Abstract
In the era of personalized medicine, insights into the molecular mechanisms that differentially contribute to disease phenotypes, such as asthma phenotypes including obesity-associated asthma, are urgently needed. Peripheral blood was drawn from 10 obese, non-atopic asthmatic adults with a high body mass index (BMI; 36.67 ± 6.90); 10 non-obese, non-atopic asthmatic adults with normal BMI (23.88 ± 2.73); and 10 healthy controls with normal BMI (23.62 ± 3.74). All asthmatic patients were considered to represent a low type-2 asthma phenotype according to selective clinical parameters. RNA sequencing (RNA-Seq) was conducted on peripheral blood CD4+ T cells. Thousands of differentially expressed genes were identified in both asthma groups compared with heathy controls. The expression of interferon (IFN)-stimulated genes associated with IFN-related signaling pathways was specifically affected in obese asthmatics, while the gap junction and G protein-coupled receptor (GPCR) ligand binding pathways were enriched in both asthma groups. Furthermore, obesity gene markers were also upregulated in CD4+ T cells from obese asthmatics compared with the two other groups. Additionally, the enriched genes of the three abovementioned pathways showed a unique correlation pattern with various laboratory and clinical parameters. The specific activation of IFN-related signaling and viral infection pathways might provide a novel view of the molecular mechanisms associated with the development of the low type-2 obesity-associated asthma phenotype, which is a step ahead in the development of new stratified therapeutic approaches.
1. Introduction
Over the last decades, non-communicable diseases (NCDs) have become the major cause of death worldwide, especially after the development of effective anti-infectious measures of prevention (vaccines) and treatment (antibiotics) [1,2,3]. Moreover, they represent a huge burden on the healthcare system and economic situation [4,5]. Prevalence and incidence of NCDs are still further increasing along with ongoing urbanization, industrialization, and globalization of unhealthy diet and lifestyles [6,7]. According to the World Health Organization (WHO), the rapid increase in the NCDs is mainly driven by various risk factors including tobacco, harmful use of alcohol, and obesity [8]. Furthermore, different NCDs and their major risk factors seem to have substantially overlapping underlying mechanisms, often involving immunometabolic alterations [9,10]. Combinations of certain NCDs and associated risk factors can create separate specific disease entities, classified as a clinical subtype or phenotype [11].
Obesity is a key risk factor underlying a variety of major NCDs, including asthma [10,12]. The comorbidity of obesity and asthma is referred to as obesity-associated asthma [13,14]. Various associated clinical characteristics of obesity and asthma have been described. The major obesity-associated asthma phenotype is characterized as “late-onset, severe and difficult to treat, type-2 low inflammation disease and presenting mostly in women” [15,16,17]. While these detailed clinical characteristics strictly define this particular phenotype of asthma, the specific underlying cellular and molecular mechanisms of this phenotype are still only poorly understood.
Gene expression alterations, especially in cells critically involved in disease development, represent a major molecular contributor to the pathophysiology of chronic inflammatory diseases such as asthma. Subsets of CD4+ T cells, such as Th1, Th2, and Th17 have been shown to differentially contribute to the initiation and perpetuation of specific asthma phenotypes [18,19]. Certain biological pathways and cellular processes play an essential role in the polarization of the CD4+ T cells subtypes [20]. Thus, deep understanding of how those molecular mechanisms underlie clinical asthma representations might help to further solve the complex disease heterogeneity puzzle, particularly also with regard to specific aspects of obesity-associated asthma phenotypes.
The current study addresses the specific role of CD4+ T cells in obesity-associated low type-2 asthma. We compared the transcriptome profiles of these cells in strictly selected, clinically characterized obese and non-obese low type-2 asthmatics and related the findings to healthy controls. In addition, functional pathway enrichment and gene clusters network analyses were conducted to comprehensively understand the association of the dysregulated genes with the respective asthma phenotype.
2. Results
2.1. Patient Selection and Clinical Parameters Comparison
In the present study we selectively focused on low type-2 asthmatics as identified by low blood eosinophil counts (<300 eos/µL) and low FeNO (<25 ppb) levels according to the latest Global Initiative for Asthma (GINA) [21], associated with low serum total IgE levels (<300 kU/L). A detailed comparison of the two asthma groups based on laboratory and clinical parameters including CRP, FeNO, age of onset, and lung function parameters are provided in Table 1. With the exception of BMI, no significant differences in clinical parameters were identified between the obese asthmatics and non-obese asthmatics groups.

Table 1.
General patient characteristics of the two low type-2 asthma patient groups.
To confirm the low type-2 state of the asthmatic patients, we checked the expression of a series of recently identified marker genes of Th2 differentiation [22] in the CD4+ T cells transcriptome data set. The majority of the tested Th2 genes showed no significant differences between either asthma group when compared with healthy controls (Table 2). Interestingly, the master transcription factor for the differentiation and activation of the Th2 cell subset, i.e., GATA binding protein 3 (GATA3), was significantly less expressed in both asthma groups compared with healthy controls, while expression of interleukin 4 (IL-4), IL-5, and IL-13 were below the detection limit for all samples.

Table 2.
Selected type-2-related genes and their corresponding fold change (FC) and false discovery rate (FDR) values in both comparisons, obese asthmatics vs. Ctrl (healthy controls) and non-obese asthmatics vs. Ctrl.
