Study on Maximum Specific Loss Power in Fe3O4 Nanoparticles Decorated with Biocompatible Gamma-Cyclodextrins for Cancer Therapy with Superparamagnetic Hyperthermia
Abstract
:1. Introduction
2. Basic Equations Used in the Calculation of Specific Loss Power in the Case of Biocompatible Magnetic Nanoparticles Dispersed in a Liquid
3. Results and Discussion
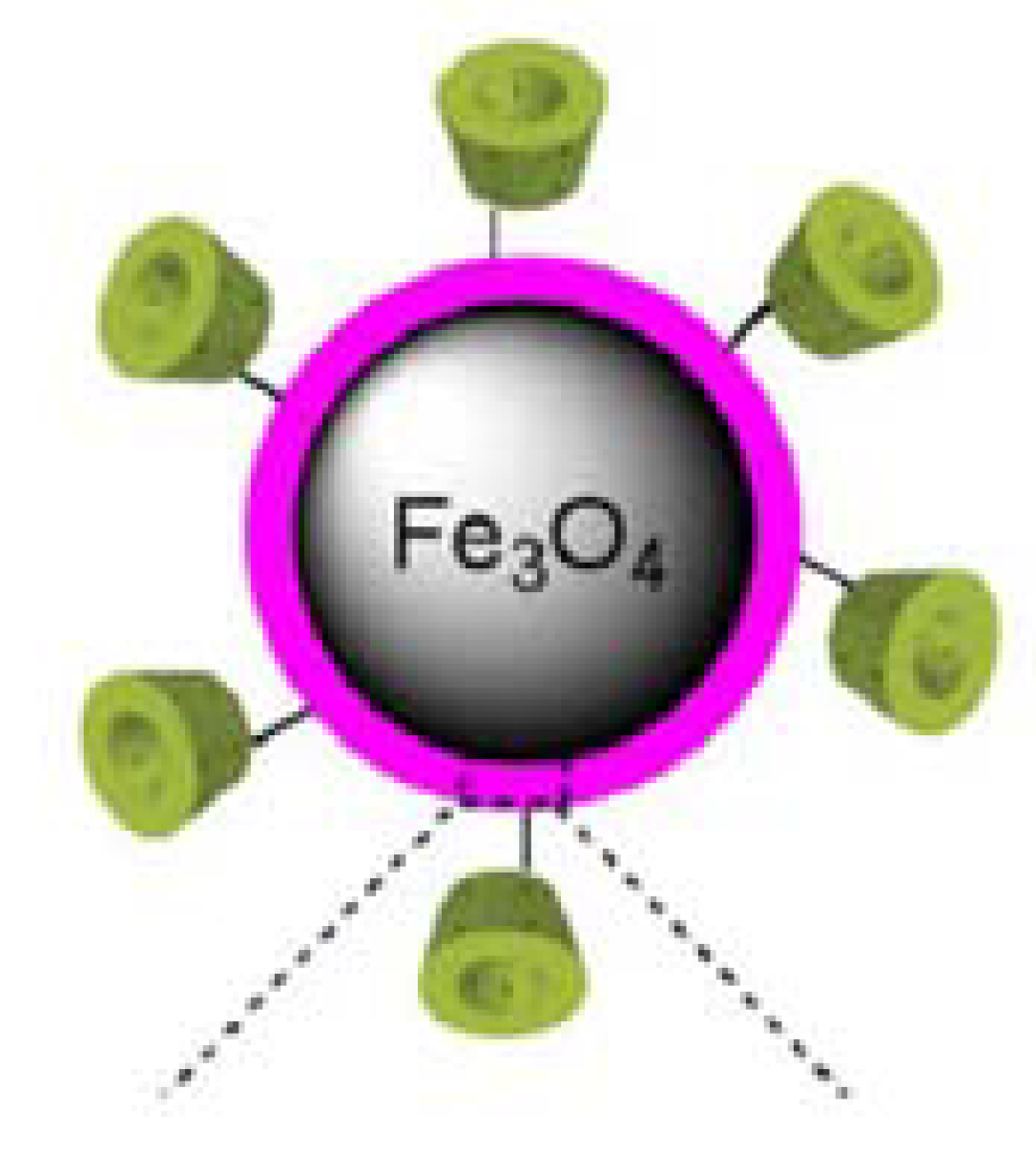
3.1. Biocompatible Fe3O4 Nanoparticles Decorated with γ-CDs and Dispersed in a Liquid to Eliminate Toxicity and Increase Efficiency in Superparamagnetic Hyperthermia
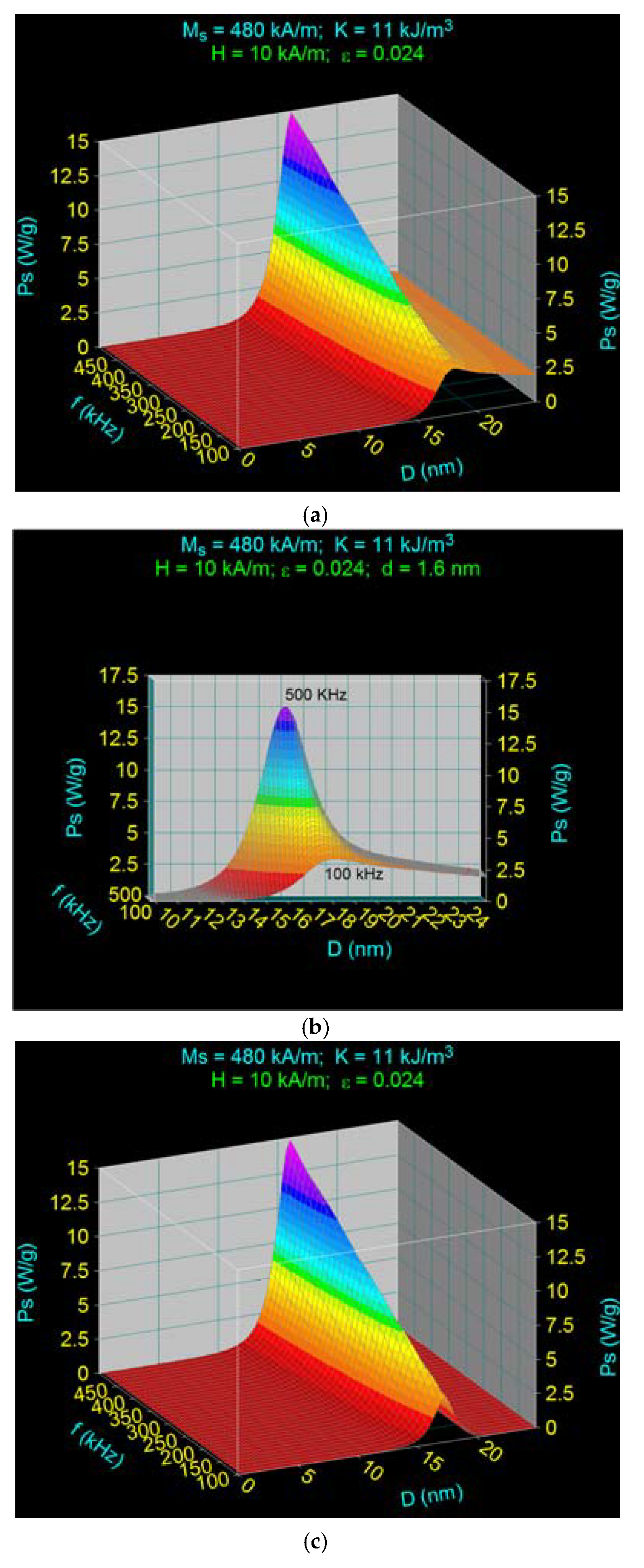
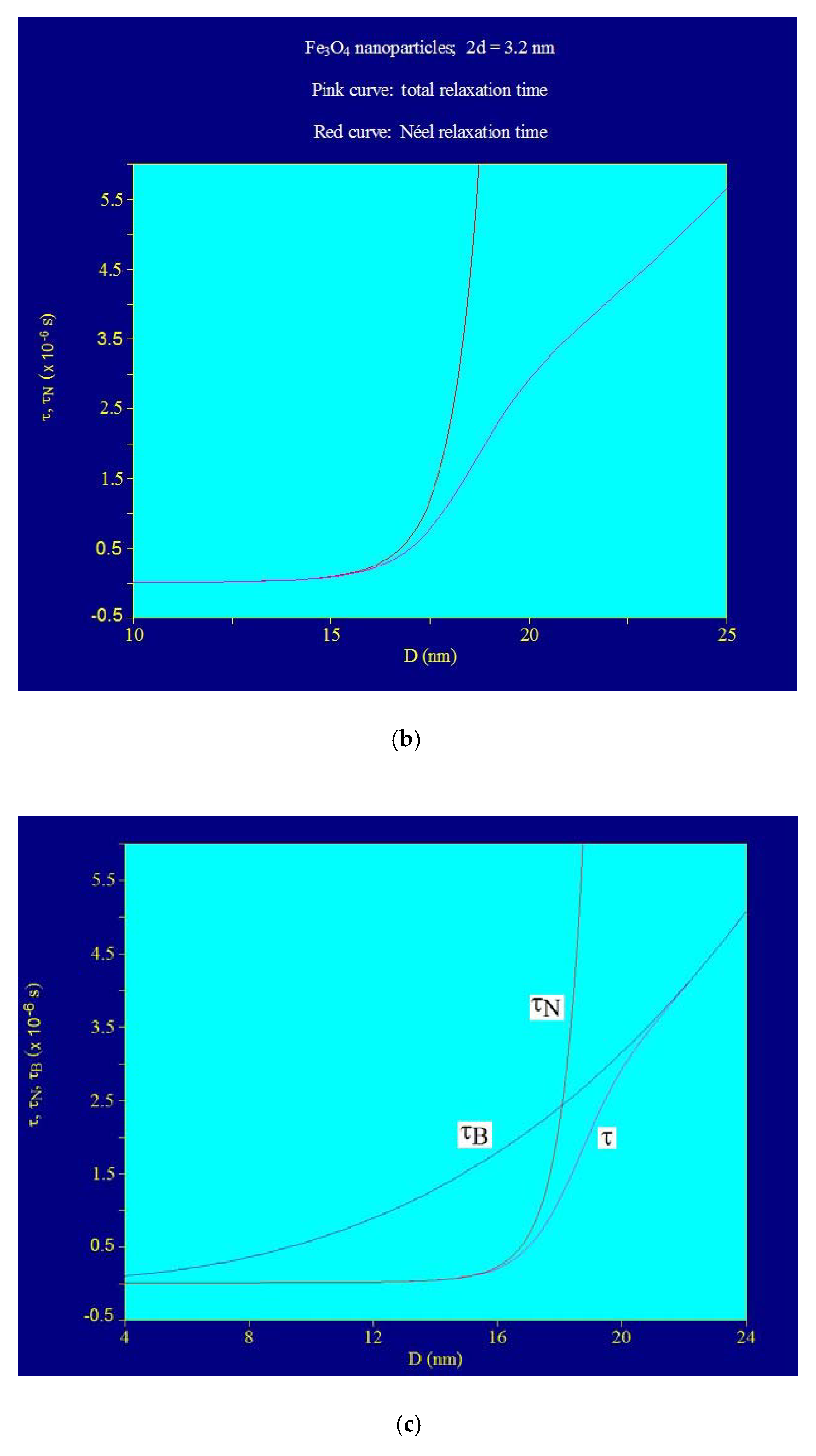
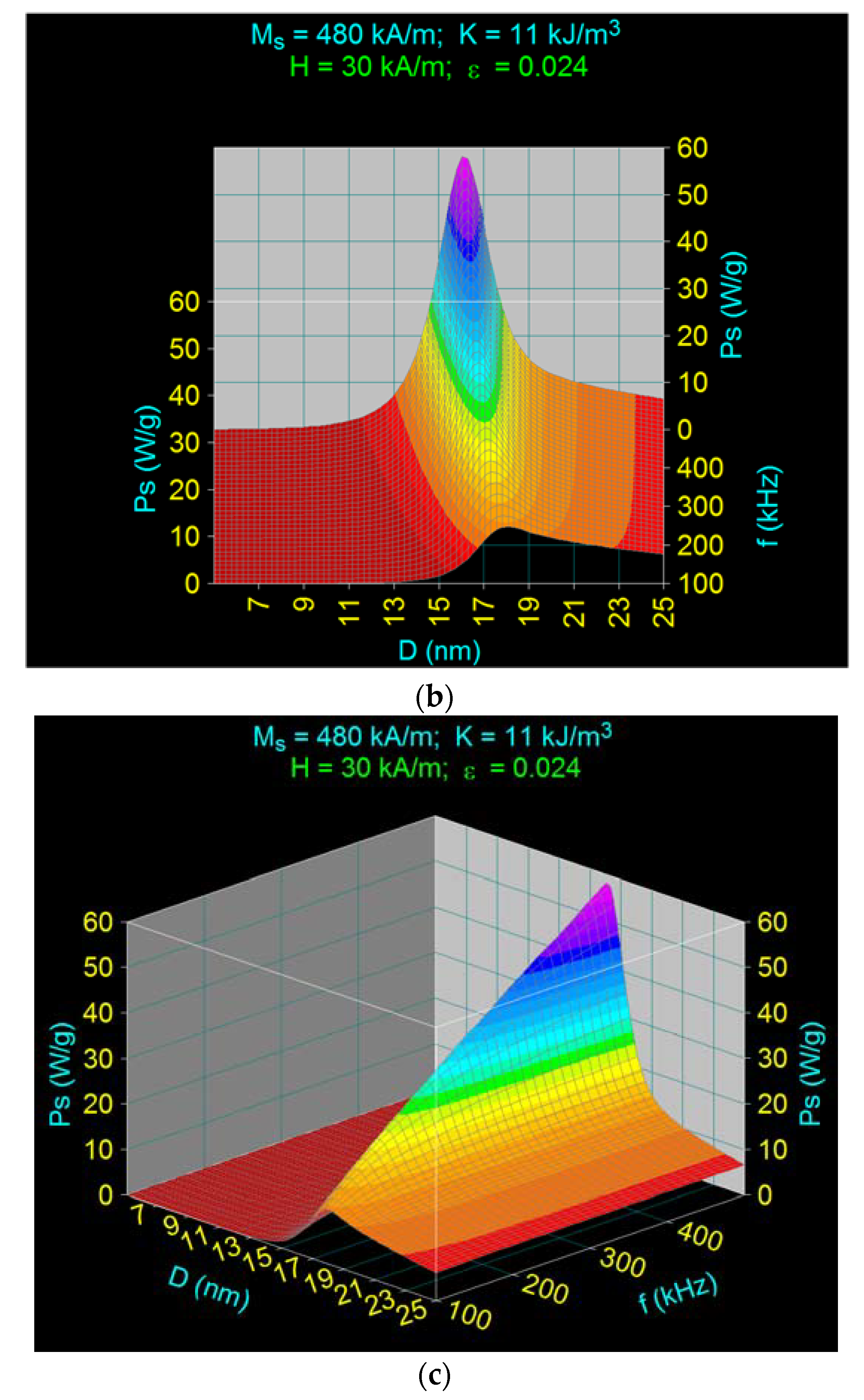
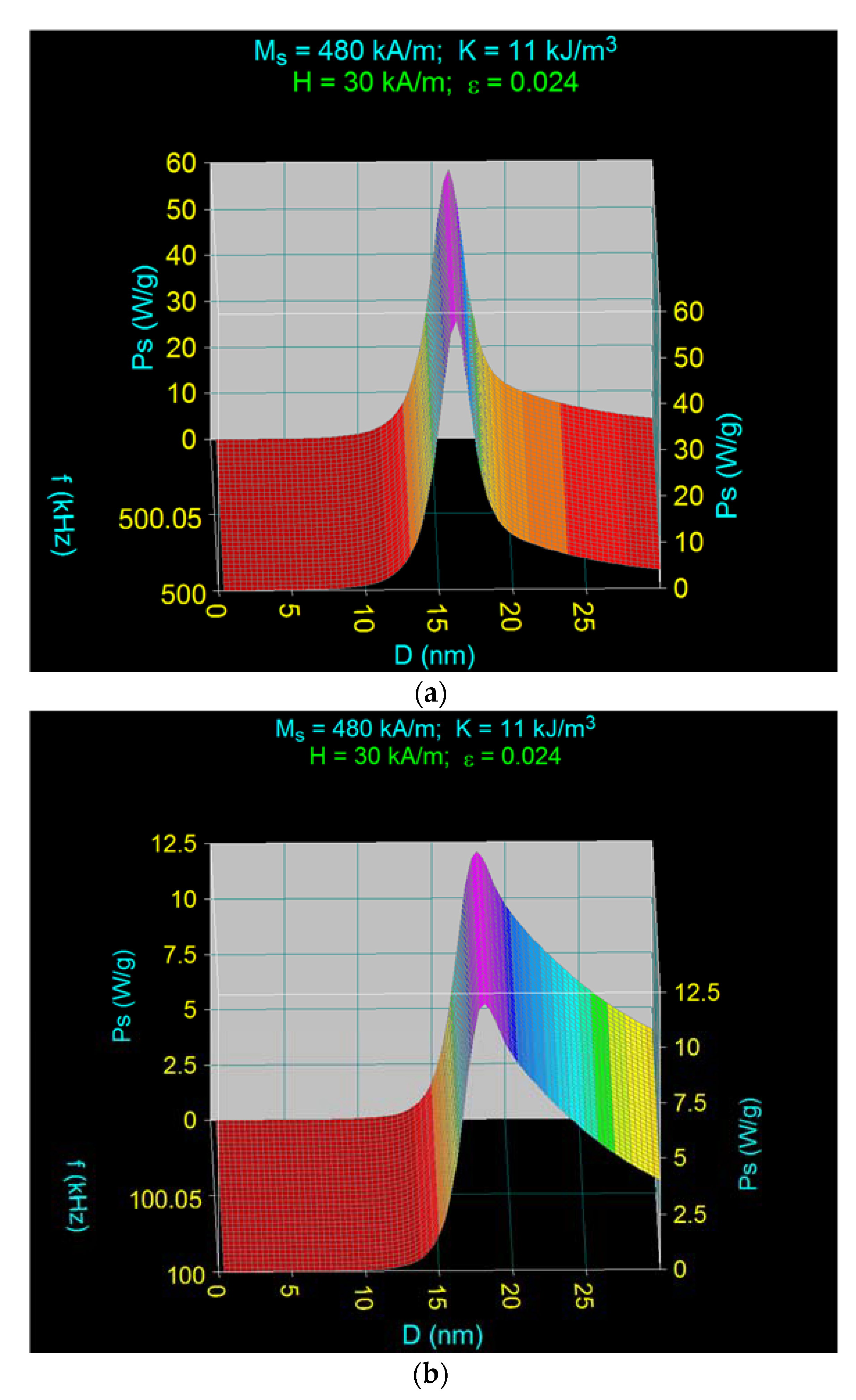
3.2. Maximum Specific Loss Power in Fe3O4 Nanoparticles Decorated with γ-CDs Dispersed in a Liquid
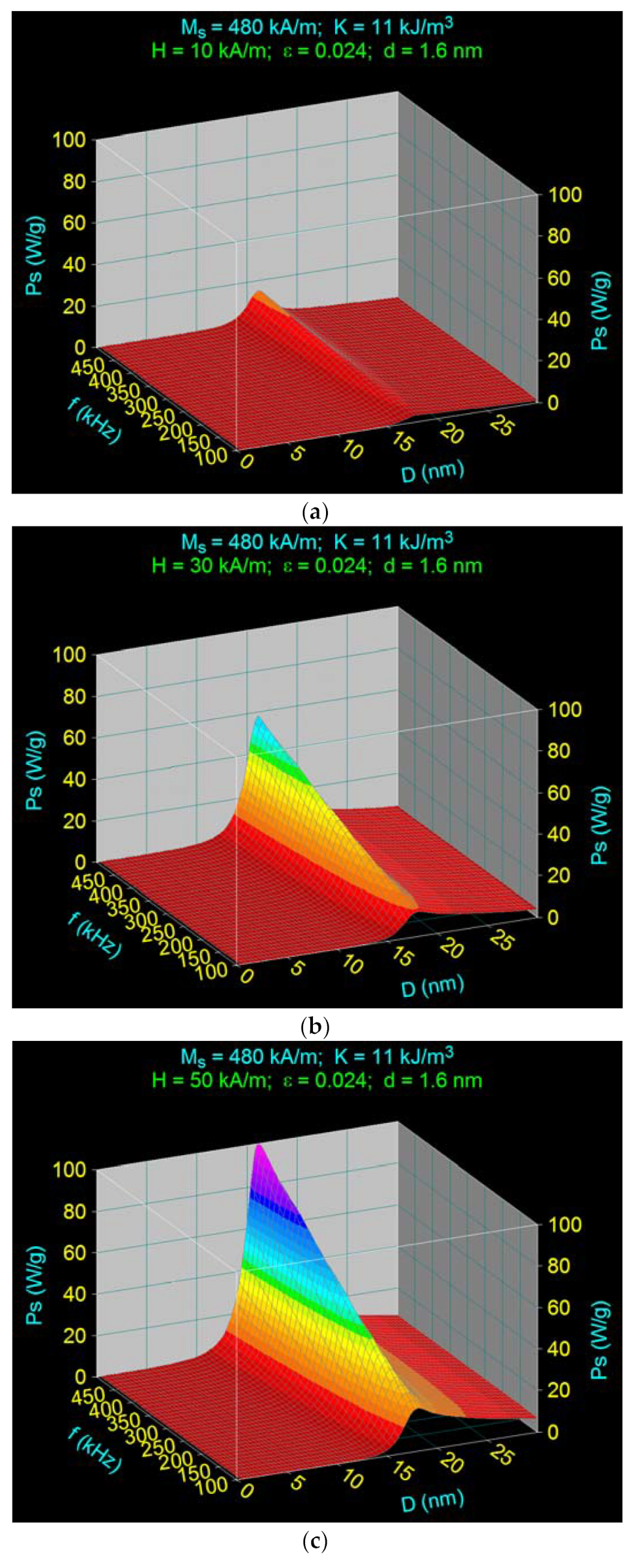
3.3. The Effect of the Amplitude and Frequency of the Magnetic Field on the Maximum Specific Loss Power in Fe3O4 Nanoparticles Decorated with Gamma-Cyclodextrins
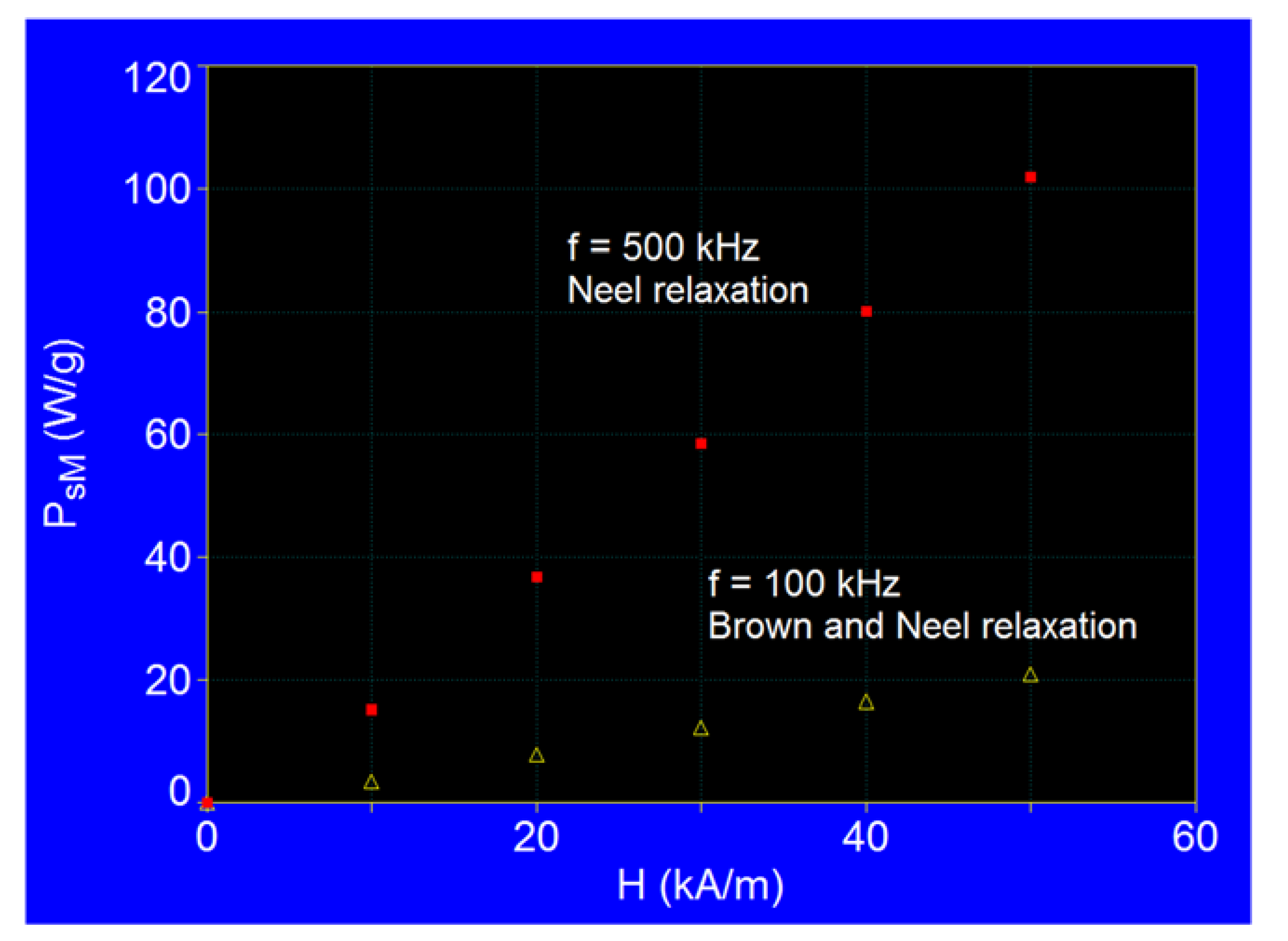
3.3.1. The Effect of the Amplitude of the Magnetic Field
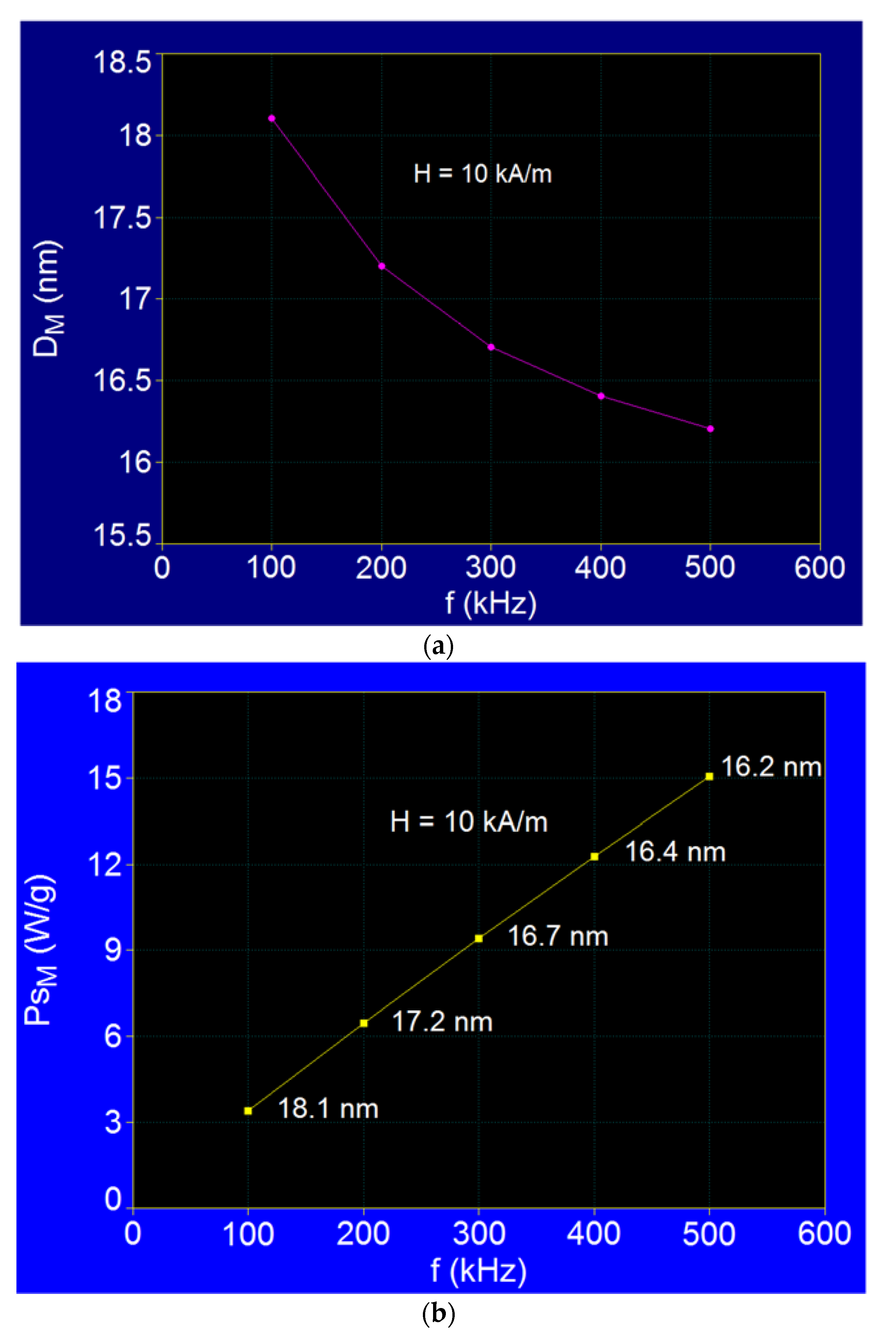
3.3.2. The Effect of the Magnetic Field Frequency
3.4. Maximum Specific Loss Power in Superparamagnetic Hyperthermia with Fe3O4–γ-CDs Bionanoparticles in Optimal Conditions for the Allowable Limit Frequency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rego, G.N.A.; Nucci, M.P.; Javier, B.M.; Fernando, A.O.; Luciana, C.M.; Igor, S.F.; João, M.F.; Caroline, C.R.; Daniele de Paula, F.; Espinha, P.L.; et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef] [Green Version]
- Caizer, C. Magnetic/Superparamagnetic hyperthermia as an effective noninvasive alternative method for therapy of malignant tumors. In Nanotheranostics: Applications and Limitations; Rai, M., Jamil, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 297–335. [Google Scholar]
