The Interplay between Non-Esterified Fatty Acids and Plasma Zinc and Its Influence on Thrombotic Risk in Obesity and Type 2 Diabetes
Abstract
1. Introduction
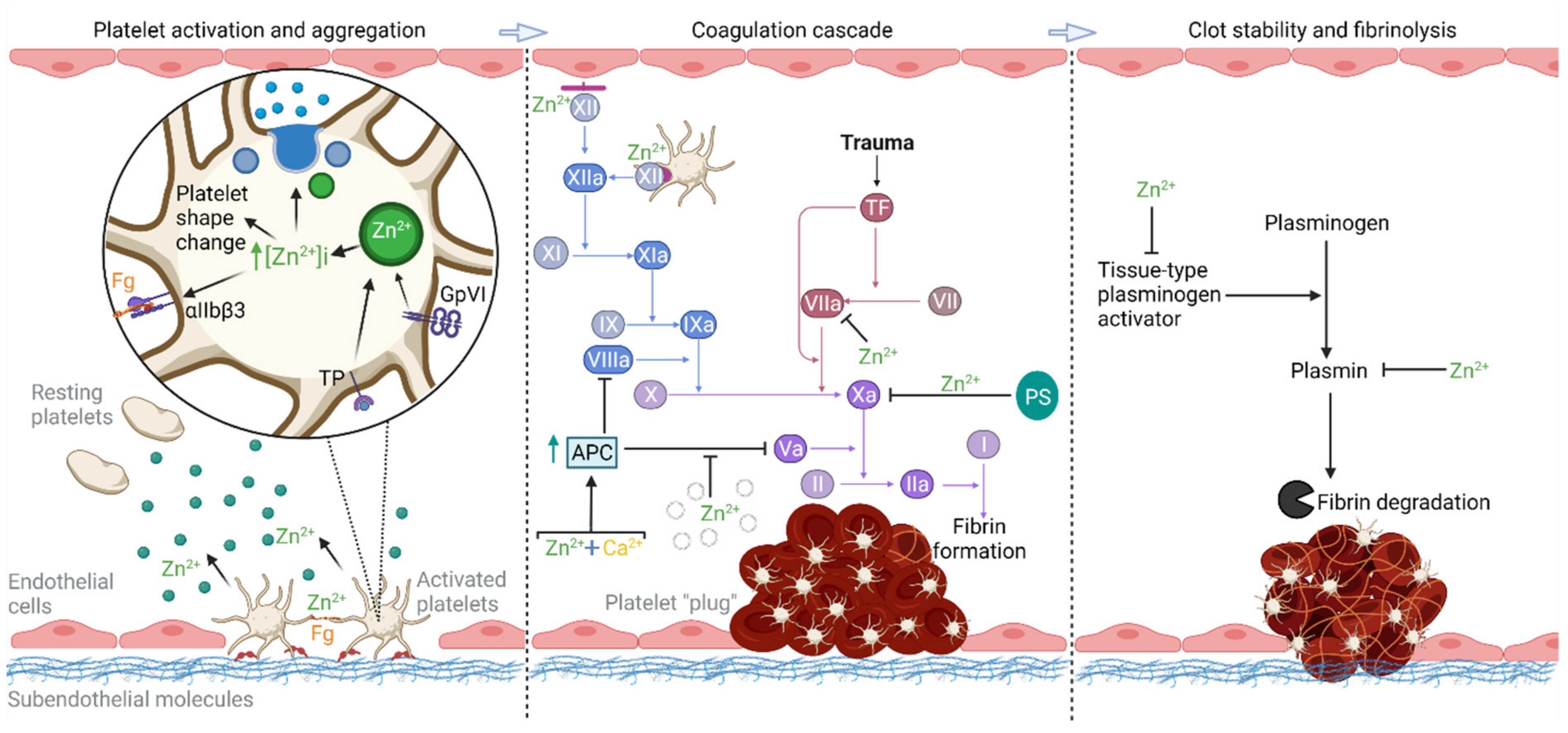
2. Zn2+ as a Regulator of Hemostasis
3. Control of Plasma Zn2+ Availability and the Impact of NEFAs
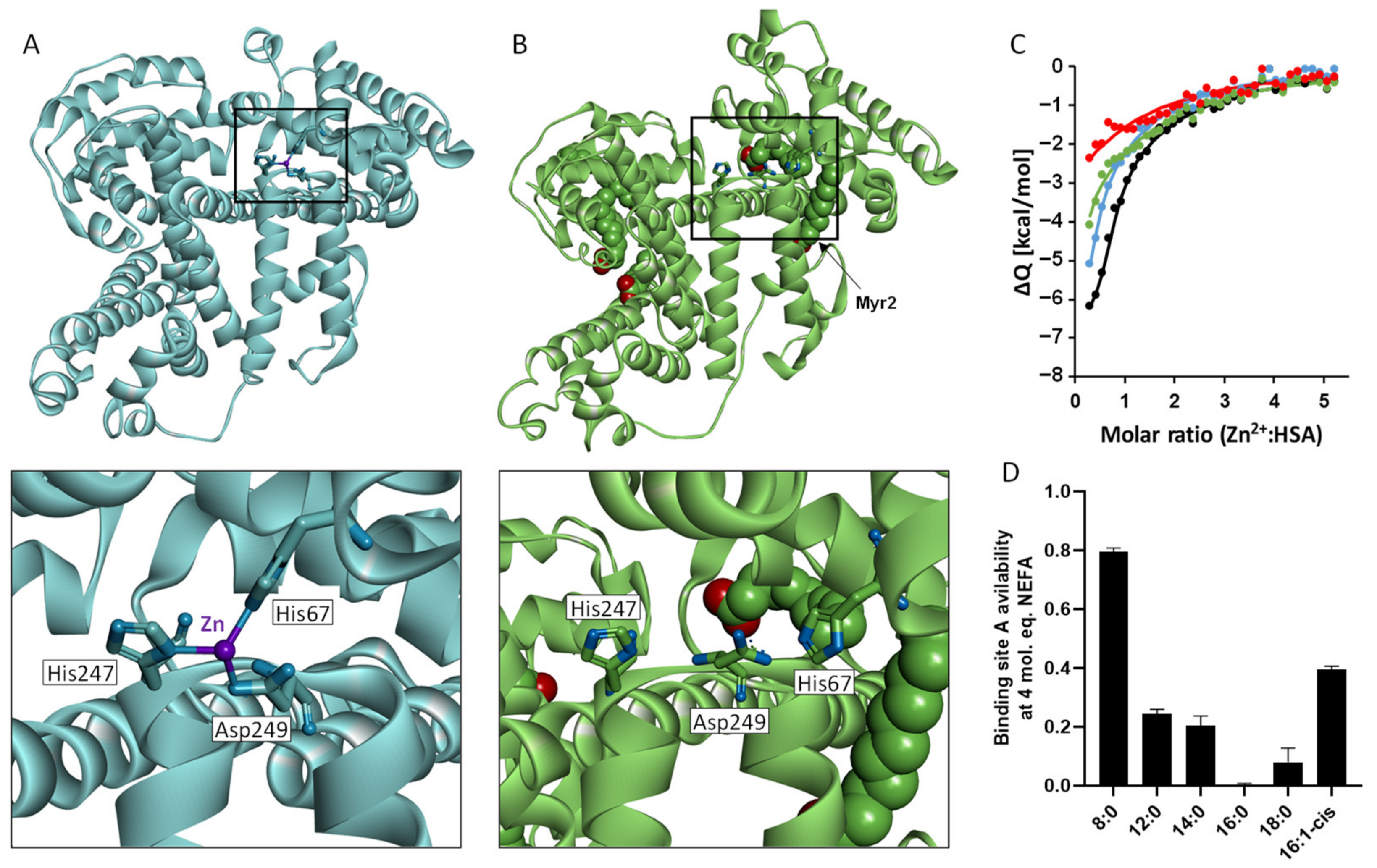
4. Impact of NEFAs on Zn2+–Protein Interactions
5. Evidence for the Zn2+-NEFA Switch as a Thrombotic Mechanism
6. NEFAs a Target for Therapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blokhin, I.O.; Lentz, S.R. Mechanisms of thrombosis in obesity. Curr. Opin. Hematol. 2013, 20, 437–444. [Google Scholar] [CrossRef]
- Erbey, J.R.; Kuller, L.H.; Becker, D.J.; Orchard, T.J. The association between a family history of type 2 diabetes and coronary artery disease in a type 1 diabetes population. Diabetes Care 1998, 21, 610–614. [Google Scholar] [CrossRef]
- Pearson, S.M.; Whittam, B.; Kulavarasalingam, K.; Mitchell-Gears, A.; James, C.; Ajjan, R.A. Reduction in cardiovascular mortality following severe hypoglycemia in individuals with type 2 diabetes: The role of a pragmatic and structured intervention: Structured intervention for community hypoglycemia. Cardiovasc. Diabetol. 2021, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Roglic, G.; Colhoun, H.M.; Stevens, L.K.; Lemkes, H.H.; Manes, C.; Fuller, J.H. Parental history of hypertension and parental history of diabetes and microvascular complications in insulin-dependent diabetes mellitus: The EURODIAB IDDM Complications Study. Diabet. Med. 1998, 15, 418–426. [Google Scholar] [CrossRef]
- Sobczak, A.I.S.; Stewart, A.J. Coagulatory Defects in Type-1 and Type-2 Diabetes. Int. J. Mol. Sci. 2019, 20, 6345. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.H.; Ajjan, R.A. Coagulation and fibrinolysis in diabetes. Diabetes Vasc. Dis. Res. 2010, 7, 260–273. [Google Scholar] [CrossRef]
- Sumaya, W.; Parker, W.A.E.; Judge, H.M.; Hall, I.R.; Orme, R.C.; Adam, Z.; Richardson, J.D.; Rothman, A.M.K.; Morgan, K.P.; Gunn, J.P.; et al. Prolonged enoxaparin therapy compared with standard-of-care antithrombotic therapy in opiate-treated patients undergoing primary percutaneous coronary intervention. Platelets 2021, 32, 555–559. [Google Scholar] [CrossRef]
- Sumaya, W.; Wallentin, L.; James, S.K.; Siegbahn, A.; Gabrysch, K.; Bertilsson, M.; Himmelmann, A.; Ajjan, R.A.; Storey, R.F. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: A PLATO substudy. Eur. Heart J. 2018, 39, 1078–1085. [Google Scholar] [CrossRef]
- Howard, B.V.; Ruotolo, G.; Robbins, D.C. Obesity and dyslipidemia. Endocrinol. Metab. Clin. N. Am. 2003, 32, 855–867. [Google Scholar] [CrossRef]
- Schofield, J.D.; Liu, Y.; Rao-Balakrishna, P.; Malik, R.A.; Soran, H. Diabetes Dyslipidemia. Diabetes Ther. 2016, 7, 203–219. [Google Scholar] [CrossRef]
- Bjorntorp, P.; Bergman, H.; Varnauskas, E. Plasma free fatty acid turnover rate in obesity. Acta Med. Scand. 1969, 185, 351–356. [Google Scholar] [CrossRef]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Wessman, Y.; Almgren, P.; Groop, L. High levels of nonesterified fatty acids are associated with increased familial risk of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1588–1594. [Google Scholar] [CrossRef]
- Frohnert, B.I.; Jacobs, D.R.; Steinberger, J.; Moran, A.; Steffen, L.M.; Sinaiko, A.R. Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes 2013, 62, 3163–3169. [Google Scholar] [CrossRef]
- Sobczak, A.I.S.; Blindauer, C.A.; Stewart, A.J. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef]
- Morigny, P.; Houssier, M.; Mouisel, E.; Langin, D. Adipocyte lipolysis and insulin resistance. Biochimie 2016, 125, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, Y.; Cederberg, H.; Vangipurapu, J.; Kangas, A.J.; Soininen, P.; Kuusisto, J.; Uusitupa, M.; Ala-Korpela, M.; Laakso, M. Glycerol and fatty acids in serum predict the development of hyperglycemia and type 2 diabetes in Finnish men. Diabetes Care 2013, 36, 3732–3738. [Google Scholar] [CrossRef]