2.2. Differentially Expressed Genes (DEGs) of CD4+ T Cells in Low Type-2 Obese and Non-Obese Asthmatics
To identify specific transcriptome patterns in CD4+ T cells associated with low type-2 obese asthma, we performed differential gene expression analyses in peripheral blood-derived CD4+ T cells of 10 obese asthmatic adults in comparison with 10 non-obese asthmatics as well as 10 healthy non-obese adults. Following RNA sequencing (RNA-Seq) and bioinformatic analysis, we first created a correlation matrix based on the 75% most abundantly expressed genes in order to observe distance and clustering of the matched samples (Figure 1A). All three study groups exhibited major unique clusters and some smaller sub-clusters. Interestingly, the obese asthmatics showed less clustering consistency than non-obese asthmatics. Further, we performed a pairwise comparison of the three studied groups at a significance threshold for the false discovery rate (FDR) < 0.1. A total of 1304 DEGs (647 up and 657 down) were identified in CD4+ T cells of obese asthmatics compared with healthy controls (Ctrl), while 869 genes (386 up and 483 down) were found to be differentially expressed in CD4+ T cells of non-obese asthmatics compared with healthy controls. A total of 283 DEGs (141 up and 142 down) were detected when comparing CD4+ T cells from obese asthmatics with those from non-obese asthmatics (Figure 1B). As shown in the Venn diagram in Figure 1B, 386 genes were found to be specifically upregulated in the comparison of obese asthmatics vs. Ctrl (green circle), while 195 and 66 upregulated genes were shared between non-obese asthmatics vs. Ctrl and obese asthmatics vs. non- obese asthmatics comparisons, respectively (Figure 1B, left). Likewise, the numbers of significantly downregulated genes for all three comparisons are shown in the right panel of (Figure 1B).
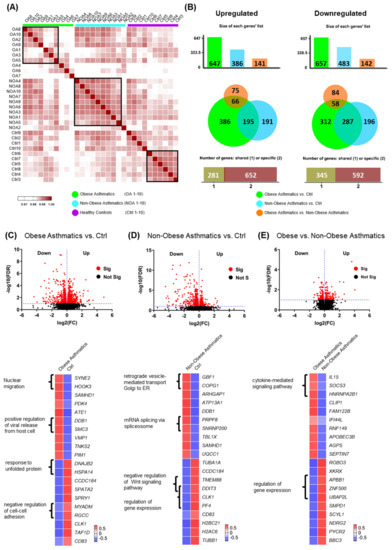
Figure 1.
Differentially expressed genes of both low type-2 obese asthma and non-obese asthma phenotypes using a significance threshold cutoff for FDR < 0.1. (A) Correlation matrix of 75% of the detected genes for 10 obese asthmatics ”OA, green”, 10 non-obese asthmatics “NOA, turquoise”, and 10 healthy controls “Ctrl, purple”. (B) Proportional Venn diagrams showing the specific and shared genes of obese asthmatics vs. Ctrl (healthy controls), non-obese asthmatics vs. Ctrl, and obese asthmatics vs. non-obese asthmatics. (C–E, up) Volcano plots presenting differentially expressed genes of the three comparisons. (C–E, down) Heatmaps showing the top significantly 10 up and downregulated genes with some of the associated biological processes (GO terms, p < 0.05).
To separately investigate each comparison in more details, we created volcano plots (Figure 1C–E, up) and heat maps depicting the top 10 significantly up and downregulated genes alongside their associated biological processes (GO terms; Figure 1C–E, down).
Our results demonstrated the positive regulation of viral release from the host cell pathway to be enriched in the obese asthmatics vs. healthy controls as represented by the upregulated genes damage-specific DNA-binding protein 1 (DDB1) and structural maintenance of chromosomes 3 (SMC3) (Figure 1C, down). The negative regulation of Wnt signaling pathway was less active in non-obese asthmatics compared with healthy controls, as represented by the downregulated transmembrane protein 88 (TMEM88) and DNA damage-inducible transcript 3 (DDIT3) genes (Figure 1D, down). In the comparison of obese asthmatics vs. non-obese asthmatics, the upregulated IL15, SOCS3, and heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1) genes were associated with the cytokine-mediated signaling pathway (Figure 1E, down). Further differentially expressed genes and their related biological processes are shown in Figure 1C–E.