- Zhou, P.; Zhao, H.; Wang, Q.; Zhou, Z.; Wang, J.; Deng, G.; Wang, X.; Liu, Q.; Yang, H.; Yang, S. Photoacoustic-enabled self-guidance in magnetic hyperthermia Fe@Fe3O4 nanoparticles for theranostics in vivo. Adv. Healthc. Mater. 2018, 7, e1701201. [Google Scholar] [CrossRef] [PubMed]
- Thorat, N.; Bohara, R.; Noor, M.R.; Dhamecha, D.; Soulimane, T.; Tofail, S. Effective cancer theranostics with polymer encapsulated superparamagnetic nanoparticles: Combined effects of magnetic hyperthermia and controlled drug release. ACS Biomater. Sci. Eng. 2017, 3, 1332–1340. [Google Scholar] [CrossRef]
- Liu, X.L.; Ng, C.T.; Chandrasekharan, P.; Yang, H.T.; Zhao, L.Y.; Peng, E.; Lv, Y.B.; Xiao, W.; Fang, J.; Yi, J.B.; et al. Synthesis of ferromagnetic Fe0.6Mn0.4O nanoflowers as a new class of magnetic theranostic platform for in vivo T1-T2 Dual-Mode magnetic resonance imaging and magnetic hyperthermia therapy. Adv. Healthc. Mater. 2016, 5, 2092–2104. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Ordóńez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.K.; Ménager, C.; Wilhelm, C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano. 2015, 9, 2904–2916. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kakimi, K.; Nakayama, E.; Jimbow, K. Antitumor immunity by magnetic nanoparticle mediated hyperthermia. Nanomedicine 2014, 9, 1715–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alphandéry, E.; Chebbi, I.; Guyot, F.; Durand-Dubief, M. Use of bacterial magnetosomes in the magnetic hyperthermia treatment of tumours: A review. Int. J. Hyperthermia 2013, 29, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Zhang, X.; Liu, X.; Xu, B.; Yang, H.; Xia, Q.; Li, L.; Chen, C.; Tang, J. Higher temperature improves the efficacy of magnetic fluid hyperthermia for Lewis lung cancer in a mouse model. Thorac. Cancer 2012, 3, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.H.; Hou, S.M.; Hsueh, Y.S.; Lin, J.; Wu, H.C.; Lin, F.H. The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 2009, 30, 3956–3960. [Google Scholar] [CrossRef]