- Salgin, B.; Ong, K.K.; Thankamony, A.; Emmett, P.; Wareham, N.J.; Dunger, D.B. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; Kim, J.Y.; Singh, M.; Shin, Y.K.; Kim, J.; Kumbrink, J.; Wu, Y.; Lee, M.J.; Kirsch, K.H.; Fried, S.K.; et al. Insulin inhibits lipolysis in adipocytes via the evolutionarily conserved mTORC1-Egr1-ATGL-mediated pathway. Mol. Cell. Biol. 2013, 33, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Kim, J.-S.; Kim, J.H.; Oh, K.; Koh, S.-B.; Seo, W.-K. High free fatty acid level is associated with recurrent stroke in cardioembolic stroke patients. Neurology 2014, 82, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.X.; Zhang, G.P.; Wang, X.B.; Yu, H.; Wu, J.L.; Liu, K.Z.; Wang, L.; Long, X. Elevated Serum and Cerebrospinal Fluid Free Fatty Acid Levels Are Associated with Unfavorable Functional Outcome in Subjects with Acute Ischemic Stroke. Mol. Neurobiol. 2017, 54, 1677–1683. [Google Scholar] [CrossRef]
- Xiong, Z.; Xu, H.; Huang, X.; Arnlov, J.; Qureshi, A.R.; Cederholm, T.; Sjogren, P.; Lindholm, B.; Riserus, U.; Carrero, J.J. Nonesterified fatty acids and cardiovascular mortality in elderly men with CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, S.; Ghanim, H.; Dandona, P. Nonesterified fatty acids, albumin, and platelet aggregation. Diabetes 2015, 64, 703–705. [Google Scholar] [CrossRef]
- Tanka-Salamon, A.; Komorowicz, E.; Szabo, L.; Tenekedjiev, K.; Kolev, K. Free Fatty Acids Modulate Thrombin Mediated Fibrin Generation Resulting in Less Stable Clots. PLoS ONE 2016, 11, e0167806. [Google Scholar] [CrossRef]
- Vu, T.T.; Fredenburgh, J.C.; Weitz, J.I. Zinc: An important cofactor in haemostasis and thrombosis. Thromb. Haemost. 2013, 109, 421–430. [Google Scholar] [CrossRef]
- Bhattacharya, A.A.; Grune, T.; Curry, S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J. Mol. Biol. 2000, 303, 721–732. [Google Scholar] [CrossRef]
- Curry, S.; Mandelkow, H.; Brick, P.; Franks, N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Struct. Biol. 1998, 5, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Petitpas, I.; Grüne, T.; Bhattacharya, A.A.; Curry, S. Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J. Mol. Biol. 2001, 314, 955–960. [Google Scholar] [CrossRef]
- Coverdale, J.P.; Barnett, J.P.; Adamu, A.H.; Griffiths, E.J.; Stewart, A.J.; Blindauer, C.A. A metalloproteomic analysis of interactions between plasma proteins and zinc: Elevated fatty acid levels affect zinc distribution. Metallomics 2019, 11, 1805–1819. [Google Scholar] [CrossRef]
- Coverdale, J.P.; Khazaipoul, S.; Arya, S.; Stewart, A.J.; Blindauer, C.A. Crosstalk between zinc and free fatty acids in plasma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 532–542. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Falati, S.; Gross, P.; Merrill-Skoloff, G.; Furie, B.C.; Furie, B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 2002, 8, 1175–1181. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, R.; Mou, X.; Blankenfeld, A.; Marx, G. Platelet multielemental composition, lability, and subcellular localization. Am. J. Hematol. 1993, 42, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.; Korner, G.; Mou, X.; Gorodetsky, R. Packaging zinc, fibrinogen, and factor XIII in platelet α-granules. J. Cell. Physiol. 1993, 156, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.R.; Haudenschild, C. Adhesion of platelets to subendothelium. Ann. N. Y. Acad. Sci. 1972, 201, 22–36. [Google Scholar] [CrossRef]
- Beumer, S.; Heijnen, H.F.; MJ, I.J.; Orlando, E.; de Groot, P.G.; Sixma, J.J. Platelet adhesion to fibronectin in flow: The importance of von Willebrand factor and glycoprotein Ib. Blood 1995, 86, 3452–3460. [Google Scholar] [CrossRef]
- Hindriks, G.; Ijsseldijk, M.; Sonnenberg, A.; Sixma, J.; De Groot, P.G. Platelet adhesion to laminin: Role of Ca2+ and Mg2+ ions, shear rate, and platelet membrane glycoproteins. Blood 1992, 79, 928–935. [Google Scholar] [CrossRef]
- Tomokiyo, K.; Kamikubo, Y.; Hanada, T.; Araki, T.; Nakatomi, Y.; Ogata, Y.; Jung, S.M.; Nakagaki, T.; Moroi, M. Von Willebrand factor accelerates platelet adhesion and thrombus formation on a collagen surface in platelet-reduced blood under flow conditions. Blood 2005, 105, 1078–1084. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H. Platelet integrin αIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 1–22. [Google Scholar] [CrossRef]