2.3. Predominant Interferon Signaling Pathway in CD4+ T Cells of Low Type-2 Obese Asthmatics
We further carried out a functional pathway enrichment analysis utilizing the genes comprising a fold change (FC) ≥ 1.5 at FDR < 0.1. The joined pathway analysis of obese and non-obese asthmatics compared with healthy controls revealed a variety of shared biological pathways at different significance levels in the two asthma groups. The top 15 significant canonical pathways are shown in Figure 2A. To elucidate the interconnection between the enriched pathways, we identified genes mutually involved in the respective pathways and performed a correlation analysis based on these genes (Supplementary Figures S1A and S1B). Three distinct pathway clusters were identified. Further, we performed pathway network analysis based on the two previous analyses. Pathways such as cytokine–cytokine receptor interaction and chemokine signaling pathway encompassed the highest number of mutual genes (size of circle) in the first pathways cluster (green) and provided a scaffold to the second pathways cluster (blue) (Figure 2B). Next, we applied the same analysis to the obese asthmatics vs. Ctrl and obese asthmatics vs. non-obese asthmatics comparisons in order to highlight the obesity asthma-related pathways. Interestingly, pathways representing interferon signaling and viral infection mechanisms were robustly overrepresented in this analysis (Figure 2C). Notably, the EIF2AK2 gene, encoding eukaryotic translation initiation factor 2 alpha kinase 2, also known as interferon-induced double-stranded RNA-activated protein kinase, coincided with most of the pathways and might thus be critical for specific pathomechanisms of the obesity-associated asthma phenotype (Supplementary Figure S1C). We detected two main clusters of pathways in the corresponding analysis, clearly demonstrating a central role for interferon signaling mechanisms (Supplementary Figures S1D and Figure 2D).

Figure 2.
Biological pathway analysis utilizing Bioplanet database with significance threshold of p < 0.05. (A) Bar plot presenting the top 15 significant pathways shared between obese asthmatics vs. Ctrl (healthy controls) and non-obese asthmatics vs. Ctrl, alongside their (B) network analysis showing the association between the previous pathways (circle color corresponds to pathways cluster, circle size to the number of mutual genes between the corresponding pathways and all other connected pathways, circles proximity to the number of mutual genes between two pathways). (C) Bar plot presenting all significant pathways shared between asthmatics vs. Ctrl and obese asthmatics vs. non-obese asthmatics with their (D) network analysis.
2.4. Further Gene Regulation Pathways Involved in Low Type-2 Asthma Phenotypes
To select candidate biological pathways from the previous analysis, we performed gene network and clustering analyses for each of the three comparisons separately and chose the clusters containing the shared pathways. These network gene clusters were built from all genes of each of the three comparisons showing a FC ≥ 1.5 at FDR < 0.1. Further, the huge STRING PPI gene networks were subdivided into clusters based on topology to find densely connected network regions. Genes of the G protein-coupled receptor (GPCR) ligand binding and gap junction pathways were identified in both comparisons of obese asthmatics vs. Ctrl and non-obese asthmatics vs. Ctrl (Figure 3A). Moreover, in the obese asthmatics vs. Ctrl and vs. non-obese asthmatics comparisons, the interferon signaling pathway was again featured as differentially affected, identifying this pathway as a quite specific mechanism in the regulation of CD4+ T cells activities of obese low type-2 asthmatics (Figure 3B). Differential expression of genes in the gene network clusters are shown in Supplementary Figure S2.
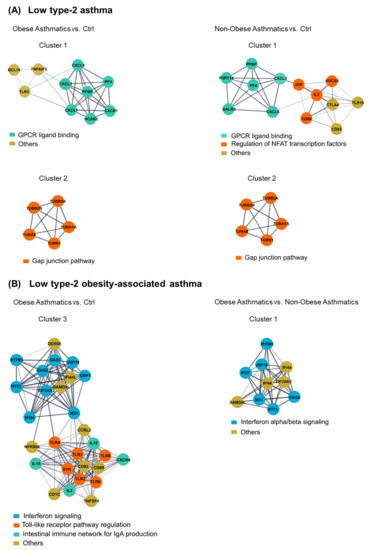
Figure 3.
Clustering of dense network regions and their associated biological pathways. Gene clusters associated with shared pathways between ((A), left) obese asthmatics vs. Ctrl (healthy controls) and ((A), right) non-obese asthmatics vs. Ctrl, as well as between ((B), left) obese asthmatics vs. Ctrl and ((B), right) obese asthmatics vs. non-obese asthmatics.
2.5. Correlation of Biological Pathway Enriched Genes with Clinical Variables
As shown in the patients’ characteristics (Table 1), asthma-related clinical parameters revealed no significant differences between obese asthmatics and non-obese asthmatics and cannot be used to distinguish between these phenotypes. However, we intended to explore the correlation between these parameters and gene expression levels in the two asthmatics populations. Thus, we exploited the enriched genes represented in the three major canonical pathways contributing to differentially regulated network clusters, i.e., IFN signaling, GPCR ligand binding, and gap junction pathways. In obese asthmatics strong positive correlations of interferon-induced transmembrane protein 3 (IFITM3) and guanylate-binding protein 3 (GBP3) with peak expiratory flow (PEF), forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), maximum vital capacity (VC max), and inspiratory capacity (IC) were identified, while some less pronounced inverse correlations were observed between further IFN signaling pathway enriched genes and FeNO (Figure 4A).
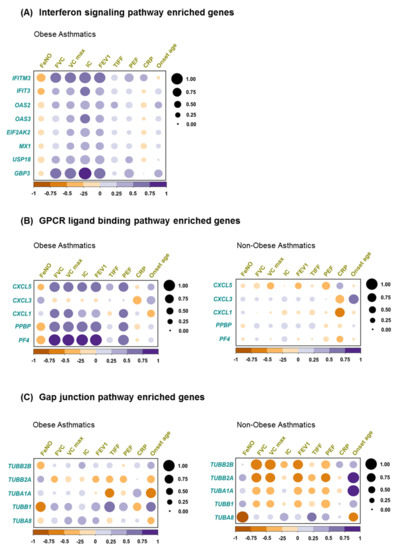
Figure 4.
