.; Laroche-Cholette, A.; Mathieu, J.; Chagnon, P.; Gauvreau, D. Specific pathological Tau protein variants characterize Pick’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 159–168. [Google Scholar] [CrossRef]
- Arai, T.; Ikeda, K.; Akiyama, H.; Shikamoto, Y.; Tsuchiya, K.; Yagishita, S.; Beach, T.; Rogers, J.; Schwab, C.; McGeer, P.L. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 2001, 101, 167–173. [Google Scholar] [CrossRef]
- Buée-Scherrer, V.; Hof, P.R.; Buée, L.; Leveugle, B.; Vermersch, P.; Perl, D.P.; Olanow, C.W.; Delacourte, A. Hyperphosphorylated tau proteins differentiate corticobasal degeneration and Pick’s disease. Acta Neuropathol. 1996, 91, 351–359. [Google Scholar] [CrossRef]
- Togo, T.; Sahara, N.; Yen, S.-H.; Cookson, N.; Ishizawa, T.; Hutton, M.; de Silva, R.; Lees, A.; Dickson, D.W. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J. Neuropathol. Exp. Neurol. 2002, 61, 547–556. [Google Scholar] [CrossRef]
- Mirbaha, H.; Chen, D.; Morazova, O.A.; Ruff, K.M.; Sharma, A.M.; Liu, X.; Goodarzi, M.; Pappu, R.V.; Colby, D.W.; Mirzaei, H.; et al. Inert and seed-competent tau monomers suggest structural origins of aggregation. Elife 2018, 7. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Falcon, B.; He, S.; Murzin, A.G.; Murshudov, G.; Garringer, H.J.; Crowther, R.A.; Ghetti, B.; Goedert, M.; Scheres, S.H.W. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017, 547, 185–190. [Google Scholar] [CrossRef]
- Falcon, B.; Zhang, W.; Murzin, A.G.; Murshudov, G.; Garringer, H.J.; Vidal, R.; Crowther, R.A.; Ghetti, B.; Scheres, S.H.W.; Goedert, M. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 2018, 561, 137–140. [Google Scholar] [CrossRef]
- Song, L.; Wells, E.A.; Robinson, A.S. Critical Molecular and Cellular Contributors to Tau Pathology. Biomedicines 2021, 9, 190. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Frost, B.; Jacks, R.L.; Diamond, M.I. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009, 284, 12845–12852. [Google Scholar] [CrossRef] [PubMed]
- Mirbaha, H.; Holmes, B.B.; Sanders, D.W.; Bieschke, J.; Diamond, M.I. Tau Trimers Are the Minimal Propagation Unit Spontaneously Internalized to Seed Intracellular Aggregation. J. Biol. Chem. 2015, 290, 14893–14903. [Google Scholar] [CrossRef]
- Sharma, A.M.; Thomas, T.L.; Woodard, D.R.; Kashmer, O.M.; Diamond, M.I. Tau monomer encodes strains. Elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Brunden, K.R.; Trojanowski, J.Q.; Lee, V.M.-Y. Evidence that non-fibrillar tau causes pathology linked to neurodegeneration and behavioral impairments. J. Alzheimers. Dis. 2008, 14, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Deters, N.; Doldissen, A.; Bokhari, L.; Ke, Y.; Wiesner, A.; Schonrock, N.; Ittner, L.M. A decade of tau transgenic animal models and beyond. Brain Pathol. 2007, 17, 91–103. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta - Mol. Basis Dis. 2005, 1739, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, P.V.; Growdon, J.H.; Hedley-Whyte, E.T.; Hyman, B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992, 42, 631–639. [Google Scholar] [CrossRef]
- Amos, L.A. Microtubule structure and its stabilisation. Org. Biomol. Chem. 2004, 2, 2153–2160. [Google Scholar] [CrossRef]
- Kar, S.; Fan, J.; Smith, M.J.; Goedert, M.; Amos, L.A. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 2003, 22, 70–77. [Google Scholar] [CrossRef]
