Oxidative Stress and NADPH Oxidase: Connecting Electromagnetic Fields, Cation Channels and Biological Effects
Abstract
1. Introduction
2. EMF Effect on Membrane Function and Cation Channels Can Induce OS
- EMFs can act on membrane-associated free ions by means of a forced-vibration mechanism, which acts on the voltage-gated channels using the vibrations that EMFs cause on the transported ions [2,3]. These are the Κ+ leak channels, the Νa+ leak channels, and the leak channels of other cations such as Ca2+. Briefly, the open or closed state of these channels is determined by the electrostatic interaction between the voltage sensors of the channels and the transmembrane voltage, and they change between their open and closed state when the electrostatic force on the electric charges of their voltage sensors exceeds a critical value. Alternating electric fields exert a periodic force on all of the free ions passing across voltage-gated channels and can distort the electrochemical equilibrium state of every oscillating ion due to its site-displacement from the developed electrochemical gradient. This results in a periodical displacement of the electric charge, which will exert force on every fixed charge, such as those existing on the voltage sensors of the voltage-gated channels. Specifically, voltage-gated channels contain transmembrane a-helices in parallel, forming pores through which ions pass in their dehydrated state. The voltage sensors of these channels are four positively charged symmetrically arranged transmembrane alpha-helical domains (designated S4). It is known that small voltage changes of about 30 mV in the membrane potential are able to gate this kind of channel [14,15]. Such a change can be caused by the displacement of a single ion by 10−12 m from the electric field of the EMF and in the vicinity S4 of the voltage-gated channels. Hence, EMF-induced oscillating ions can disturb the electrochemical balance of the membrane via the gating of such channels, and those ions crossing such channels can change their normal positions and can produce a false signal for the gating such channels with their charge. This mechanism can also explain the biological action of oscillating magnetic fields by replacing the force of the electric field with the force exerted by an alternating magnetic field and also by accounting for the induced electric field, which is always generated by the pulsed magnetic one. The mechanism concludes that oscillating electric or magnetic fields with frequencies lower than 1.6 × 104 Hz (ELF and VLF fields) can be bioactive, even at very low intensities [2,16]. It is also claimed that pulsed EMFs can even further amplify their biological action compared to continuous EMFs [16,17,18].
- In the other mechanism [7,8], low intensity EMFs cause abnormal hypersensitivity on voltage-gated calcium channels (VGCC), with consequent increases in intracellular Ca2+ (and OS) acting on the structure of the aforementioned voltage sensor (and its 20 electrically charged groups that are equally distributed on its 4 alpha-helixes), which actually regulates the opening of the channel. They transverse the lipid bilayer of the membrane and their disturbance by the EMFs relies on the following very important and distinct reasons: The EMF forces on the voltage sensor are 20 times higher since they act on all 20 charges, and according to Coulomb’s Law of physics, they are inversely proportional to the dielectric constant of the fatty part of the membrane, which is about 120 times less than the forces on the charges in the aqueous parts of the cell. Thus, the forces exerted on the 20 electrically charged groups of the VGCC’s voltage sensor by the electric field of the RF EMF will also be about 120 times stronger (or 2400 times stronger, accounting for all 20 charges), which is only because of the dielectric constant of the fatty region of the cell membrane where the voltage sensor’s 20 charges are located. A third, even more important, reason is based on the fact that the cell membrane has a very high electrical resistance, which acts as amplifier of the electrical gradient (the difference in electrical charge across the cell membrane), amplifying it by about 3000 times. Combining these three distinct reasons, it is implied that the total amplification of the exerted forces by the RF EMF electric fields on the VGCC voltage sensor’s 20 electrical charges is equal to 20 × 120 times (due to the dielectric constant of the fatty inner space of the membrane) × 3000 times (due to the electrical gradient of the membrane), totaling 7,200,000 times. That is, the forces exerted on the VGCC voltage sensor by the RF EMFs are about 7.2 million times stronger than those in the electrically charged groups that are in the hydrophilic environment of our cells, which is where the safety guidelines for the RF EMF are set by ICNIRP.