Correlation between clinical variables and biological pathways enriched genes. Pearson correlation between clinical assessments and (A) interferon signaling pathway enriched genes in obese asthma, (B) gap junction pathway enriched genes in both obese asthmatics and non-obese asthmatics, and (C) GPCR ligand binding enriched genes in both obese asthmatics and non-obese asthmatics.
Additionally, in obese asthmatics, except C-X-C motif chemokine ligand 3 (CXCL3), GPCR binding ligand enriched genes correlated positively with FVC, VC max, IC, FEV1, and PEF (Figure 4B, left), while this correlation pattern was not observed in non-obese asthmatics (Figure 4B, right). This might indicate the presence of a superior clinical-molecular link specifically in the obese asthmatics.
Eventually, in case of gap junction pathway enriched genes, tubulin alpha 1a (TUBA1A) gene showed a robust negative correlation with TIFF and onset age, whereas tubulin beta 1 class VI (TUBB1) gene correlated negatively with FeNO in obese asthmatics (Figure 4C, left). In contrast, in non-obese asthmatics, most of the gap junction pathway enriched genes exhibited a negative correlation with PEF, FEV1, FVC, and VC max (Figure 4C, right).
3. Discussion
Even though our understanding of asthma has substantially increased within the last several decades, the progress made is not sufficient to fully understand the molecular mechanisms behind various clinical phenotypes of the disease, including obesity-associated asthma, i.e., to identify the relevant underlying molecular endotypes [23,24]. In the age of personalized medicine, this may represent an important basis for the establishment of highly detailed methods of molecular endotyping. This would enable the identification of asthmatics benefiting the most from specific treatment modalities or the selection of stratified therapeutic options for specific patients [25,26]. Therefore, we here performed a gene expression analysis in patients with the low type-2 obesity-associated asthma phenotype. To perform this task in a comprehensive and systematic way, we (i) conducted mRNA analyses on a genome-wide level, (ii) applied a stepwise bioinformatics analysis from DEGs through enriched pathways to select the pathways with the highest biological relevance, (iii) compared results with low type-2 asthmatics with no accompanying obesity and included a healthy control group, and (iv) performed all the analyses in a highly purified population of CD4+ T cells. These cells are known to play a central regulatory role in asthma development by orchestrating the actions of a variety of effector cells [27,28,29]. Thus, understanding their differential biology based on gene expression patterns is a major step forward in understanding the pathophysiology of asthma, and more specifically of the obesity-related asthma phenotype.
Although standard laboratory and clinical measurements such as lung function parameters, CRP, FeNO, and age of onset were not able to distinguish between the obese and non-obese asthma groups, there was an obvious difference between those two groups by hundreds of differentially expressed genes in CD4+ T cells. Generally, we observed a greater variability in gene expression of CD4+ T cells of obese asthmatics compared with non-obese asthmatics. This might be due to varying stimulating signals triggered by adipose tissue or other potential factors that exert immunomodulatory effects (Figure 1A) [30,31]. Among the top 10 significant DEGs, IL15 and SOCS3 were found to be upregulated in obese asthmatics compared with non-obese asthmatics, which have been previously reported as obesity-associated marker genes [32,33,34,35,36,37,38].
Most importantly, IFN-related signaling pathways were overrepresented in obese asthmatics, compared with both healthy controls and non-obese asthmatics and induced by various IFN-stimulated genes (ISGs) (Figure 3B). Specifically, the involvement of the interferon-gamma signaling pathway may indicate that CD4+ T cells in obese asthmatics or at least a bigger proportion of them have been skewed in a Th1 polarization manner.
The lipid metabolism-associated hormone leptin is one principal factor that is able to drive CD4+ T cells to produce IFN proteins and might thus, among others, be responsible for this finding [39,40]. This notion is further supported by the observation of the negative correlation between the IFN signaling genes expression and FeNO, an established marker of type-2 inflammation in asthma (Figure 4A) [41].
The topological interconnection between IFN signaling and viral infection pathways (e.g., influenza factor interaction with host) in obese asthmatics could define a complex mechanism underlying this asthma phenotype (Figure 2D). Predominant upregulation of Toll-like receptor pathway regulation genes such as Toll-like receptor 1 (TLR-1), TLR-2, TLR-3, TLR-4, TLR-6, and TLR-8 in obese asthmatics compared with healthy controls could be an additional proof of the potential role of viral infections in the low type-2 obesity asthma phenotype (Figure 3B) [42,43]. Tang et al. demonstrated that obesity could foster respiratory tract infections and subsequently contribute to asthma exacerbations in adults [44,45]. Similar findings of the association between obesity and viral infection were reported by Maccioni et al. [46]. Notably, this association was a high spot during the current COVID-19 pandemic because various studies have implied that obesity can worsen the outcomes of the SARS-CoV-2 viral infection [47,48].
Altered regulation of a variety of GPCR ligand binding associated genes has been observed in both groups of low type-2 asthmatics compared with healthy controls (Figure 3A) [49,50]. Approximately 34% of currently marketed drugs directly or indirectly target G-protein-coupled receptors [51,52]. The discrete robust correlation between GPCR ligand binding enriched genes and most of the lung function parameters in obese asthmatics indicates a key role of the GPCR signaling pathway specifically in this asthma phenotype (Figure 4B).
