Radiolabeled Gold Nanoseeds Decorated with Substance P Peptides: Synthesis, Characterization and In Vitro Evaluation in Glioblastoma Cellular Models
Abstract
1. Introduction
2. Results
2.1. Synthesis and Characterization of AuNPs Carrying SP Peptides
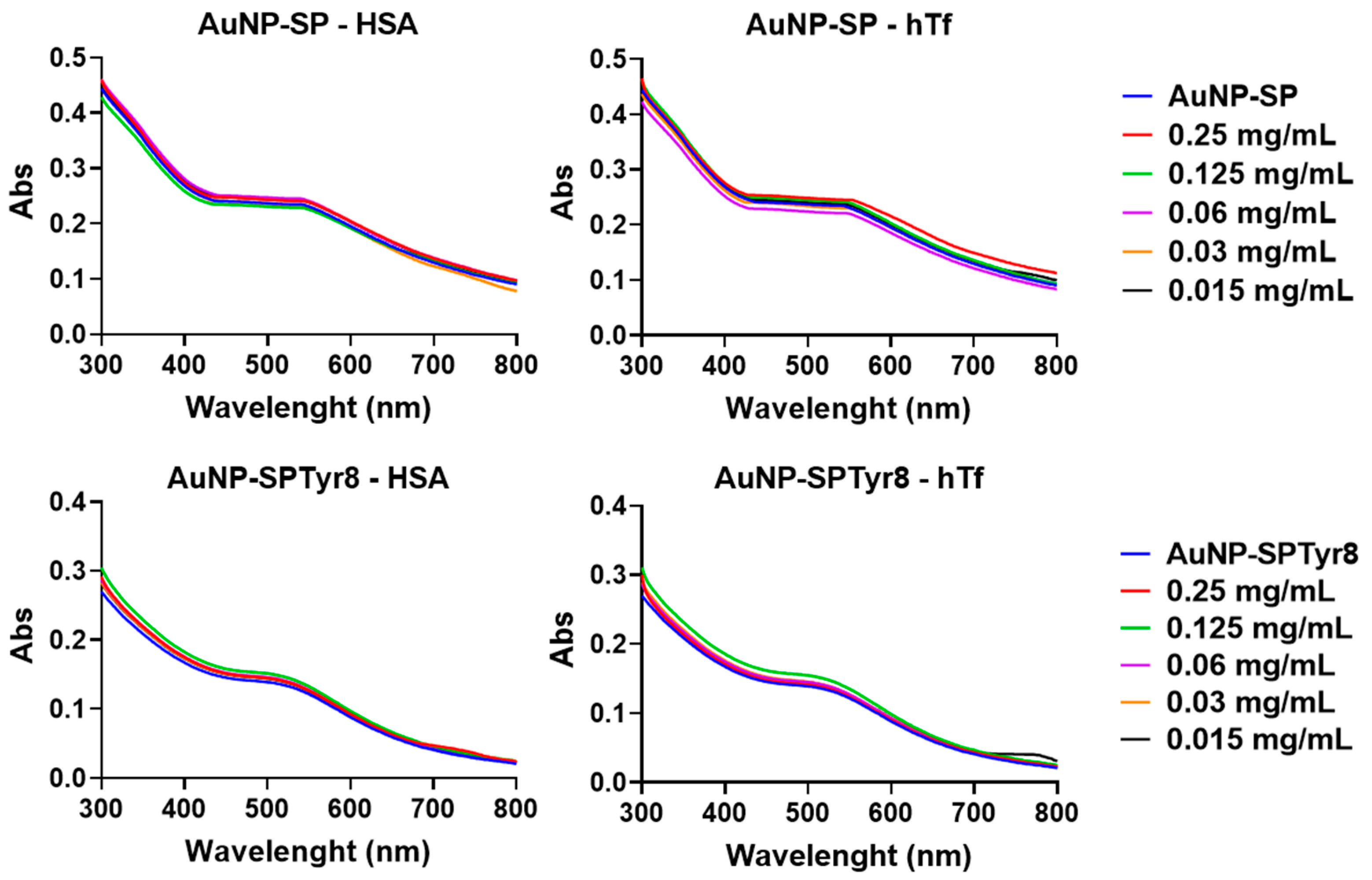
2.2. Interaction with Plasma Proteins
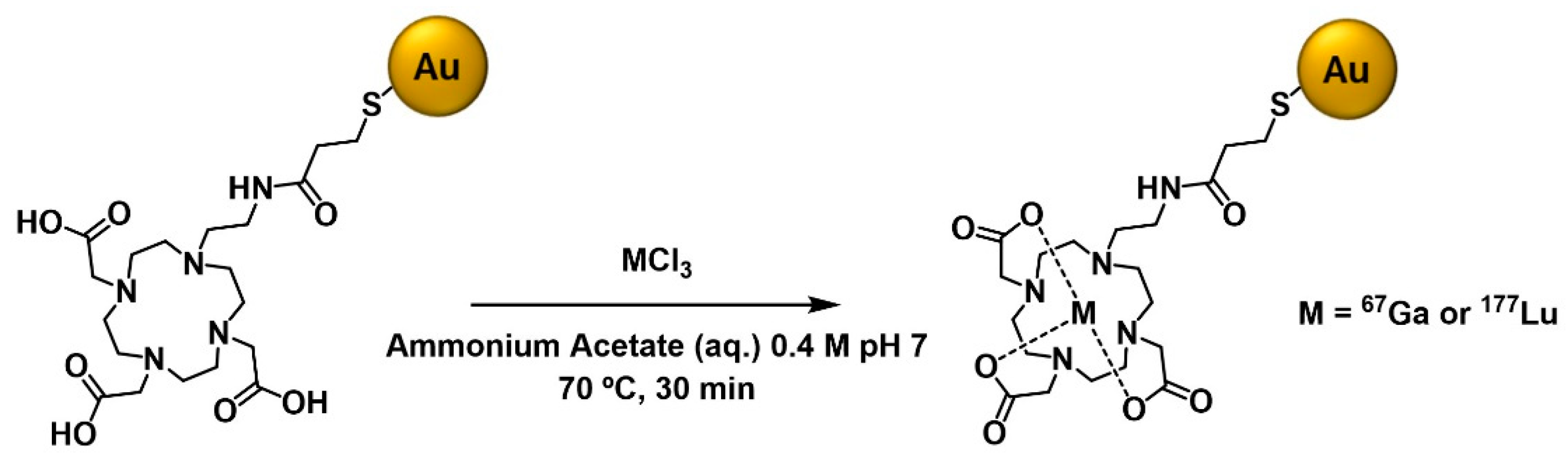
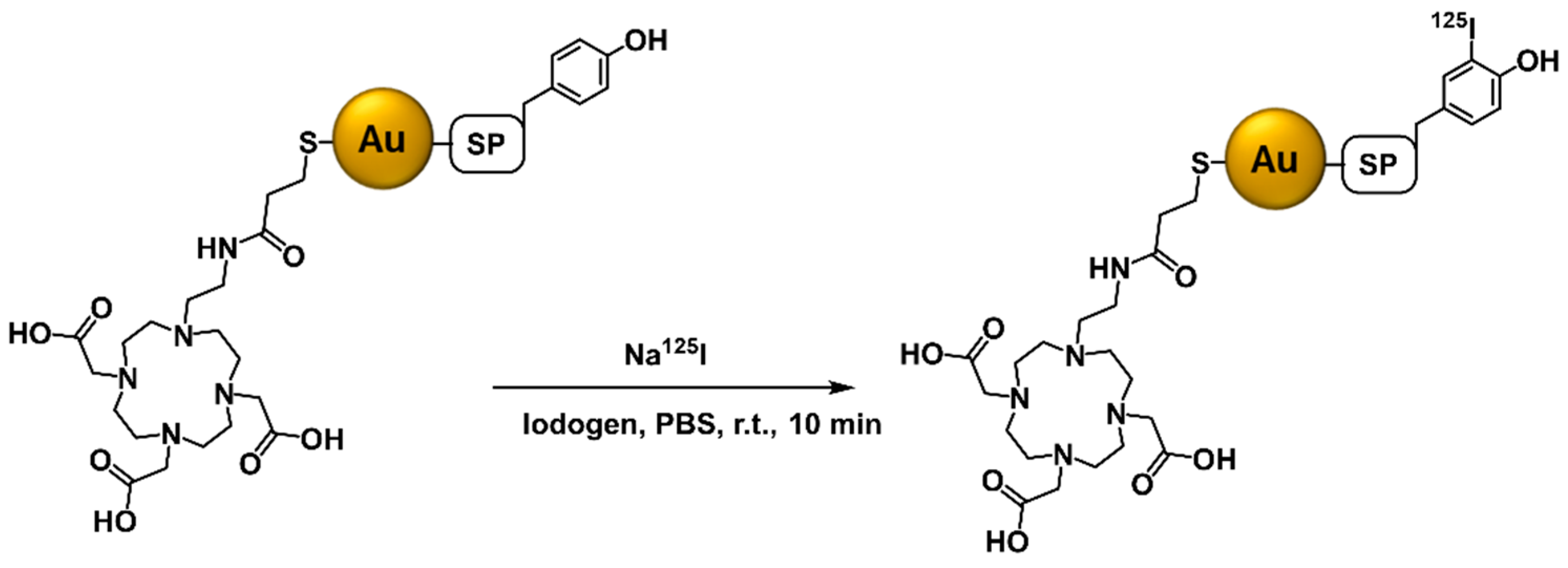
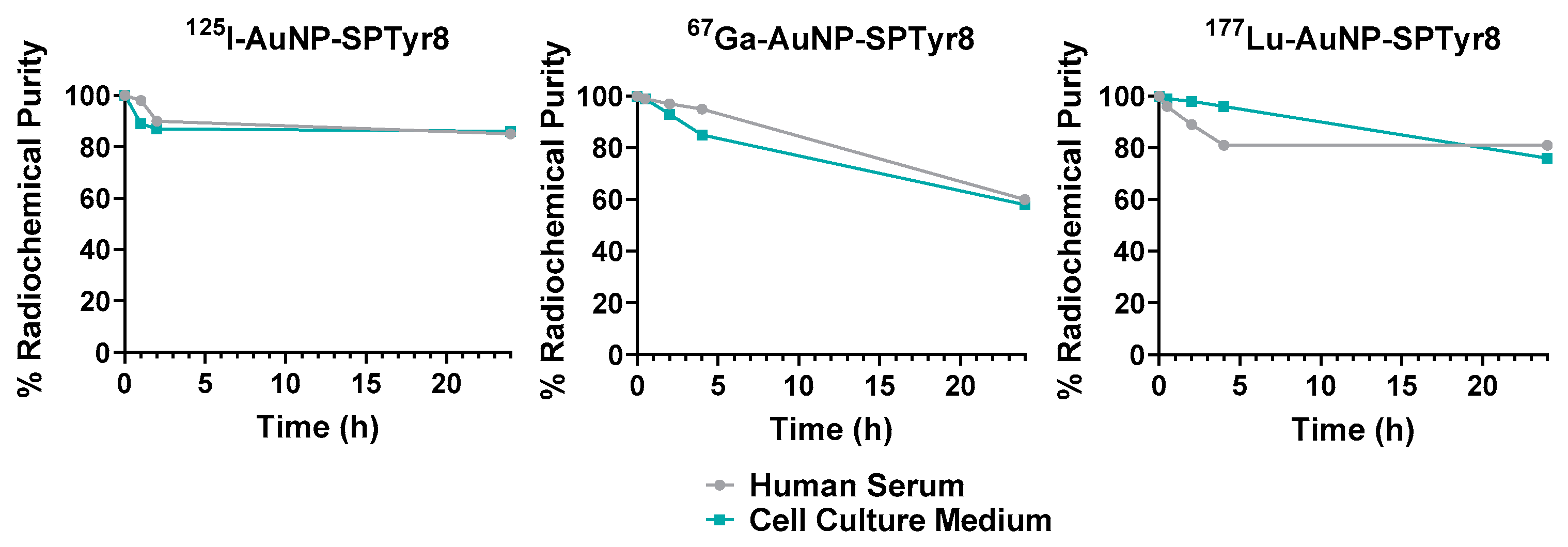
2.3. Radioiodination (125I) and Radiometallation (67Ga and 177Lu) of the AuNPs
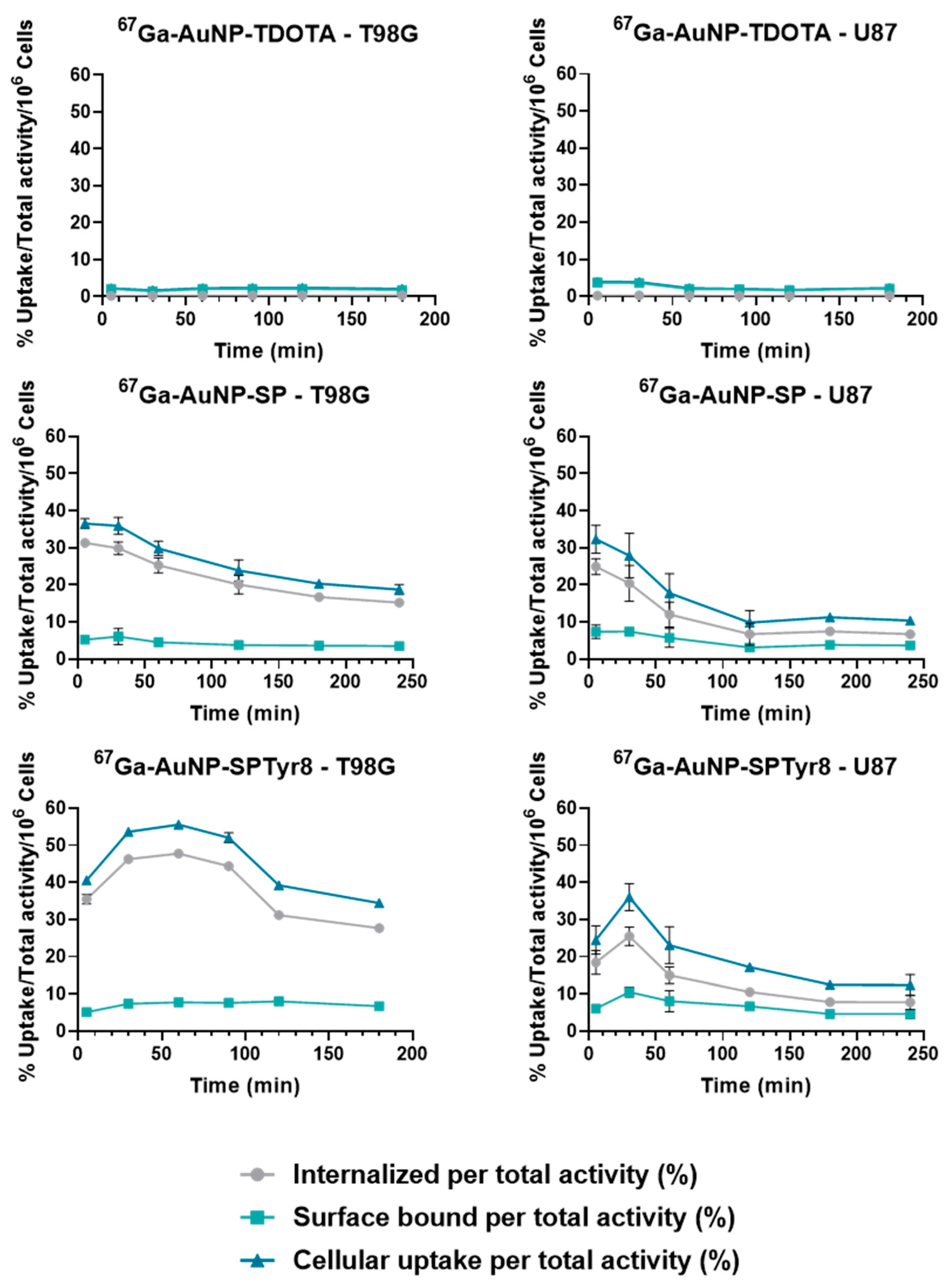
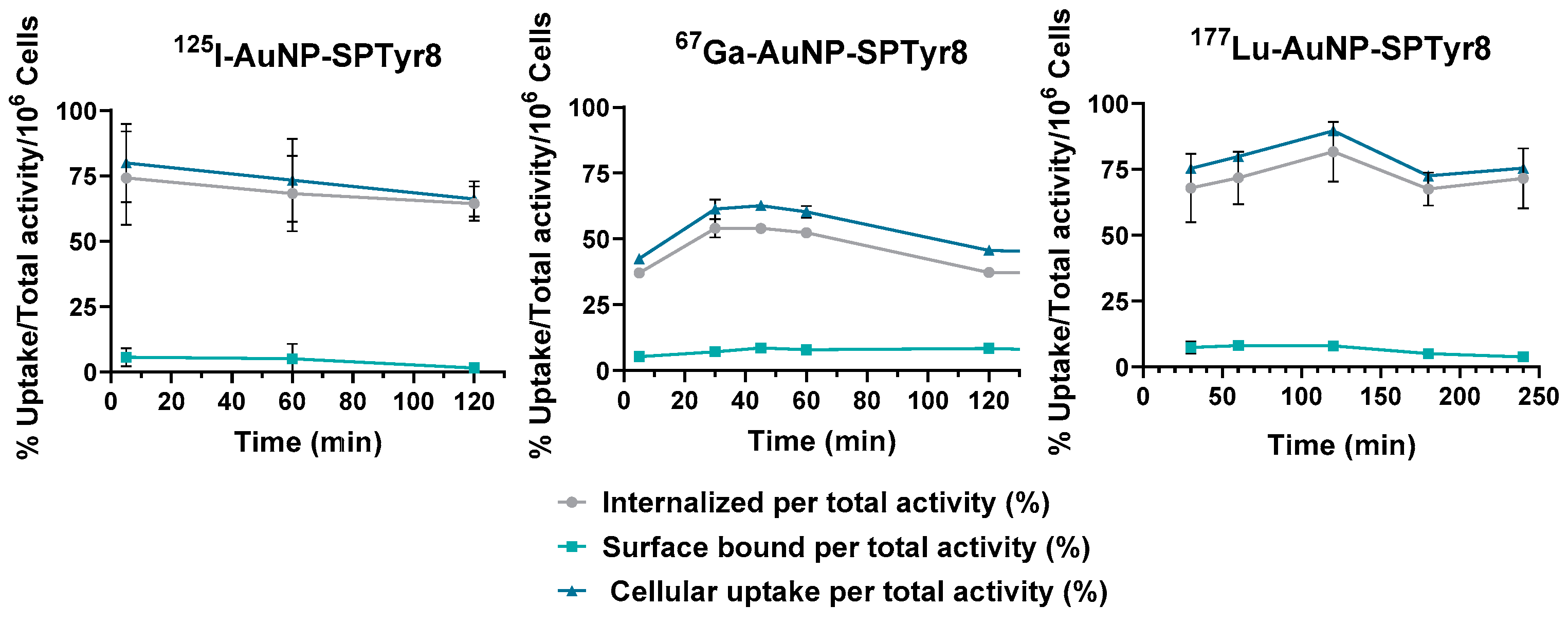
2.4. Cellular Studies
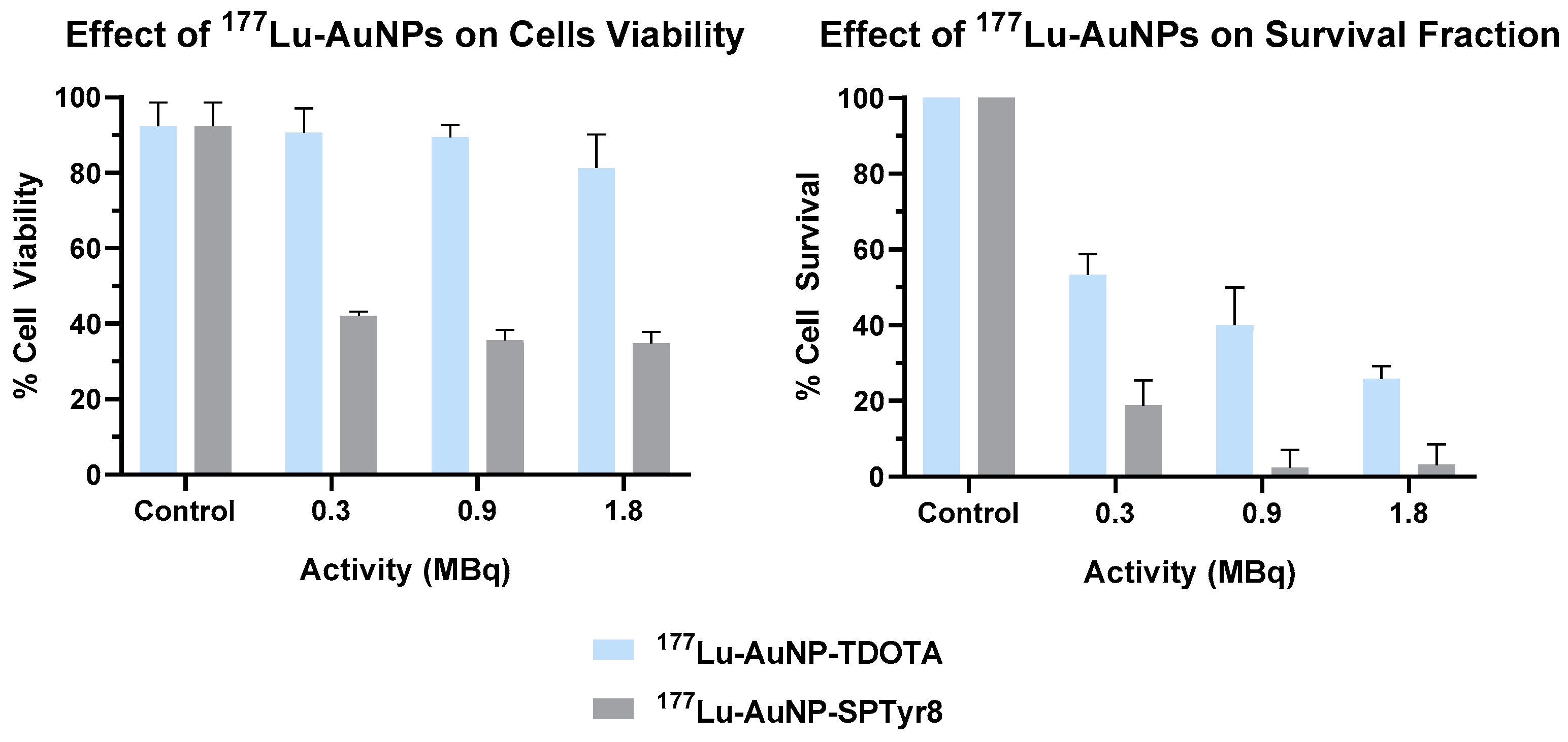
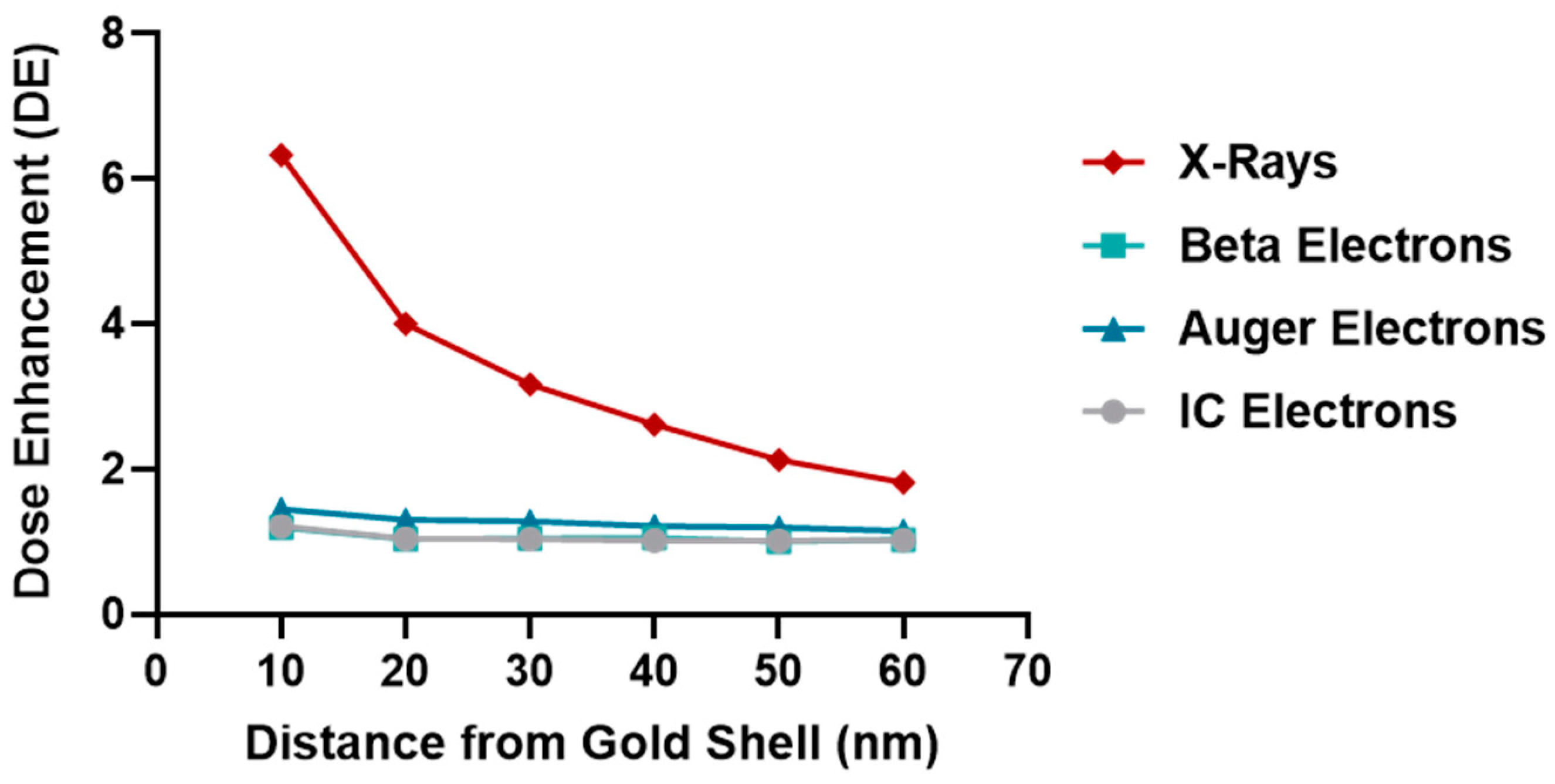
2.5. Microdosimetry Studies
- • Scenario 1