3. Biochemistry of ROS
4. EMF-Induced OS, and Biological Effects
5. Conclusions
6. Theory and Research Perspectives for a Conclusive Linking of EMFs with ROS/RNS
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Schuermann, D.; Mevissen, M. Manmade electromagnetic fields and oxidative stress—Biological effects and consequences for health. Int. J. Mol. Sci. 2021, 22, 3772. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Karabarbounis, A.; Margaritis, L.H. Mechanism for action of electromagnetic fields on cells. Biochem. Biophys. Res. Commun. 2002, 298, 95–102. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Margaritis, L.H. Theoretical considerations for the biological effects of electromagnetic fields. In Biological Effects of Electromagnetic Fields; Stavroulakis, P., Ed.; Springer Science & Business Media: Heidelberg, Germany; London, UK, 2003. [Google Scholar]
- Kıvrak, E.G.; Yurt, K.K.; Kaplan, A.A.; Alkan, I.; Altun, G. Effects of electromagnetic fields exposure on the antioxidant defense system. J. Microsc. Ultrastruct. 2017, 5, 167–176. [Google Scholar] [CrossRef]
- Pall, M.L. Scientific evidence contradicts findings and assumptions of Canadian Safety Panel 6: Microwaves act through voltage-gated calcium channel activation to induce biological impacts at non-thermal levels, supporting a paradigm shift for microwave/lower frequency electromagnetic field action. Rev. Environ. Health 2015, 30, 99–116. [Google Scholar]
- Pall, M.L. Electromagnetic fields act similarly in plants as in animals: Probable activation of calcium channels via their voltage sensor. Curr. Chem. Biol. 2016, 10, 74–82. [Google Scholar] [CrossRef]
- Pall, M.L. How cancer can be caused by microwave frequency electromagnetic field (EMF) exposures: EMF activation of voltage-gated calcium channels (VGCCs) can cause cancer including tumor promotion, tissue invasion and metastasis via 15 mechanisms. In Mobile Communications and Public Health; Markov, M., Ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Pall, M.L. 5G Risk: The Scientific Perspective; The 5G Summit: Washington, DC, USA, 2020. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Membrane Transport of Small Molecules and the Electrical Properties of Membranes-Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Kiselyov, K.; Muallem, S. ROS and intracellular ion channels. Cell Calcium 2016, 60, 108–114. [Google Scholar] [CrossRef]
- Ramírez, A.; Vázquez-Sánchez, A.Y.; Carrión-Robalino, H.; Camacho, J. Ion channels and oxidative stress as a potential link for the diagnosis or treatment of liver diseases. Oxid. Med. Cel. Longev. 2016, 2016, 3928714. [Google Scholar] [CrossRef]
- Patel, R.; Sesti, F. Oxidation of ion channels in the aging nervous system. Brain Res. 2016, 1639, 174–185. [Google Scholar] [CrossRef]
- Bachmann, M.; Li, W.; Edwards, M.J.; Ahmad, S.A.; Patel, S.; Szabo, I.E.G. Voltage-gated potassium channels as regulators of cell death. Front. Cell Dev. Biol. 2020, 8, 611853. [Google Scholar] [CrossRef]
- Bezanilla, F.; White, M.M.; Taylor, R.E. Gating currents associated with potassium channel activation. Nature 1982, 296, 657–659. [Google Scholar] [CrossRef]
