Antimicrobial Peptides from Plants: A cDNA-Library Based Isolation, Purification, Characterization Approach and Elucidating Their Modes of Action
Abstract
1. Introduction
2. Expression Systems Applied for AMP Production
2.1. Bacterial Expression System
2.2. Yeast Expression System
2.3. Plant Expression System
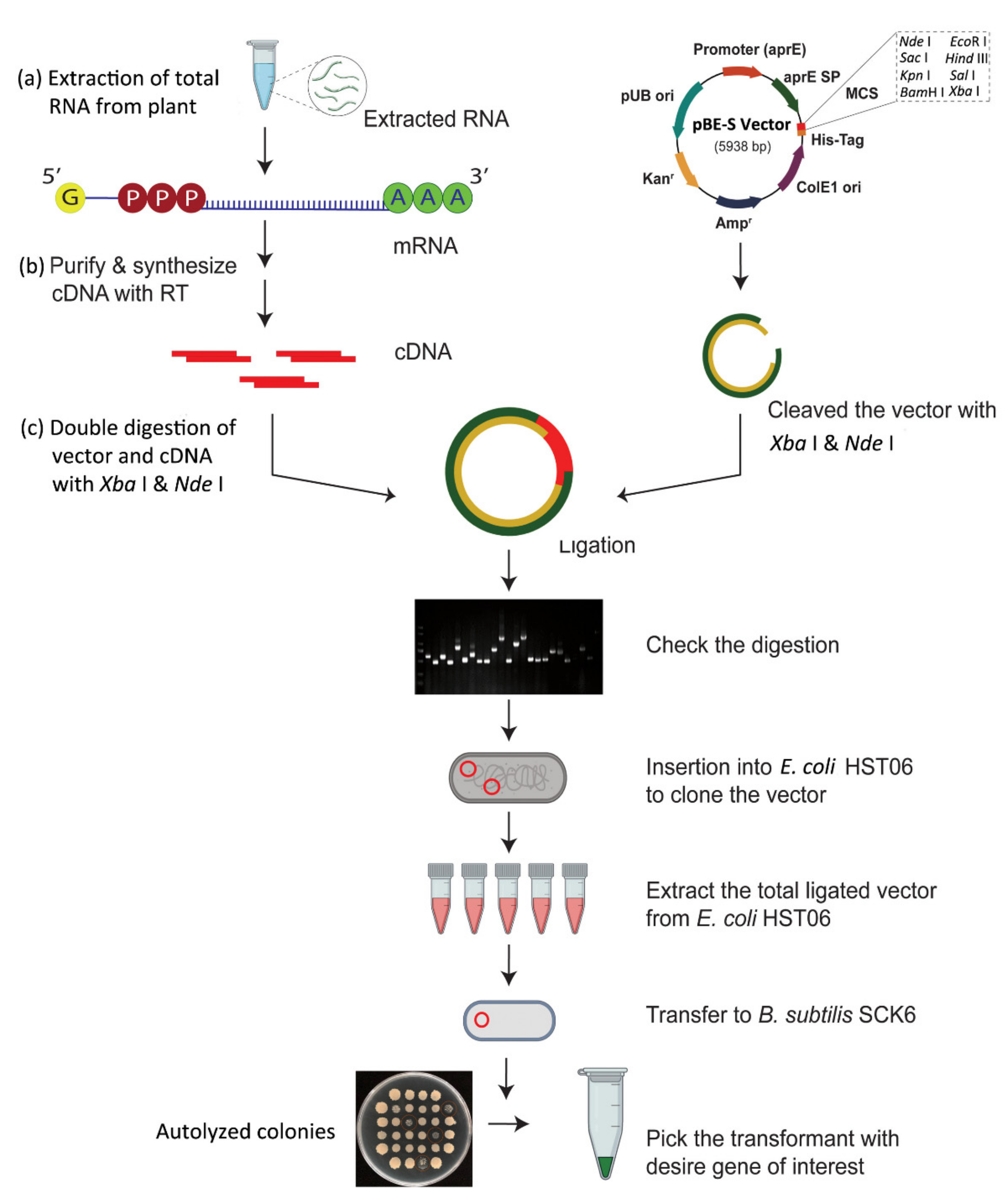
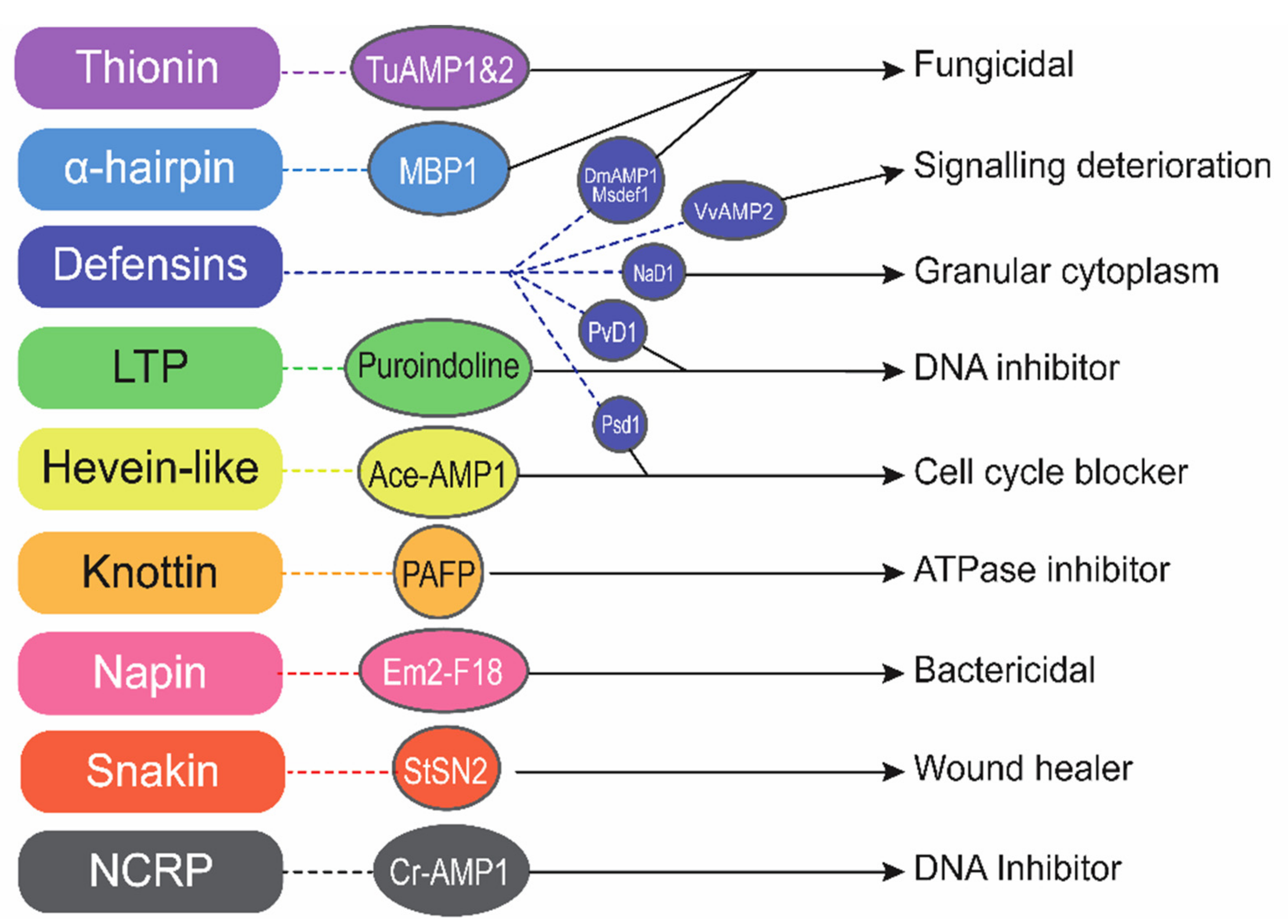
3. CDNA Library Based Plant AMPs Isolation
3.1. CDNA Library Construction
3.2. CDNA Sequencing Analysis
3.3. Bioinformatics Analysis of Candidate Peptides
3.3.1. In Silico Prediction Analysis
3.3.2. Prediction of Physiochemical Properties of AMPs
3.3.3. 3D Structure Prediction
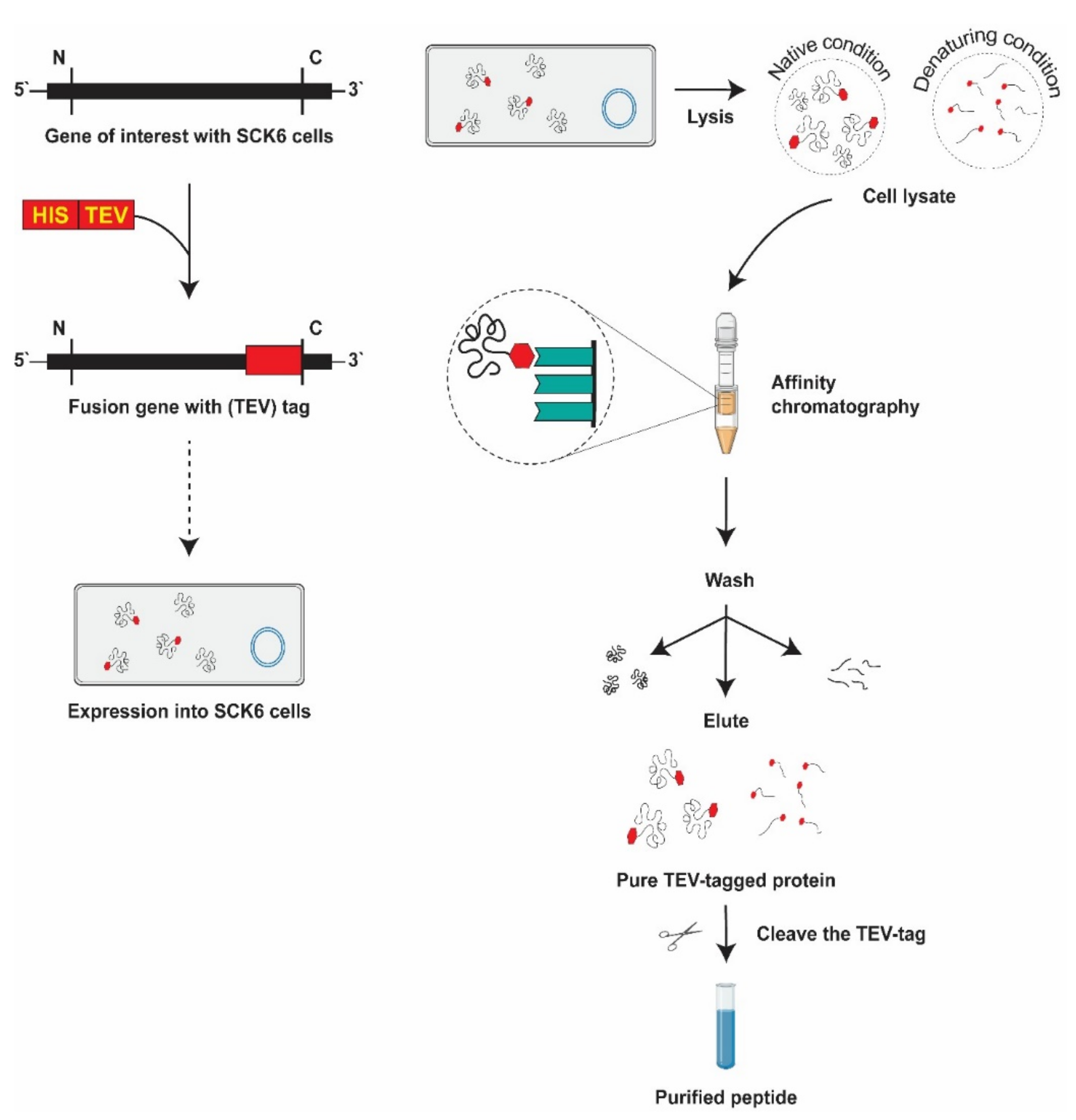
3.4. Candidate Protein Extraction from the B. subtilis Expression System
3.5. His-Tag Fusion Peptide Purification
3.5.1. Immobilized Metal Affinity Chromatography
3.5.2. Elimination of Tag
3.6. Tris-Tricine SDS-PAGE and Western Blotting
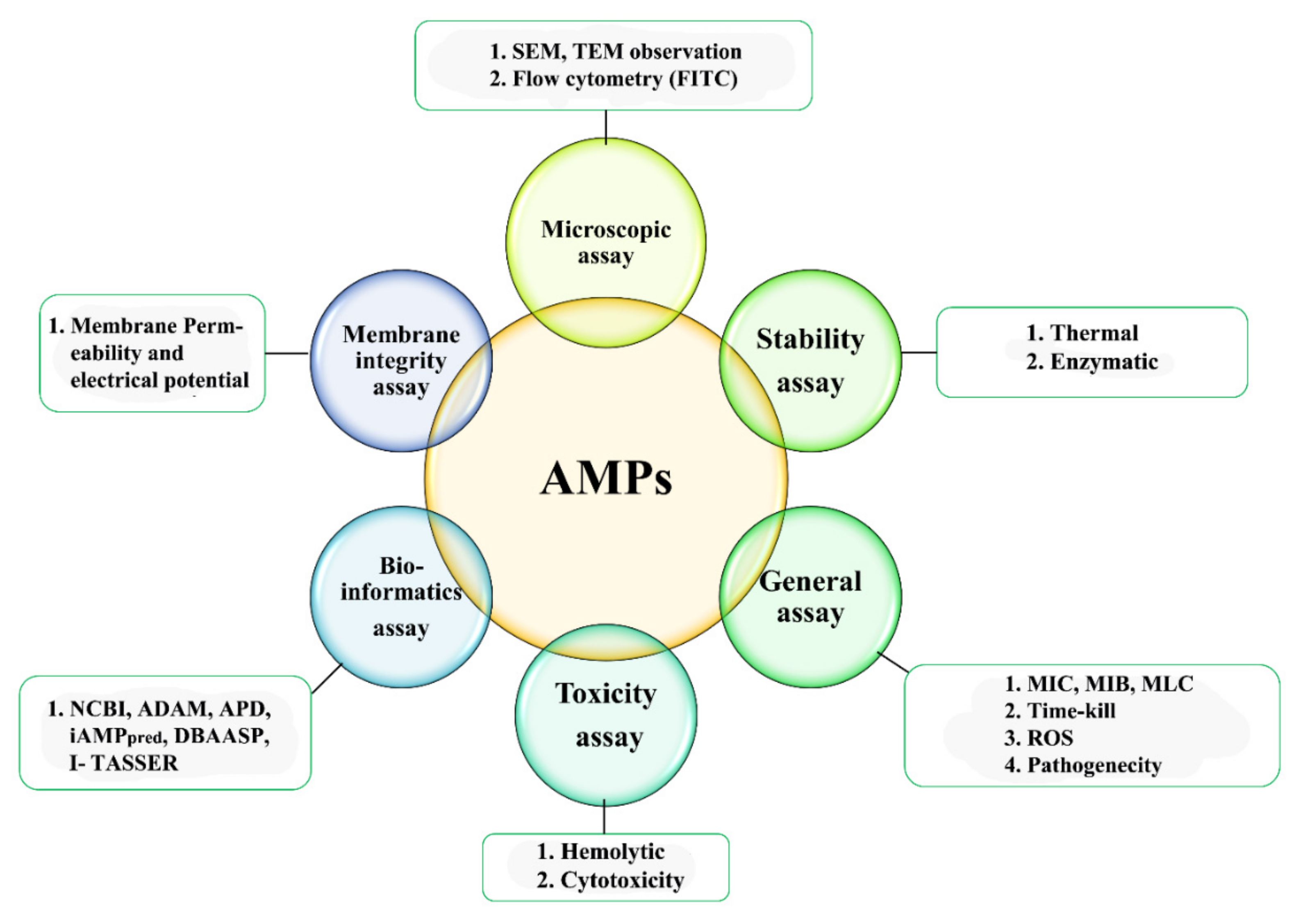
4. Characterization of AMPs
4.1. Microscopic Studies
4.2. Hemolytic Activity
4.3. Minimum Inhibitory Concentration (MIC)
4.4. AMPs Stability Determination
4.5. Cell Membrane Integrity
4.6. Reactive Oxygen Species
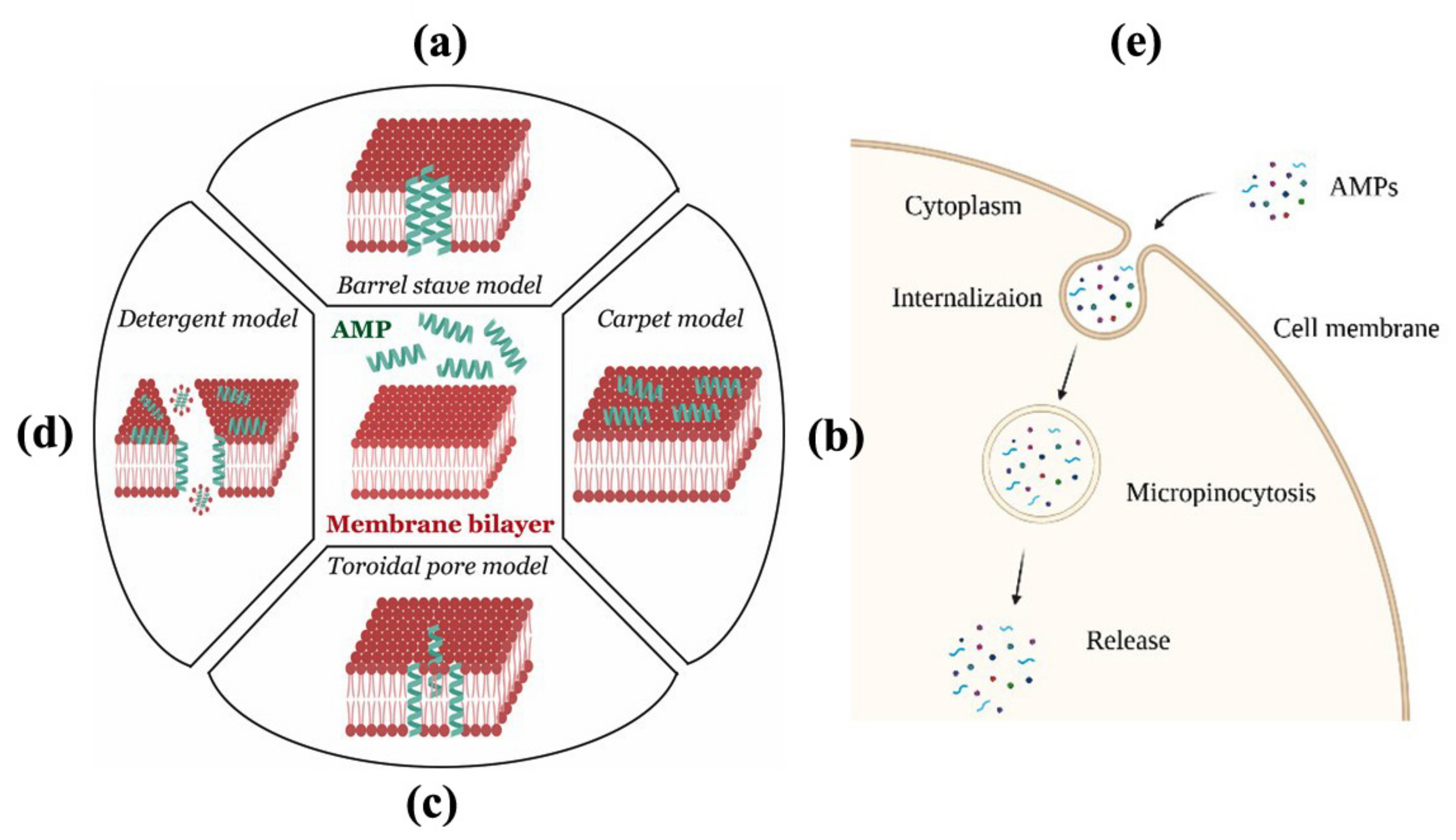
5. Mode of Action of AMPs
5.1. Barrel-Stave Model
5.2. Carpet Model
5.3. Toroidal Pore Model
5.4. Disordered/Detergent Toroidal Pore Model
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Hancock, R.E.W. Cationic Host Defence Peptides: Innate Immune Regulatory Peptides as a Novel Approach for Treating Infections. Cell. Mol. Life Sci. 2007, 64, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Reassessing the Host Defense Peptide Landscape. Front. Chem. 2019, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, E.; Bardaji, E. Synthetic Antimicrobial Peptides as Agricultural Pesticides for Plant-disease Control. Chem. Biodivers. 2008, 5, 1225–1237. [Google Scholar] [CrossRef]
- Benko-Iseppon, A.M.; Crovella, S. Ethnobotanical Bioprospection of Candidates for Potential Antimicrobial Drugs from Brazilian Plants: State of Art and Perspectives. Curr. Protein Pept. Sci. 2010, 11, 189–194. [Google Scholar] [CrossRef]
- Kaas, Q.; Westermann, J.-C.; Craik, D.J. Conopeptide Characterization and Classifications: An Analysis Using ConoServer. Toxicon 2010, 55, 1491–1509. [Google Scholar] [CrossRef]
- Zanetti, M. Cathelicidins, Multifunctional Peptides of the Innate Immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chang, T.-Y.; Lee, Y.-L. Adsorption Behavior of 11-Mercapto-1-Undecanol on Au (111) Electrode in an Electrochemical System. J. Phys. Chem. C 2007, 111, 4014–4020. [Google Scholar] [CrossRef]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Lee, H.-T.; Lee, C.-C.; Yang, J.-R.; Lai, J.Z.C.; Chang, K.Y. A Large-Scale Structural Classification of Antimicrobial Peptides. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, B.L.; Marshall, A.T.; Gillon, A.D.; Craik, D.J.; Anderson, M.A. Plant Cyclotides Disrupt Epithelial Cells in the Midgut of Lepidopteran Larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 1221–1225. [Google Scholar] [CrossRef]
- Barbosa Pelegrini, P.; Del Sarto, R.P.; Silva, O.N.; Franco, O.L.; Grossi-de-Sa, M.F. Antibacterial Peptides from Plants: What They Are and How They Probably Work. Biochem. Res. Int. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Segura, A.; Moreno, M.; López, G.; Garcıa-Olmedo, F.; Molina, A. Snakin-2, an Antimicrobial Peptide from Potato Whose Gene Is Locally Induced by Wounding and Responds to Pathogen Infection. Plant. Physiol. 2002, 128, 951–961. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular Mechanisms of Membrane Targeting Antibiotics. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Brandenburg, K.; Heinbockel, L.; Correa, W.; Lohner, K. Peptides with Dual Mode of Action: Killing Bacteria and Preventing Endotoxin-Induced Sepsis. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 971–979. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-Positive Bacterial Cell Envelopes: The Impact on the Activity of Antimicrobial Peptides. Biochim. Biophys. Acta BBA Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Poth, A.G.; Colgrave, M.L.; Lyons, R.E.; Daly, N.L.; Craik, D.J. Discovery of an Unusual Biosynthetic Origin for Circular Proteins in Legumes. Proc. Natl. Acad. Sci. USA 2011, 108, 10127–10132. [Google Scholar] [CrossRef]
- Herbel, V.; Sieber-Frank, J.; Wink, M. The Antimicrobial Peptide Snakin-2 Is Upregulated in the Defense Response of Tomatoes (Solanum lycopersicum) as Part of the Jasmonate-Dependent Signaling Pathway. J. Plant. Physiol. 2017, 208, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Hong, J.K.; Kim, Y.J.; Hwang, B.K. Pepper Gene Encoding Thionin Is Differentially Induced by Pathogens, Ethylene and Methyl Jasmonate. Physiol. Mol. Plant. Pathol. 2000, 56, 207–216. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune Modulation by Multifaceted Cationic Host Defense (Antimicrobial) Peptides. Nat. Chem. Biol. 2013, 9, 761. [Google Scholar] [CrossRef]
- Campos, M.L.; de Souza, C.M.; de Oliveira, K.B.S.; Dias, S.C.; Franco, O.L. The Role of Antimicrobial Peptides in Plant Immunity. J. Exp. Bot. 2018, 69, 4997–5011. [Google Scholar] [CrossRef]
- Fjell, C.D.; Jenssen, H.; Cheung, W.A.; Hancock, R.E.W.; Cherkasov, A. Optimization of Antibacterial Peptides by Genetic Algorithms and Cheminformatics. Chem. Biol. Drug Des. 2011, 77, 48–56. [Google Scholar] [CrossRef]
