Nasal Rifampicin Improves Cognition in a Mouse Model of Dementia with Lewy Bodies by Reducing α-Synuclein Oligomers
Abstract
:1. Introduction
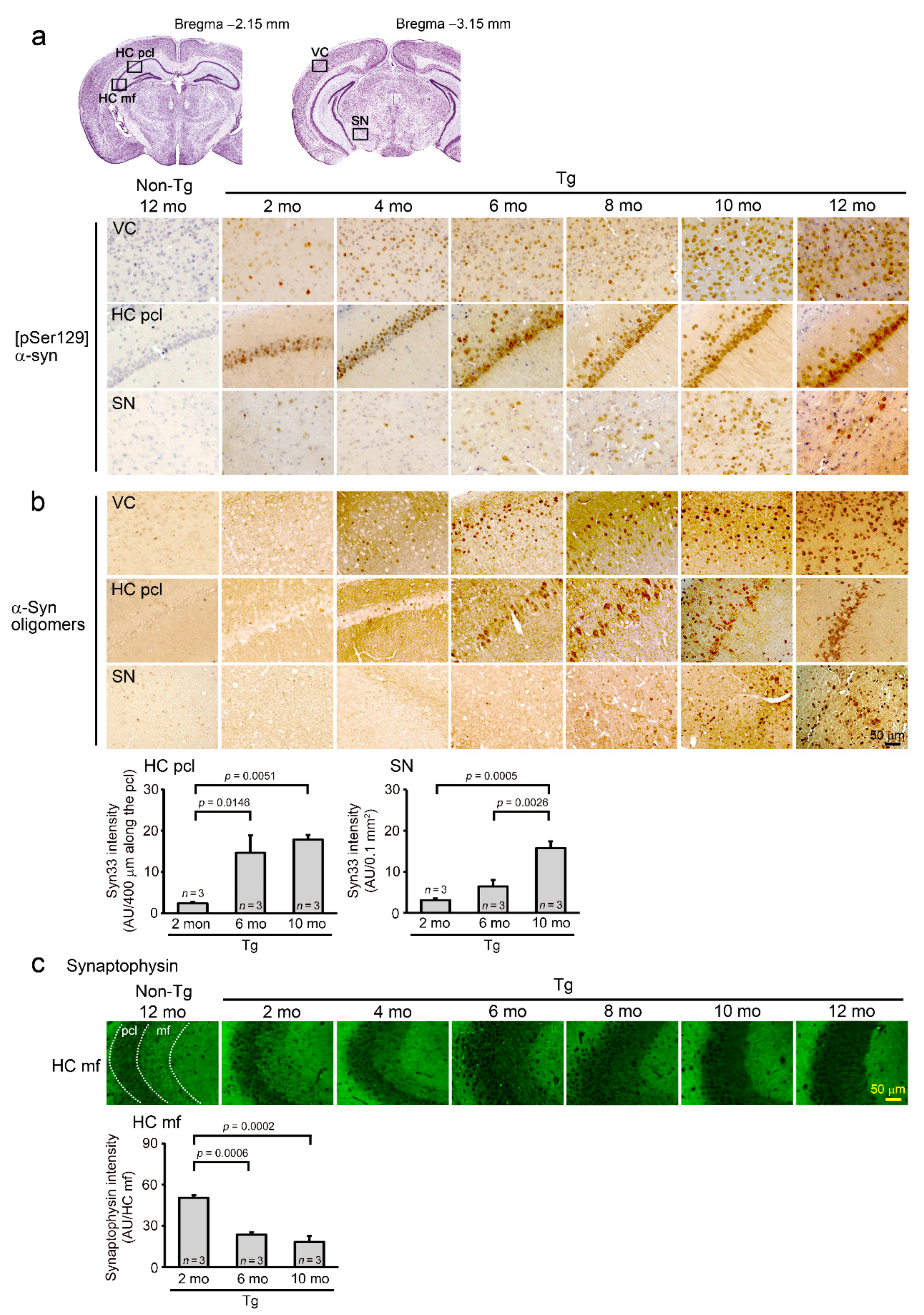