- Liman, E.R.; Hess, P.; Weaver, F.; Koren, G. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature 1991, 353, 752–756. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Messini, N.; Karabarbounis, A.; Philippetis, A.L.; Margaritis, L.H. A mechanism for action of oscillating electric fields on cells. Biochem. Biophys. Res. Commun. 2000, 272, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J.; Johansson, O.; Carlo, G.L. Polarization: A key difference between man-made and natural electromagnetic fields, in regard to biological activity. Sci. Rep. 2015, 5, 14914. [Google Scholar] [CrossRef]
- Panagopoulos, D.J. Comparing DNA damage induced by mobile telephony and other types of man-made electromagnetic fields. Mutat. Res. 2019, 781, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Hurst, J.K. What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 2012, 53, 508–520. [Google Scholar] [CrossRef]
- Friedman, J.; Kraus, S.; Hauptman, Y.; Schiff, Y.; Seger, R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem. J. 2007, 405, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.N.; Weir, E.K.; Peers, C. Diphenylamine iodonium blocks K+ and Ca2+ currents in type I cells isolated from the rat carotid body. Neurosci. Lett. 1994, 172, 63–66. [Google Scholar] [CrossRef]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef]
- DeCoursey, T.E. Interactions between NADPH oxidase and voltage-gated proton channels: Why electron transport depends on proton transport. FEBS Lett. 2003, 555, 57–61. [Google Scholar] [CrossRef]
- DeCoursey, T.; Morgan, D.; Cherny, V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 2003, 422, 531–534. [Google Scholar] [CrossRef] [PubMed]
- DeCoursey, T.E. Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology 2010, 25, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Musset, B.; Cherny, V.V.; Morgan, D.; DeCoursey, T.E. The intimate and mysterious relationship between proton channels and NADPH oxidase. FEBS Lett. 2009, 583, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, I.S.; Ruchti, E.; Kaczmarek, J.S.; Clapham, D.E. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc. Natl. Acad. Sci. USA 2009, 106, 7642–7647. [Google Scholar] [CrossRef]
- Seredenina, T.; Demaurex, N.; Krause, K.H. Voltage-gated proton channels as novel drug targets: From NADPH oxidase regulation to sperm biology. Antioxid. Redox Signal. 2015, 23, 490–513. [Google Scholar] [CrossRef]
- Rada, B.K.; Geiszt, M.; Hably, C.; Ligeti, E. Consequences of the electrogenic function of the phagocytic NADPH oxidase. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.M. NADPH oxidase subunit gp91phox: A proton pathway. Protoplasma 2001, 217, 37–42. [Google Scholar] [CrossRef]
- O’Rourke, B.; Cortassa, S.; Aon, M.A. Mitochondrial ion channels: Gatekeepers of life and death. Physiology 2005, 20, 303–315. [Google Scholar] [CrossRef]
- O-Uchi, J.; Ryu, S.Y.; Jhun, B.S.; Hurst, S.; Sheu, S.S. Mitochondrial ion channels/transporters as sensors and regulators of cellular redox signaling. Antioxid. Redox Signal. 2014, 21, 987–1006. [Google Scholar] [CrossRef]