Additional regulation in both asthma groups was observed in genes associated with the gap junction pathway, representing another mechanism of cell–cell communication, which plays also a key role in T cell activation (Figure 3A) [53,54,55,56,57]. Shutting down such conventional cellular communication paths could contribute to the persistent inflammatory response in the low type-2 asthma phenotype [57,58]. Of interest, in a mouse model of allergic airway inflammation mimicking asthma, carbenoxolone, a gap junction uncoupling agent, was found to reduce infiltration of inflammatory cells and interleukin production, thereby decreasing lung inflammation [59].
To the best of our knowledge, it is the first study investigating gene expression in obesity-associated asthma on the level of isolated CD4+ T cells. Although, the study may be limited by the rather small sample size, it is compensated for by the careful selection of patients representing the phenotype of interest out of a larger cohort. The focus was on a single blood cell population and the immediate isolation of these cells of interest directly after blood drawing and finally deep transcriptome sequencing, resulting in meaningful, solid data and robust, unbiased results.
In conclusion, deciphering the underlying molecular mechanism and thus deeper endo/phenotyping of the unique low type-2 obesity-associated asthma phenotype is a step ahead in the development of modern personalized medicine approaches. Stratified therapy strategies targeting the IFN signaling pathway or some of its central genes, such as the eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2), could be a specific treatment option for low type-2 obese asthmatics, whereas conventional or novel anti-viral treatments could be another feasibility. Moreover, certain lung function parameters including FVC, VC max, IC, and FEV1 can be utilized as positive indicators of therapy response in low type-2 obese asthmatics.
4. Material and Methods
4.1. Study Population and Clinical Assessments
One hundred forty-four asthmatic adults were recruited into the study by the Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Centre Giessen and Marburg. All patients were physician-diagnosed with asthma according to recent clinical guideline diagnostics [21]. Within this cohort, we identified patient sub-groups based on their body mass index (BMI), blood eosinophil counts, and total serum immunoglobulin E (IgE) levels. To selectively focus on low type-2 asthmatics, only patients with low blood eosinophil counts (<300 eos/µL), low fractional exhaled nitric oxide (FeNO; <25 ppb), and low serum total IgE levels (<300 kU/L) were included in this study. Out of those, 10 patients representing the >75% quartile in BMI were selected as obese asthmatics and compared with 10 patients with normal body weight. Detailed patients’ characteristics are shown in Table 1. For comparison, 10 healthy controls were recruited to the Comprehensive Biobank Marburg at the Medical Faculty of the Philipps University of Marburg. Laboratory and clinical parameters including C-reactive protein (CRP), blood cell counts, serum IgE levels, and lung function parameters were measured as part of routine clinical assessments. The study was approved by the local ethics committee (approval No. 155/08/; 202/12), and all participants gave written informed consent.
4.2. Blood CD4+ T Cells Isolation
For the study purpose, 9 mL of peripheral blood was drawn from all subjects into S-Monovette K3 EDTA collection tubes (Sarstedt, Nümbrecht, Germany). Within 2 h following blood drawing, CD4+ T cells were isolated using the StraightFrom® Whole Blood CD4 MicroBeads, human kit (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer’s protocol. Quality control was performed by flow cytometry analysis and revealed >95% CD4+ T cell purity. Isolated CD4+ T cells were lysed in RLT buffer (Qiagen, Hilden, Germany) supplemented with 1% 2-mercaptoethanol (Carl Roth, Karlsruhe, Germany) and preserved at −80 °C until further use.
4.3. RNA Extraction and Sequencing
Total RNA was extracted using the miRNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. RNA quality and quantity were assessed by Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) and Qubit 3 (Thermo Fisher Scientific, Foster City, CA, USA) analyses, respectively. RNA sequencing was performed using the BGISEQ-500 platform (BGI, Shenzhen, China). Briefly, mRNAs molecules were purified from total RNA using oligo(dT)-coupled magnetic beads and fragmented into small pieces using fragmentation reagent. Afterwards, first-strand cDNA was generated using random hexamer-primed reverse transcription, followed by a second-strand cDNA synthesis. The synthesized cDNA was subjected to end-repair and was then 3′ adenylated. Adapters were ligated to the ends of these 3′ adenylated cDNA fragments, which were then amplified using adapter-specific primers. Resulting PCR products were purified with Ampure XP Beads (Beckmann Coulter, Brea, CA, USA) and dissolved in elution buffer. Library quality was validated on an Agilent 2100 Bioanalyzer, and 50 base-pair, paired-end sequencing was performed.
