Efficient Neutrophil Activation Requires Two Simultaneous Activating Stimuli
Abstract
:1. Introduction
2. Results
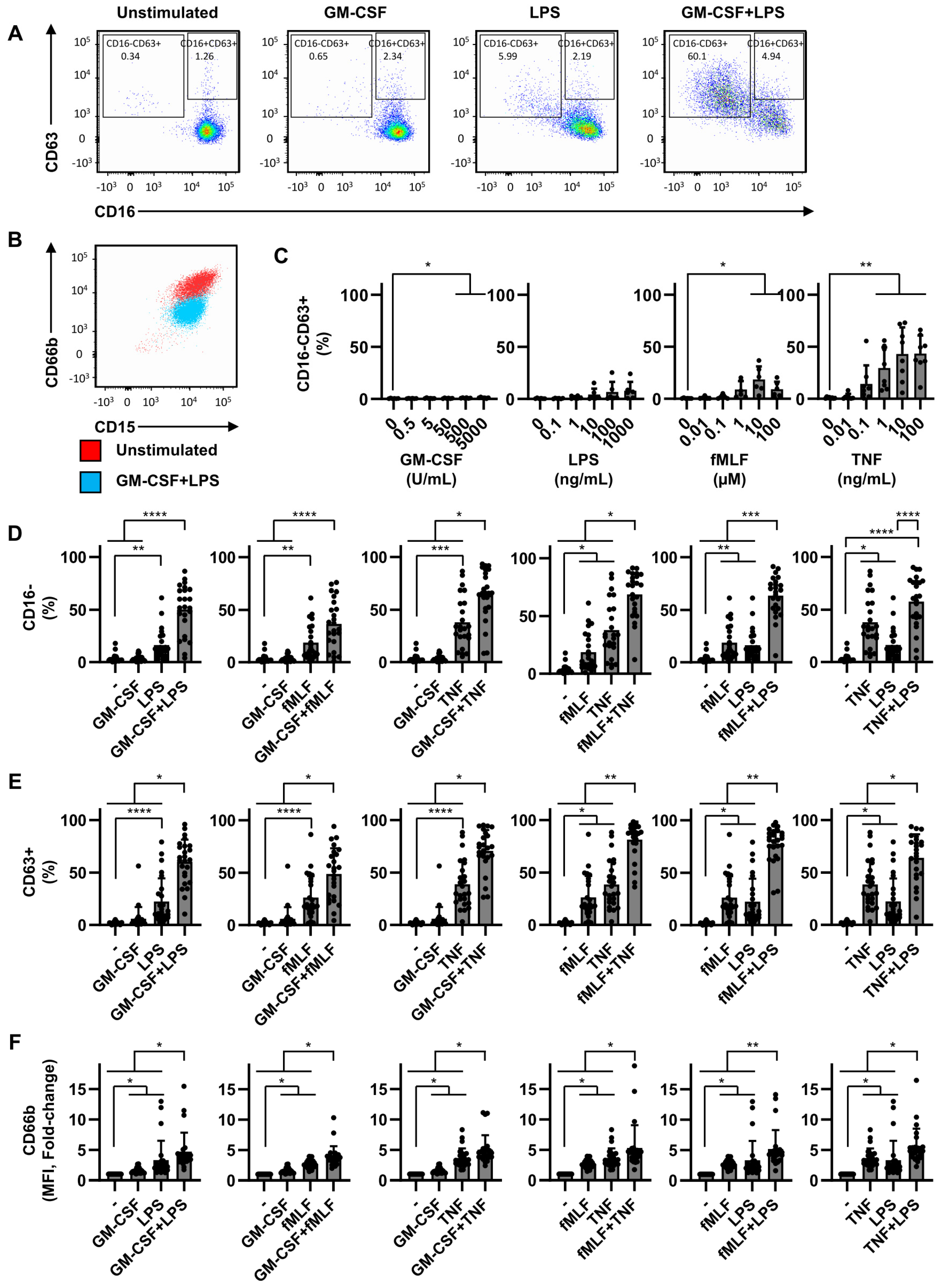
2.1. Dual Stimulation of Neutrophils Is Necessary for Efficient Degranulation
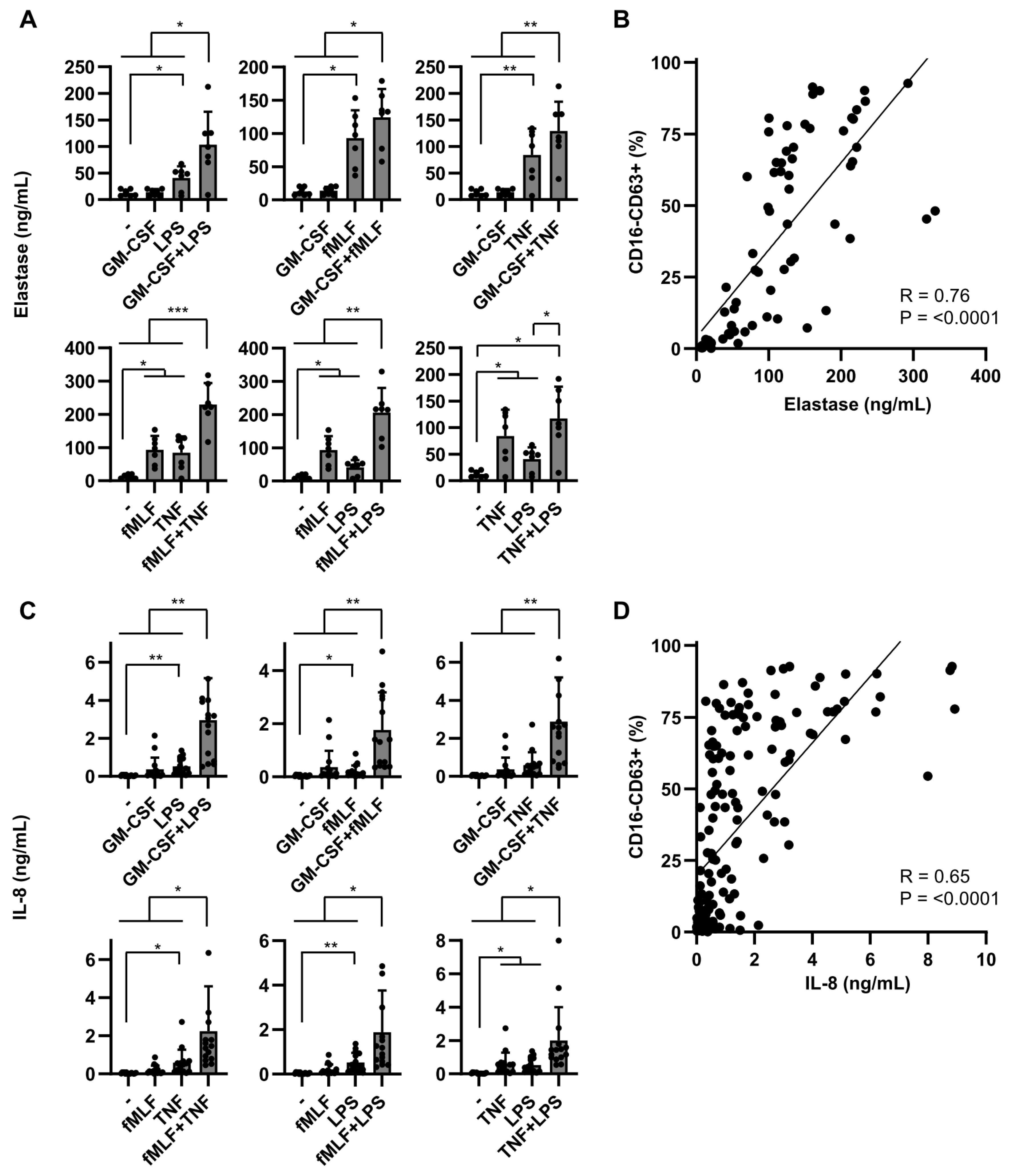
2.2. Dual Stimulation of Neutrophils Is Required for Optimal Mediator Release
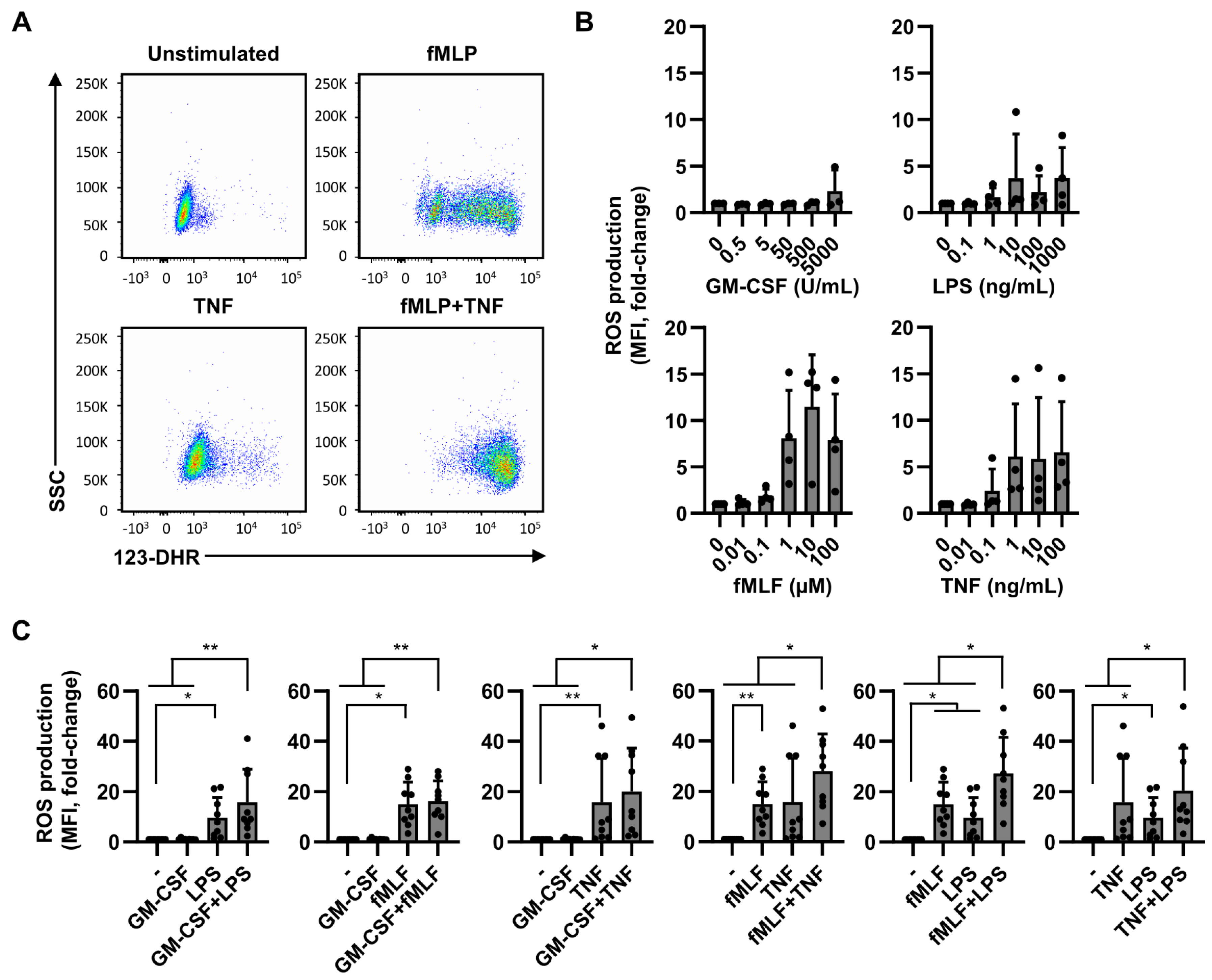
2.3. ROS Production Is Less Dependent on Dual Stimulation
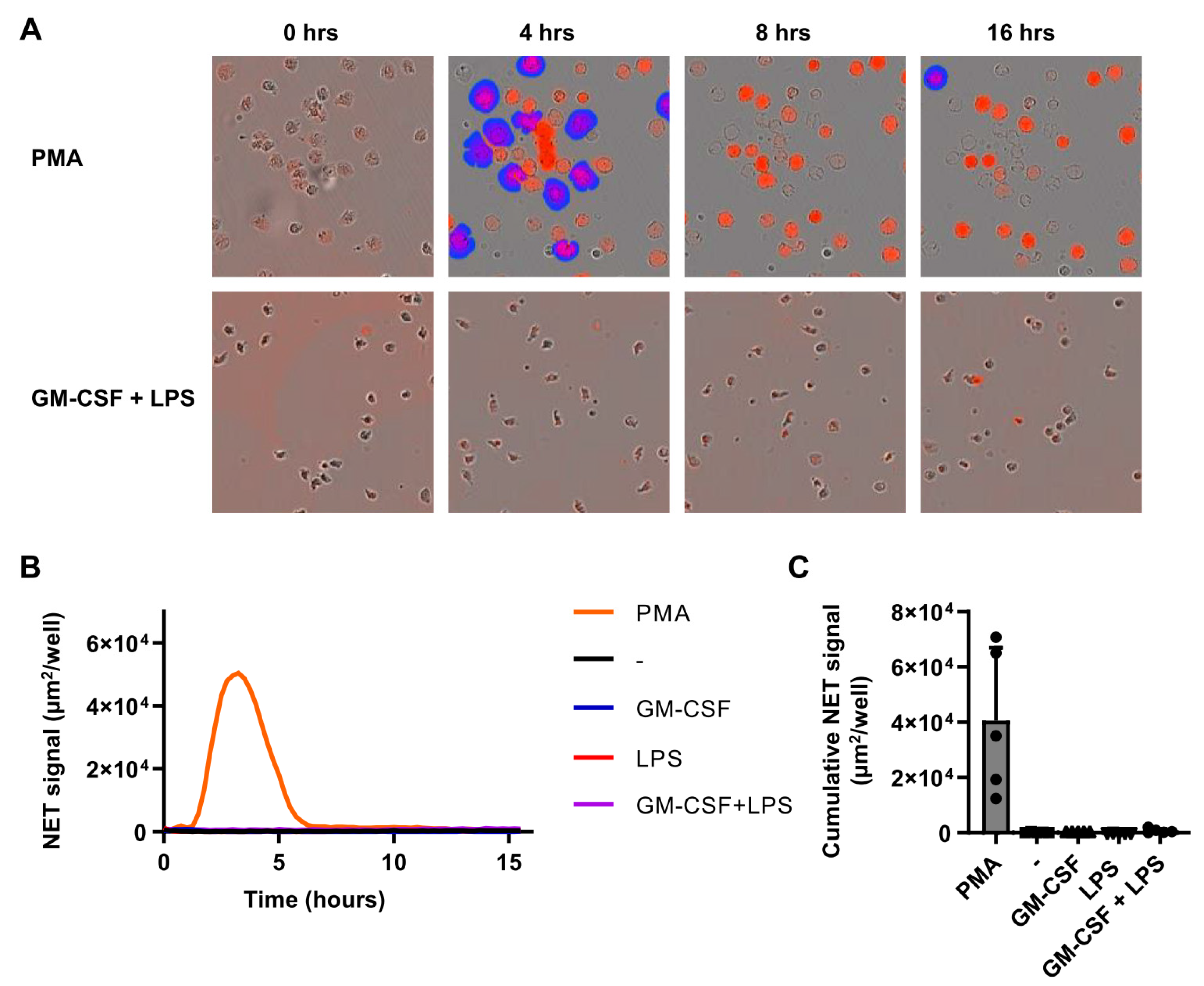
2.4. Individual Stimuli or Combinations Do Not Induce NETosis
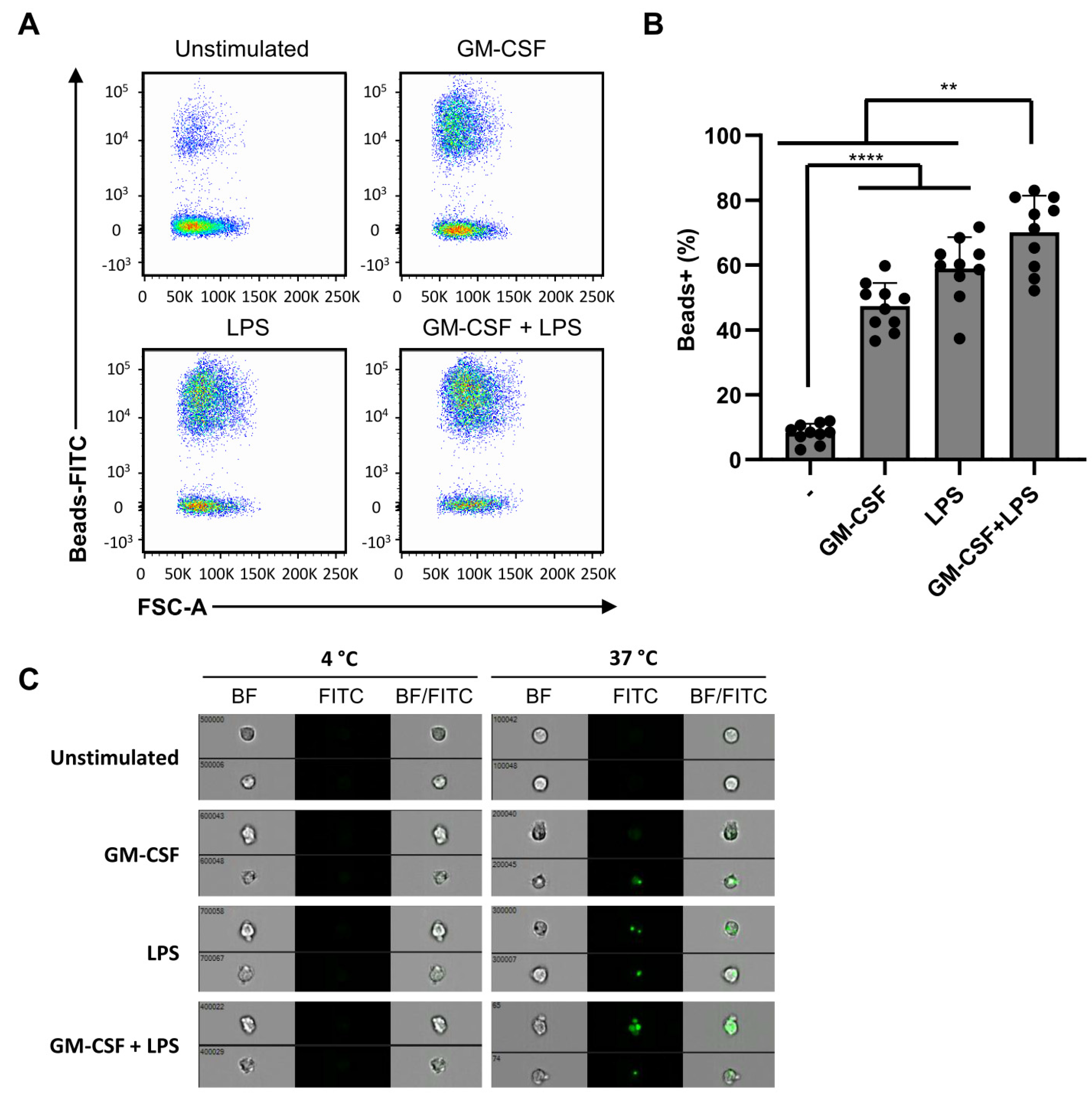
2.5. Phagocytosis Is Independent of Dual Stimulation
2.6. Dual Stimulation of Neutrophils Increases the Release of EVs
3. Discussion
4. Materials and Methods
4.1. Neutrophil Isolation
4.2. Neutrophil Culture, Stimulation and Flow Cytometric Analysis
4.3. IL-8 ELISA
4.4. Neutrophil Elastase ELISA
4.5. NETosis Assay
4.6. Phagocytosis Assay
4.7. EV Isolation and Fluorescent Labeling
4.8. Western Blotting
4.9. Neutrophil EV Quantification by High-Resolution Flow Cytometry
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 123-DHR | 123-dihydrorhodamine |
| BSA | Bovine serum albumin |
| DAPI | DAPI dihydrochloride |
| EV | Extracellular vesicles |
| fMLF | 0N-formyl-methionyl-leucyl-fenylalanine |
| FBS | Fetal bovine serum |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HBSS | Hanks balanced salt solution |
| HI | Heat inactivated |
| LPS | Lipopolysaccharide |
| MFI | Mean fluorescence intensity |
| NE | Neutrophil elastase |
| NET | Neutrophil extracellular trap |
| PAMP | Pathogen associated molecular patterns |
| PBMC | Peripheral blood mononuclear cells |
| PI | Propidium iodide |
| PMA | Phorbol myristate acetate |
| ROS | Reactive oxygen species |
| SLE | Systemic lupus erythematosus |
| TMB | Tetramethylbenzidine |
| TNF | Tumor necrosis factor |
References