- Akbarali, H.I. Oxidative stress and ion channels. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Sahoo, N.; Hoshi, T.; Heinemann, S.H. Oxidative modulation of voltage-gated potassium channels. Antioxid. Redox Signal. 2014, 21, 933–952. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–911. [Google Scholar] [CrossRef]
- Phillips, J.L.; Singh, N.P.; Lai, H. Electromagnetic fields and DNA damage. Pathophysiology 2009, 16, 79–88. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. Roy. Soc. A 1934, 147, 332–351. [Google Scholar]
- Barb, W.G.; Baxendale, J.H.; George, P.; Hargrave, K.R. Reactions of ferrous and ferric ions with hydrogen peroxide. Part I.—The ferrous ion reaction. Trans. Faraday Soc. 1951, 47, 462–500. [Google Scholar] [CrossRef]
- Barb, W.G.; Baxendale, J.H.; George, P.; Hargrave, K.R. Reactions of ferrous and ferric ions with hydrogen peroxide. Part II.—The ferric ion reaction. Trans. Faraday Soc. 1951, 47, 591–616. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Hsieh, Y.P. Kinetics of Fe(III) reduction by ascorbic acid in aqueous solutions. J. Agric. Food Chem. 2000, 48, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Illés, E.; Mizrahi, A.; Marks, V.; Meyerstein, D. Carbonate-radical-anions, and not hydroxyl radicals, are the products of the Fenton reaction in neutral solutions containing bicarbonate. Free Radic. Biol. Med. 2019, 131, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. On the irrelevancy of hydroxyl radical to DNA damage from oxidative stress and implications for epigenetics. Chem. Soc. Rev. 2020, 2020. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E. Hydroxyl radical production by ascorbate and hydrogen peroxide. Neurotox. Res. 2000, 2, 1029–8428. [Google Scholar] [CrossRef]
- Blough, N.V.; Zafiriou, O.C. Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg. Chem. 1985, 24, 3502–3504. [Google Scholar] [CrossRef]
- Gunaydin, H.; Houk, K.N. Molecular dynamics simulation of the HOONO decomposition and the HO*/NO2* caged radical pair in water. J. Am. Chem. Soc. 2008, 130, 10036–10037. [Google Scholar] [CrossRef]
- Clément, M.-V.; Pervaiz, S. Intracellular superoxide and hydrogen peroxide concentrations: A critical balance that determines survival or death. Redox Rep. 2001, 6, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; Goodman, R. A mechanism for stimulation of biosynthesis by electromagnetic fields: Charge transfer in DNA and base pair separation. J. Cell Physiol. 2008, 214, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Challis, L.J. Mechanisms for interaction between RF fields and biological tissue. Bioelectromagnetics 2005, 26 (Suppl. 7), 98–106. [Google Scholar] [CrossRef]
- McNamee, J.P.; Chauhan, V. Radiofrequency radiation and gene/protein expression: A review. Radiat. Res. 2009, 172, 265–287. [Google Scholar] [CrossRef]
- Lee, K.S.; Choi, J.S.; Hong, S.Y.; Son, T.H.; Yu, K. Mobile phone electromagnetic radiation activates MAPK signaling and regulates viability in Drosophila. Bioelectromagnetics 2008, 29, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Minelli, T.A.; Balduzzo, M.; Milone, F.F.; Nofrate, V. Modeling cell dynamics under mobile phone radiation. Nonlin. Dyn. Psychol. Life Sci. 2007, 11, 197–218. [Google Scholar]