- Gazeau, F.; Lévy, M.; Wilhelm, C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine 2008, 3, 831–844. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neuro-Oncol. 2007, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Wust, P.; Schirra, H.; Schiestel, T.; Schmidt, H.; Felix, R.J. Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J. Magn. Magn. Mater. 1999, 194, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Johannsen, M.; Gneveckow, U.; Thiesen, B.; Taymoorian, K.; Cho, C.H.; Waldofner, N.; Scholz, R.; Jordan, A.; Loening, S.A.; Wust, P. Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur. Urol. 2007, 52, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Kawai, N.; Ito, A.; Nakahara, Y.; Honda, H.; Kobayashi, T.; Futakuchi, M.; Shirai, T.; Tozawa, K.; Kohri, K. Complete regression of experimental prostate cancer in nude mice by repeated hyperthermia using magnetite cationic liposomes and a newly developed solenoid containing a ferrite core. Prostate 2006, 66, 718–727. [Google Scholar] [CrossRef]
- Hilger, I.; Hergt, R.; Kaiser, W.A. Towards breast cancer treatment by magnetic heating. J. Magn. Magn. Mater. 2005, 293, 314–319. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Caizer, C. Nanoparticle size effect on some magnetic properties. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Caizer, C. Computational study on superparamagnetic hyperthermia with biocompatible SPIONs to destroy the cancer cells. J. Phys. Conf. Ser. 2014, 521, 012015. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, G.; Sudame, A.; Bhati, P.; Chakrabarty, A.; Maity, D. Systematic investigations on heating effects of carboxyl-amine functionalized superparamagnetic iron oxide nanoparticles (SPIONs) based ferrofluids for in vitro cancer hyperthermia therapy. J. Mol. Liq. 2018, 256, 224–237. [Google Scholar] [CrossRef]