- Clonogenic assay: 70% 177Lu in the nucleus for a decay of 14 days + 4 h, 30% 177Lu in the culture medium for 4 h decay;
- MTT assay: 70% 177Lu in the nucleus for a decay of 72 h + 4 h, 30% 177Lu in the culture medium for 4 h decay.
- • Scenario 2
- Clonogenic assay: 70% 177Lu in the cytoplasm for a decay of 14 days + 4 h, 30% 177Lu in the culture medium for 4 h decay;
- MTT assay: 70% 177Lu in the cytoplasm for a decay of 72 h + 4 h, 30% 177Lu in the culture medium for 4 h decay.
- • Scenario 1/efflux
- Clonogenic assay: 70% 177Lu in the nucleus for a decay of 4 h, 30% 177Lu in the culture media decaying for 4 h (considering efflux, 70% 177Lu in the culture media decaying for 14 days);
- MTT assay: 70% 177Lu in the nucleus for a decay of 4 h, 30% 177Lu in the culture media decaying for 4 h (considering efflux, 70% 177Lu in the culture media decaying for 72 h).
- • Scenario 2/efflux
- Clonogenic assay: 70% 177Lu in the cytoplasm for a decay of 4 h, 30% 177Lu in the culture medium for 4 h decay (considering efflux, 70% 177Lu in the culture media decaying for 14 days);
- MTT assay: 70% 177Lu in the cytoplasm for a decay of 4h, 30% 177Lu in the culture medium for 4 h decay (considering efflux, 70% 177Lu in the culture media decaying for 72 h).
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Synthesis of Thioctic Acid (TA)-Terminated SP Derivatives: TA-SP and TA-SPTyr8
4.3. Synthesis of the AuNPs Functionalized with SP Peptides: AuNP-SP and AuNP-SPTyr8
4.4. Electrospray Ionization Mass Spectrometry (ESI-MS)
4.5. Dynamic Light Scattering (DLS) and Zeta Potential Determination