- Kolarova, M.; García-Sierra, F.; Bartos, A.; Ricny, J.; Ripova, D. Structure and pathology of tau protein in Alzheimer disease. Int. J. Alzheimers. Dis. 2012, 2012, 731526. [Google Scholar] [CrossRef]
- Trinczek, B.; Ebneth, A.; Mandelkow, E.M.; Mandelkow, E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J. Cell Sci. 1999, 112 Pt 1, 2355–2367. [Google Scholar] [CrossRef] [PubMed]
- Beevers, J.E.; Lai, M.C.; Collins, E.; Booth, H.D.E.; Zambon, F.; Parkkinen, L.; Vowles, J.; Cowley, S.A.; Wade-Martins, R.; Caffrey, T.M. MAPT Genetic Variation and Neuronal Maturity Alter Isoform Expression Affecting Axonal Transport in iPSC-Derived Dopamine Neurons. Stem Cell Reports 2017, 9, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Ross, J.L.; Goldman, Y.E.; Holzbaur, E.L.F. Differential regulation of dynein and kinesin motor proteins by tau. Science 2008, 319, 1086–1089. [Google Scholar] [CrossRef]
- Magnani, E.; Fan, J.; Gasparini, L.; Golding, M.; Williams, M.; Schiavo, G.; Goedert, M.; Amos, L.A.; Spillantini, M.G. Interaction of tau protein with the dynactin complex. EMBO J. 2007, 26, 4546–4554. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-Z.; Rasenick, M.M. Tau associates with actin in differentiating PC12 cells. FASEB J. 2006, 20, 1452–1461. [Google Scholar] [CrossRef]
- Leugers, C.J.; Lee, G. Tau potentiates nerve growth factor-induced mitogen-activated protein kinase signaling and neurite initiation without a requirement for microtubule binding. J. Biol. Chem. 2010, 285, 19125–19134. [Google Scholar] [CrossRef] [PubMed]
- Frandemiche, M.L.; De Seranno, S.; Rush, T.; Borel, E.; Elie, A.; Arnal, I.; Lante, F.; Buisson, A. Activity-Dependent Tau Protein Translocation to Excitatory Synapse Is Disrupted by Exposure to Amyloid-Beta Oligomers. J. Neurosci. 2014, 34, 6084–6097. [Google Scholar] [CrossRef]
- Tang, Z.; Ioja, E.; Bereczki, E.; Hultenby, K.; Li, C.; Guan, Z.; Winblad, B.; Pei, J.-J. mTor mediates tau localization and secretion: Implication for Alzheimer’s disease. Biochim. Biophys. Acta - Mol. Cell Res. 2015, 1853, 1646–1657. [Google Scholar] [CrossRef]
- Loomis, P.A.; Howard, T.H.; Castleberry, R.P.; Binder, L.I. Identification of nuclear tau isoforms in human neuroblastoma cells. Proc. Natl. Acad. Sci. USA 1990, 87, 8422–8426. [Google Scholar] [CrossRef]
- Alonso, A.D.; Grundke-Iqbal, I.; Barra, H.S.; Iqbal, K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc. Natl. Acad. Sci. USA 1997, 94, 298–303. [Google Scholar] [CrossRef]
- Ebneth, A.; Godemann, R.; Stamer, K.; Illenberger, S.; Trinczek, B.; Mandelkow, E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: Implications for Alzheimer’s disease. J. Cell Biol. 1998, 143, 777–794. [Google Scholar] [CrossRef]
- Futerman, A.H.; Banker, G.A. The economics of neurite outgrowth—The addition of new membrane to growing axons. Trends Neurosci. 1996, 19, 144–149. [Google Scholar] [CrossRef]
- Trojanowski, J.Q.; Lee, V.M. Phosphorylation of paired helical filament tau in Alzheimer’s disease neurofibrillary lesions: Focusing on phosphatases. FASEB J. 1995, 9, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.J.; Huber, B.R.; Silverman, M.A.; Cline, M.M.; Gill, T.B.; Cross, C.G.; Cook, D.G.; Minoshima, S. Intranasal Paclitaxel Alters Alzheimer’s Disease Phenotypic Features in 3xTg-AD Mice. J. Alzheimers. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Soeda, Y.; Takashima, A. New Insights Into Drug Discovery Targeting Tau Protein. Front. Mol. Neurosci. 2020, 13, 590896. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Huang, Y.; Gao, Q.; Zhou, Q. Stabilization of microtubules improves cognitive functions and axonal transport of mitochondria in Alzheimer’s disease model mice. Neurobiol. Aging 2020, 96, 223–232. [Google Scholar] [CrossRef]