- Caizer, C.; Stefanescu, M. Magnetic characterization of nanocrystalline Ni–Zn ferrite powder prepared by the glyoxylate precursor method. J. Phys. D Appl. Phys. 2002, 35, 3035–3040. [Google Scholar] [CrossRef]
- Caizer, C.; Stefanescu, M. Nanocrystallite size effect on σs and Hc in nanoparticle assemblies. Phys. B Condens. Matter 2003, 327, 129–134. [Google Scholar] [CrossRef]
- Bean, C.P.; Livingston, L.D. Superparamagnetism. J. Appl. Phys. 1959, 30, S120–S129. [Google Scholar] [CrossRef]
- Néel, L. Théorie du traînage magnétique des ferromagnétiques en grains fins avec application aux terres cuites. Ann. Geophys. 1949, 5, 99–136. [Google Scholar]
- Brown, W.F., Jr. Thermal fluctuations of a single-domain particle. Phys. Rev. 1963, 130, 1677–1686. [Google Scholar] [CrossRef]
- Caizer, C. Magnetic Nanofluids; Eurobit: Timisoara, Romannia, 2004. (In Romanian) [Google Scholar]
- Aharoni, A. Thermal agitation of single domain particles. Phys. Rev. 1964, 135, 447–449. [Google Scholar] [CrossRef]
- Caizer, C. Magnetic hyperthermia-using magnetic metal/oxide nanoparticles with potential in cancer therapy. In Metal Nanoparticles in Pharma; Rai, M., Shegokar, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Bellia, F.; La Mendola, D.; Pedone, C.; Rizzarelli, E.; Saviano, M.; Vecchio, G. Selectively functionalized cyclodextrins and their metal complexes. Chem. Soc. Rev. 2009, 38, 2756. [Google Scholar] [CrossRef] [PubMed]