- Fang, H.; Ataker, F.; Hedin, G.; Dornbusch, K. Molecular Epidemiology of Extended-Spectrum β-Lactamases among Escherichia coli Isolates Collected in a Swedish Hospital and Its Associated Health Care Facilities from 2001 to 2006. J. Clin. Microbiol. 2008, 46, 707–712. [Google Scholar] [CrossRef]
- Kolbert, C.P.; Arruda, J.; Varga-Delmore, P.; Zheng, X.; Lewis, M.; Kolberg, J.; Persing, D.H. Branched-DNA Assay for Detection of ThemecA Gene in Oxacillin-Resistant and Oxacillin-Sensitive Staphylococci. J. Clin. Microbiol. 1998, 36, 2640–2644. [Google Scholar] [CrossRef]
- Patel, R.; Uhl, J.R.; Kohner, P.; Hopkins, M.K.; Cockerill, F.R. Multiplex PCR Detection of VanA, VanB, VanC-1, and VanC-2/3 Genes in Enterococci. J. Clin. Microbiol. 1997, 35, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, R.J.; Morris, D.; Chee, M.; Hubbell, E.; Kozal, M.J.; Shah, N.; Shen, N.; Yang, R.; Fodor, S.P. Using Oligonucleotide Probe Arrays to Access Genetic Diversity. Biotechniques 1995, 19, 442. [Google Scholar] [PubMed]
- Christiansen, M.T.; Kaas, R.S.; Chaudhuri, R.R.; Holmes, M.A.; Hasman, H.; Aarestrup, F.M. Genome-Wide High-Throughput Screening to Investigate Essential Genes Involved in Methicillin-Resistant Staphylococcus aureus Sequence Type 398 Survival. PLoS ONE 2014, 9, e89018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nairn, B.L.; Eliasson, O.S.; Hyder, D.R.; Long, N.J.; Majumdar, A.; Chakravorty, S.; McDonald, P.; Roy, A.; Newton, S.M.; Klebba, P.E. Fluorescence High-Throughput Screening for Inhibitors of TonB Action. J. Bacteriol. 2017, 199, e00889-16. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant Protein Expression in Escherichia coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Khow, O.; Suntrarachun, S. Strategies for Production of Active Eukaryotic Proteins in Bacterial Expression System. Asian Pac. J. Trop. Biomed. 2012, 2, 159–162. [Google Scholar] [CrossRef]
- He, Q.; Fu, A.; Li, T. Expression and One-Step Purification of the Antimicrobial Peptide Cathelicidin-BF Using the Intein System in Bacillus subtilis. J. Ind. Microbiol. Biotechnol. 2015, 42, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Z.; Du, C.; Wang, J.; Li, S. Improved Expression and Characterization of a Multidomain Xylanase from Thermoanaerobacterium aotearoense SCUT27 in Bacillus subtilis. J. Agric. Food Chem. 2015, 63, 6430–6439. [Google Scholar] [CrossRef]
- Heng, C.; Chen, Z.; Du, L.; Lu, F. Expression and Secretion of an Acid-Stable α-Amylase Gene in Bacillus subtilis by SacB Promoter and Signal Peptide. Biotechnol. Lett. 2005, 27, 1731–1737. [Google Scholar] [CrossRef]
- Fu, T.; Islam, M.S.; Ali, M.; Wu, J.; Dong, W. Two Antimicrobial Genes from Aegilops tauschii Cosson Identified by the Bacillus subtilis Expression System. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Hassan, M.F.; Qutb, A.M.; Dong, W. Prediction and Activity of a Cationic α-Helix Antimicrobial Peptide ZM-804 from Maize. Int. J. Mol. Sci. 2021, 22, 2643. [Google Scholar] [CrossRef]
- Kong, X.; Yang, M.; Abbas, H.M.K.; Wu, J.; Li, M.; Dong, W. Antimicrobial Genes from Allium sativum and Pinellia ternata Revealed by a Bacillus subtilis Expression System. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Islam, S.; Guo, P.; Hu, X.; Dong, W. Isolation of Antimicrobial Genes from Oryza rufipogon Griff by Using a Bacillus subtilis Expression System with Potential Antimicrobial Activities. Int. J. Mol. Sci. 2020, 21, 8722. [Google Scholar] [CrossRef]
- Wenzel, M.; Müller, A.; Siemann-Herzberg, M.; Altenbuchner, J. Self-Inducible Bacillus subtilis Expression System for Reliable and Inexpensive Protein Production by High-Cell-Density Fermentation. Appl. Environ. Microbiol. 2011, 77, 6419–6425. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Pan, J.-G.; Park, S.-H.; Choi, S.-K. Development of a Stationary Phase-Specific Auto Inducible Expression System in Bacillus subtilis. J. Biotechnol. 2010, 149, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, P.; Ng, T.B.; Yang, J.; Lin, J.; Yan, F.; Ye, X. Highly Efficient Expression and Characterization of a β-mannanase from Bacillus subtilis in Pichia pastoris. Biotechnol. Appl. Biochem. 2015, 62, 64–70. [Google Scholar] [CrossRef]
- Islam, M.S.; Mahmud, S.; Sultana, R.; Dong, W. Identification and in Silico Molecular Modelling Study of Newly Isolated Bacillus subtilis SI-18 Strain against S9 Protein of Rhizoctonia solani. Arab. J. Chem. 2020, 13, 8600–8612. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Yamanaka, K.; Xu, Y.; Zhang, W.; Vlamakis, H.; Kolter, R.; Moore, B.S.; Qian, P.-Y. Directed Natural Product Biosynthesis Gene Cluster Capture and Expression in the Model Bacterium Bacillus subtilis. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Parachin, N.S.; Mulder, K.C.; Viana, A.A.B.; Dias, S.C.; Franco, O.L. Expression Systems for Heterologous Production of Antimicrobial Peptides. Peptides 2012, 38, 446–456. [Google Scholar] [CrossRef]
- Li, C.; Haug, T.; Styrvold, O.B.; Jørgensen, T.Ø.; Stensvåg, K. Strongylocins, Novel Antimicrobial Peptides from the Green Sea Urchin, Strongylocentrotus droebachiensis. Dev. Comp. Immunol. 2008, 32, 1430–1440. [Google Scholar] [CrossRef]
- Li, C.; Blencke, H.-M.; Paulsen, V.; Haug, T.; Stensvåg, K. Powerful Workhorses for Antimicrobial Peptide Expression and Characterization. Bioeng. Bugs 2010, 1, 217–220. [Google Scholar] [CrossRef]
- Oard, S.V.; Enright, F.M. Expression of the Antimicrobial Peptides in Plants to Control Phytopathogenic Bacteria and Fungi. Plant. Cell Rep. 2006, 25, 561–572. [Google Scholar] [CrossRef]
- Desai, P.N.; Shrivastava, N.; Padh, H. Production of Heterologous Proteins in Plants: Strategies for Optimal Expression. Biotechnol. Adv. 2010, 28, 427–435. [Google Scholar] [CrossRef]