- Novak, P.; Kontsekova, E.; Zilka, N.; Novak, M. Ten Years of Tau-Targeted Immunotherapy: The Path Walked and the Roads Ahead. Front. Neurosci. 2018, 12, 1–14. [Google Scholar] [CrossRef]
- Vitale, F.; Giliberto, L.; Ruiz, S.; Steslow, K.; Marambaud, P.; D’Abramo, C. Anti-tau conformational scFv MC1 antibody efficiently reduces pathological tau species in adult JNPL3 mice. Acta Neuropathol. Commun. 2018, 6, 82. [Google Scholar] [CrossRef]
- West, T.; Hu, Y.; Verghese, P.B.; Bateman, R.J.; Braunstein, J.B.; Fogelman, I.; Budur, K.; Florian, H.; Mendonca, N.; Holtzman, D.M. Preclinical and Clinical Development of ABBV-8E12, a Humanized Anti-Tau Antibody, for Treatment of Alzheimer’s Disease and Other Tauopathies. J. Prev. Alzheimers Dis. 2017, 4, 236–241. [Google Scholar]
- DeVos, S.L.; Goncharoff, D.K.; Chen, G.; Kebodeaux, C.S.; Yamada, K.; Stewart, F.R.; Schuler, D.R.; Maloney, S.E.; Wozniak, D.F.; Rigo, F.; et al. Antisense Reduction of Tau in Adult Mice Protects against Seizures. J. Neurosci. 2013, 33, 12887–12897. [Google Scholar] [CrossRef] [PubMed]
- Holth, J.K.; Bomben, V.C.; Reed, J.G.; Inoue, T.; Younkin, L.; Younkin, S.G.; Pautler, R.G.; Botas, J.; Noebels, J.L. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J. Neurosci. 2013, 33, 1651–1659. [Google Scholar] [CrossRef]
- Ahmed, T.; Van der Jeugd, A.; Blum, D.; Galas, M.-C.; D’Hooge, R.; Buee, L.; Balschun, D. Cognition and hippocampal synaptic plasticity in mice with a homozygous tau deletion. Neurobiol. Aging 2014, 35, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Shipton, O.A.; Leitz, J.R.; Dworzak, J.; Acton, C.E.J.; Tunbridge, E.M.; Denk, F.; Dawson, H.N.; Vitek, M.P.; Wade-Martins, R.; Paulsen, O.; et al. Tau protein is required for amyloid {beta}-induced impairment of hippocampal long-term potentiation. J. Neurosci. 2011, 31, 1688–1692. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Wei, Y.-P.; Xiong, Y.; Wang, X.-C.; Xie, A.-J.; Wang, X.-L.; Yang, Y.; Wang, Q.; Lu, Y.-M.; Liu, R.; et al. Synaptic Released Zinc Promotes Tau Hyperphosphorylation by Inhibition of Protein Phosphatase 2A (PP2A). J. Biol. Chem. 2012, 287, 11174–11182. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, Q.; Liu, F.-F.; Hu, F.; Xie, A.-J.; Zhu, L.-Q.; Liu, D. Synaptic Dysfunction in Alzheimer’s Disease: Aβ, Tau, and Epigenetic Alterations. Mol. Neurobiol. 2018, 55, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Krstic, D.; Pfister, S.; Notter, T.; Knuesel, I. Decisive role of Reelin signaling during early stages of Alzheimer’s disease. Neuroscience 2013, 246, 108–116. [Google Scholar] [CrossRef]
- Dorostkar, M.M.; Zou, C.; Blazquez-Llorca, L.; Herms, J. Analyzing dendritic spine pathology in Alzheimer’s disease: Problems and opportunities. Acta Neuropathol. 2015, 130, 1–19. [Google Scholar] [CrossRef]
- Fá, M.; Puzzo, D.; Piacentini, R.; Staniszewski, A.; Zhang, H.; Baltrons, M.A.; Li Puma, D.D.; Chatterjee, I.; Li, J.; Saeed, F.; et al. Extracellular Tau Oligomers Produce An Immediate Impairment of LTP and Memory. Sci. Rep. 2016, 6, 19393. [Google Scholar] [CrossRef]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; D’Adamio, L.; Grassi, C.; Devanand, D.P.; Honig, L.S.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimers. Dis. 2018, 64, S611–S631. [Google Scholar] [CrossRef]