- Caizer, C.; Dehelean, C.; Soica, C. Classical magnetoliposomes vs current magnetociclodextrins with ferrimagnetic nanoparticle for high efficiency and low toxicity in alternative therapy of cancer by magnetic/ superparamagnetic hyperthermia. Ch 13. In Magnetic Nanoparticles in Human Health and Medicine: Current Medical Applications and Alternative Therapy of Cancer; Caizer, C., Rai, M., Eds.; Wiley: Hoboken, NJ, USA, 2021; accepted. [Google Scholar]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 1890. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Tanaka, K.; Honda, H.; Abe, S.; Yamaguchi, H.; Kobayaschi, T. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. J. Biosci. Bioeng. 2003, 96, 364–369. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [Green Version]
- Back, C.H.; Weller, D.; Heidmann, J.; Mauri, D.; Guarisco, D.; Garwin, E.L.; Siegmann, H.C. Magnetization reversal in ultrashort magnetic field pulses. Phys. Rev. Lett. 1998, 81, 3251. [Google Scholar] [CrossRef] [Green Version]
- Raikher, Y.I.; Shliomis, M.I. The effective field method in the orientational kinetics of magnetic fluids and liquid crystals. In Relaxation Phenomena in Condensed Matter; Advances in Chemical Physics Series; Coffey, W.I., Ed.; Wiley: Hoboken, NJ, USA, 2008; Volume 97. [Google Scholar]
- Caizer, C. Optimization study on specific loss power in superparamagnetic hyperthermia with magnetite nanoparticles for high efficiency in alternative cancer therapy. Nanomaterials 2021, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Langevin, P. Magnétisme et théorie des électrons. Ann. Chim. Phys. 1905, 5, 70–127. [Google Scholar]
- Jacobs, I.S.; Bean, C.P. Fine particles, thin films and exchange anisotropy. In Magnetism; Rado, G.T., Suhl, H., Eds.; Academic Press: Cambridge, MA, USA, 1963; Volume III, pp. 271–350. [Google Scholar]