- Manta, A.K.; Papadopoulou, D.; Polyzos, A.P.; Fragopoulou, A.F.; Skouroliakou, A.S.; Thanos, D.; Stravopodis, D.J.; Margaritis, L.H. Mobile-phone radiation-induced perturbation of gene-expression profiling, redox equilibrium and sporadic-apoptosis control in the ovary of Drosophila melanogaster. Fly 2017, 11, 75–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barteri, M.; Pala, A.; Rotella, S. Structural and kinetic effects of mobile phone microwaves on acetylcholinesterase activity. Biophys. Chem. 2005, 113, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Nelson, C.; Parekh, A.B. Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J. Biol. Chem. 2011, 286, 14795–14803. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, A.F.; Samara, A.; Antonelou, M.H.; Xanthopoulou, A.; Papadopoulou, A.; Vougas, K.; Koutsogiannopoulou, E.; Anastasiadou, E.; Stravopodis, D.J.; Tsangaris, G.T.; et al. Brain proteome response following whole body exposure of mice to mobile phone or wireless DECT base radiation. Electromagn. Biol. Med. 2012, 31, 250–274. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Galati, S.; Boni, C.; Gerra, M.C.; Lazzaretti, M.; Buschini, A. Autophagy: A player in response to oxidative stress and DNA damage. Oxid. Med. Cell. Longev. 2019, 2019, 5692958. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef] [PubMed]
- Kanofsky, J.R. Singlet oxygen production by biological systems. Chem. Biol. Interact. 1989, 70, 1–28. [Google Scholar] [CrossRef]
- Onyango, A.N. Endogenous generation of singlet oxygen and ozone in human and animal tissues: Mechanisms, biological significance, and influence of dietary components. Oxid. Med. Cell Longev. 2016, 2016, 2398573. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA strand breaking by hydroxyl radical is governed by the accessible surface area of the hydrogen atom of the DNA backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9738–9743. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.P.; Ravanat, J.L.; Sauvaigo, S. Hydroxyl radicals and DNA base damage. Mutat. Res. 1999, 1424, 9–21. [Google Scholar] [CrossRef]
- Tsunoda, M.; Sakaue, T.; Naito, S.; Sunami, T.; Abe, N.; Ueno, Y.; Matsuda, A.; Takénaka, A. Insights into the structures of DNA damaged by hydroxyl radical: Crystal structures of DNA duplexes containing 5-formyluracil. J. Nucleic Acids 2010, 2010, 107289. [Google Scholar] [CrossRef]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Wada, K.; Kabuta, T. Lysosomal degradation of intracellular nucleic acids—multiple autophagic pathways. J. Biochem. 2017, 161, 145–154. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Lai, H.; Singh, N.P. Single- and double-strand DNA breaks in rat brain cells after acute exposure to radiofrequency electromagnetic radiation. Int. J. Radiat. Biol. 1996, 69, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Guler, G.; Tomruk, A.; Ozgur, E.; Seyhan, N. The effect of radiofrequency radiation on DNA and lipid damage in non-pregnant and pregnant rabbits and their newborns. Gen. Physiol. Biophys. 2010, 29, 59–66. [Google Scholar] [CrossRef]