- Cowan, C.M.; Mudher, A. Are tau aggregates toxic or protective in tauopathies? Front. Neurol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Moreno, H.; Morfini, G.; Buitrago, L.; Ujlaki, G.; Choi, S.; Yu, E.; Moreira, J.E.; Avila, J.; Brady, S.T.; Pant, H.; et al. Tau pathology-mediated presynaptic dysfunction. Neuroscience 2016, 325, 30–38. [Google Scholar] [CrossRef][Green Version]
- Medeiros, R.; Kitazawa, M.; Chabrier, M.A.; Cheng, D.; Baglietto-Vargas, D.; Kling, A.; Moeller, A.; Green, K.N.; LaFerla, F.M. Calpain Inhibitor A-705253 Mitigates Alzheimer’s Disease–Like Pathology and Cognitive Decline in Aged 3xTgAD Mice. Am. J. Pathol. 2012, 181, 616–625. [Google Scholar] [CrossRef]
- Roy, S.M.; Minasov, G.; Arancio, O.; Chico, L.W.; Van Eldik, L.J.; Anderson, W.F.; Pelletier, J.C.; Watterson, D.M. A Selective and Brain Penetrant p38αMAPK Inhibitor Candidate for Neurologic and Neuropsychiatric Disorders That Attenuates Neuroinflammation and Cognitive Dysfunction. J. Med. Chem. 2019, 62, 5298–5311. [Google Scholar] [CrossRef]
- Tracy, T.E.; Sohn, P.D.; Minami, S.S.; Wang, C.; Min, S.-W.; Li, Y.; Zhou, Y.; Le, D.; Lo, I.; Ponnusamy, R.; et al. Acetylated Tau Obstructs KIBRA-Mediated Signaling in Synaptic Plasticity and Promotes Tauopathy-Related Memory Loss. Neuron 2016, 90, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, D.L.; Brautigam, H.; Stockton, S.D.; Schmeidler, J.; Hof, P.R. Changes in dendritic complexity and spine morphology in transgenic mice expressing human wild-type tau. Brain Struct. Funct. 2010, 214, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Polydoro, M.; Acker, C.M.; Duff, K.; Castillo, P.E.; Davies, P. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J. Neurosci. 2009, 29, 10741–10749. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, K.; Lewis, J.; Spires, T.; Paulson, J.; Kotilinek, L.; Ingelsson, M.; Guimaraes, A.; DeTure, M.; Ramsden, M.; McGowan, E.; et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.-M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M.-Y. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Leroy, K.; Bretteville, A.; Schindowski, K.; Gilissen, E.; Authelet, M.; De Decker, R.; Yilmaz, Z.; Buée, L.; Brion, J.-P. Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice. Am. J. Pathol. 2007, 171, 976–992. [Google Scholar] [CrossRef] [PubMed]
- Lasagna-Reeves, C.A.; Castillo-Carranza, D.L.; Sengupta, U.; Guerrero-Munoz, M.J.; Kiritoshi, T.; Neugebauer, V.; Jackson, G.R.; Kayed, R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep. 2012, 2, 700. [Google Scholar] [CrossRef]
- Rady, R.M.; Zinkowski, R.P.; Binder, L.I. Presence of tau in isolated nuclei from human brain. Neurobiol. Aging 1995, 16, 479–486. [Google Scholar] [CrossRef]
- Fernández-Nogales, M.; Lucas, J.J. Altered Levels and Isoforms of Tau and Nuclear Membrane Invaginations in Huntington’s Disease. Front. Cell. Neurosci. 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Paonessa, F.; Evans, L.D.; Solanki, R.; Larrieu, D.; Wray, S.; Hardy, J.; Jackson, S.P.; Livesey, F.J. Microtubules Deform the Nuclear Membrane and Disrupt Nucleocytoplasmic Transport in Tau-Mediated Frontotemporal Dementia. Cell Rep. 2019, 26, 582–593.e5. [Google Scholar] [CrossRef]