- White, J.G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood 1968, 31, 604–622. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Lopes Pires, M.E.; Taylor, K.A.; Pugh, N. Agonist-Evoked Increases in Intra-Platelet Zinc Couple to Functional Responses. Thromb. Haemost. 2019, 119, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Sharda, A.; Flaumenhaft, R. The life cycle of platelet granules. F1000Res. 2018, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Sharir, H.; Zinger, A.; Nevo, A.; Sekler, I.; Hershfinkel, M. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Biol. Chem. 2010, 285, 26097–26106. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.R.; White, N.A.; Taylor, K.A.; Howes, J.-M.; Malcor, J.-D.M.; Bihan, D.; Sage, S.O.; Farndale, R.W.; Pugh, N. Zinc is a transmembrane agonist that induces platelet activation in a tyrosine phosphorylation-dependent manner. Metallomics 2016, 8, 91–100. [Google Scholar] [CrossRef]
- Podolnikova, N.P.; Yakovlev, S.; Yakubenko, V.P.; Wang, X.; Gorkun, O.V.; Ugarova, T.P. The interaction of integrin αIIbβ3 with fibrin occurs through multiple binding sites in the αIIb β-propeller domain. J. Biol. Chem. 2014, 289, 2371–2383. [Google Scholar] [CrossRef]
- Heyns, A.d.P.; Eldor, A.; Yarom, R.; Marx, G. Zinc-induced platelet aggregation is mediated by the fibrinogen receptor and is not accompanied by release or by thromboxane synthesis. Blood 1985, 66, 213–219. [Google Scholar] [CrossRef]
- Kowalska, M.A.; Juliano, D.; Trybulec, M.; Lu, W.; Niewiarowski, S. Zinc ions potentiate adenosine diphosphate-induced platelet aggregation by activation of protein kinase C. J. Lab. Clin. Med. 1994, 123, 102–109. [Google Scholar]
- Trybulec, M.; Kowalska, M.A.; McLane, M.A.; Silver, L.; Lu, W.; Niewiarowski, S. Exposure of platelet fibrinogen receptors by zinc ions: Role of protein kinase C. Proc. Soc. Exp. Biol. Med. 1993, 203, 108–116. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Braun, A. Zinc homeostasis in platelet-related diseases. Int. J. Mol. Sci. 2019, 20, 5258. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Serrata, M.; Tomczak, L.; Higgins, S.; Ryu, J.; Laprise, D.; Enjyoji, K.; Bekendam, R.; Kaushik, V.; Flaumenhaft, R. Cationic zinc is required for factor XII recruitment and activation by stimulated platelets and for thrombus formation in vivo. J. Thromb. Haemost. 2020, 18, 2318–2328. [Google Scholar] [CrossRef]
- Schousboe, I. Contact activation in human plasma is triggered by zinc ion modulation of factor XII (Hageman factor). Blood Coagul. Fibrinolysis 1993, 4, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, I. Endothelial cells express a matrix protein which binds activated factor XII in a zinc-independent manner. Thromb. Haemost. 2006, 95, 312–319. [Google Scholar] [CrossRef]
- Mahdi, F.; Madar, Z.S.; Figueroa, C.D.; Schmaier, A.H. Factor XII interacts with the multiprotein assembly of urokinase plasminogen activator receptor, gC1qR, and cytokeratin 1 on endothelial cell membranes. Blood 2002, 99, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiu, Q.; Mahdi, F.; Shariat-Madar, Z.; Røjkjær, R.; Schmaier, A.H. Assembly and activation of HK-PK complex on endothelial cells results in bradykinin liberation and NO formation. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1821–H1829. [Google Scholar] [CrossRef]
- Baglia, F.A.; Gailani, D.; Lopez, J.A.; Walsh, P.N. Identification of a binding site for glycoprotein Ibalpha in the Apple 3 domain of factor XI. J. Biol. Chem. 2004, 279, 45470–45476. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Marx, G. Zinc binding to fibrinogen and fibrin. Arch. Biochem. Biophys. 1988, 266, 285–288. [Google Scholar] [CrossRef]
- Gailani, D.; Renne, T. The intrinsic pathway of coagulation: A target for treating thromboembolic disease? J. Thromb. Haemost. 2007, 5, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.J.; Stafford, A.R.; Leslie, B.A.; Kim, P.Y.; Vaezzadeh, N.; Ni, R.; Fredenburgh, J.C.; Weitz, J.I. Zinc delays clot lysis by attenuating plasminogen activation and plasmin-mediated fibrin degradation. Thromb. Haemost. 2015, 113, 1278–1288. [Google Scholar] [CrossRef]