- Ye, G.; Wu, H.; Huang, J.; Wang, W.; Ge, K.; Li, G.; Zhong, J.; Huang, Q. LAMP2: A Major Update of the Database Linking Antimicrobial Peptides. Database 2020, 2020, baaa061. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, H.P.; Mortensen, K.K. Advanced Genetic Strategies for Recombinant Protein Expression in Escherichia coli. J. Biotechnol. 2005, 115, 113–128. [Google Scholar] [CrossRef]
- Li, Y. Recombinant Production of Antimicrobial Peptides in Escherichia coli: A Review. Protein Expr. Purif. 2011, 80, 260–267. [Google Scholar] [CrossRef]
- Westers, L.; Westers, H.; Quax, W.J. Bacillus subtilis as Cell Factory for Pharmaceutical Proteins: A Biotechnological Approach to Optimize the Host Organism. Biochim. Biophys. Acta BBA Mol. Cell Res. 2004, 1694, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Tjalsma, H.; Bolhuis, A.; Jongbloed, J.D.H.; Bron, S.; van Dijl, J.M. Signal Peptide-Dependent Protein Transport in Bacillus subtilis: A Genome-Based Survey of the Secretome. Microbiol. Mol. Biol. Rev. 2000, 64, 515–547. [Google Scholar] [CrossRef]
- Mattanovich, D.; Branduardi, P.; Dato, L.; Gasser, B.; Sauer, M.; Porro, D. Recombinant Protein Production in Yeasts. Recomb. Gene Expr. 2012, 824, 329–358. [Google Scholar]
- Unrean, P. Pathway Analysis of Pichia Pastoris to Elucidate Methanol Metabolism and Its Regulation for Production of Recombinant Proteins. Biotechnol. Prog. 2014, 30, 28–37. [Google Scholar] [CrossRef]
- De, S.; Mattanovich, D.; Ferrer, P.; Gasser, B. Established Tools and Emerging Trends for the Production of Recombinant Proteins and Metabolites in Pichia pastoris. Essays Biochem. 2021, 65, 293–307. [Google Scholar]
- Cereghino, G.P.L.; Cereghino, J.L.; Ilgen, C.; Cregg, J.M. Production of Recombinant Proteins in Fermenter Cultures of the Yeast Pichia pastoris. Curr. Opin. Biotechnol. 2002, 13, 329–332. [Google Scholar] [CrossRef]
- Delaunois, B.; Cordelier, S.; Conreux, A.; Clément, C.; Jeandet, P. Molecular Engineering of Resveratrol in Plants. Plant. Biotechnol. J. 2009, 7, 2–12. [Google Scholar] [CrossRef]
- Bakker, H.; Bardor, M.; Molthoff, J.W.; Gomord, V.; Elbers, I.; Stevens, L.H.; Jordi, W.; Lommen, A.; Faye, L.; Lerouge, P. Galactose-Extended Glycans of Antibodies Produced by Transgenic Plants. Proc. Natl. Acad. Sci. USA 2001, 98, 2899–2904. [Google Scholar] [CrossRef]
- Hu, X.; Reddy, A.S.N. Cloning and Expression of a PR5-like Protein from Arabidopsis: Inhibition of Fungal Growth by Bacterially Expressed Protein. Plant. Mol. Biol. 1997, 34, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.A.; Silva, M.S.; Magalhães, C.P.; Ribeiro, S.G.; Sarto, R.P.D.; Vieira, E.A.; de Sá, M.F.G. Expression in Escherichia coli, Purification, Refolding and Antifungal Activity of an Osmotin from Solanum nigrum. Microb. Cell Fact. 2008, 7, 1–10. [Google Scholar] [CrossRef]
- Harrison, S.J.; McManus, A.M.; Marcus, J.P.; Goulter, K.C.; Green, J.L.; Nielsen, K.J.; Craik, D.J.; Maclean, D.J.; Manners, J.M. Purification and Characterization of a Plant Antimicrobial Peptide Expressed in Escherichia coli. Protein Expr. Purif. 1999, 15, 171–177. [Google Scholar] [CrossRef]
- Tavares, L.S.; Rettore, J.V.; Freitas, R.M.; Porto, W.F.; do Nascimento Duque, A.P.; de Lacorte Singulani, J.; Silva, O.N.; de Lima Detoni, M.; Vasconcelos, E.G.; Dias, S.C. Antimicrobial Activity of Recombinant Pg-AMP1, a Glycine-Rich Peptide from Guava Seeds. Peptides 2012, 37, 294–300. [Google Scholar] [CrossRef]
- De Beer, A.; Vivier, M.A. Vv-AMP1, a Ripening Induced Peptide from Vitis vinifera Shows Strong Antifungal Activity. BMC Plant. Biol. 2008, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, R.; Ventimiglia, I.; Palumbo, D.; Nicodemo, D.; Salvatore, P.; Amoroso, M.G.; Iannaccone, M. Expression of Recombinant Puroindolines for the Treatment of Staphylococcal Skin Infections (Acne vulgaris). J. Biotechnol. 2007, 128, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.J.; Lightfoot, D.A. Soybean Disease Resistance Protein RHG1-LRR Domain Expressed, Purified and Refolded from Escherichia coli Inclusion Bodies: Preparation for a Functional Analysis. Protein Expr. Purif. 2007, 53, 346–355. [Google Scholar] [CrossRef]
- Carvajal-Vallejos, P.K.; Campos, A.; Fuentes-Prior, P.; Villalobos, E.; Almeida, A.M.; Barbera, E.; Torné, J.M.; Santos, M. Purification and in Vitro Refolding of Maize Chloroplast Transglutaminase Over-Expressed in Escherichia coli. Biotechnol. Lett. 2007, 29, 1255–1262. [Google Scholar] [CrossRef]
- Iwai, T.; Kaku, H.; Honkura, R.; Nakamura, S.; Ochiai, H.; Sasaki, T.; Ohashi, Y. Enhanced Resistance to Seed-Transmitted Bacterial Diseases in Transgenic Rice Plants Overproducing an Oat Cell-Wall-Bound Thionin. Mol. Plant Microbe Interact. 2002, 15, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Abbas, H.M.K.; Li, J.; Yuan, Y.; Liu, Y.; Wang, G.; Dong, W. Cell Membrane-Interrupting Antimicrobial Peptides from Isatis indigotica Fortune Isolated by a Bacillus subtilis Expression System. Biomolecules 2020, 10, 30. [Google Scholar] [CrossRef]
- Chan, Y.-L.; Yang, A.-H.; Chen, J.-T.; Yeh, K.-W.; Chan, M.-T. Heterologous Expression of Taro Cystatin Protects Transgenic Tomato against Meloidogyne Incognita Infection by Means of Interfering Sex Determination and Suppressing Gall Formation. Plant. Cell Rep. 2010, 29, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Qutb, A.M.; Wei, F.; Dong, W. Prediction and Characterization of Cationic Arginine-Rich Plant Antimicrobial Peptide SM-985 From Teosinte (Zea Mays Ssp. Mexicana). Front. Microbiol. 2020, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Hammes, U.; Thor, K.; Suter-Grotemeyer, M.; Flückiger, R.; Slusarenko, A.J.; Ward, J.M.; Rentsch, D. AtPTR1, a Plasma Membrane Peptide Transporter Expressed during Seed Germination and in Vascular Tissue of Arabidopsis. Plant. J. 2004, 40, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Maresova, L.; Sychrova, H. Arabidopsis thaliana CHX17 Gene Complements the Kha1 Deletion Phenotypes in Saccharomyces cerevisiae. Yeast 2006, 23, 1167–1171. [Google Scholar] [CrossRef]