- Ruediger, H.W. Genotoxic effects of radiofrequency electromagnetic fields. Pathophysiology 2009, 16, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Yakymenko, I.; Tsybulin, O.; Sidorik, E.; Henshel, D.; Kyrylenko, O.; Kyrylenko, S. Oxidative mechanisms of biological activity of low-intensity radiofrequency radiation. Electromagn. Biol. Med. 2016, 35, 186–202. [Google Scholar] [CrossRef]
- Agarwal, A.; Desai, N.R.; Makker, K.; Varghese, A.; Mouradi, R.; Sabanegh, E.; Sharma, R. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: An in vitro pilot study. Fertil. Steril. 2009, 92, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- De Luliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef]
- Irmak, M.K.; Fadillioğlu, E.; Güleç, M.; Erdoğan, H.; Yağmurca, M.; Akyol, O. Effects of electromagnetic radiation from a cellular telephone on the oxidant and antioxidant levels in rabbits. Cell Biochem. Funct. 2002, 20, 279–283. [Google Scholar] [CrossRef]
- Manta, A.K.; Stravopodis, D.J.; Papassideri, I.S.; Margaritis, L.H. Reactive oxygen species elevation and recovery in Drosophila bodies and ovaries following short-term and long-term exposure to DECT base EMF. Electromagn. Biol. Med. 2014, 33, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J.; Karabarbounis, A.; Margaritis, L.H. Effect of GSM 900-MHz mobile phone radiation on the reproductive capacity of Drosophila melanogaster. Electromagn. Biol. Med. 2004, 23, 29–43. [Google Scholar] [CrossRef]
- Margaritis, L.H.; Manta, A.K.; Kokkaliaris, K.D.; Schiza, D.; Alimisis, K.; Barkas, G.; Georgiou, E.; Giannakopoulou, O.; Kollia, I.; Kontogianni, G.; et al. Drosophila oogenesis as a bio-marker responding to EMF sources. Electromagn. Biol. Med. 2014, 33, 165–189. [Google Scholar] [CrossRef]
- Martin-Romero, F.J.; Gutiérrez-Martín, Y.; Henao, F.; Gutiérrez-Merino, C. The NADH oxidase activity of the plasma membrane of synaptosomes is a major source of superoxide anion and is inhibited by peroxynitrite. J. Neurochem. 2002, 82, 604–614. [Google Scholar] [CrossRef]
- Stefi, A.L.; Margaritis, L.H.; Skouroliakou, A.S.; Vassilacopoulou, D. Mobile phone electromagnetic radiation affects Amyloid Precursor Protein and α-synuclein metabolism in SH-SY5Y cells. Pathophysiology 2019, 26, 203–212. [Google Scholar] [CrossRef]
- Ntzouni, M.P.; Skouroliakou, A.; Kostomitsopoulos, N.; Margaritis, L.H. Transient and cumulative memory impairments induced by GSM 1.8 GHz cell phone signal in a mouse model. Electromagn. Biol. Med. 2013, 32, 95–120. [Google Scholar] [CrossRef]
- Ntzouni, M.P.; Stamatakis, A.; Stylianopoulou, F.; Margaritis, L.H. Short-term memory in mice is affected by mobile phone radiation. Pathophysiology 2011, 18, 193–199. [Google Scholar] [CrossRef]
- Maskey, D.; Kim, M.; Aryal, B.; Pradhan, J.; Choi, I.-Y.; Park, K.-S.; Son, T.; Hong, S.-Y.; Kim, S.B.; Kim, H.G.; et al. Effect of 835MHz radiofrequency radiation exposure on calcium binding proteins in the hippocampus of the mouse brain. Brain Res. 2010, 1313, 232–241. [Google Scholar] [CrossRef]