- Rousseaux, M.W.C.; de Haro, M.; Lasagna-Reeves, C.A.; de Maio, A.; Park, J.; Jafar-Nejad, P.; Al-Ramahi, I.; Sharma, A.; See, L.; Lu, N.; et al. TRIM28 regulates the nuclear accumulation and toxicity of both alpha-synuclein and tau. Elife 2016, 5, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Diez, L.; Wegmann, S. Nuclear Transport Deficits in Tau-Related Neurodegenerative Diseases. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Farmer, K.M.; Ghag, G.; Puangmalai, N.; Montalbano, M.; Bhatt, N.; Kayed, R. P53 aggregation, interactions with tau, and impaired DNA damage response in Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 1–21. [Google Scholar] [CrossRef]
- Dickson, J.R.; Yoon, H.; Frosch, M.P.; Hyman, B.T. Cytoplasmic Mislocalization of RNA Polymerase II Subunit RPB1 in Alzheimer Disease Is Linked to Pathologic Tau. J. Neuropathol. Exp. Neurol. 2021, 80, 530–540. [Google Scholar] [CrossRef]
- Mahoney, R.; Ochoa Thomas, E.; Ramirez, P.; Miller, H.E.; Beckmann, A.; Zuniga, G.; Dobrowolski, R.; Frost, B. Pathogenic Tau Causes a Toxic Depletion of Nuclear Calcium. Cell Rep. 2020, 32. [Google Scholar] [CrossRef]
- Chauderlier, A.; Gilles, M.; Spolcova, A.; Caillierez, R.; Chwastyniak, M.; Kress, M.; Drobecq, H.; Bonnefoy, E.; Pinet, F.; Weil, D.; et al. Tau/DDX6 interaction increases microRNA activity. Biochim. Biophys. Acta. Gene Regul. Mech. 2018, 1861, 762–772. [Google Scholar] [CrossRef]
- Liu, C.; Götz, J. Profiling Murine Tau with 0N, 1N and 2N Isoform-Specific Antibodies in Brain and Peripheral Organs Reveals Distinct Subcellular Localization, with the 1N Isoform Being Enriched in the Nucleus. PLoS One 2013, 8, e84849. [Google Scholar] [CrossRef]
- Sultan, A.; Nesslany, F.; Violet, M.; Bégard, S.; Loyens, A.; Talahari, S.; Mansuroglu, Z.; Marzin, D.; Sergeant, N.; Humez, S.; et al. Nuclear Tau, a key player in neuronal DNA protection. J. Biol. Chem. 2011, 286, 4566–4575. [Google Scholar] [CrossRef]
- Qi, H.; Cantrelle, F.-X.; Benhelli-Mokrani, H.; Smet-Nocca, C.; Buée, L.; Lippens, G.; Bonnefoy, E.; Galas, M.-C.; Landrieu, I. Nuclear Magnetic Resonance Spectroscopy Characterization of Interaction of Tau with DNA and Its Regulation by Phosphorylation. Biochemistry 2015, 54, 1525–1533. [Google Scholar] [CrossRef]
- Ulrich, G.; Salvadè, A.; Boersema, P.; Calì, T.; Foglieni, C.; Sola, M.; Picotti, P.; Papin, S.; Paganetti, P. Phosphorylation of nuclear Tau is modulated by distinct cellular pathways. Sci. Rep. 2018, 8, 17702. [Google Scholar] [CrossRef]
- Violet, M.; Delattre, L.; Tardivel, M.; Sultan, A.; Chauderlier, A.; Caillierez, R.; Talahari, S.; Nesslany, F.; Lefebvre, B.; Bonnefoy, E.; et al. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front. Cell. Neurosci. 2014, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Federico, C.; Pinedo, F.; Bruno, F.; Rebolledo, A.B.; Montoya, J.J.; Olazabal, I.M.; Ferrer, I.; Saccone, S. Aging dependent effect of nuclear tau. Brain Res. 2017, 1677, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; He, R.Q. Tau could protect DNA double helix structure. Biochim. Biophys. Acta - Proteins Proteomics 2003, 1645, 205–211. [Google Scholar] [CrossRef]
- Vasudevaraju, P.; Guerrero, E.; Hegde, M.L.; Collen, T.B.; Britton, G.B.; Rao, K.S. New evidence on α-synuclein and Tau binding to conformation and sequence specific GC* rich DNA: Relevance to neurological disorders. J. Pharm. Bioallied Sci. 2012, 4, 112–117. [Google Scholar] [PubMed]
- Sjöberg, M.K.; Shestakova, E.; Mansuroglu, Z.; Maccioni, R.B.; Bonnefoy, E. Tau protein binds to pericentromeric DNA: A putative role for nuclear tau in nucleolar organization. J. Cell Sci. 2006, 119, 2025–2034. [Google Scholar] [CrossRef]