- Caizer, C.; Rai, M. Magnetic nanoparticles in alternative tumors therapy: Biocompatibility, toxicity and safety compared with classical methods. Ch 16. In Magnetic Nanoparticles in Human Health and Medicine: Current Medical Applications and Alternative Therapy of Cancer; Caizer, C., Rai, M., Eds.; Wiley: Hoboken, NJ, USA, 2021; accepted. [Google Scholar]
- Caizer, C.; Buteica, A.S.; Mindrila, I. Biocompatible magnetic oxide nanoparticles with metal ions coated with organic shell as potential therapeutic agents in cancer. In Metal Nanoparticles in Pharma; Rai, M., Shegokar, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Caizer, C.; Dehelean, C.; Coricovac, D.E.; Caizer, I.S.; Soica, C. Magnetic nanoparticles nanoformulations for alternative therapy of cancer by magnetic/superparamagnetic hyperthermia. In Nanoformulations in Human Health; Talegaonkar, S., Rai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Oroujenia, M.; Kaboudin, B.; Xia, W.; Jönsson, P.; Ossipov, D.A. Conjugation of cyclodextrin to magnetic Fe3O4 nanoparticles via polydopamine coating for drug delivery. Prog. Org. Coat. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Caizer, C. Obtaining superparamagnetic nanoparticles of (Fe-Co)f and 2D/3D computational study on superparamagnetic hyperthermia in these nanosystems and bioconjugates with gamma-cyclodextrins. Sci. Res. Rep. UEFISCDI, 2020; Non-published. [Google Scholar]
- Caizer, C. Magnetic behavior of Mn0.6Fe0.4Fe2O4 nanopartciles in ferrofluid at low temperatures. J. Magn. Magn. Mater. 2002, 251, 304–315. [Google Scholar] [CrossRef]
- Caizer, C. Magnetic properties of the novel nanocomposite (Zn0.15Ni0.85Fe2O4)0.15/(SiO2)0.85 at room temperature. J. Magn. Magn. Mater. 2008, 320, 1056–1062. [Google Scholar] [CrossRef]
- Caizer, C. T2 law for magnetite-based ferrofluids. J. Phys. Condens. Matter 2003, 15, 765–776. [Google Scholar] [CrossRef]
- Ito, A.; Kuga, Y.; Honda, H.; Kikkawa, H.; Horiuchi, A.; Watanabe, Y.; Kobayashi, T. Magnetite nanoparticle-loaded anti-HER2 immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett. 2004, 212, 167–175. [Google Scholar] [CrossRef]