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwick, J.A.; Mills, R.; Kay, O.; Michail, K.; Stephen, J.; Rossi, A.G.; Dransfield, I.; Hirani, N. Neutrophils induce macrophage anti-inflammatory reprogramming by suppressing NF-κB activation article. Cell Death Dis. 2018, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Souwer, Y.; Groot Kormelink, T.; Taanman-Kueter, E.W.; Muller, F.J.; van Capel, T.M.; Varga, D.V.; Bar-Ephraim, Y.E.; Teunissen, M.B.; van Ham, S.M.; Kuijpers, T.W.; et al. Human TH17 cell development requires processing of dendritic cell–derived CXCL8 by neutrophil elastase. J. Allergy Clin. Immunol. 2018, 141, 2286–2289.e5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yuan, Y.; Xu, Q.; Jiang, Z.; Chu, C.Q. Contribution of neutrophils in the pathogenesis of rheumatoid arthritis. J. Biomed. Res. 2020, 34, 86–93. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from Marrow to Microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Linden, M.; Meyaard, L. Fine-tuning neutrophil activation: Strategies and consequences. Immunol. Lett. 2016, 178, 3–9. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Groot Kormelink, T.; Mol, S.; De Jong, E.C.; Wauben, M.H.M. The role of extracellular vesicles when innate meets adaptive. Semin. Immunopathol. 2018, 40, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Alarcon, M.F.; McLaren, Z.; Wright, H.L. Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O. Front. Immunol. 2021, 12, 301. [Google Scholar]
- Vogt, K.L.; Summers, C.; Chilvers, E.R.; Condliffe, A.M. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur. J. Clin. Investig. 2018, 48, 161. [Google Scholar] [CrossRef] [PubMed]
- Miralda, I.; Uriarte, S.M.; McLeish, K.R. Multiple Phenotypic Changes Define Neutrophil Priming. Front. Cell. Infect. Microbiol. 