- Benhelli-Mokrani, H.; Mansuroglu, Z.; Chauderlier, A.; Albaud, B.; Gentien, D.; Sommer, S.; Schirmer, C.; Laqueuvre, L.; Josse, T.; Buée, L.; et al. Genome-wide identification of genic and intergenic neuronal DNA regions bound by Tau protein under physiological and stress conditions. Nucleic Acids Res. 2018, 1, 1–18. [Google Scholar] [CrossRef]
- Wei, Y.; Qu, M.H.; Wang, X.S.; Chen, L.; Wang, D.L.; Liu, Y.; Hua, Q.; He, R.Q. Binding to the minor groove of the double-strand, Tau protein prevents DNA damage by peroxidation. PLoS One 2008, 3. [Google Scholar] [CrossRef]
- Rossi, G.; Redaelli, V.; Contiero, P.; Fabiano, S.; Tagliabue, G.; Perego, P.; Benussi, L.; Bruni, A.C.; Filippini, G.; Farinotti, M.; et al. Tau mutations serve as a novel risk factor for cancer. Cancer Res. 2018, 78, 3731–3739. [Google Scholar] [CrossRef]
- Rossi, G.; Conconi, D.; Panzeri, E.; Redaelli, S.; Piccoli, E.; Paoletta, L.; Dalprà, L.; Tagliavini, F. Mutations in MAPT gene cause chromosome instability and introduce copy number variations widely in the genome. J. Alzheimer’s Dis. 2013, 33, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Conconi, D.; Panzeri, E.; Paoletta, L.; Piccoli, E.; Ferretti, M.G.; Mangieri, M.; Ruggerone, M.; Dalprà, L.; Tagliavini, F. Mutations in MAPT give rise to aneuploidy in animal models of tauopathy. Neurogenetics 2014, 15, 31–40. [Google Scholar] [CrossRef]
- Gómez de Barreda, E.; Dawson, H.N.; Vitek, M.P.; Avila, J. Tau deficiency leads to the upregulation of BAF-57, a protein involved in neuron-specific gene repression. FEBS Lett. 2010, 584, 2265–2270. [Google Scholar] [CrossRef]
- Oyama, F.; Kotliarova, S.; Harada, A.; Ito, M.; Miyazaki, H.; Ueyama, Y.; Hirokawa, N.; Nukina, N.; Ihara, Y. Gem GTPase and tau: Morphological changes induced by gem GTPase in cho cells are antagonized by tau. J. Biol. Chem. 2004, 279, 27272–27277. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Hemberg, M.; Lewis, J.; Feany, M.B. Tau promotes neurodegeneration through global chromatin relaxation. Nat. Neurosci. 2014, 17, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Samimi, H.; Gamez, M.; Zare, H.; Frost, B. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat. Neurosci. 2018, 21, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Jeong, H.H.; Hsieh, Y.C.; Klein, H.U.; Bennett, D.A.; De Jager, P.L.; Liu, Z.; Shulman, J.M. Tau Activates Transposable Elements in Alzheimer’s Disease. Cell Rep. 2018, 23, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- Maina, M.B.; Bailey, L.J.; Doherty, A.J.; Serpell, L.C. The Involvement of Aβ42 and Tau in Nucleolar and Protein Synthesis Machinery Dysfunction. Front. Cell. Neurosci. 2018, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maina, M.B.; Bailey, L.J.; Wagih, S.; Biasetti, L.; Pollack, S.J.; Quinn, J.P.; Thorpe, J.R.; Doherty, A.J.; Serpell, L.C. The involvement of tau in nucleolar transcription and the stress response. Acta Neuropathol. Commun. 2018, 6, 70. [Google Scholar] [CrossRef]
- Portillo, M.; Eremenko, E.; Kaluski, S.; Garcia-Venzor, A.; Onn, L.; Stein, D.; Slobodnik, Z.; Zaretsky, A.; Ueberham, U.; Einav, M.; et al. SIRT6-CBP-dependent nuclear Tau accumulation and its role in protein synthesis. Cell Rep. 2021, 35, 109035. [Google Scholar] [CrossRef]
- Siano, G.; Varisco, M.; Caiazza, M.C.; Quercioli, V.; Mainardi, M.; Ippolito, C.; Cattaneo, A.; Di Primio, C. Tau Modulates VGluT1 Expression. J. Mol. Biol. 2019, 431, 873–884. [Google Scholar] [CrossRef]