- Smit, J.; Wijin, H.P.J. Les Ferites; Bibliotheque Technique Philips: Paris, France, 1961. [Google Scholar]
- Valenzuela, R. Magnetic Ceramics; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Caizer, C. Theoretical study on specific loss power and heating temperature in cofe2o4 nanoparticles as possible candidate for alternative cancer therapy by superparamagnetic hyperthemia. Appl. Sci. 2021, 11, 5505. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia–biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Prasad, N.K.; Rathinasamy, K.; Panda, D.; Bahadur, D. Mechanism of cell death induced by magnetic hyperthermia with nanoparticles of -MnxFe2–xO3 synthesized by a single step process. J. Mat. Chem. 2007, 17, 5042–5051. [Google Scholar] [CrossRef]
- Shinkai, M.; Ito, A. Functional magnetic particles for medical application. Adv. Biochem. Eng./Biotechnol. 2004, 91, 191–220. [Google Scholar]




| Observables | D (nm) | d (nm) | Ms * (kA/m) | K * (kJ/m3) | ρ * (×103 kg/m3) | ε | η (kg/ms) | f (kHz) | H (kA/m) |
|---|---|---|---|---|---|---|---|---|---|
| Values | 1–30 | 1.6 | 480 | 11 | 5.24 | 0.024 | 7 × 10−4 | 100–1000 | 5–50 |
| Frequency | 500 kHz | 100 kHz | ||||
|---|---|---|---|---|---|---|
| Observables | H (kA/m) | PsM (W/g) | DM (nm) | PsM (W/g) | DM (nm) | |
| Nr. | ||||||
| 1 | 10 | 15.04 | 16.2 | 3.38 | 18.1 | |
| 2 | 30 | 58.32 | 16.1 | 12.05 | 18.0 | |
| 3 | 50 | 101.72 | 16.1 | 20.73 | 18.0 | |
| Nanoparticles | Fe3O4–γ-CDs | |||
|---|---|---|---|---|
| Observables | f (kHz) |
PsM (W/g) |
DM (nm) | |
| Nr. | ||||
| 1 | 100 | 3.38 | 18.1 | |
| 2 | 200 | 6.44 | 17.2 | |
| 3 | 300 | 9.38 | 16.7 | |
| 4 | 400 | 12.25 | 16.4 | |
| 5 | 500 | 15.04 | 16.2 | |
| Observables |
H (kA/m) |
fl (kHz) |
H × f (AHz/m) |