- Rubio, F.; Gassmann, W.; Schroeder, J.I. Sodium-Driven Potassium Uptake by the Plant Potassium Transporter HKT1 and Mutations Conferring Salt Tolerance. Science 1995, 270, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Eskandari, S.; Grallath, S.; Rentsch, D. AtGAT1, a High Affinity Transporter for γ-Aminobutyric Acid in Arabidopsis thaliana. J. Biol. Chem. 2006, 281, 7197–7204. [Google Scholar] [CrossRef]
- Schneider, S.; Schneidereit, A.; Udvardi, P.; Hammes, U.; Gramann, M.; Dietrich, P.; Sauer, N. Arabidopsis INOSITOL TRANSPORTER2 Mediates H+ Symport of Different Inositol Epimers and Derivatives across the Plasma Membrane. Plant. Physiol. 2007, 145, 1395–1407. [Google Scholar] [CrossRef]
- López-García, B.; Moreno, A.B.; San Segundo, B.; De los Ríos, V.; Manning, J.M.; Gavilanes, J.G.; Martínez-del-Pozo, Á. Production of the Biotechnologically Relevant AFP from Aspergillus giganteus in the Yeast Pichia pastoris. Protein Expr. Purif. 2010, 70, 206–210. [Google Scholar] [CrossRef]
- Kant, P.; Liu, W.-Z.; Pauls, K.P. PDC1, a Corn Defensin Peptide Expressed in Escherichia coli and Pichia pastoris Inhibits Growth of Fusarium graminearum. Peptides 2009, 30, 1593–1599. [Google Scholar] [CrossRef]
- Cabral, K.M.S.; Almeida, M.S.; Valente, A.P.; Almeida, F.C.L.; Kurtenbach, E. Production of the Active Antifungal Pisum sativum Defensin 1 (Psd1) in Pichia pastoris: Overcoming the Inefficiency of the STE13 Protease. Protein Expr. Purif. 2003, 31, 115–122. [Google Scholar] [CrossRef]
- Diatloff, E.; Forde, B.G.; Roberts, S.K. Expression and Transport Characterisation of the Wheat Low-Affinity Cation Transporter (LCT1) in the Methylotrophic Yeast Pichia pastoris. Biochem. Biophys. Res. Commun. 2006, 344, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.L.; Beames, B.; Summers, M.D.; Park, W.D. Characterization of the Lipid Acyl Hydrolase Activity of the Major Potato (Solanum tuberosum) Tuber Protein, Patatin, by Cloning and Abundant Expression in a Baculovirus Vector. Biochem. J. 1988, 252, 199–206. [Google Scholar] [CrossRef]
- Harashima, H.; Shinmyo, A.; Sekine, M. Phosphorylation of Threonine 161 in Plant Cyclin-dependent Kinase A Is Required for Cell Division by Activation of Its Associated Kinase. Plant. J. 2007, 52, 435–448. [Google Scholar] [CrossRef]
- Hayashi, H.; De Bellis, L.; Hayashi, Y.; Nito, K.; Kato, A.; Hayashi, M.; Hara-Nishimura, I.; Nishimura, M. Molecular Characterization of an Arabidopsis Acyl-Coenzyme a Synthetase Localized on Glyoxysomal Membranes. Plant. Physiol. 2002, 130, 2019–2026. [Google Scholar] [CrossRef][Green Version]
- Furman-Matarasso, N.; Cohen, E.; Du, Q.; Chejanovsky, N.; Hanania, U.; Avni, A. A Point Mutation in the Ethylene-Inducing Xylanase Elicitor Inhibits the β-1–4-Endoxylanase Activity but Not the Elicitation Activity. Plant. Physiol. 1999, 121, 345–352. [Google Scholar] [CrossRef][Green Version]
- Rivero, M.; Furman, N.; Mencacci, N.; Picca, P.; Toum, L.; Lentz, E.; Bravo-Almonacid, F.; Mentaberry, A. Stacking of Antimicrobial Genes in Potato Transgenic Plants Confers Increased Resistance to Bacterial and Fungal Pathogens. J. Biotechnol. 2012, 157, 334–343. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Zhang, Z.; Ren, L.; Du, L.; Zhang, B.; Xu, H.; Xin, Z. Expression of a Radish Defensin in Transgenic Wheat Confers Increased Resistance to Fusarium graminearum and Rhizoctonia cerealis. Funct. Integr. Genom. 2011, 11, 63–70. [Google Scholar] [CrossRef]
- Choi, M.-S.; Kim, Y.-H.; Park, H.-M.; Seo, B.-Y.; Jung, J.-K.; Kim, S.-T.; Kim, M.-C.; Shin, D.-B.; Yun, H.-T.; Choi, I.-S. Expression of BrD1, a Plant Defensin from Brassica rapa, Confers Resistance against Brown Planthopper (Nilaparvata lugens) in Transgenic Rices. Mol. Cells 2009, 28, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Portieles, R.; Ayra, C.; Gonzalez, E.; Gallo, A.; Rodriguez, R.; Chacón, O.; López, Y.; Rodriguez, M.; Castillo, J.; Pujol, M. NmDef02, a Novel Antimicrobial Gene Isolated from Nicotiana megalosiphon Confers High-level Pathogen Resistance under Greenhouse and Field Conditions. Plant. Biotechnol. J. 2010, 8, 678–690. [Google Scholar] [CrossRef]
- Abdallah, N.A.; Shah, D.; Abbas, D.; Madkour, M. Stable Integration and Expression of a Plant Defensin in Tomato Confers Resistance to Fusarium wilt. GM Crops 2010, 1, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Rončević, T.; Gerdol, M.; Spazzali, F.; Florian, F.; Mekinić, S.; Tossi, A.; Pallavicini, A. Parallel Identification of Novel Antimicrobial Peptide Sequences from Multiple Anuran Species by Targeted DNA Sequencing. BMC Genom. 2018, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, L.; Lu, J.; Cheng, Y.; You, Q.; Wang, L.; Song, X.; Zhou, X.; Jiao, Y. Large-Scale Investigation of Soybean Gene Functions by Overexpressing a Full-Length Soybean CDNA Library in Arabidopsis. Front. Plant. Sci. 2018, 9, 631. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.S.; de Souza, V.C.; Nunes, V.S.; Silva, O.N.; de Souza, G.T.; Marques, L.F.; Goliatt, P.V.Z.C.; Viccini, L.F.; Franco, O.L.; de Oliveira Santos, M. Antimicrobial Peptide Selection from Lippia Spp Leaf Transcriptomes. Peptides 2020, 129, 170317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, L.; Ullah, A.; Jin, X.; Yang, X.; Zhang, X. Identification of Multiple Stress Responsive Genes by Sequencing a Normalized cDNA Library from Sea-Land Cotton (Gossypium barbadense L.). PLoS ONE 2016, 11, e0152927. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Mishra, A.K.; Mehraj, V.; Duraisamy, G.S. Advances and Applications of Molecular Cloning in Clinical Microbiology. Biotechnol. Genet. Eng. Rev. 2014, 30, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Oumer, O.J.; Abate, D. Characterization of Pectinase from Bacillus subtilis Strain Btk 27 and Its Potential Application in Removal of Mucilage from Coffee Beans. Enzyme Res. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N.; Pathania, S.; Handa, S. Purification and Characterization of Lipase by Bacillus methylotrophicus PS3 under Submerged Fermentation and Its Application in Detergent Industry. J. Genet. Eng. Biotechnol. 2017, 15, 369–377. [Google Scholar] [CrossRef]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the Use of Bacillus Species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Ramos, K.R.M.; Valdehuesa, K.N.G.; Cabulong, R.B.; Moron, L.S.; Nisola, G.M.; Hong, S.-K.; Lee, W.-K.; Chung, W.-J. Overexpression and Secretion of AgaA7 from Pseudoalteromonas hodoensis Sp. Nov in Bacillus subtilis for the Depolymerization of Agarose. Enzyme Microb. Technol. 2016, 90, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Waghu, F.H.; Idicula-Thomas, S. Collection of Antimicrobial Peptides Database and Its Derivatives: Applications and Beyond. Protein Sci. 2020, 29, 36–42. [Google Scholar] [CrossRef]
- Meher, P.K.; Sahu, T.K.; Saini, V.; Rao, A.R. Predicting Antimicrobial Peptides with Improved Accuracy by Incorporating the Compositional, Physico-Chemical and Structural Features into Chou’s General PseAAC. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Lata, S.; Sharma, B.K.; Raghava, G.P.S. Analysis and Prediction of Antibacterial Peptides. BMC Bioinform. 2007, 8, 1–10. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Vishnepolsky, B.; Pirtskhalava, M. Prediction of Linear Cationic Antimicrobial Peptides Based on Characteristics Responsible for Their Interaction with the Membranes. J. Chem. Inf. Model. 2014, 54, 1512–1523. [Google Scholar] [CrossRef]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A Web Server to Screen Sequences with Specific α-Helical Properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Buchan, D.W.A.; Jones, D.T. The PSIPRED Protein Analysis Workbench: 20 Years On. Nucleic Acids Res. 2019, 47, W402–W407. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A Unified Platform for Automated Protein Structure and Function Prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de Novo Structure Prediction for Linear Peptides in Solution and in Complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-Web: Interactive Web Service for the Recognition of Errors in Three-Dimensional Structures of Proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-Atom Structure Validation for Macromolecular Crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Crowe, J.; Dobeli, H.; Gentz, R.; Hochuli, E.; Stiiber, D.; Henco, K. 6xffis-ni-nta chromatography as a superior technique in recombinant protein expression/purification. In Protocols for Gene Analysis; Springer: Totowa, NJ, USA, 1994; pp. 371–387. [Google Scholar]
- Schoonen, L.; van Esterik, K.S.; Zhang, C.; Ulijn, R.V.; Nolte, R.J.M.; van Hest, J.C.M. Alternative Application of an Affinity Purification Tag: Hexahistidines in Ester Hydrolysis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Pourhassan-Moghaddam, M.; Wood, D.W.; Majdi, H.; Zarghami, N. Current Affinity Approaches for Purification of Recombinant Proteins. Cogent Biol. 2019, 5, 1665406. [Google Scholar] [CrossRef]
- Lilius, G.; Persson, M.; Bülow, L.; Mosbach, K. Metal Affinity Precipitation of Proteins Carrying Genetically Attached Polyhistidine Affinity Tails. Eur. J. Biochem. 1991, 198, 499–504. [Google Scholar] [CrossRef]
- Katti, S.K.; LeMaster, D.M.; Eklund, H. Crystal Structure of Thioredoxin from Escherichia coli at 1.68 Å Resolution. J. Mol. Biol. 1990, 212, 167–184. [Google Scholar] [CrossRef]
- Smith, D.B.; Johnson, K.S. Single-Step Purification of Polypeptides Expressed in Escherichia coli as Fusions with Glutathione S-Transferase. Gene 1988, 67, 31–40. [Google Scholar] [CrossRef]
- Di Guana, C.; Lib, P.; Riggsa, P.D.; Inouyeb, H. Vectors That Facilitate the Expression and Purification of Foreign Peptides in Escherichia coli by Fusion to Maltose-Binding Protein. Gene 1988, 67, 21–30. [Google Scholar] [CrossRef]
- Pestov, N.B.; Rydström, J. Purification of Recombinant Membrane Proteins Tagged with Calmodulin-Binding Domains by Affinity Chromatography on Calmodulin-Agarose: Example of Nicotinamide Nucleotide Transhydrogenase. Nat. Protoc. 2007, 2, 198–202. [Google Scholar] [CrossRef]
- Zhao, X.; Li, G.; Liang, S. Several Affinity Tags Commonly Used in Chromatographic Purification. J. Anal. Methods Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Chong, S.; Mersha, F.B.; Comb, D.G.; Scott, M.E.; Landry, D.; Vence, L.M.; Perler, F.B.; Benner, J.; Kucera, R.B.; Hirvonen, C.A. Single-Column Purification of Free Recombinant Proteins Using a Self-Cleavable Affinity Tag Derived from a Protein Splicing Element. Gene 1997, 192, 271–281. [Google Scholar] [CrossRef]
- Einhauer, A.; Jungbauer, A. The FLAGTM Peptide, a Versatile Fusion Tag for the Purification of Recombinant Proteins. J. Biochem. Biophys. Methods 2001, 49, 455–465. [Google Scholar] [CrossRef]