4.4. Bioinformatics Analysis
Sequenced reads were mapped with Salmon v1.3.0 in quantification mode against the human transcriptome of cDNA and ncRNA sequences (GRCh38) with the respective genome background as decoys [60]. Resulting read count estimates were rounded to integers, and differential gene expression analysis was performed by DEseq2 [61]. Differential expression analysis was performed with standard parameters, using either asthma status or asthma status + weight as the experimental design. Genes with low counts (<10) were filtered out. For sample–sample comparison and data visualization, cell count data were transformed via Variance stabilizing transformation (vst). iDEP v.0.91 was used to create the correlation matrix of the matched samples as a default option [62]. Heatmaps were created based on Z-score values of gene expression. Functional pathway and gene ontology analyses were conducted by Enrichr Bioplanet and GO biological Process [63,64,65]. Pathway network analyses were built with the R package (ggraph v2.0.5), and gene network analyses were performed by STRING PPI v.11.0 using default settings [66]. Gene networks were further processed by Cytoscape v.23 add-ins MCODE and Clustermaker. Correlation plots of clinical and molecular parameters were generated by the R package (Corrplot v.0.84), implemented with default Pearson correlation.
4.5. Statistical Analysis
Unpaired t-tests applying a significance threshold of p < 0.05 were performed for comparisons of numerical data using the GraphPad Prism 7 software package (GraphPad Software Inc., San Diego, CA, USA).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms221810144/s1.
Author Contributions
H.G., D.P.P., and T.G. created the study design; T.G., P.I.P., F.P., and T.B. recruited and characterized the study participants; F.A. and B.A.A. performed the laboratory study analyses; F.A., L.M.M., and C.T. conducted the bioinformatics analysis; F.A., D.P.P., and H.G. drafted the manuscript, and all coauthors provided their revision points. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the German Center for Lung Research (DZL); the German Academic Exchange Service (DAAD; F.A., personal reference number: 91726294); the HessenFonds, World University Service (WUS; F.A.); the Hessen State Ministry for Higher Education, Research and the Arts (HMWK; F.A.); and the German Research Foundation (DFG; B.A.A., Grant 512416910386–GRK 2573/1).
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Philipps University of Marburg (approval No. 155/08/; 202/12).
Informed Consent Statement
Written informed consent was given by all individual participants included in the study.
Data Availability Statement
The data presented in this study and underlying raw data are available on reasonable request from the corresponding author.
Acknowledgments
We thank Sarah Miethe and Florian Michel for technical support, Ho-Ryun Chung for bioinformatics analysis assistance, and all colleagues who provided critical discussion points.
Conflicts of Interest
All authors declare that they have no conflicts of interest and/or competing interests.
References
- Pearce, N.; Asher, I.; Billo, N.; Bissell, K.; Ellwood, P.; El Sony, A.; Garcia-Marcos, L.; Chiang, C.-Y.; Mallol, J.; Marks, G.; et al. Asthma in the global NCD agenda: A neglected epidemic. Lancet Respir. Med. 2013, 1, 96–98. [Google Scholar] [CrossRef]
- The Top 10 Causes of Death. 14 July 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 27 July 2021).
- Habib, S.H.; Saha, S. Burden of non-communicable disease: Global overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2010, 4, 41–47. [Google Scholar] [CrossRef]
- Nugent, R.A.; Husain, M.J.; Kostova, D.; Chaloupka, F. Introducing the PLOS special collection of economic cases for NCD prevention and control: A global perspective. PLoS ONE 2020, 15, e0228564. [Google Scholar] [CrossRef] [PubMed]
- Kankeu, H.T.; Saksena, P.; Xu, K.; Evans, D.B. The financial burden from non-communicable diseases in low- and middle-income countries: A literature review. Health Res. Policy Syst. 2013, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Gowshall, M.; Taylor-Robinson, S.D. The increasing prevalence of non-communicable dis-eases in low-middle income countries: The view from Malawi. Int. J. Gen. Med. 2018, 11, 255–264. [Google Scholar] [CrossRef]
- Sivanantham, P.; Sahoo, J.; Lakshminarayanan, S.; Bobby, Z.; Kar, S.S. Profile of risk factors for Non-Communicable Diseases (NCDs) in a highly urbanized district of India: Findings from Puducherry district-wide STEPS Survey, 2019–2020. PLoS ONE 2021, 16, e0245254. [Google Scholar] [CrossRef]
- Noncommunicable Diseases. 16 July 2021. Available online: https://www.who.int/data/gho/data/themes/noncommunicable-diseases (accessed on 27 July 2021).