4.6. Transmission Electron Microscopy (TEM)
4.7. Interaction with Plasma Proteins
4.8. Radiolabeling of AuNPs with 67Ga, 177Lu and 125I
4.8.1. Radioactive Activity Measurements and Radiochromatography Analysis
4.8.2. Labeling with 67Ga
4.8.3. Labeling with 177Lu
4.8.4. Labeling with 125I
4.9. In Vitro Stability Assays
4.10. Cell Studies
4.10.1. Cell Culture
4.10.2. Western Blot Analysis
4.10.3. Cellular Uptake
4.10.4. Cell Viability Assay (MTT Assay)
4.10.5. Clonogenic Assay
4.11. Cellular Absorbed Dose Assessment
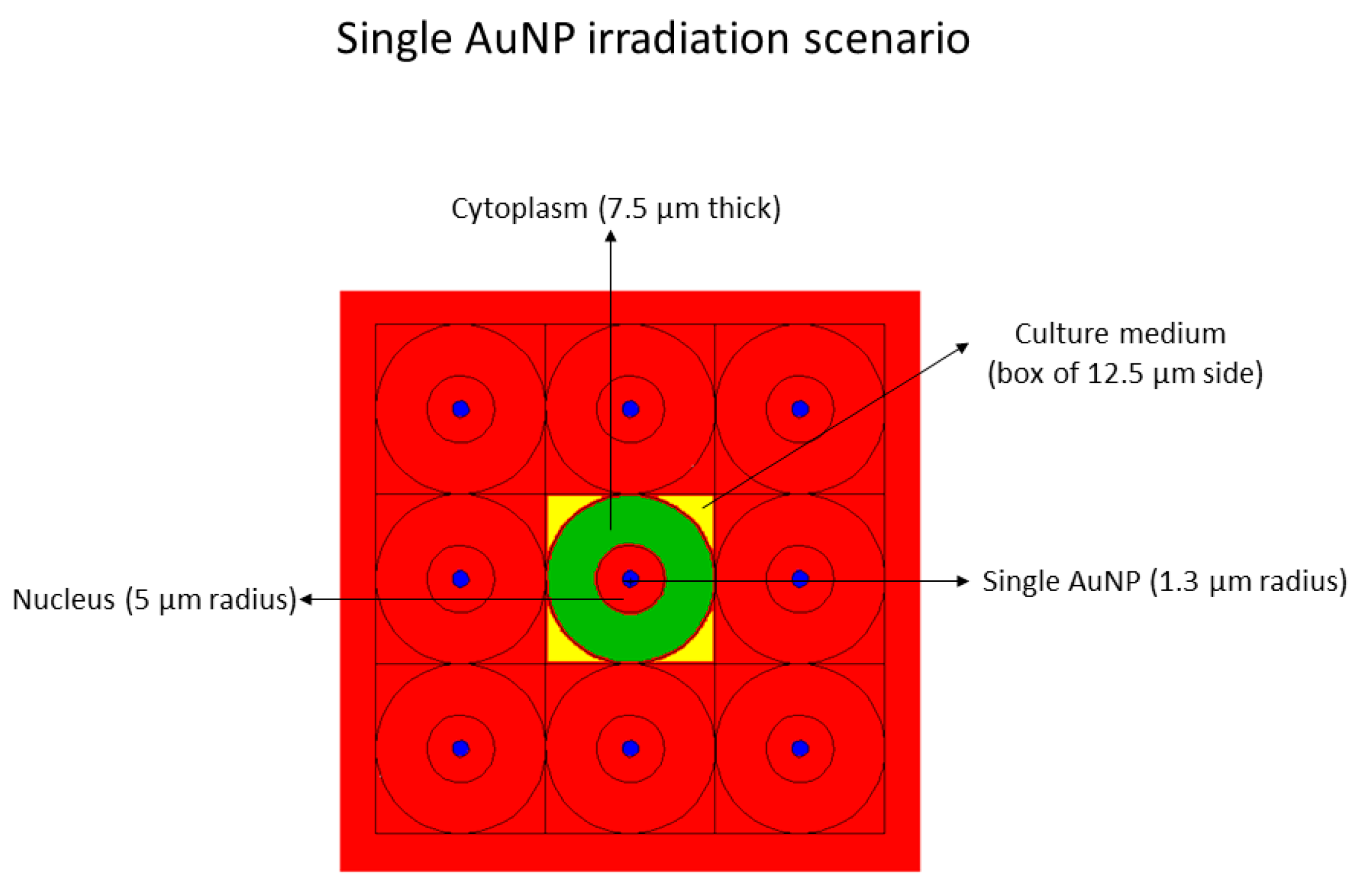
- Single AuNP at the center in the nucleus, with a radius of 1.3 µm, mimicking 2.83E8 AuNPs, each of 4 nm diameter, with a homogenous radionuclide emitting in the nucleus (see Figure 9).