- Los, G.V.; Encell, L.P.; McDougall, M.G.; Hartzell, D.D.; Karassina, N.; Zimprich, C.; Wood, M.G.; Learish, R.; Ohana, R.F.; Urh, M. HaloTag: A Novel Protein Labeling Technology for Cell Imaging and Protein Analysis. ACS Chem. Biol. 2008, 3, 373–382. [Google Scholar] [CrossRef]
- Da Costa, S.J.M. Development of a Novel Fusion System for Recombinant Protein Production and Purification in Escherichia coli. Ph.D. Thesis, Universidade do Minho, Braga, Portugal, 31 May 2013. [Google Scholar]
- Hillman, M.C.; Yang, L.S.; Sun, S.; Duke, J.L.; O’Neil, K.T.; Kochie, J.E.; Karjoo, A.; Nath, P.; Breth, L.A.; Murphy, K. A Comprehensive System for Protein Purification and Biochemical Analysis Based on Antibodies to C-Myc Peptide. Protein Expr. Purif. 2001, 23, 359–368. [Google Scholar] [CrossRef]
- Hus, C.H.; Rad, N.F.; Hackbarth, J.S.; Lee, S.; Meng, X.W.; Vroman, B.T.; Kaufmann, S.H.; Karnitz, L.M. S-Peptide Epitope Tagging for Protein Purification, Expression Monitoring, and Localization in Mammalian Cells. Biotechniques 2004, 37, 835–839. [Google Scholar]
- Spriestersbach, A.; Kubicek, J.; Schäfer, F.; Block, H.; Maertens, B. Purification of His-Tagged Proteins. Methods Enzymol. 2015, 559, 1–15. [Google Scholar] [PubMed]
- Carson, M.; Johnson, D.H.; McDonald, H.; Brouillette, C.; DeLucas, L.J. His-Tag Impact on Structure. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Skala, W.; Goettig, P.; Brandstetter, H. Do-It-Yourself Histidine-Tagged Bovine Enterokinase: A Handy Member of the Protein Engineer’s Toolbox. J. Biotechnol. 2013, 168, 421–425. [Google Scholar] [CrossRef]
- Jenny, R.J.; Mann, K.G.; Lundblad, R.L. A Critical Review of the Methods for Cleavage of Fusion Proteins with Thrombin and Factor Xa. Protein Expr. Purif. 2003, 31, 1–11. [Google Scholar] [CrossRef]
- Kimple, M.E.; Brill, A.L.; Pasker, R.L. Overview of Affinity Tags for Protein Purification. Curr. Protoc. Protein Sci. 2013, 73, 9. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, D.P.; Smith, B.J.; King, L.M.; West, S.M.; Reeks, D.G.; Stephens, P.E. Efficient Site Specific Removal of a C-Terminal FLAG Fusion from a Fab′ Using Copper (II) Ion Catalysed Protein Cleavage. Protein Eng. 1999, 12, 179–184. [Google Scholar] [CrossRef][Green Version]
- Arnau, J.; Lauritzen, C.; Petersen, G.E.; Pedersen, J. Current Strategies for the Use of Affinity Tags and Tag Removal for the Purification of Recombinant Proteins. Protein Expr. Purif. 2006, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.-M.; Lin, L.-C.; Wang, C.-F.; Lee, Y.-J.; Chen, Y.-T.; Liao, Y.-D. Antimicrobial Properties of an Immunomodulator-15 KDa Human Granulysin. PLoS ONE 2016, 11, e0156321. [Google Scholar] [CrossRef]
- Kuester, M.; Becker, G.L.; Hardes, K.; Lindberg, I.; Steinmetzer, T.; Than, M.E. Purification of the Proprotein Convertase Furin by Affinity Chromatography Based on PC-Specific Inhibitors. Biol. Chem. 2011, 392, 973–981. [Google Scholar] [CrossRef][Green Version]
- Sarpong, K.; Bose, R. Efficient Sortase-Mediated N-Terminal Labeling of TEV Protease Cleaved Recombinant Proteins. Anal. Biochem. 2017, 521, 55–58. [Google Scholar] [CrossRef][Green Version]
- Topilina, N.I.; Mills, K.V. Recent Advances in in Vivo Applications of Intein-Mediated Protein Splicing. Mob. DNA 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Gramespacher, J.A.; Stevens, A.J.; Nguyen, D.P.; Chin, J.W.; Muir, T.W. Intein Zymogens: Conditional Assembly and Splicing of Split Inteins via Targeted Proteolysis. J. Am. Chem. Soc. 2017, 139, 8074–8077. [Google Scholar] [CrossRef]
- Frey, S.; Görlich, D. A New Set of Highly Efficient, Tag-Cleaving Proteases for Purifying Recombinant Proteins. J. Chromatogr. A 2014, 1337, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Hwang, D.C.; Choi, K.Y.; Song, B.D. Proteolytic Processing of Oligopeptides Containing the Target Sequences by the Recombinant Tobacco Vein Mottling Virus NIa Proteinase. Mol. Cells 2000, 10, 213–219. [Google Scholar] [CrossRef]
- Waugh, D.S. An Overview of Enzymatic Reagents for the Removal of Affinity Tags. Protein Expr. Purif. 2011, 80, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N.; Kurn-Abramowitz, N.; Sokolovsky, M. Basic and Non-Basic Substrates of Carboxypeptidase, B. Eur. J. Biochem. 1973, 35, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Kumar Purbey, P.; Cyril Jayakumar, P.; Deepalakshmi, P.D.; Patole, M.S.; Galande, S. GST Fusion Vector with Caspase-6 Cleavage Site for Removal of Fusion Tag during Column Purification. Biotechniques 2005, 38, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Ryge, T.S.; Doisy, X.; Ifrah, D.; Olsen, J.E.; Hansen, P.R. New Indolicidin Analogues with Potent Antibacterial Activity. J. Pept. Res. 2004, 64, 171–185. [Google Scholar] [CrossRef]
- Krishnakumari, V.; Sharadadevi, A.; Sitaram, N.; Nagaraj, R. Consequences of Introducing a Disulfide Bond into an Antibacterial and Hemolytic Peptide. J. Pept. Res. 1999, 54, 528–535. [Google Scholar] [CrossRef]
- Dennison, S.R.; Phoenix, D.A. Susceptibility of Sheep, Human, and Pig Erythrocytes to Haemolysis by the Antimicrobial Peptide Modelin 5. Eur. Biophys. J. 2014, 43, 423–432. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Wu, Z.; Xie, F. Antimicrobial Peptides from the Skin of the Asian Frog, Odorrana Jingdongensis: De Novo Sequencing and Analysis of Tandem Mass Spectrometry Data. J. Proteom. 2012, 75, 5807–5821. [Google Scholar] [CrossRef]
- Belokoneva, O.S.; Villegas, E.; Corzo, G.; Dai, L.; Nakajima, T. The Hemolytic Activity of Six Arachnid Cationic Peptides Is Affected by the Phosphatidylcholine-to-Sphingomyelin Ratio in Lipid Bilayers. Biochim. Biophys. Acta BBA Biomembr. 2003, 1617, 22–30. [Google Scholar] [CrossRef]