(PsM)l (W/g) |
(DM)o (nm) | Relaxation Type | |
|---|---|---|---|---|---|---|---|
| Nr. | |||||||
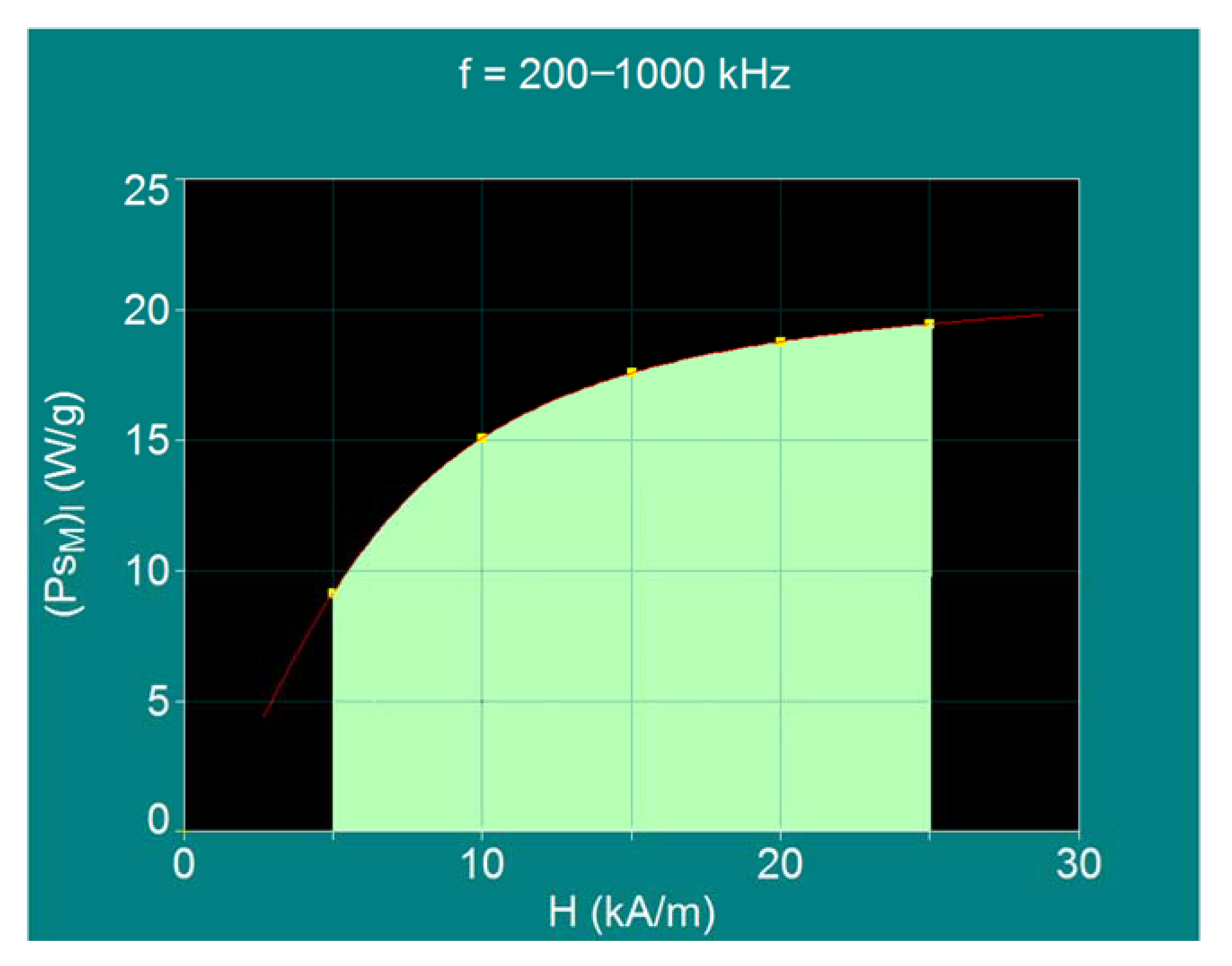
| 1 | 5 | 1000 | 5 × 109 | 9.09 | 15.5 | Neel relaxation prevails | |
| 2 | 10 | 500 | 5 × 109 | 15.04 | 16.2 | Neel >>> Brown | |
| 3 | 15 | 334 | 5 × 109 | 17.57 | 16.6 | Neel >> Brown | |
| 4 | 20 | 250 | 5 × 109 | 18.74 | 16.9 | Neel > Brown | |
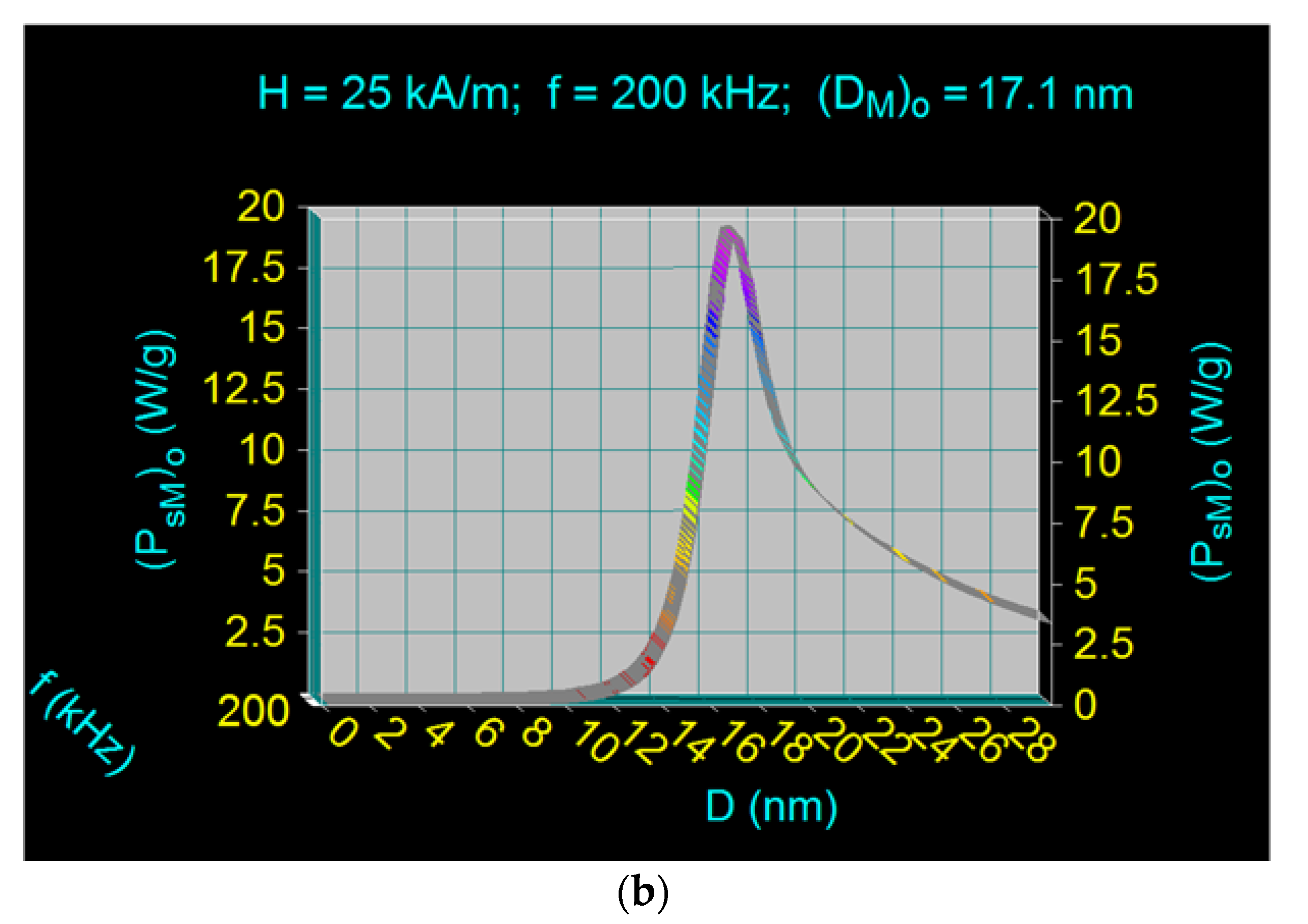
| 5 | 25 | 200 | 5 × 109 | 19.44 | 17.1 | Neel and Brown relaxations in proportion approx. equal | |
| 6 | 50 | 100 | 5 × 109 | 20.73 | 18.1 | Brown relaxation prevails | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caizer, C.; Caizer, I.S. Study on Maximum Specific Loss Power in Fe3O4 Nanoparticles Decorated with Biocompatible Gamma-Cyclodextrins for Cancer Therapy with Superparamagnetic Hyperthermia. Int. J. Mol. Sci. 2021, 22, 10071. https://doi.org/10.3390/ijms221810071
Caizer C, Caizer IS. Study on Maximum Specific Loss Power in Fe3O4 Nanoparticles Decorated with Biocompatible Gamma-Cyclodextrins for Cancer Therapy with Superparamagnetic Hyperthermia. International Journal of Molecular Sciences. 2021; 22(18):10071. https://doi.org/10.3390/ijms221810071
Chicago/Turabian StyleCaizer, Costica, and Isabela Simona Caizer. 2021. "Study on Maximum Specific Loss Power in Fe3O4 Nanoparticles Decorated with Biocompatible Gamma-Cyclodextrins for Cancer Therapy with Superparamagnetic Hyperthermia" International Journal of Molecular Sciences 22, no. 18: 10071. https://doi.org/10.3390/ijms221810071
APA StyleCaizer, C., & Caizer, I. S. (2021). Study on Maximum Specific Loss Power in Fe3O4 Nanoparticles Decorated with Biocompatible Gamma-Cyclodextrins for Cancer Therapy with Superparamagnetic Hyperthermia. International Journal of Molecular Sciences, 22(18), 10071. https://doi.org/10.3390/ijms221810071