- Henderson, S.J.; Xia, J.; Wu, H.; Stafford, A.R.; Leslie, B.A.; Fredenburgh, J.C.; Weitz, D.A.; Weitz, J.I. Zinc promotes clot stability by accelerating clot formation and modifying fibrin structure. Thromb. Haemost. 2016, 115, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.; Stafford, A.R.; Leslie, B.A.; Fredenburgh, J.C.; Weitz, J.I. Zinc Enhances the Protection of Fibrin-Bound Thrombin from Antithro Inhibition. Blood 2007, 110, 2691. [Google Scholar] [CrossRef]
- Bradford, H.N.; Pixley, R.A.; Colman, R.W. Human factor XII binding to the glycoprotein Ib-IX-V complex inhibits thrombin-induced platelet aggregation. J. Biol. Chem. 2000, 275, 22756–22763. [Google Scholar] [CrossRef] [PubMed]
- Lundblad, R.L.; White, G.C. The interaction of thrombin with blood platelets. Platelets 2005, 16, 373–385. [Google Scholar] [CrossRef]
- Bal, W.; Sokolowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Yaseen, Z.; Aswal, V.; Zhou, X.; Haider, S. Morphological changes in human serum albumin in the presence of cationic amphiphilic drugs. New J. Chem. 2018, 42, 2270–2277. [Google Scholar] [CrossRef]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. Albumin as a zinc carrier: Properties of its high-affinity zinc-binding site. Biochem. Soc. Trans. 2008, 36, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Blindauer, C.A.; Harvey, I.; Bunyan, K.E.; Stewart, A.J.; Sleep, D.; Harrison, D.J.; Berezenko, S.; Sadler, P.J. Structure, properties, and engineering of the major zinc binding site on human albumin. J. Biol. Chem. 2009, 284, 23116–23124. [Google Scholar] [CrossRef]
- Handing, K.B.; Shabalin, I.G.; Kassaar, O.; Khazaipoul, S.; Blindauer, C.A.; Stewart, A.J.; Chruszcz, M.; Minor, W. Circulatory zinc transport is controlled by distinct interdomain sites on mammalian albumins. Chem. Sci. 2016, 7, 6635–6648. [Google Scholar] [CrossRef]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Paar, M.; Fengler, V.H.; Rosenberg, D.J.; Krebs, A.; Stauber, R.E.; Oettl, K.; Hammel, M. Albumin in patients with liver disease shows an altered conformation. Commun. Biol. 2021, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Qais, F.A.; Alam, M.M.; Naseem, I. Effect of glycation on human serum albumin-zinc interaction: A biophysical study. J. Biol. Inorg. Chem. 2018, 23, 447–458. [Google Scholar] [CrossRef]
- Bhat, S.; Jagadeeshaprasad, M.G.; Venkatasubramani, V.; Kulkarni, M.J. Abundance matters: Role of albumin in diabetes, a proteomics perspective. Expert Rev. Proteom. 2017, 14, 677–689. [Google Scholar] [CrossRef]
- Coussons, P.J.; Jacoby, J.; McKay, A.; Kelly, S.M.; Price, N.C.; Hunt, J.V. Glucose modification of human serum albumin: A structural study. Free Radic. Biol. Med. 1997, 22, 1217–1227. [Google Scholar] [CrossRef]
- Dolhofer, R.; Wieland, O.H. Increased glycosylation of serum albumin in diabetes mellitus. Diabetes 1980, 29, 417–422. [Google Scholar] [CrossRef]
- Rondeau, P.; Bourdon, E. The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Sattarahmady, N.; Moosavi-Movahedi, A.A.; Habibi-Rezaei, M.; Ahmadian, S.; Saboury, A.A.; Heli, H.; Sheibani, N. Detergency effects of nanofibrillar amyloid formation on glycation of human serum albumin. Carbohydr. Res. 2008, 343, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Kohzuma, T.; Ohnishi, A. Changes in the albumin glycation site, plasma pentosidine and esRAGE concentrations before and after intensive diabetic treatment in patients with abnormally high glycated albumin levels. Ann. Clin. Biochem. 2018, 55, 84–91. [Google Scholar] [CrossRef]
- Jacobs, M.J.; Pinger, C.W.; Castiaux, A.D.; Maloney, K.J.; Spence, D.M. A novel 3D-printed centrifugal ultrafiltration method reveals in vivo glycation of human serum albumin decreases its binding affinity for zinc. Metallomics 2020, 12, 1036–1043. [Google Scholar] [CrossRef]
- Chuang, V.T.G.; Otagiri, M. How do fatty acids cause allosteric binding of drugs to human serum albumin? Pharm. Res. 2002, 19, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Crossey, M.J. Elevated free fatty acid concentrations in lipemic sera reduce protein binding of valproic acid significantly more than phenytoin. Am. J. Med Sci. 1997, 313, 75–79. [Google Scholar]