- Straniero, V.; Pallavicini, M.; Chiodini, G.; Zanotto, C.; VolontŔ, L.; Radaelli, A.; Bolchi, C.; Fumagalli, L.; Sanguinetti, M.; Menchinelli, G. 3-(Benzodioxan-2-Ylmethoxy)-2, 6-Difluorobenzamides Bearing Hydrophobic Substituents at the 7-Position of the Benzodioxane Nucleus Potently Inhibit Methicillin-Resistant Sa and Mtb Cell Division. Eur. J. Med. Chem. 2016, 120, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Ma, Q.; Shan, A.; Lv, Y.; Hu, W.; Gu, Y.; Li, Y. Strand Length-Dependent Antimicrobial Activity and Membrane-Active Mechanism of Arginine-and Valine-Rich β-Hairpin-like Antimicrobial Peptides. Antimicrob. Agents Chemother. 2012, 56, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Comparative Mode of Action of the Antimicrobial Peptide Melimine and Its Derivative Mel4 against Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The Expanding Scope of Antimicrobial Peptide Structures and Their Modes of Action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Hammami, R.; Ben Hamida, J.; Vergoten, G.; Fliss, I. PhytAMP: A Database Dedicated to Antimicrobial Plant Peptides. Nucleic Acids Res. 2009, 37, D963–D968. [Google Scholar] [CrossRef]
- Parisi, K.; Shafee, T.M.A.; Quimbar, P.; van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. The Evolution, Function and Mechanisms of Action for Plant Defensins. Semin. Cell Dev. Biol. 2019, 88, 107–118. [Google Scholar] [CrossRef]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The Interaction of Antimicrobial Peptides with Membranes. Adv. Colloid Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the Binding, Insertion and Destabilization of Phospholipid Bilayer Membranes by α-Helical Antimicrobial and Cell Non-Selective Membrane-Lytic Peptides. Biochim. Biophys. Acta BBA Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Järvå, M.; Lay, F.T.; Phan, T.K.; Humble, C.; Poon, I.K.H.; Bleackley, M.R.; Anderson, M.A.; Hulett, M.D.; Kvansakul, M. X-Ray Structure of a Carpet-like Antimicrobial Defensin–phospholipid Membrane Disruption Complex. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, V.; Bukhteeva, I.; Kit, O.Y.; Nesmelova, I.V. Plant Defensins from a Structural Perspective. Int. J. Mol. Sci. 2020, 21, 5307. [Google Scholar] [CrossRef] [PubMed]
- Clifton, L.A.; Sanders, M.; Kinane, C.; Arnold, T.; Edler, K.J.; Neylon, C.; Green, R.J.; Frazier, R.A. The Role of Protein Hydrophobicity in Thionin–phospholipid Interactions: A Comparison of A1 and A2-Purothionin Adsorbed Anionic Phospholipid Monolayers. Phys. Chem. Chem. Phys. 2012, 14, 13569–13579. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Lazaridis, T. Antimicrobial Peptides in Toroidal and Cylindrical Pores. Biochim. Biophys. Acta BBA Biomembr. 2010, 1798, 1485–1493. [Google Scholar] [CrossRef]
- Cirac, A.D.; Moiset, G.; Mika, J.T.; Koçer, A.; Salvador, P.; Poolman, B.; Marrink, S.J.; Sengupta, D. The Molecular Basis for Antimicrobial Activity of Pore-Forming Cyclic Peptides. Biophys. J. 2011, 100, 2422–2431. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef]
- Wilmes, M.; Cammue, B.P.A.; Sahl, H.-G.; Thevissen, K. Antibiotic Activities of Host Defense Peptides: More to It than Lipid Bilayer Perturbation. Nat. Prod. Rep. 2011, 28, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Vriens, K.; Cammue, B.; Thevissen, K. Antifungal Plant Defensins: Mechanisms of Action and Production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef]
- Khan, R.S.; Iqbal, A.; Malak, R.; Shehryar, K.; Attia, S.; Ahmed, T.; Khan, M.A.; Arif, M.; Mii, M. Plant Defensins: Types, Mechanism of Action and Prospects of Genetic Engineering for Enhanced Disease Resistance in Plants. 3 Biotech 2019, 9, 1–12. [Google Scholar]
- Bechinger, B.; Gorr, S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, S.; Jian, W.; Xie, C.; Yang, X. Plant Antimicrobial Peptides: Structures, Functions, and Applications. Bot. Stud. 2021, 62, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Mattoo, A.K. Plant antimicrobial peptides. In Host Defense Peptides and Their Potential as Therapeutic Agents; Springer: Cham, Switzerland, 2016; pp. 111–136. [Google Scholar]
| Expression System-AMPs/Recombinant Protein | Source | Specific Role | References |
|---|---|---|---|
| Escherichia coli | |||
| Thaumatin-like protein (ATLP3) | Arabidopsis thaliana | Fungal growth inhibition | [61] |
| Osmotin-like protein (SnOLP) | Solanum nigrum | Mycelium growth inhibition (Phytophthora nicotiana, Fusarium solani, Colletotrichum gossypii) | [62] |
| Mi AMP1 | Macadamia integrifolia | Spore and mycelium growth inhibition | [63] |
| Pg-AMP1 | Guava psidium | Gram-positive (Staphylococcus sp.) and Gram-negative (Pseudomonas sp.) bacterial growth inhibition | [64] |
| Vv-AMP1 | Plant Vitis vinifera | Fungal growth inhibition | [65] |
| Puroindoline A | Wheat seed | Staphylococcus epidermidis growth inhibition | [66] |
| RHG1-LRR | Glycine max | Fungal mycelium growth reduction | [67] |
| Transglutaminase (TGZ) | Zea mays | Resistance to phytopathogens | [68] |
| Bacillus subtilis | |||
| Thionin | Oat | Inhibition of bacterial infection | [69] |
| β-purothionin | Z. mays | Controlling bacterial and fungal infection | [48] |
| AsR416 | Allium sativum | Bacterial infection reduction | [38] |
| AsR498 | A. sativum | Bacterial infection reduction | [38] |
| IiR515 | Isatis indigo | Bacterial and fungal growth inhibition | [70] |
| liR915 | I. indigo | Bacterial and fungal growth inhibition | [70] |