2017, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Hazeldine, J.; Harris, P.; Chapple, I.L.; Grant, M.; Greenwood, H.; Livesey, A.; Sapey, E.; Lord, J. Impaired neutrophil extracellular trap formation: A novel defect in the innate immune system of aged individuals. Aging Cell 2014, 13, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Nakamura, M.; McLeish, K.R. Priming of the Neutrophil Respiratory Burst Involves p38 Mitogen-activated Protein Kinase-dependent Exocytosis of Flavocytochrome b558-containing Granules. J. Biol. Chem. 2000, 275, 36713–36719. [Google Scholar] [CrossRef] [Green Version]
- Eken, C.; Gasser, O.; Zenhaeusern, G.; Oehri, I.; Hess, C.; Schifferli, J.A. Polymorphonuclear Neutrophil-Derived Ectosomes Interfere with the Maturation of Monocyte-Derived Dendritic Cells. J. Immunol. 2008, 180, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karnad, A.B.; Hartshorn, K.L.; Wright, J.; Myers, J.B.; Schwartz, J.H.; Tauber, A.I. Priming of human neutrophils with N-formyl-methionyl-leucyl-phenylalanine by a calcium-independent, pertussis toxin-insensitive pathway. Blood. 1989, 74, 2519–2526. [Google Scholar] [CrossRef] [Green Version]
- Uriarte, S.M.; Rane, M.J.; Luerman, G.C.; Barati, M.T.; Ward, R.A.; Nauseef, W.M.; McLeish, K.R. Granule Exocytosis Contributes to Priming and Activation of the Human Neutrophil Respiratory Burst. J. Immunol. 2011, 187, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Almkvist, J.; Fäldt, J.; Dahlgren, C.; Leffler, H.; Karlsson, A. Lipopolysaccharide-Induced Gelatinase Granule Mobilization Primes Neutrophils for Activation by Galectin-3 and Formylmethionyl-Leu-Phe. Infect. Immun. 2001, 69, 832–837. [Google Scholar] [CrossRef] [Green Version]
- Doerfler, M.E.; Danner, R.L.; Shelhamer, J.H.; Parrillo, J.E. Bacterial lipopolysaccharides prime human neutrophils for enhanced production of leukotriene B4. J. Clin. Investig. 1989, 83, 970–977. [Google Scholar] [CrossRef] [Green Version]