- Takamura, N.; Shinozawa, S.; Maruyama, T.; Suenaga, A.; Otagiri, M. Effects of fatty acids on serum binding between furosemide and valproic acid. Biol. Pharm. Bull. 1998, 21, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Vorum, H.; Honore, B. Influence of fatty acids on the binding of warfarin and phenprocoumon to human serum albumin with relation to anticoagulant therapy. J. Pharm. Pharmacol. 1996, 48, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.P.; Blindauer, C.A.; Kassaar, O.; Khazaipoul, S.; Martin, E.M.; Sadler, P.J.; Stewart, A.J. Allosteric modulation of zinc speciation by fatty acids. Biochim. Biophys. Acta 2013, 1830, 5456–5464. [Google Scholar] [CrossRef]
- Sobczak, A.I.S.; Katundu, K.G.H.; Phoenix, F.A.; Khazaipoul, S.; Yu, R.; Lampiao, F.; Stefanowicz, F.; Blindauer, C.A.; Pitt, S.J.; Smith, T.K.; et al. Albumin-mediated alteration of plasma zinc speciation by fatty acids modulates blood clotting in type-2 diabetes. Chem. Sci. 2021, 12, 4079–4093. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. A guide to human zinc absorption: General overview and recent advances of in vitro intestinal models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef]
- Jones, A.L.; Hulett, M.D.; Parish, C.R. Histidine-rich glycoprotein: A novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems. Immunol. Cell Biol. 2005, 83, 106–118. [Google Scholar] [CrossRef]
- Leung, L.; Harpel, P.C.; Nachman, R.L.; Rabellino, E.M. Histidine-rich glycoprotein is present in human platelets and is released following thrombin stimulation. Blood 1983, 62, 1016–1021. [Google Scholar] [CrossRef]
- Castaman, G.; Ruggeri, M.; Burei, F.; Rodeghiero, F. High levels of histidine-rich glycoprotein and thrombotic diathesis. Report of two unrelated families. Thromb. Res. 1993, 69, 297–305. [Google Scholar] [CrossRef]
- Siudut, J.; Natorska, J.; Son, M.; Plens, K.; Undas, A. Increased levels of histidine-rich glycoprotein are associated with the development of post-thrombotic syndrome. Sci. Rep. 2020, 10, 14419. [Google Scholar] [CrossRef] [PubMed]
- Kassaar, O.; Schwarz-Linek, U.; Blindauer, C.A.; Stewart, A.J. Plasma free fatty acid levels influence Zn2+-dependent histidine-rich glycoprotein–heparin interactions via an allosteric switch on serum albumin. J. Thromb. Haemost. 2015, 13, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kluszynski, B.A.; Kim, C.; Faulk, W.P. Zinc as a cofactor for heparin neutralization by histidine-rich glycoprotein. J. Biol. Chem. 1997, 272, 13541–13547. [Google Scholar] [CrossRef] [PubMed]
- Priebatsch, K.M.; Kvansakul, M.; Poon, I.K.; Hulett, M.D. Functional regulation of the plasma protein histidine-rich glycoprotein by Zn2+ in settings of tissue injury. Biomolecules 2017, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Stafford, A.R.; Leslie, B.A.; Kim, P.Y.; Fredenburgh, J.C.; Weitz, J.I. Histidine-rich glycoprotein binds fibrin(ogen) with high affinity and competes with thrombin for binding to the gamma′-chain. J. Biol. Chem. 2011, 286, 30314–30323. [Google Scholar] [CrossRef] [PubMed]
- Fredenburgh, J.C.; Leslie, B.A.; Stafford, A.R.; Lim, T.; Chan, H.H.; Weitz, J.I. Zn2+ mediates high affinity binding of heparin to the alphaC domain of fibrinogen. J. Biol. Chem. 2013, 288, 29394–29402. [Google Scholar] [CrossRef] [PubMed]
- Sussulini, A.; Kratzin, H.; Jahn, O.; Banzato, C.E.; Arruda, M.A.; Becker, J.S. Metallomics studies of human blood serum from treated bipolar disorder patients. Anal. Chem. 2010, 82, 5859–5864. [Google Scholar] [CrossRef]
- Robinson, N.; Waldron, K.; Tottey, S.; Bessant, C. Metalloprotein metal pools: Identification and quantification by coupling native and non-native separations through principal component analysis. Protoc. Exch. 2008. [Google Scholar] [CrossRef]
- Koot, B.G.; Houwen, R.; Pot, D.J.; Nauta, J. Congenital analbuminaemia: Biochemical and clinical implications. A case report and literature review. Eur. J. Pediatr. 2004, 163, 664–670. [Google Scholar] [CrossRef]
- Frizzell, N.; Baynes, J.W. Chelation therapy: Overlooked in the treatment and prevention of diabetes complications? Future Med. Chem. 2013, 5, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Logie, L.; Harthill, J.; Patel, K.; Bacon, S.; Hamilton, D.L.; Macrae, K.; McDougall, G.; Wang, H.H.; Xue, L.; Jiang, H.; et al. Cellular responses to the metal-binding properties of metformin. Diabetes 2012, 61, 1423–1433. [Google Scholar] [CrossRef]