| CeCPI | Colocasia esculenta | Suppression of gall formation in tomato | [71] |
| AtR100 | A. tauschii | Bacterial (Clavibacter fangii., Clavibacter michigenesis) and fungal (B. cinerea) infection reduction | [36] |
| AtR472 | Aegilops tauschii | Bacterial (C. fangii., C. michigenesis) and fungal (B. cinerea) infection reduction | [36] |
| ZM-985 | Z. mays | Bacterial and fungal growth inhibition | [72] |
| ZM-804 | Z. mays | Bacterial and fungal growth inhibition | [37] |
| OrR214 | Oryza rufipogon Griff | Resistant to bacterial and fungal infection | [39] |
| Saccharomyces cerevisiae | |||
| AtPTR1 | A. thaliana | Reduced phytopathogens infection | [73] |
| AtChx17 | A. thaliana | Transcriptional inhibitor | [74] |
| HKT1 | Tritichum aestivum | Salt and stress-tolerant | [75] |
| AtGAT1 | A. thaliana | Plasma membrane integrating role | [76] |
| AtINT2 | A. thaliana | Encoded truncated protein to inhibit transmembrane helices | [77] |
| Pichia pastoris | |||
| Antifungal Protein (AFP) | Aspergillus | Antifungal activity | [78] |
| Defensin (Pdc1) | Corn | Fungal mycelium and conidial growth reduction | [79] |
| Defensin (Psd1) | Pisum sativum | Resistant to fungal infection | [80] |
| LCT1 | Tritichum aestivum | Cationic promoter | [81] |
| Baculovirus-mediated insect cell | |||
| Patatin | Solanum tuberosum | Exhibit enzymatic activity | [82] |
| Cyclin-dependent kinase A (CDKA) | A. thaliana | Control cell cycle activation | [83] |
| Acyl-CoA synthetase | A. thaliana | Provides energy during germination | [84] |
| Ethylene-inducing xylanase | Nicotiana tabacum | Not clear | [85] |
| Plants system | |||
| AP24 osmotine | N. tabacum | Resistance to Phytophthora infestens, Rhizoctonia solani, Fusarium solani | [86] |
| RsAFP2 | Raphanus sativus | Resistance to Fusarium graminearum and Rhizoctonia cerealis | [87] |
| Defensin 1 (BrD1) | Brassica rapa | Resistance to Nilaparvata lugens | [88] |
| NmDEF02 | N. tabacum | Enhanced crop improvement | [89] |
| MsDef1 | Medicago sativa | Resistance to Fusarium oxysporum | [90] |
| Protein | Tag Name | Size (kDa) | Length (aa) | Matrix | References |
|---|---|---|---|---|---|
| Hexahistidine | His-tag (6x) | 1 | 6–10 | Immobilized metal ions (Ni2+, Co2+, Cu2+, Zn2+, Fe3+) | [39,114] |
| His-patch thio-fusion | HP- thioredoxin | 11.7 | 100 | Metal chelating agents | [115] |
| Glutathione S-transferase | GST | 26 | 211 | Glutathione | [116] |
| Maltose-binding protein | MBP | 42 | 396 | Amylose | [117] |
| Calmodulin-binding peptide | CBP | 2 | 26 | Ca2+ chelating agents | [118,119] |
| Intein-Chitin binding domain | CBD | 5.6 | 51 | Chitin | [120] |
| FLAg tag peptide | FLAG | 1.01 | 8 | Anti-FLAG mAb | [121] |
| Streptavidin/Biotin-binding peptide | SBP | 4.3 | 38 | Streptavidin | [119] |
| Strep-tag peptide | Strep-II | 1.06 | 8 | Strep-Tactin | [119] |
| Halo protein tag | Halo | 33 | 297 | Halo-link resin | [122] |
| Fasciola hepatica8-Da antigen | Fh8 | 8 | 69 | EDTA | [123] |
| Myc protein | c-Myc | 1.2 | 11 | Anti-Myc epitope mAb | [124] |
| S protein | S | 1.75 | 15 | S-protein RNase A | [125] |
| Protease | Source | Cleavage Sites | Molecular Weight (kDa) | References |
|---|---|---|---|---|
| TEV | Tobacco Etch Virus | ENLYFQ- | 27 | [39,70] |
| Enterokinase | E. coli | DDDDK- | 31 | [128] |
| Factor Xa | Bovine plasma | IDGR- | 42 + 17 | [129] |
| Genenase | Bacillus amyloliquefaciens | PGAAHY- | 28 | [130,131] |
| Thrombin | Bovine plasma | LVPR-GS | 6 | [132] |
| PreScission | Human rhinovirus (HRV) 3C | LEVLFQ-GP | 46 | [133] |
| Furin | Spodoptera frugiperda (Sf9) cells | RXK/RR- | 52.7 | [134] |
| Sortase A | Staphylococcus aureus | LPET-G | 12 | [135] |
| Intein | S. cerevisiae (vma gene) | Self-cleavable | 51 | [136,137] |
| SUMO | E. coli | Conformation (Requires His-tag) | 12 | [138] |
| TVMV | Tobacco vein mottling virus | ETVRFQ-S | 77.9 | [139] |
| TAGZyme system | S. frugiperda (baculovirus) | Exoproteolysis | 23 + 16 + 6 | [140] |
| Exoproteases Carboxypeptidase A | Pancreas E. coli S. cerevisiae | C-terminal amino acids except Pro, Lys, and Arg | 33 | [140] |
| Carboxypeptidase B | Pancreas E. coli P. pastoris | C-terminal Lys and Arg | 35 | [140,141] |
| CasP6 | E. coli | VEID- | 26 | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S.; Mohamed, G.; Polash, S.A.; Hasan, M.A.; Sultana, R.; Saiara, N.; Dong, W. Antimicrobial Peptides from Plants: A cDNA-Library Based Isolation, Purification, Characterization Approach and Elucidating Their Modes of Action. Int. J. Mol. Sci. 2021, 22, 8712. https://doi.org/10.3390/ijms22168712
Islam MS, Mohamed G, Polash SA, Hasan MA, Sultana R, Saiara N, Dong W. Antimicrobial Peptides from Plants: A cDNA-Library Based Isolation, Purification, Characterization Approach and Elucidating Their Modes of Action. International Journal of Molecular Sciences. 2021; 22(16):8712. https://doi.org/10.3390/ijms22168712
Chicago/Turabian StyleIslam, Md. Samiul, Gamarelanbia Mohamed, Shakil Ahmed Polash, Md. Amit Hasan, Razia Sultana, Noshin Saiara, and Wubei Dong. 2021. "Antimicrobial Peptides from Plants: A cDNA-Library Based Isolation, Purification, Characterization Approach and Elucidating Their Modes of Action" International Journal of Molecular Sciences 22, no. 16: 8712. https://doi.org/10.3390/ijms22168712
APA StyleIslam, M. S., Mohamed, G., Polash, S. A., Hasan, M. A., Sultana, R., Saiara, N., & Dong, W. (2021). Antimicrobial Peptides from Plants: A cDNA-Library Based Isolation, Purification, Characterization Approach and Elucidating Their Modes of Action. International Journal of Molecular Sciences, 22(16), 8712. https://doi.org/10.3390/ijms22168712