- Peters, R.; Ee, N.; Peters, J.; Beckett, N.; Booth, A.; Rockwood, K.; Anstey, K.J. Common risk factors for major noncommunicable disease, a systematic overview of reviews and commentary: The implied potential for targeted risk reduction. Ther. Adv. Chronic Dis. 2019, 10, 2040622319880392. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.T.; Batty, G.; Pentti, J.; Virtanen, M.; Alfredsson, L.; Fransson, E.I.; Goldberg, M.; Heikkila, K.; Jokela, M.; Knutsson, A.; et al. Obesity and loss of disease-free years owing to major non-communicable diseases: A multicohort study. Lancet Public Health 2018, 3, e490–e497. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.A. Asthma is a major noncommunicable disease affecting over 230 million people worldwide and represents the most common chronic disease among children. Int. Immunopharmacol. 2014, 23, 315. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Miethe, S.; Schindler, V.; Alhamdan, F.; Garn, H. Role of airway epithelial cells in the development of different asthma phenotypes. Cell. Signal. 2020, 69, 109523. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Ulrik, C.S. Obesity and asthma: A coincidence or a causal relationship? A systematic review. Respir. Med. 2013, 107, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Miethe, S.; Guarino, M.; Alhamdan, F.; Simon, H.-U.; Renz, H.; Dufour, J.-F.; Potaczek, D.P.; Garn, H. Effects of obesity on asthma: Immunometabolic links. Polish Arch. Intern. Med. 2018, 128, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef]
- McAlees, J.W.; Lajoie, S.; Dienger, K.; Sproles, A.A.; Richgels, P.K.; Yang, Y.; Khodoun, M.; Azuma, M.; Yagita, H.; Fulkerson, P.C.; et al. Differential control of CD4(+) T-cell subsets by the PD-1/PD-L1 axis in a mouse model of allergic asthma. Eur. J. Immunol. 2015, 45, 1019–1029. [Google Scholar] [CrossRef]
- Muehling, L.M.; Lawrence, M.G.; Woodfolk, J.A. Pathogenic CD4+ T cells in patients with asthma. J. Allergy Clin. Immunol. 2017, 140, 1523–1540. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Wong, N.; Rao, G.; Nguyen, A.; Avery, D.T.; Payne, K.; Torpy, J.; O’Young, P.; Deenick, E.; Bustamante, J.; et al. Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets. J. Exp. Med. 2016, 213, 1589–1608. [Google Scholar] [CrossRef]
- Global Initiative for Asthma—GINA. Global Initiative for Asthma—Global Initiative for Asthma—GINA. 29 July 2021. Available online: https://ginasthma.org/ (accessed on 30 July 2021).
- Radens, C.M.; Blake, D.; Jewell, P.; Barash, Y.; Lynch, K.W. Meta-analysis of transcriptomic varia-tion in T-cell populations reveals both variable and consistent signatures of gene expres-sion and splicing. RNA 2020, 26, 1320–1333. [Google Scholar] [CrossRef]
- Gelfand, E.W.; Schedel, M. Molecular Endotypes Contribute to the Heterogeneity of Asthma. Immunol. Allergy Clin. 2018, 38, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Oriss, T.B.; Wenzel, S.E. Emerging molecular phenotypes of asthma. American journal of physiology. Lung Cell. Mol. Physiol. 2015, 308, L130–L140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef]
- Schoettler, N.; Strek, M.E. Recent Advances in Severe Asthma: From Phenotypes to Personalized Medicine. Chest 2020, 157, 516–528. [Google Scholar] [CrossRef]
- Miura, K.; Inoue, K.; Ogura, A.; Kaminuma, O. Role of CD4+ T Cells in Allergic Airway Diseases: Learning from Murine Models. Int. J. Mol. Sci. 2020, 21, 7480. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.N.; Renz, H.; Casale, T.B.; Garn, H. Biologic Therapy and Novel Molecular Targets of Severe Asthma. J. Allergy Clin. Immunol. Pract. 2017, 5, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.W.; Morris, D.; DelProposto, J.L.; Geletka, L.; Zamarron, B.; Martinez-Santibanez, G.; Meyer, K.A.; Singer, K.; O’Rourke, R.; Lumeng, C.N. An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Rep. 2014, 9, 605–617. [Google Scholar] [CrossRef]
- Endo, Y.; Asou, H.K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, D.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 2015, 12, 1042–1055. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Wang, W.; Guo, Q.; Wen, C.; Li, Y.; Yin, Y. Interleukin-15 in obesity and metabolic dysfunction: Current understanding and future perspectives. Obes. Rev. 2017, 18, 1147–1158. [Google Scholar] [CrossRef]
- Barra, N.G.; Reid, S.; MacKenzie, R.; Werstuck, G.; Trigatti, B.L.; Richards, C.; Holloway, A.C.; Ashkar, A.A. Interleukin-15 contributes to the regulation of murine adipose tissue and human adipocytes. Obesity 2010, 18, 1601–1607. [Google Scholar] [CrossRef]
- Reyes, J.L.; Vannan, D.T.; Vo, T.; Gulamhusein, A.; Beck, P.L.; Reimer, R.A.; Eksteen, B. Neutralization of IL-15 abrogates experimental immune-mediated cholangitis in diet-induced obese mice. Sci. Rep. 2018, 8, 3127. [Google Scholar] [CrossRef]
- Zampieri, T.T.; Da Silva, T.E.O.; de Paula Romeu, D.; Da Silva Torrão, A.; Donato, J., Jr. SOCS3 expression within leptin receptor-expressing cells regulates food intake and leptin sensitivity but does not affect weight gain in pregnant mice consuming a high-fat diet. Physiol. Behav. 2016, 157, 109–115. [Google Scholar] [CrossRef]