- McLeish, K.R.; Merchant, M.L.; Creed, T.M.; Tandon, S.; Barati, M.T.; Uriarte, S.M.; Ward, R.A. Frontline Science: Tumor necrosis factor-α stimulation and priming of human neutrophil granule exocytosis. J. Leukoc. Biol. 2017, 102, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Potera, R.M.; Jensen, M.J.; Hilkin, B.M.; South, G.K.; Hook, J.S.; Gross, E.A.; Moreland, J.G. Neutrophil azurophilic granule exocytosis is primed by TNF-α and partially regulated by NADPH oxidase. Innate Immun. 2016, 22, 635–646. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.H.; Roberts, A.I.; Das, J.; Xu, G.; Ren, G.; Zhang, Y.; Zhang, L.; Yuan, Z.R.; Tan, H.S.; et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 2006, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Lacy, P. Mechanisms of Degranulation in Neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borregaard, N.; Sørensen, O.E.; Theilgaard-Mönch, K. Neutrophil granules: A library of innate immunity proteins. Trends Immunol. 2007, 28, 340–345. [Google Scholar] [CrossRef]
- Amulic, B.; Cazalet, C.; Hayes, G.L.; Metzler, K.D.; Zychlinsky, A. Neutrophil Function: From Mechanisms to Disease. Annu. Rev. Immunol. 2012, 30, 459–489. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Newton, R.; Bahaie, N.S.; Long, C.; Walcheck, B. ADAM17 cleaves CD16b (FcγRIIIb) in human neutrophils. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 680–685. [Google Scholar] [CrossRef] [Green Version]
- Fortunati, E.; Kazemier, K.M.; Grutters, J.C.; Koenderman, L.; van den Bosch, J.M.M. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin. Exp. Immunol. 2009, 155, 559–566. [Google Scholar] [CrossRef]
- Pellmé, S.; Mörgelin, M.; Tapper, H.; Mellqvist, U.H.; Dahlgren, C.; Karlsson, A. Localization of human neutrophil interleukin-8 (CXCL-8) to organelle(s) distinct from the classical granules and secretory vesicles. J. Leukoc. Biol. 2005, 79, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Stanley, A.C.; Lacy, P. Pathways for Cytokine Secretion. Physiology 2010, 25, 218–229. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Neubert, E.; Meyer, D.; Rocca, F.; Günay, G.; Kwaczala-Tessmann, A.; Grandke, J.; Senger-Sander, S.; Geisler, C.; Egner, A.; Schön, M.P.; et al. Chromatin swelling drives neutrophil extracellular trap release. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van der Vlist, E.J.; Nolte-‘t Hoen, E.N.M.; Stoorvogel, W.; Arkesteijn, G.J.A.; Wauben, M.H.M. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat. Protoc. 2012, 7, 1311–1326. [Google Scholar] [CrossRef]
- Drew, W.; Wilson, D.V.; Sapey, E. Inflammation and neutrophil immunosenescence in health and disease: Targeted treatments to improve clinical outcomes in the elderly. Exp. Gerontol. 2018, 105, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Spijkerman, R.; Hesselink, L.; Hellebrekers, P.; Vrisekoop, N.; Hietbrink, F.; Leenen, L.P.H.; Koenderman, L. Automated flow cytometry enables high performance point-of-care analysis of leukocyte phenotypes. J. Immunol. Methods 2019, 474, 112646. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 655. [Google Scholar] [CrossRef] [Green Version]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [Green Version]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil Extracellular Traps (NETs) Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines Induced Neutrophil Extracellular Traps Formation: Implication for the Inflammatory Disease Condition. PLoS ONE 2012, 7, e48111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, S.; Behnen, M.; Bieber, K.; Möller, S.; Hellberg, L.; Witte, M.; Hänsel, M.; Zillikens, D.; Solbach, W.; Laskay, T.; et al. Dimethylfumarate Impairs Neutrophil Functions. J. Investig. Dermatol. 2016, 136, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, E.; Rother, N.; Yanginlar, C.; Hilbrands, L.B.; van der Vlag, J. Neutrophils Discriminate between Lipopolysaccharides of Different Bacterial Sources and Selectively Release Neutrophil Extracellular Traps. Front. Immunol. 2016, 7, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubert, E.; Senger-Sander, S.N.; Manzke, V.S.; Busse, J.; Polo, E.; Scheidmann, S.E.F.; Schön, M.P.; Kruss, S.; Erpenbeck, L. Serum and Serum Albumin Inhibit in vitro Formation of Neutrophil Extracellular Traps (NETs). Front. Immunol. 