- Santini, M.T.; Ferrante, A.; Rainaldi, G.; Indovina, P.; Indovina, P.L. Extremely low frequency (ELF) magnetic fields and apoptosis: A review. Int. J. Radiat. Biol. 2005, 81, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sagioglou, N.E.; Manta, A.K.; Giannarakis, I.K.; Skouroliakou, A.S.; Margaritis, L.H. Apoptotic cell death during Drosophila oogenesis is differentially increased by electromagnetic radiation depending on modulation, intensity and duration of exposure. Electromagn. Biol. Med. 2016, 35, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, C.D. Oxidative stress-induced biological damage by low-level EMFs: Mechanism of free radical pair electron spin-polarization and biochemical amplification. In Non-Thermal Effects and Mechanisms of Interaction between Electromagnetic Fields and Living Matter; Giuliani, L., Soffritti, M., Eds.; Collegium Ramazzini (European J. Oncology Library): Bologna, Italy, 2010; Volume 5, pp. 63–113. [Google Scholar]
- Adams, B.; Sinayskiy, I.; Petruccione, F. An open quantum system approach to the radical pair mechanism. Sci. Rep. 2018, 8, 15719. [Google Scholar] [CrossRef] [PubMed]
- IARC, International Agency for Research on Cancer (IARC) Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2013; Volume 102. Available online: https://www.ncbi.nlm.nih.gov/books/NBK304630/ (accessed on 16 September 2021).
- Georgiou, D.C.; Papapostolou, I.; Patsoukis, N.; Tsegenidis, T.; Sideris, T. An ultrasensitive fluorescent assay for the in vivo quantification of superoxide radical in organisms. Anal. Biochem. 2005, 347, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, C.D.; Papapostolou, I.; Grintzalis, K. Superoxide radical detection in cells, tissues, organisms (animals, plants, insects, microorganisms) and soils. Nat. Protoc. 2008, 3, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
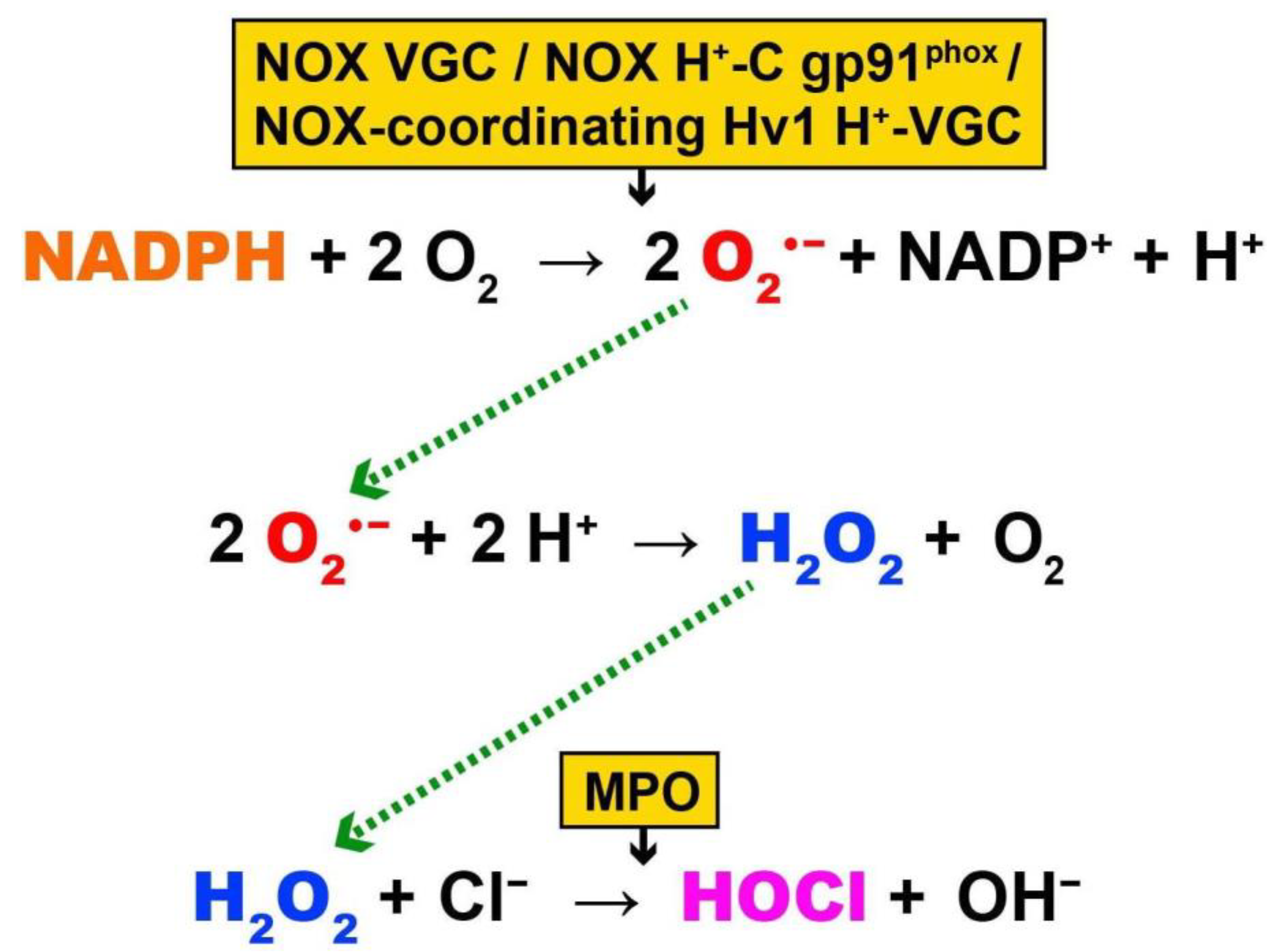
| Fe2+/Cu+ + H2O2 → Fe3+/Cu2+ + •OH + HO− (1) |
| Fe3+ + O2•− → Fe2+ + O2 (2) |
| Fe3+ + H2O2 → Fe2+ + H+ + HO2• (3) |
| 2 Fe3+ + AH2 → 2 Fe2+ + A + 2 H+ (4) |
| AH− + H2O2 → A•− + H2O + •OH (5) |
| NO + O2•− + H+ → ONOOH (6) |
| ONOOH → •OH + •NO2 (7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiou, C.D.; Margaritis, L.H. Oxidative Stress and NADPH Oxidase: Connecting Electromagnetic Fields, Cation Channels and Biological Effects. Int. J. Mol. Sci. 2021, 22, 10041. https://doi.org/10.3390/ijms221810041
Georgiou CD, Margaritis LH. Oxidative Stress and NADPH Oxidase: Connecting Electromagnetic Fields, Cation Channels and Biological Effects. International Journal of Molecular Sciences. 2021; 22(18):10041. https://doi.org/10.3390/ijms221810041
Chicago/Turabian StyleGeorgiou, Christos D., and Lukas H. Margaritis. 2021. "Oxidative Stress and NADPH Oxidase: Connecting Electromagnetic Fields, Cation Channels and Biological Effects" International Journal of Molecular Sciences 22, no. 18: 10041. https://doi.org/10.3390/ijms221810041
APA StyleGeorgiou, C. D., & Margaritis, L. H. (2021). Oxidative Stress and NADPH Oxidase: Connecting Electromagnetic Fields, Cation Channels and Biological Effects. International Journal of Molecular Sciences, 22(18), 10041. https://doi.org/10.3390/ijms221810041