- Pedroso, J.; Silveira, M.; Lima, L.B.; Furigo, I.C.; Zampieri, T.T.; Ramos-Lobo, A.M.; Buonfiglio, D.C.; Teixeira, P.; Frazao, R.; Donato, J. Changes in Leptin Signaling by SOCS3 Modulate Fasting-Induced Hyperphagia and Weight Regain in Mice. Endocrinology 2016, 157, 3901–3914. [Google Scholar] [CrossRef]
- Pedroso, J.A.B.; Ramos-Lobo, A.M.; Donato, J. SOCS3 as a future target to treat metabolic dis-orders. Hormones 2019, 18, 127–136. [Google Scholar] [CrossRef]
- Palanivel, R.; Fullerton, M.D.; Galic, S.; Honeyman, J.; Hewitt, K.A.; Jorgensen, S.B.; Steinberg, G.R. Reduced Socs3 expression in adipose tissue protects female mice against obesi-ty-induced insulin resistance. Diabetologia 2012, 55, 3083–3093. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, F. Regulation, Communication, and Functional Roles of Adipose Tissue-Resident CD4+ T Cells in the Control of Metabolic Homeostasis. Front. Immunol. 2018, 9, 1961. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, K.; MacIver, N.J. The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease. Front. Immunol. 2020, 11, 622468. [Google Scholar] [CrossRef] [PubMed]
- Korn, S.; Wilk, M.; Voigt, S.; Weber, S.; Keller, T.; Buhl, R. Measurement of Fractional Exhaled Nitric Oxide: Comparison of Three Different Analysers. RES 2020, 99, 1–8. [Google Scholar] [CrossRef]
- Carty, M.; Bowie, A.G. Recent insights into the role of Toll-like receptors in viral infection. Clin. Exp. Immunol. 2010, 161, 397–406. [Google Scholar] [CrossRef]
- Zakeri, A.; Russo, M. Dual Role of Toll-like Receptors in Human and Experimental Asthma Models. Front. Immunol. 2018, 9, 1027. [Google Scholar] [CrossRef]
- Tang, M.; Henderson, R.J.; Holbrook, J.T.; Que, L.G.; Mathews, A.M.; Wise, R.A.; Dixon, A.E.; Peters, S.P.; Rogers, L.; Smith, L.J.; et al. Does Obesity Increase Respiratory Tract Infections in Patients with Asthma? J. Allergy Clin. Immunol. Pract. 2019, 7, 954–961.e6. [Google Scholar] [CrossRef]
- Jartti, T.; Bønnelykke, K.; Elenius, V.; Feleszko, W. Role of viruses in asthma. Semin. Immunopathol. 2020, 42, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, L.; Weber, S.; Elgizouli, M.; Stoehlker, A.-S.; Geist, I.; Peter, H.-H.; Vach, W.; Nieters, A. Obesity and risk of respiratory tract infections: Results of an infection-diary based cohort study. BMC Public Health 2018, 18, 271. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Piernas, C.; Astbury, N.M.; Hippisley-Cox, J.; O’Rahilly, S.; Aveyard, P.; Jebb, S.A. Associa-tions between body-mass index and COVID-19 severity in 6·9 million people in England: A prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021, 9, 350–359. [Google Scholar] [CrossRef]
- Yates, T.; Razieh, C.; Zaccardi, F.; Rowlands, A.V.; Seidu, S.; Davies, M.J.; Khunti, K. Obesity, walking pace and risk of severe COVID-19 and mortality: Analysis of UK Biobank. Int. J. Obes. 2021, 45, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Wendell, S.G.; Fan, H.; Zhang, C. G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol. Rev. 2020, 72, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.A.; Penn, R.B. Targeting G protein-coupled receptor signaling in asthma. Cell. Signal. 2006, 18, 2105–2120. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Tobar, J.A.; Shoji, K.F.; De Calisto, J.; Kalergis, A.; Bono, M.R.; Rosemblatt, M.; Sáez, J.C. Gap junctions at the dendritic cell-T cell interface are key elements for antigen-dependent T cell activation. J. Immunol. 2009, 183, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.; Verlaan, I.; Hengeveld, T.; Janssen, H.; Calafat, J.; Falk, M.M.; Moolenaar, W.H. Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 2001, 11, 1364–1368. [Google Scholar] [CrossRef]
- Bermudez-Fajardo, A.; Ylihärsilä, M.; Evans, W.H.; Newby, A.C.; Oviedo-Orta, E. CD4+ T lympho-cyte subsets express connexin 43 and establish gap junction channel communication with macrophages in vitro. J. Leukoc. Biol. 2007, 82, 608–612. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.-S.; Kim, H.; Jang, J.H.; Choung, Y.-H. Gap Junction-Mediated Intercellular Commu-nication of cAMP Prevents CDDP-Induced Ototoxicity via cAMP/PKA/CREB Pathway. Int. J. Mol. Sci. 2021, 22, 6327. [Google Scholar] [CrossRef]
- Ring, S.; Karakhanova, S.; Johnson, T.; Enk, A.H.; Mahnke, K. Gap junctions between regulatory T cells and dendritic cells prevent sensitization of CD8(+) T cells. J. Allergy Clin. Immunol. 2010, 125, 237–246. [Google Scholar] [CrossRef]
- Bopp, T.; Becker, C.; Klein, M.; Klein-Heßling, S.; Palmetshofer, A.; Serfling, E.; Heib, V.; Becker, M.; Kubach, J.; Schmitt, S.; et al. Cyclic adeno-sine monophosphate is a key component of regulatory T cell–mediated suppression. J. Exp. Med. 2007, 204, 1303–1310. [Google Scholar] [CrossRef]
- Ram, A.; Singh, S.K.; Singh, V.P.; Kumar, S.; Ghosh, B. Inhaled carbenoxolone prevents allergic airway inflammation and airway hyperreactivity in a mouse model of asthma. Int. Arch. Allergy Immunol. 2009, 149, 38–46. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’Ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).