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.D.; Lee, M.C.; Saleh, R.; Khiew, H.W.; Christensen, A.D.; Achuthan, A.; Fleetwood, A.J.; Lacey, D.C.; Smith, J.E.; Förster, I.; et al. TNF and granulocyte macrophage-colony stimulating factor interdependence mediates inflammation via CCL17. JCI Insight 2018, 3, e99249. [Google Scholar] [CrossRef] [Green Version]
- Lotfi, N.; Thome, R.; Rezaei, N.; Zhang, G.X.; Rezaei, A.; Rostami, A.; Esmaeil, N. Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front. Immunol. 2019, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Idborg, H.; Eketjäll, S.; Pettersson, S.; Gustafsson, J.T.; Zickert, A.; Kvarnström, M.; Oke, V.; Jakobsson, P.J.; Gunnarsson, I.; Svenungsson, E. TNF-α and plasma albumin as biomarkers of disease activity in systemic lupus erythematosus. Lupus Sci. Med. 2018, 5, e000260. [Google Scholar] [CrossRef]
- Merza, M.; Hartman, H.; Rahman, M.; Hwaiz, R.; Zhang, E.; Renström, E.; Luo, L.; Mörgelin, M.; Regner, S.; Thorlacius, H. Neutrophil Extracellular Traps Induce Trypsin Activation, Inflammation, and Tissue Damage in Mice with Severe Acute Pancreatitis. Gastroenterology 2015, 149, 1920–1931.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radermecker, C.; Detrembleur, N.; Guiot, N.; Cavalier, E.; Henket, M.; d’Emal, C.; Vanwinge, C.; Cataldo, D.; Oury, C.; Delvenne, P.; et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020, 217, e20201012. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, T.A.P.; Mol, S.; De Bruin, S.; Peters, A.L.; Zhang, X.; Lindenbergh, M.F.S.; Beuger, B.M.; van Stalborch, A.D.; Spaan, T.; De Jong, E.C.; et al. Y--RNA subtype ratios in plasma extracellular vesicles are cell type-- specific and are candidate biomarkers for inflammatory diseases. J. Extracell. Vesicles 2020, 9, 1764213. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Burbidge, K.; Zwikelmaier, V.; Cook, B.; Long, M.M.; Balva, B.; Lonigro, M.; Ispas, G.; Rademacher, D.J.; Campbell, E.M. Cargo and cell--specific differences in extracellular vesicle populations identified by multiplexed immunofluorescent analysis. J. Extracell. Vesicles 2020, 9, 1789326. [Google Scholar] [CrossRef]
- Groot Kormelink, T.; Arkesteijn, G.J.A.; van de Lest, C.H.A.; Geerts, W.J.C.; Goerdayal, S.S.; Altelaar, M.A.F.; Redegeld, F.A.; Nolte-‘t Hoen, E.N.M.; Wauben, M.H.M. Mast Cell Degranulation Is Accompanied by the Release of a Selective Subset of Extracellular Vesicles That Contain Mast Cell–Specific Proteases. J. Immunol. 2016, 197, 3382–3392. [Google Scholar] [CrossRef] [Green Version]
- Shopova, I.A.; Belyaev, I.; Dasari, P.; Jahreis, S.; Stroe, M.C.; Cseresnyés, Z.; Zimmermann, A.K.; Medyukhina, A.; Svensson, C.M.; Krüger, T.; et al. Human Neutrophils Produce Antifungal Extracellular Vesicles against Aspergillus fumigatus. mBio 2020, 11, e00596-20. [Google Scholar] [CrossRef] [Green Version]
- Timar, C.I.; Lőrincz, A.M.; Csépányi-Kömi, R.; Vályi-Nagy, A.; Nagy, G.; Buzás, E.I.; Iványi, Z.; Kittel, A.; Powell, D.W.; McLeish, K.R.; et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood 2013, 121, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Snijders, A.; Kalinski, P.; Hilkens, C.M.; Kapsenberg, M.L. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 1998, 10, 1593–1598. [Google Scholar] [CrossRef] [Green Version]
- Vogelpoel, L.T.C.; Baeten, D.L.P.; De Jong, E.C.; Den Dunnen, J. Control of Cytokine Production by Human Fc Gamma Receptors: Implications for Pathogen Defense and Autoimmunity. Front. Immunol. 2015, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- De Morales, J.M.G.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Gupta, S.; Chan, D.W.; Zaal, K.J.; Kaplan, M.J. A High-Throughput Real-Time Imaging Technique To Quantify NETosis and Distinguish Mechanisms of Cell Death in Human Neutrophils. J. Immunol. 2017, 200, 869–879. [Google Scholar] [CrossRef]