- 2.
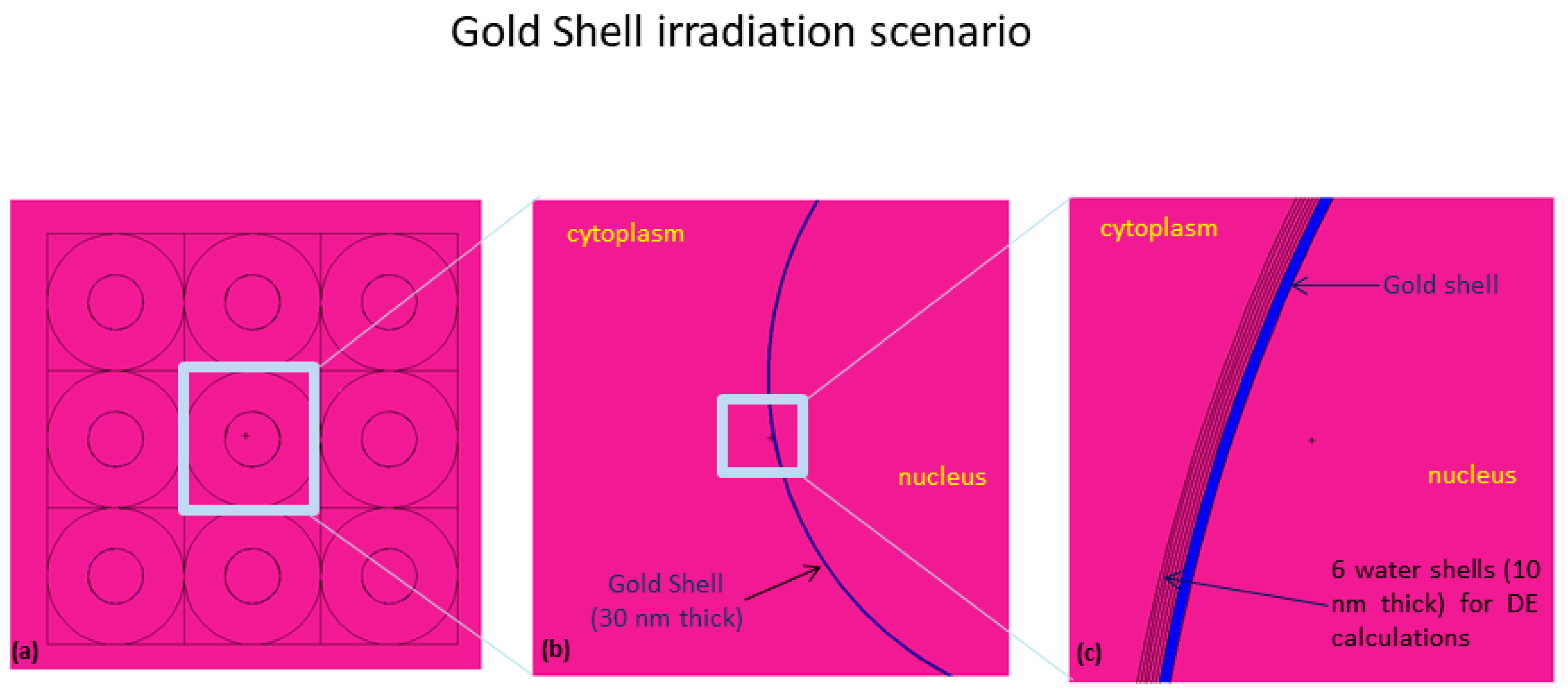
- Shell of gold, 30 nm thick, around the nucleus, mimicking 2.83E8 AuNPs, each of 4 nm diameter, with a homogeneous radionuclide distribution emitting in the cytoplasm (see Figure 10).