- Siano, G.; Caiazza, M.C.; Varisco, M.; Calvello, M.; Quercioli, V.; Cattaneo, A.; Di Primio, C. Modulation of tau subcellular localization as a tool to investigate the expression of disease-related genes. J. Vis. Exp. 2019, 154, e59988. [Google Scholar] [CrossRef]
- Khadrawyb YA, E.H. Glutamate Excitotoxicity and Neurodegeneration. J. Mol. Genet. Med. 2014, 8, 8–11. [Google Scholar] [CrossRef]
- Crescenzi, R.; DeBrosse, C.; Nanga, R.P.R.; Byrne, M.D.; Krishnamoorthy, G.; D’Aquilla, K.; Nath, H.; Morales, K.H.; Iba, M.; Hariharan, H.; et al. Longitudinal imaging reveals sub-hippocampal dynamics in glutamate levels associated with histopathologic events in a mouse model of tauopathy and healthy mice. Hippocampus 2017, 27, 285–302. [Google Scholar] [CrossRef]
- Kazim, S.F.; Chuang, S.C.; Zhao, W.; Wong, R.K.S.; Bianchi, R.; Iqbal, K. Early-onset network hyperexcitability in presymptomatic Alzheimer’s disease transgenic mice is suppressed by passive immunization with anti-human APP/Aβ antibody and by mGluR5 blockade. Front. Aging Neurosci. 2017, 9, 1–17. [Google Scholar] [CrossRef]
- Angulo, S.L.; Orman, R.; Neymotin, S.A.; Liu, L.; Buitrago, L.; Cepeda-Prado, E.; Stefanov, D.; Lytton, W.W.; Stewart, M.; Small, S.A.; et al. Tau and amyloid-related pathologies in the entorhinal cortex have divergent effects in the hippocampal circuit. Neurobiol. Dis. 2017, 108, 261–276. [Google Scholar] [CrossRef]
- Sánchez, M.P.; García-Cabrero, A.M.; Sánchez-Elexpuru, G.; Burgos, D.F.; Serratosa, J.M. Tau-induced pathology in epilepsy and dementia: Notions from patients and animal models. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Revett, T.J.; Baker, G.B.; Jhamandas, J.; Kar, S. Glutamate system, amyloid β peptides and tau protein: Functional interrelationships and relevance to Alzheimer disease pathology. J. Psychiatry Neurosci. 2013, 38, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Siano, G.; Varisco, M.; Scarlatti, A.; Caiazza, M.C.; Dunville, K.; Cremisi, F.; Costa, M.; Pancrazi, L.; Di Primio, C.; Cattaneo, A. Gene Expression of Disease-related Genes in Alzheimer’s Disease is Impaired by Tau Aggregation. J. Mol. Biol. 2020, 432, 166675. [Google Scholar] [CrossRef]
- Poirel, O.; Mella, S.; Videau, C.; Ramet, L.; Davoli, M.A.; Herzog, E.; Katsel, P.; Mechawar, N.; Haroutunian, V.; Epelbaum, J.; et al. Moderate decline in select synaptic markers in the prefrontal cortex (BA9) of patients with Alzheimer’s disease at various cognitive stages. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Hoshi, A.; Tsunoda, A.; Yamamoto, T.; Tada, M.; Kakita, A.; Ugawa, Y. Altered expression of glutamate transporter-1 and water channel protein aquaporin-4 in human temporal cortex with Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2018, 44, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Kirvell, S.L.; Esiri, M.; Francis, P.T. Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer’s disease. J. Neurochem. 2006, 98, 939–950. [Google Scholar] [CrossRef]
- Kashani, A.; Lepicard, E.; Poirel, O.; Videau, C.; David, J.P.; Fallet-Bianco, C.; Simon, A.; Delacourte, A.; Giros, B.; Epelbaum, J.; et al. Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol. Aging 2008, 29, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Berger, Z.; Roder, H.; Hanna, A.; Carlson, A.; Rangachari, V.; Yue, M.; Wszolek, Z.; Ashe, K.; Knight, J.; Dickson, D.; et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J. Neurosci. 2007, 27, 3650–3662. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).