- Nolte-‘t Hoen, E.N.M.; van der Vlist, E.J.; Aalberts, M.; Mertens, H.C.; Bosch, B.J.; Bartelink, W.; Mastrobattista, E.; van Gaal, E.V.; Stoorvogel, W.; Arkesteijn, G.J.A.; et al. Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomed. Nanotechnol. Biol. Med. 2011, 8, 712–720. [Google Scholar] [CrossRef] [Green Version]
- Groot Kormelink, T.; Arkesteijn, G.J.A.; Nauwelaers, F.A.; van den Engh, G.; Nolte-‘t Hoen, E.N.M.; Wauben, M.H.M. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytom. Part A 2015, 89, 135–147. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mol, S.; Hafkamp, F.M.J.; Varela, L.; Simkhada, N.; Taanman-Kueter, E.W.; Tas, S.W.; Wauben, M.H.M.; Groot Kormelink, T.; de Jong, E.C. Efficient Neutrophil Activation Requires Two Simultaneous Activating Stimuli. Int. J. Mol. Sci. 2021, 22, 10106. https://doi.org/10.3390/ijms221810106
Mol S, Hafkamp FMJ, Varela L, Simkhada N, Taanman-Kueter EW, Tas SW, Wauben MHM, Groot Kormelink T, de Jong EC. Efficient Neutrophil Activation Requires Two Simultaneous Activating Stimuli. International Journal of Molecular Sciences. 2021; 22(18):10106. https://doi.org/10.3390/ijms221810106
Chicago/Turabian StyleMol, Sanne, Florianne M. J. Hafkamp, Laura Varela, Neena Simkhada, Esther W. Taanman-Kueter, Sander W. Tas, Marca H. M. Wauben, Tom Groot Kormelink, and Esther C. de Jong. 2021. "Efficient Neutrophil Activation Requires Two Simultaneous Activating Stimuli" International Journal of Molecular Sciences 22, no. 18: 10106. https://doi.org/10.3390/ijms221810106
APA StyleMol, S., Hafkamp, F. M. J., Varela, L., Simkhada, N., Taanman-Kueter, E. W., Tas, S. W., Wauben, M. H. M., Groot Kormelink, T., & de Jong, E. C. (2021). Efficient Neutrophil Activation Requires Two Simultaneous Activating Stimuli. International Journal of Molecular Sciences, 22(18), 10106. https://doi.org/10.3390/ijms221810106