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Krolicki, L.; Kunikowska, J.; Bruchertseifer, F.; Koziara, H.; Krolicki, B.; Jakucinski, M.; Pawlak, D.; Rola, R.; Morgenstern, A.; Rosiak, E.; et al. 225AC-and 213BiSubstance P Analogues for Glioma Therapy. Semin. Nucl. Med. 2020, 50, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Han, I.; Wu, L.Q.; Zeng, X. Targeted Nanotechnology in Glioblastoma Multiforme. Front. Pharmacol. 2017, 8, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.; Antosh, M.P. Effect of size on gold nanoparticles in radiation therapy: Uptake and survival effects. J. Nanomed. 2019, 2, 1013. [Google Scholar]
- Schuemann, J.; Berbeco, R.; Chithrani, D.B.; Cho, S.H.; Kumar, R.; McMahon, S.J.; Sridhar, S.; Krishnan, S. Roadmap to Clinical Use of Gold Nanoparticles for Radiation Sensitization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 189–205. [Google Scholar] [CrossRef]
- Joh, D.Y.; Sun, L.; Stangl, M.; Al Zaki, A.; Murty, S.; Santoiemma, P.P.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Bhang, D.; et al. Selective Targeting of Brain Tumors with Gold Nanoparticle-Induced Radiosensitization. PLoS ONE 2013, 8, e62425. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, M.F.; Wang, J.Z.; Wang, T.Q.; Yao, Y.; Zhang, X.M.; Zhang, C.; Zhang, N. Thermosensitive Hydrogel Co-loaded with Gold Nanoparticles and Doxorubicin for Effective Chemoradiotherapy. AAPS J. 2016, 18, 146–155. [Google Scholar] [CrossRef]
- Silva, F.; Campello, M.P.C.; Paulo, A. Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer. Materials 2021, 14. [Google Scholar] [CrossRef]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef]
- Moeendarbari, S.; Tekade, R.; Mulgaonkar, A.; Christensen, P.; Ramezani, S.; Hassan, G.; Jiang, R.; Oz, O.K.; Hao, Y.W.; Sun, X.K. Theranostic Nanoseeds for Efficacious Internal Radiation Therapy of Unresectable Solid Tumors. Sci. Rep. 2016, 6, 20614. [Google Scholar] [CrossRef]
- Yook, S.; Cai, Z.L.; Lu, Y.J.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Radiation Nanomedicine for EGFR-Positive Breast Cancer: Panitumumab-Modified Gold Nanoparticles Complexed to the beta-Particle-Emitter, Lu-177. Mol. Pharm. 2015, 12, 3963–3972. [Google Scholar] [CrossRef]
- Yook, S.; Cai, Z.L.; Lu, Y.J.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Intratumorally Injected Lu-177-Labeled Gold Nanoparticles: Gold Nanoseed Brachytherapy with Application for Neoadjuvant Treatment of Locally Advanced Breast Cancer. J. Nucl. Med. 2016, 57, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Majkowska-Pilip, A.; Gaweda, W.; Zelechowska-Matysiak, K.; Wawrowicz, K.; Bilewicz, A. Nanoparticles in Targeted Alpha Therapy. Nanomaterials 2020, 10, 1366. [Google Scholar] [CrossRef]
- Dziawer, L.; Kozminski, P.; Meczynska-Wielgosz, S.; Pruszynski, M.; Lyczko, M.; Was, B.; Celichowski, G.; Grobelny, J.; Jastrzebski, J.; Bilewicz, A. Gold nanoparticle bioconjugates labelled with At-211 for targeted alpha therapy. RSC Adv. 2017, 7, 41024–41032. [Google Scholar] [CrossRef]
- Piotrowska, A.; Meczynska-Wielgosz, S.; Majkowska-Pilip, A.; Kozminski, P.; Wojciuk, G.; Cedrowska, E.; Bruchertseifer, F.; Morgenstern, A.; Kruszewski, M.; Bilewicz, A. Nanozeolite bioconjugates labeled with Ra-223 for targeted alpha therapy. Nucl. Med. Biol. 2017, 47, 10–18. [Google Scholar] [CrossRef]
- Cedrowska, E.; Pruszynski, M.; Majkowska-Pilip, A.; Meczynska-Wielgosz, S.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Functionalized TiO2 nanoparticles labelled with Ac-225 for targeted alpha radionuclide therapy. J. Nanoparticle Res. 2018, 20, 1–10. [Google Scholar] [CrossRef]
- Salvanou, E.A.; Stellas, D.; Tsoukalas, C.; Mavroidi, B.; Paravatou-Petsotas, M.; Kalogeropoulos, N.; Xanthopoulos, S.; Denat, F.; Laurent, G.; Bazzi, R.; et al. A Proof-of-Concept Study on the Therapeutic Potential of Au Nanoparticles Radiolabeled with the Alpha-Emitter Actinium-225. Pharmaceutics 2020, 12, 188. [Google Scholar] [CrossRef]
- Hennrich, U.; Kopka, K. Lutathera (R): The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharmaceuticals 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA Theranostics: Review of the Current Status of PSMA-Targeted Imaging and Radioligand Therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Broaddus, W.C.; Dorn, H.C.; Fatouros, P.P.; Chalfant, C.E.; Shultz, M.D. Metallofullerene-Nanoplatform-Delivered Interstitial Brachytherapy Improved Survival in a Murine Model of Glioblastoma Multiforme. Bioconjugate Chem. 2012, 23, 1873–1880. [Google Scholar] [CrossRef][Green Version]
- Shi, Y.; Van der Meel, R.; Chen, X.Y.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Vilchis-Juarez, A.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Avila, E.; Ocampo-Garcia, B.; Diaz-Nieto, L.; Luna-Gutierrez, M.; Jimenez-Mancilla, N.; Pedraza-Lopez, M.; Gomez-Olivan, L. Molecular Targeting Radiotherapy with Cyclo-RGDfK(C) Peptides Conjugated to Lu-177-Labeled Gold Nanoparticles in Tumor-Bearing Mice. J. Biomed. Nanotechnol. 2014, 10, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Hennig, I.M.; Laissue, J.A.; Horisberger, U.; Reubi, J.C. Substance-P receptors in human primary neoplasms—Tumoral and vascular localization. Int. J. Cancer 1995, 61, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Rizk, S.S.; Luchniak, A.; Uysal, S.; Brawley, C.M.; Rock, R.S.; Kossiakoff, A.A. An engineered substance P variant for receptor-mediated delivery of synthetic antibodies into tumor cells. Proc. Natl. Acad. Sci. USA 2009, 106, 11011–11015. [Google Scholar] [CrossRef] [PubMed]
- Cordier, D.; Forrer, F.; Kneifel, S.; Sailer, M.; Mariani, L.; Macke, H.; Muller-Brand, J.; Merlo, A. Neoadjuvant targeting of glioblastoma multiforme with radiolabeled DOTAGA-substance P-results from a phase I study. J. Neuro-Oncol. 2010, 100, 129–136. [Google Scholar] [CrossRef]