- Nagai, R.; Murray, D.B.; Metz, T.O.; Baynes, J.W. Chelation: A fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 2012, 61, 549–559. [Google Scholar] [CrossRef]
- Wihler, C.; Schafer, S.; Schmid, K.; Deemer, E.K.; Munch, G.; Bleich, M.; Busch, A.E.; Dingermann, T.; Somoza, V.; Baynes, J.W.; et al. Renal accumulation and clearance of advanced glycation end-products in type 2 diabetic nephropathy: Effect of angiotensin-converting enzyme and vasopeptidase inhibition. Diabetologia 2005, 48, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A. Chelation therapy: A new look at an old treatment for heart disease, particularly in diabetics. Circulation 2015, 131, e505–e506. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.D.; Escolar, E.; Lamas, G.A. Chelation therapy after the trial to assess chelation therapy: Results of a unique trial. Curr. Opin. Cardiol. 2014, 29, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Goertz, C.; Boineau, R.; Mark, D.B.; Rozema, T.; Nahin, R.L.; Lindblad, L.; Lewis, E.F.; Drisko, J.; Lee, K.L.; et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: The TACT randomized trial. JAMA 2013, 309, 1241–1250. [Google Scholar] [CrossRef]
- Escolar, E.; Lamas, G.A.; Mark, D.B.; Boineau, R.; Goertz, C.; Rosenberg, Y.; Nahin, R.L.; Ouyang, P.; Rozema, T.; Magaziner, A.; et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ. Cardiovasc. Qual. Outcomes 2014, 7, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Stewart, A.J.; Sleep, D.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. A molecular mechanism for modulating plasma Zn speciation by fatty acids. J. Am. Chem. Soc. 2012, 134, 1454–1457. [Google Scholar] [CrossRef]
- Buttar, H.S.; Li, T.; Ravi, N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp. Clin. Cardiol. 2005, 10, 229–249. [Google Scholar] [PubMed]
- Kim, M.K.; Jang, E.H.; Hong, O.K.; Chun, H.J.; Yoo, S.J.; Baek, K.H.; Kim, W.; Kim, E.K.; Song, K.H.; Kwon, H.S. Changes in serum levels of bone morphogenic protein 4 and inflammatory cytokines after bariatric surgery in severely obese korean patients with type 2 diabetes. Int. J. Endocrinol. 2013, 2013, 681205. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Garcia-Serrano, S.; Garcia-Almeida, J.M.; Garrido-Sanchez, L.; Garcia-Arnes, J.; Tinahones, F.J.; Cardona, I.; Rivas-Marin, J.; Gallego-Perales, J.L.; Garcia-Fuentes, E. Changes in the serum composition of free-fatty acids during an intravenous glucose tolerance test. Obesity 2009, 17, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, W.; Kwon, H.S.; Baek, K.H.; Kim, E.K.; Song, K.H. Effects of bariatric surgery on metabolic and nutritional parameters in severely obese K orean patients with type 2 diabetes: A prospective 2-year follow up. J. Diabetes Investig. 2014, 5, 221–227. [Google Scholar] [CrossRef]
- Fabbrini, E.; Tamboli, R.A.; Magkos, F.; Marks–Shulman, P.A.; Eckhauser, A.W.; Richards, W.O.; Klein, S.; Abumrad, N.N. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 2010, 139, 448–455. [Google Scholar] [CrossRef]
- Patti, A.M.; Giglio, R.V.; Papanas, N.; Rizzo, M.; Rizvi, A.A. Future perspectives of the pharmacological management of diabetic dyslipidemia. Expert Rev. Clin. Pharmacol. 2019, 12, 129–143. [Google Scholar] [CrossRef]
- Barter, P.J.; Rye, K.A. New Era of Lipid-Lowering Drugs. Pharmacol. Rev. 2016, 68, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Simental-Mendia, L.E.; Pedone, C.; Ferretti, G.; Nachtigal, P.; Bo, S.; Derosa, G.; Maffioli, P.; Watts, G.F. Statin therapy and plasma free fatty acids: A systematic review and meta-analysis of controlled clinical trials. Br. J. Clin. Pharmacol. 2016, 81, 807–818. [Google Scholar] [CrossRef]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, I.J.; de Man, F.H.; van der Laarse, A.; Frolich, M.; Gevers Leuven, J.A.; Kamper, A.M.; Blauw, G.J.; Smelt, A.H. Bezafibrate reduces heart rate and blood pressure in patients with hypertriglyceridemia. J. Hypertens. 2001, 19, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Li, Y.; Zhang, N.N.; Xie, Y.H.; Shi, Y.Q. Combination therapy with metformin and fenofibrate for insulin resistance in obesity. J. Int. Med. Res. 2011, 39, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Sane, T.; Knudsen, P.; Vuorinen-Markkola, H.; Yki-Järvinen, H.; Taskinen, M.-R. Decreasing triglyceride by gemfibrozil therapy does not affect the glucoregulatory or antilipolytic effect of insulin in nondiabetic subjects with mild hypertriglyceridemia. Metabolism 1995, 44, 589–596. [Google Scholar] [CrossRef]