- Reulen, H.J.; Poepperl, G.; Goetz, C.; Gildehaus, F.J.; Schmidt, M.; Tatsch, K.; Pietsch, T.; Kraus, T.; Rachinger, W. Long-term outcome of patients with WHO Grade III and IV gliomas treated by fractionated intracavitary radioimmunotherapy. J. Neurosurg. 2015, 123, 760–770. [Google Scholar] [CrossRef]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Krolicki, B.; Jakucinski, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Safety and efficacy of targeted alpha therapy with Bi-213-DOTA-substance P in recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 614–622. [Google Scholar] [CrossRef]
- Silva, F.; Zambre, A.; Campello, M.P.C.; Gano, L.; Santos, I.; Ferraria, A.M.; Ferreira, M.J.; Singh, A.; Upendran, A.; Paulo, A.; et al. Interrogating the Role of Receptor-Mediated Mechanisms: Biological Fate of Peptide-Functionalized Radiolabeled Gold Nanoparticles in Tumor Mice. Bioconjugate Chem. 2016, 27, 1153–1164. [Google Scholar] [CrossRef]
- Locarno, S.; Bucci, R.; Impresari, E.; Gelmi, M.L.; Pellegrino, S.; Clerici, F. Ultrashort Peptides and Gold Nanoparticles: Influence of Constrained Amino Acids on Colloidal Stability. Front. Chem. 2021, 9, 823. [Google Scholar] [CrossRef]
- Lévy, R.; Thanh, N.T.K.; Doty, R.C.; Hussain, I.; Nichols, R.J.; Schiffrin, D.J.; Brust, M.; Fernig, D.G. Rational and Combinatorial Design of Peptide Capping Ligands for Gold Nanoparticles. J. Am. Chem. Soc. 2004, 126, 10076–10084. [Google Scholar] [CrossRef] [PubMed]
- Avvakumova, S.; Galbiati, E.; Sironi, L.; Locarno, S.A.; Gambini, L.; Macchi, C.; Pandolfi, L.; Ruscica, M.; Magni, P.; Collini, M.; et al. Theranostic Nanocages for Imaging and Photothermal Therapy of Prostate Cancer Cells by Active Targeting of Neuropeptide-Y Receptor. Bioconjugate Chem. 2016, 27, 2911–2922. [Google Scholar] [CrossRef]
- Silva, F.; Paulo, A.; Pallier, A.; Meme, S.; Toth, E.; Gano, L.; Marques, F.; Geraldes, C.; Castro, M.; Cardoso, A.M.; et al. Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics. Materials 2020, 13, 17. [Google Scholar] [CrossRef]
- Fisher, G.H.; Folkers, K.; Pernow, B.; Bowers, C.Y. Hypothalamic Hormones.71. Synthesis and Some Biological-Activities of Tyrosine-8 Analog of Substance-p. J. Med. Chem. 1976, 19, 325–328. [Google Scholar] [CrossRef]
- Kitagawa, K.; Ban, Y.; Ujita, K.; Akita, T.; Segawa, T.; Nakata, Y.; Yajima, H. Synthesis of 2 Substance-P Analogs 8-Tyr And 5-Asn Substance-P. Chem. Pharm. Bull. 1978, 26, 2899–2903. [Google Scholar] [CrossRef][Green Version]
- Carmine, A.; Domoto, Y.; Sakai, N.; Matile, S. Comparison of Lipoic and Asparagusic Acid for Surface-Initiated Disulfide-Exchange Polymerization. Chem.—A Eur. J. 2013, 19, 11558–11563. [Google Scholar] [CrossRef] [PubMed]
- Trzciński, J.W.; Morillas-Becerril, L.; Scarpa, S.; Tannorella, M.; Muraca, F.; Rastrelli, F.; Castellani, C.; Fedrigo, M.; Angelini, A.; Tavano, R.; et al. Poly(lipoic acid)-Based Nanoparticles as Self-Organized, Biocompatible, and Corona-Free Nanovectors. Biomacromolecules 2021, 22, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Vitali, M.; Casals, E.; Canals, F.; Colome, N.; Puntes, V. Simple spectroscopic determination of the hard protein corona composition in AuNPs: Albumin at 75%. Nanoscale 2020, 12, 15832–15844. [Google Scholar] [CrossRef]
- Terracciano, R.; Zhang, A.B.; Butler, E.B.; Demarchi, D.; Hafner, J.H.; Grattoni, A.; Filgueira, C.S. Effects of Surface Protein Adsorption on the Distribution and Retention of Intratumorally Administered Gold Nanoparticles. Pharmaceutics 2021, 13, 216. [Google Scholar] [CrossRef]
- Ban, Z.; Yuan, P.; Yu, F.B.; Peng, T.; Zhou, Q.X.; Hu, X.G. Machine learning predicts the functional composition of the protein corona and the cellular recognition of nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 10492–10499. [Google Scholar] [CrossRef]
- Li, S.H.; Peng, Z.L.; Leblanc, R.M. Method To Determine Protein Concentration in the Protein Nanoparticle Conjugates Aqueous Solution Using Circular Dichroism Spectroscopy. Anal. Chem. 2015, 87, 6455–6459. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Development and application of a label-free fluorescence method for determining the composition of gold nanoparticle-protein conjugates. Int. J. Mol. Sci. 2014, 16, 907–923. [Google Scholar] [CrossRef]
- Cui, M.H.; Liu, R.X.; Deng, Z.Y.; Ge, G.L.; Liu, Y.; Xie, L.M. Quantitative study of protein coronas on gold nanoparticles with different surface modifications. Nano Res. 2014, 7, 345–352. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Kneipp, J. SERS Probing of Proteins in Gold Nanoparticle Agglomerates. Front. Chem. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Bailey, G.S. The Iodogen Method for Radiolabeling Protein. Protein Protoc. Handb. 1996, 5, 673–674. [Google Scholar]
- Buch, K.; Peters, T.; Nawroth, T.; Sanger, M.; Schmidberger, H.; Langguth, P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT Assay—A comparative study. Radiat. Oncol. 2012, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Subiel, A.; Ashmore, R.; Schettino, G. Standards and Methodologies for Characterizing Radiobiological Impact of High-Z Nanoparticles. Theranostics 2016, 6, 1651–1671. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, B.; Wu, H.; Dhawan, A.P.; Du, P.; Howell, R.W. MIRD pamphlet No. 25: MIRDcell V2.0 software tool for dosimetric analysis of biologic response of multicellular populations. J. Nucl. Med. 2014, 55, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.L. Recent progress in Monte Carlo simulation on gold nanoparticle radiosensitization. AIMS Biophys. 2018, 5, 231–244. [Google Scholar] [CrossRef]
- Gholami, Y.H.; Maschmeyer, R.; Kuncic, Z. Radio-enhancement effects by radiolabeled nanoparticles. Sci. Rep. 2019, 9, 13. [Google Scholar] [CrossRef]
- Li, W.B.; Beuve, M.; Di Maria, S.; Friedland, W.; Heide, B.; Klapproth, A.P.; Li, C.Y.; Poignant, F.; Rabus, H.; Rudek, B.; et al. Intercomparison of dose enhancement ratio and secondary electron spectra for gold nanoparticles irradiated by X-rays calculated using multiple Monte Carlo simulation codes. Phys. Med. 2020, 69, 147–163. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 27. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Saraee, K.R.E.; Sani, S.F.A.; Bradley, D.A. Metallic nanoparticle radiosensitization: The role of Monte Carlo simulations towards progress. Radiat. Phys. Chem. 2021, 180, 15. [Google Scholar] [CrossRef]
- Yook, S.; Lu, Y.J.; Jeong, J.J.; Cai, Z.L.; Tong, L.; Alwarda, R.; Pignol, J.P.; Winnik, M.A.; Reilly, R.M. Stability and Biodistribution of Thiol-Functionalized and Lu-177-Labeled Metal Chelating Polymers Bound to Gold Nanoparticles. Biomacromolecules 2016, 17, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Seniwal, B.; Thipe, V.C.; Singh, S.; Fonseca, T.C.F.; Freitas, F.d. Recent Advances in Brachytherapy Using Radioactive Nanoparticles: An Alternative to Seed-Based Brachytherapy. Front. Oncol. 2021, 11, 766407. [Google Scholar] [CrossRef] [PubMed]
- Kneifel, S.; Cordier, D.; Good, S.; Ionescu, M.C.S.; Ghaffari, A.; Hofer, S.; Kretzschmar, M.; Tolnay, M.; Apostolidis, C.; Waser, B.; et al. Local targeting of malignant gliomas by the diffusible peptidic vector 1,4,7,10-tetraazacyclododecane-1-glutaric acid-4,7,10-triacetic acid-substance P. Clin. Cancer Res. 2006, 12, 3843–3850. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Su, N.; Dang, Y.J.; Liang, G.L.; Liu, G.Z. Iodine-125-labeled cRGD-gold nanoparticles as tumor-targeted radiosensitizer and imaging agent. Nanoscale Res. Lett. 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeon, J.; Hong, S.H.; Rhim, W.K.; Lee, Y.S.; Youn, H.; Chung, J.K.; Lee, M.C.; Lee, D.S.; Kang, K.W.; et al. Tumor Targeting and Imaging Using Cyclic RGD-PEGylated Gold Nanoparticle Probes with Directly Conjugated Iodine-125. Small 2011, 7, 2052–2060. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 15. [Google Scholar] [CrossRef]
- Tsopelas, C. A study of radiogallium aqueous chemistry: In vitro and in vivo characterisation of Ga-67-hydrolysed-stannous fluoride particles. J. Label. Compd. Radiopharm. 2016, 59, 197–204. [Google Scholar] [CrossRef]
- Guerreiro, J.F.; Alves, V.; Abrunhosa, A.J.; Paulo, A.; Gil, O.M.; Mendes, F. Radiobiological Characterization of (CuCl2)-Cu-64 as a Simple Tool for Prostate Cancer Theranostics. Molecules 2018, 23, 2944. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Goorley, J.T.; James, M.R.; Booth, T.E.; Brown, F.B.; Bull, J.S.; Cox, L.J.; Durkee, J.W., Jr.; Elson, J.S.; Fensin, M.L.; Forster, R.A., III; et al. Initial MCNP6 Release Overview—MCNP6 Version 1.0; Los Alamos National Lab. (LANL): Los Alamos, NM, USA, 2013. [Google Scholar] [CrossRef]
- Jung, S.; Sung, W.; Lee, J.; Ye, S.J. MCNP6.1 simulations for low-energy atomic relaxation: Code-to-code comparison with GATEv7.2, PENELOPE2014, and EGSnrc. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2018, 415, 117–126. [Google Scholar] [CrossRef]
- Engels, E.; Bakr, S.; Bolst, D.; Sakata, D.; Li, N.; Lazarakis, P.; McMahon, S.J.; Ivanchenko, V.; Rosenfeld, A.B.; Incerti, S.; et al. Advances in modelling gold nanoparticle radiosensitization using new Geant4-DNA physics models. Phys. Med. Biol. 2020, 65, 225017. [Google Scholar] [CrossRef] [PubMed]
- Eckerman, K.; Endo, A. Nuclear decay data for dosimetric calculations. Ann. ICRP 2008, 38, 7–96. [Google Scholar]

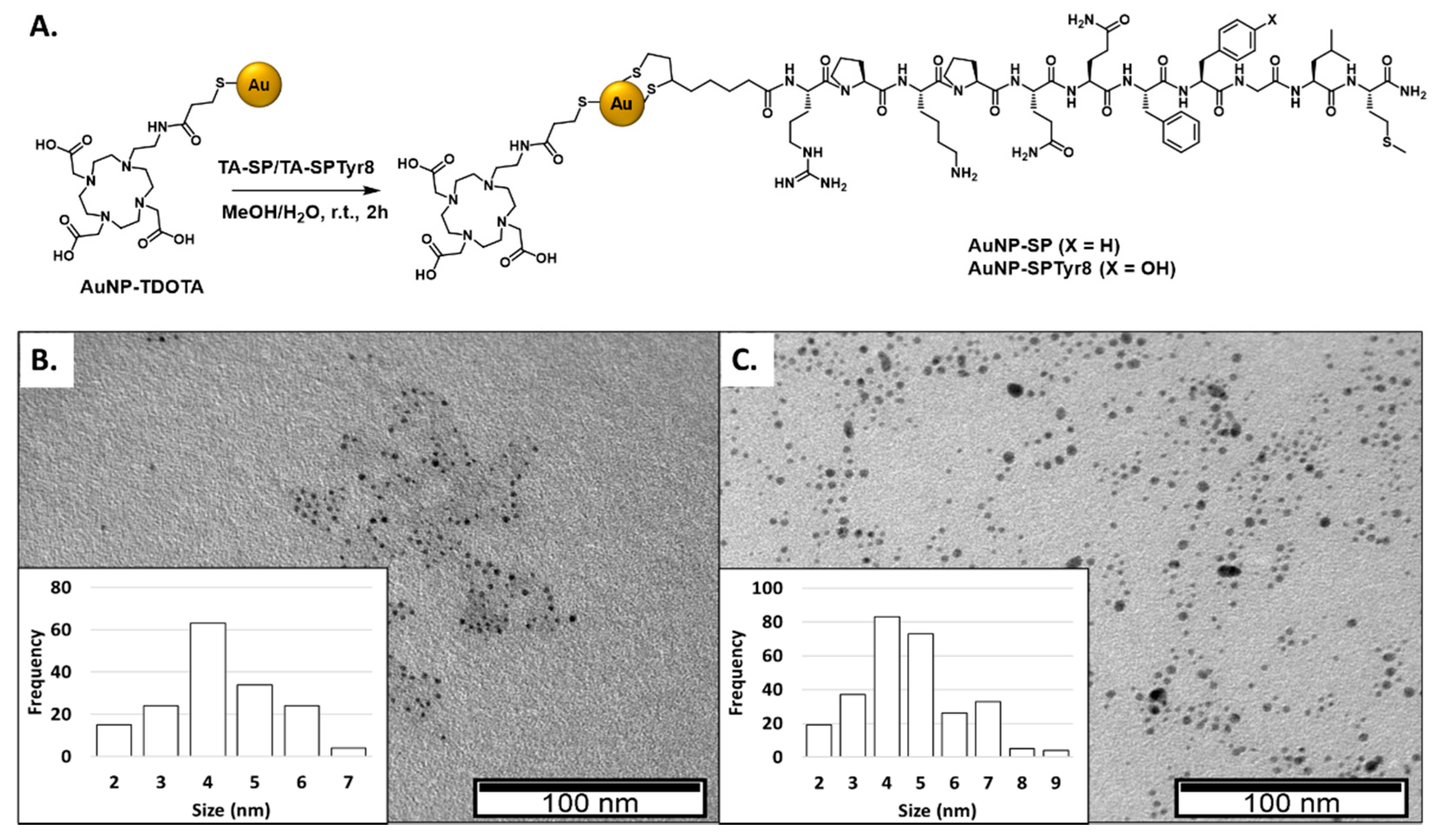

| AuNP | TEM (nm) | Hydrodynamic Size (PDI) (nm) | Zeta Potential (ζ) (mV) | UV-Vis (nm) |
|---|---|---|---|---|
| AuNP-TDOTA [28] | 4.29 ± 1.60 | 20.6 (0.3) | −62.6 ± 18.6 | 520 |
| AuNP-SP | 4.24 ± 1.21 | 93.9 (0.8)) | −40.9 ± 10.8 | 520 |
| AuNP-SPTyr8 | 4.68 ± 1.51 | 96.2 (0.7) | −38.9 ± 14.6 | 520 |
| Applied Activity (MBq) | Average Cell Dose–Scenario 1 (Gy) | Average Cell Dose–Scenario 2 (Gy) | Average Cell Dose-Scenario 1/Efflux (Gy) | Average Cell Dose-Scenario 2/Efflux (Gy) |
|---|---|---|---|---|
| 0.03 | 6.40 a 2.33 b | 1.51 a 0.55 b | 0.59 a 0.31 b | 0.48 a 0.20 b |
| 0.9 | 19.20 a 7.00 b | 4.52 a 1.66 b | 1.78 a 0.93 b | 1.45 a 0.60 b |
| 1.8 | 38.39 a 14.00 b | 9.05 a 3.31 b | 3.56 a 1.86 b | 2.90 a 1.20 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.; D’Onofrio, A.; Mendes, C.; Pinto, C.; Marques, A.; Campello, M.P.C.; Oliveira, M.C.; Raposinho, P.; Belchior, A.; Di Maria, S.; et al. Radiolabeled Gold Nanoseeds Decorated with Substance P Peptides: Synthesis, Characterization and In Vitro Evaluation in Glioblastoma Cellular Models. Int. J. Mol. Sci. 2022, 23, 617. https://doi.org/10.3390/ijms23020617
Silva F, D’Onofrio A, Mendes C, Pinto C, Marques A, Campello MPC, Oliveira MC, Raposinho P, Belchior A, Di Maria S, et al. Radiolabeled Gold Nanoseeds Decorated with Substance P Peptides: Synthesis, Characterization and In Vitro Evaluation in Glioblastoma Cellular Models. International Journal of Molecular Sciences. 2022; 23(2):617. https://doi.org/10.3390/ijms23020617
Chicago/Turabian StyleSilva, Francisco, Alice D’Onofrio, Carolina Mendes, Catarina Pinto, Ana Marques, Maria Paula Cabral Campello, Maria Cristina Oliveira, Paula Raposinho, Ana Belchior, Salvatore Di Maria, and et al. 2022. "Radiolabeled Gold Nanoseeds Decorated with Substance P Peptides: Synthesis, Characterization and In Vitro Evaluation in Glioblastoma Cellular Models" International Journal of Molecular Sciences 23, no. 2: 617. https://doi.org/10.3390/ijms23020617
APA StyleSilva, F., D’Onofrio, A., Mendes, C., Pinto, C., Marques, A., Campello, M. P. C., Oliveira, M. C., Raposinho, P., Belchior, A., Di Maria, S., Marques, F., Cruz, C., Carvalho, J., & Paulo, A. (2022). Radiolabeled Gold Nanoseeds Decorated with Substance P Peptides: Synthesis, Characterization and In Vitro Evaluation in Glioblastoma Cellular Models. International Journal of Molecular Sciences, 23(2), 617. https://doi.org/10.3390/ijms23020617