- Alberti, K.G.; Jones, I.R.; Laker, M.F.; Swai, A.B.; Taylor, R. Effect of bezafibrate on metabolic profiles in non-insulin-dependent diabetes mellitus. J. Cardiovasc. Pharmacol. 1990, 16 (Suppl. S9), S21–S24; S24–S25. [Google Scholar] [CrossRef]
- Fenderson, R.W., Jr.; Sekowski, I.; Mohan, N.C.; Deutsch, S.; Benjamin, F.; Samuel, P. Effect of clofibrate on plasma glucose and serum immunoreactive insulin in patients with hyperlipoproteinemia. Am. J. Clin. Nutr. 1974, 27, 22–28. [Google Scholar] [CrossRef]
- Avogaro, A.; Piliego, T.; Catapano, A.; Miola, M.; Tiengo, A. The effect of gemfibrozil on lipid profile and glucose metabolism in hypertriglyceridaemic well-controlled non-insulin-dependent diabetic patients. For the Gemfibrozil Study Group. Acta Diabetol. 1999, 36, 27–33. [Google Scholar] [CrossRef]
- Matsuba, I.; Matsuba, R.; Ishibashi, S.; Yamashita, S.; Arai, H.; Yokote, K.; Suganami, H.; Araki, E. Effects of a novel selective peroxisome proliferator-activated receptor-alpha modulator, pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance. J. Diabetes Investig. 2018, 9, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Calvert, G.D.; Blight, L.; Franklin, J.; Oliver, J.; Wise, P.; Gallus, A.S. The effects of clofibrate on plasma glucose, lipoproteins, fibrinogen, and other biochemical and haematological variables in patients with mature onset diabetes mellitus. Eur. J. Clin. Pharmacol. 1980, 17, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Mussoni, L.; Mannucci, L.; Sirtori, C.; Pazzucconi, F.; Bonfardeci, G.; Cimminiello, C.; Notarbartolo, A.; Scafidi, V.; Bon, G.B.; Alessandrini, P. Effects of gemfibrozil on insulin sensitivity and on haemostatic variables in hypertriglyceridemic patients. Atherosclerosis 2000, 148, 397–406. [Google Scholar] [CrossRef]
- Vega, G.L.; Cater, N.B.; Hadizadeh III, D.R.; Meguro, S.; Grundy, S.M. Free fatty acid metabolism during fenofibrate treatment of the metabolic syndrome. Clin. Pharmacol. Ther. 2003, 74, 236–244. [Google Scholar] [CrossRef]
- Jeng, C.Y.; Sheu, W.H.; Fuh, M.M.; Shieh, S.M.; Chen, Y.D.; Reaven, G.M. Gemfibrozil treatment of endogenous hypertriglyceridemia: Effect on insulin-mediated glucose disposal and plasma insulin concentrations. J. Clin. Endocrinol. Metab. 1996, 81, 2550–2553. [Google Scholar]
- Avogaro, A.; Beltramello, P.; Marin, R.; Zambon, S.; Bonanome, A.; Biffanti, S.; Confortin, L.; Manzato, E.; Crepaldi, G.; Tiengo, A. Insulin action and glucose metabolism are improved by gemfibrozil treatment in hypertriglyceridemic patients. Atherosclerosis 1995, 113, 117–124. [Google Scholar] [CrossRef]
- Jones, I.R.; Swai, A.; Taylor, R.; Miller, M.; Laker, M.F.; Alberti, K.G. Lowering of plasma glucose concentrations with bezafibrate in patients with moderately controlled NIDDM. Diabetes Care 1990, 13, 855–863. [Google Scholar] [CrossRef]
- Vuorinen-Markkola, H.; Yki-Järvinen, H.; Taskinen, M.-R. Lowering of triglycerides by gemfibrozil affects neither the glucoregulatory nor antilipolytic effect of insulin in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993, 36, 161–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Capell, W.H.; DeSouza, C.A.; Poirier, P.; Bell, M.L.; Stauffer, B.L.; Weil, K.M.; Hernandez, T.L.; Eckel, R.H. Short-term triglyceride lowering with fenofibrate improves vasodilator function in subjects with hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 307–313. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Fruehwald-Schultes, B.; Oltmanns, K.M.; Toschek, B.; Sopke, S.; Kern, W.; Born, J.; Fehm, H.L.; Peters, A. Short-term treatment with metformin decreases serum leptin concentration without affecting body weight and body fat content in normal-weight healthy men. Metabolism 2002, 51, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Gormsen, L.C.; Søndergaard, E.; Christensen, N.L.; Jakobsen, S.; Nielsen, E.H.; Munk, O.L.; Tolbod, L.P.; Jessen, N.; Nielsen, S. Metformin does not affect postabsorptive hepatic free fatty acid uptake, oxidation or resecretion in humans: A 3-month placebo-controlled clinical trial in patients with type 2 diabetes and healthy controls. Diabetes Obes. Metab. 2018, 20, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta, M.; Forsen, B.; Gullstrom, M.; Haggblom, M.; Eriksson, J.G.; Taskinen, M.R.; Groop, L. Metabolic effects of metformin in patients with impaired glucose tolerance. Diabet. Med. 2001, 18, 578–583. [Google Scholar] [CrossRef]
- Pentikäinen, P.J.; Voutilainen, E.; Aro, A.; Uusitupa, M.; Penttilä, I.; Vapaatalo, H. Cholesterol lowering effect of metformin in combined hyperlipidemia: Placebo controlled double blind trial. Ann. Med. 1990, 22, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Boussageon, R.; Supper, I.; Bejan-Angoulvant, T.; Kellou, N.; Cucherat, M.; Boissel, J.P.; Kassai, B.; Moreau, A.; Gueyffier, F.; Cornu, C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: A meta-analysis of randomised controlled trials. PLoS Med. 2012, 9, e1001204. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef]
- Gurgle, H.E.; White, K.; McAdam-Marx, C. SGLT2 inhibitors or GLP-1 receptor agonists as second-line therapy in type 2 diabetes: Patient selection and perspectives. Vasc. Health Risk Manag. 2016, 12, 239. [Google Scholar]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Strong, J. The Pleiotropic Effects of Sodium–Glucose Cotransporter-2 Inhibitors: Beyond the Glycemic Benefit. Diabetes Ther. 2019, 10, 1771–1792. [Google Scholar] [CrossRef]
- Bramante, C.T.; Raatz, S.; Bomberg, E.M.; Oberle, M.M.; Ryder, J.R. Cardiovascular risks and benefits of medications used for weight loss. Front. Endocrinol. 2020, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wu, S.; Guo, S.; Yu, K.; Yang, Z.; Li, L.; Zhang, Y.; Quan, X.; Ji, L.; Zhan, S. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Res. Clin. Pract. 2015, 110, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Plutzky, J.; Garber, A.; Falahati, A.; Toft, A.D.; Poulter, N.R. Reductions in lipids and CV risk markers in patients with type 2 diabetes treated with liraglutide: A meta-analysis. Can. J. Diabetes 2009, 33, 209–210. [Google Scholar] [CrossRef]
- Roder, M.E. Major adverse cardiovascular event reduction with GLP-1 and SGLT2 agents: Evidence and clinical potential. Ther. Adv. Chronic Dis. 2018, 9, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; Zinman, B.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar]
- Osataphan, S.; Macchi, C.; Singhal, G.; Chimene-Weiss, J.; Sales, V.; Kozuka, C.; Dreyfuss, J.M.; Pan, H.; Tangcharoenpaisan, Y.; Morningstar, J.; et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight 2019, 4, e123130. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hierons, S.J.; Marsh, J.S.; Wu, D.; Blindauer, C.A.; Stewart, A.J. The Interplay between Non-Esterified Fatty Acids and Plasma Zinc and Its Influence on Thrombotic Risk in Obesity and Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 10140. https://doi.org/10.3390/ijms221810140
Hierons SJ, Marsh JS, Wu D, Blindauer CA, Stewart AJ. The Interplay between Non-Esterified Fatty Acids and Plasma Zinc and Its Influence on Thrombotic Risk in Obesity and Type 2 Diabetes. International Journal of Molecular Sciences. 2021; 22(18):10140. https://doi.org/10.3390/ijms221810140
Chicago/Turabian StyleHierons, Stephen J., Jordan S. Marsh, Dongmei Wu, Claudia A. Blindauer, and Alan J. Stewart. 2021. "The Interplay between Non-Esterified Fatty Acids and Plasma Zinc and Its Influence on Thrombotic Risk in Obesity and Type 2 Diabetes" International Journal of Molecular Sciences 22, no. 18: 10140. https://doi.org/10.3390/ijms221810140
APA StyleHierons, S. J., Marsh, J. S., Wu, D., Blindauer, C. A., & Stewart, A. J. (2021). The Interplay between Non-Esterified Fatty Acids and Plasma Zinc and Its Influence on Thrombotic Risk in Obesity and Type 2 Diabetes. International Journal of Molecular Sciences, 22(18), 10140. https://doi.org/10.3390/ijms221810140