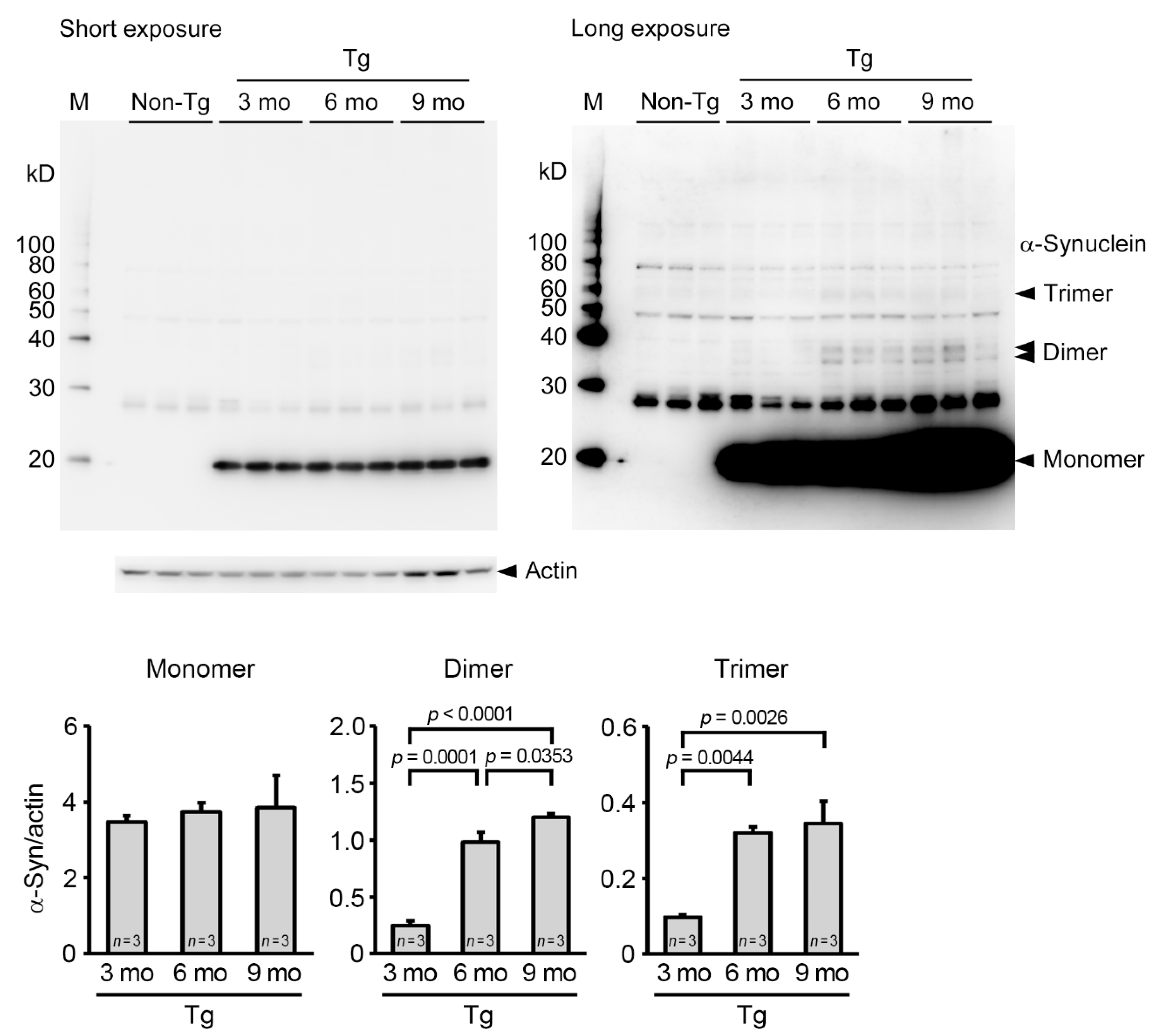
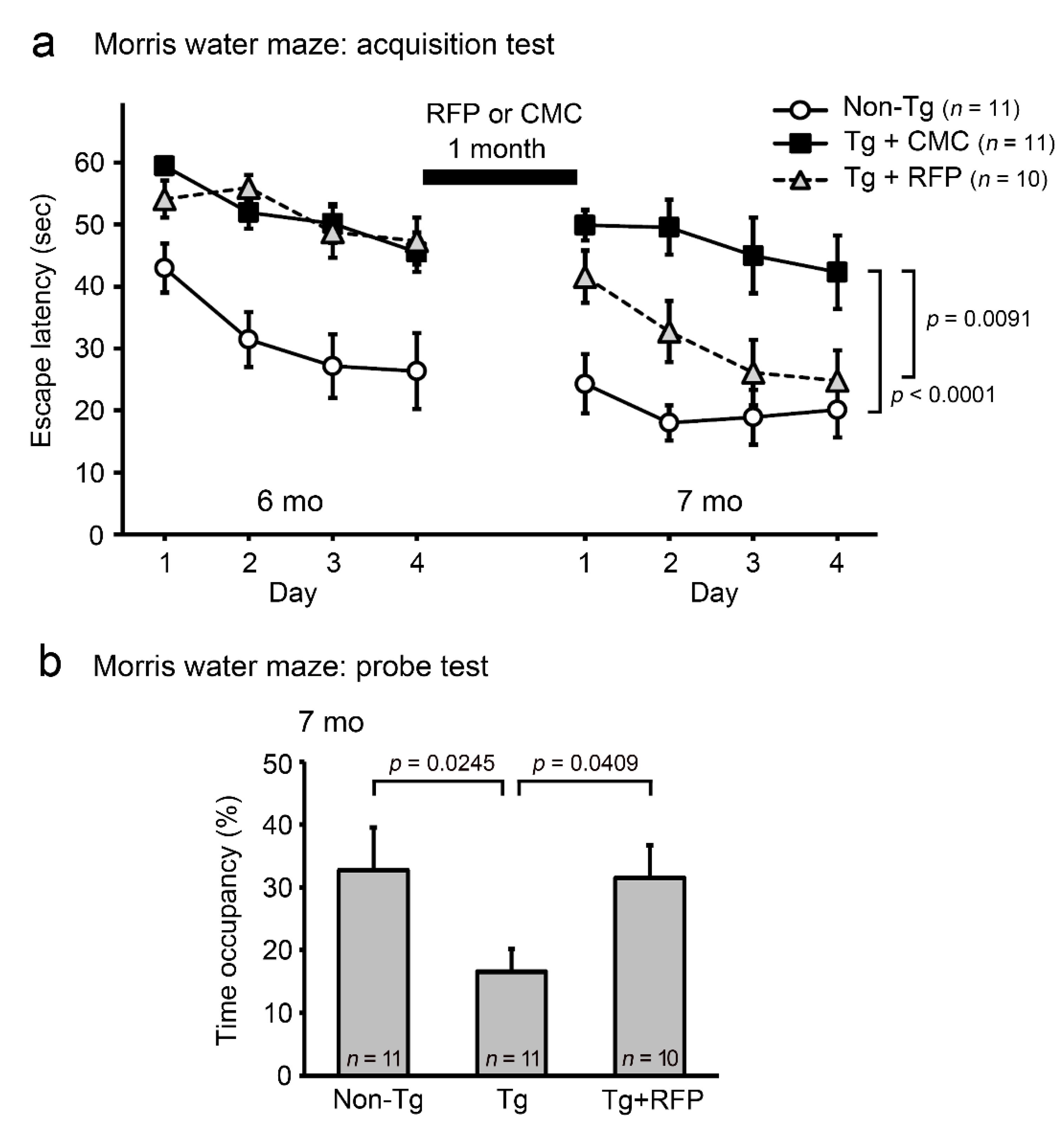
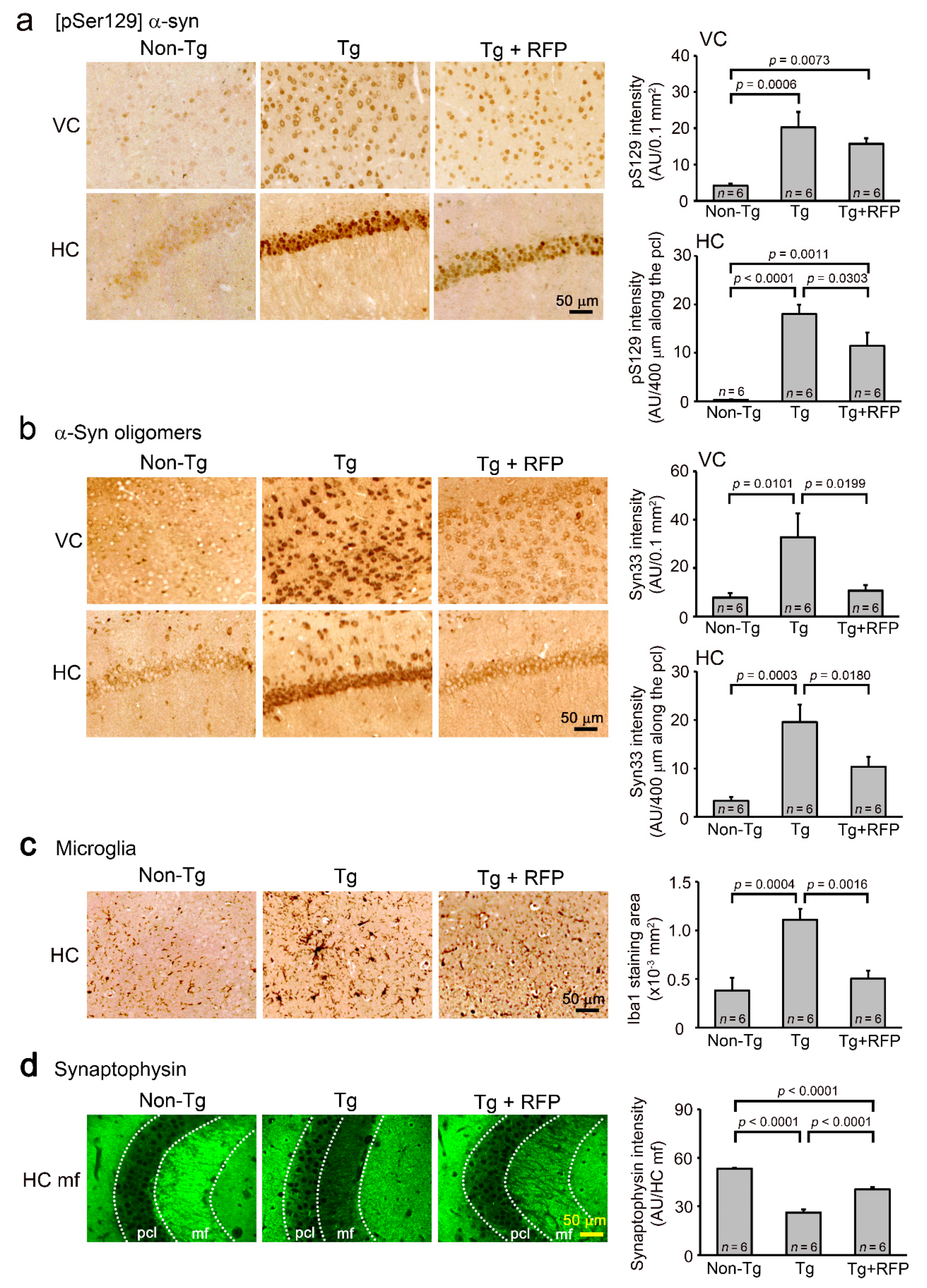
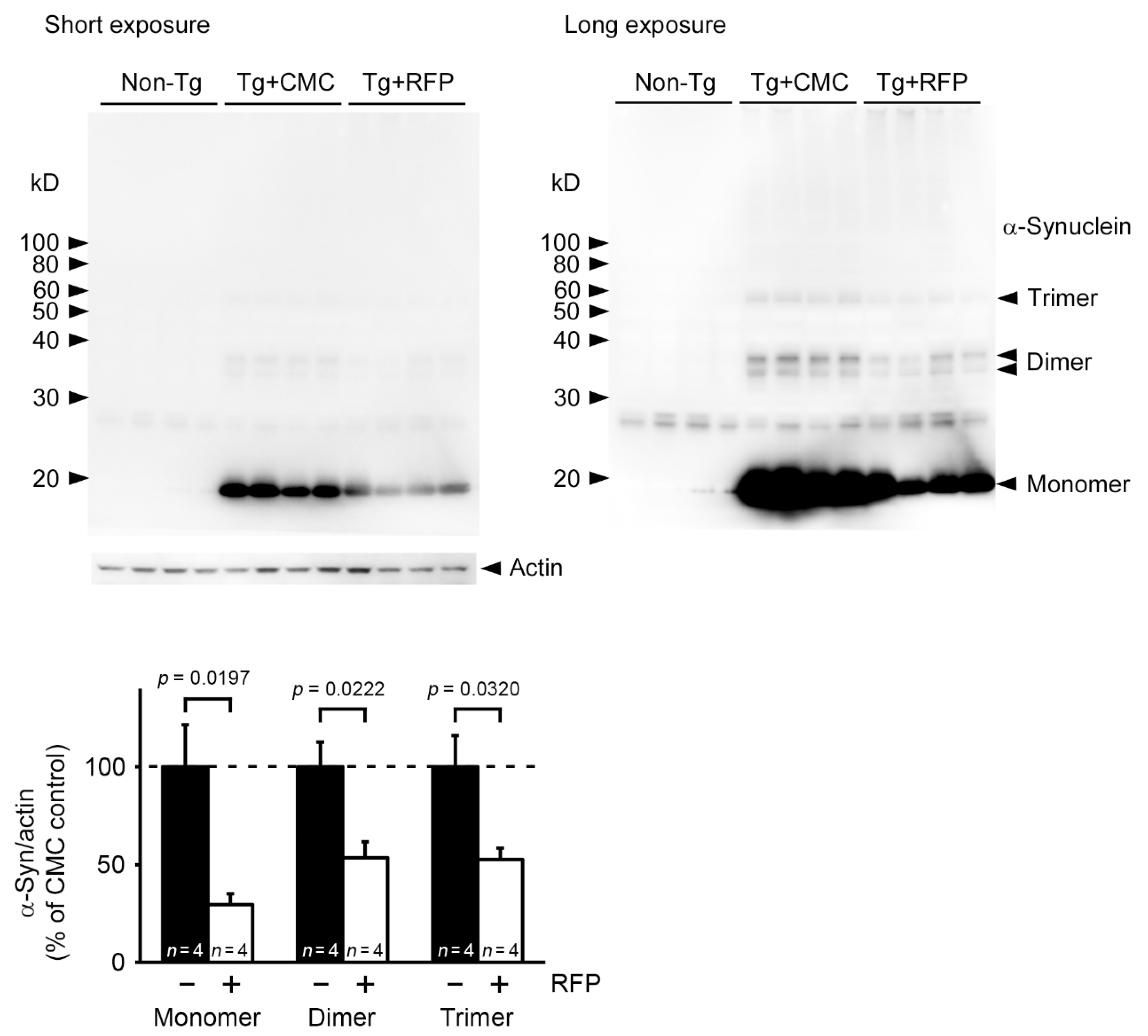
2. Results
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Behavioral Tests
4.3. Rifampicin Treatment
4.4. Immunohistochemical Analysis
4.5. Western Blot Analysis of α-Synuclein Oligomers
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCann, H.; Stevens, C.; Cartwright, H.; Halliday, G. α-Synucleinopathy phenotypes. Park. Relat. Disord. 2014, 20, S62–S67. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gao, G.; Duan, C.; Yang, H. Progress of immunotherapy of anti-α-synuclein in Parkinson’s disease. Biomed. Pharmacother. 2019, 115, 108843. [Google Scholar] [CrossRef]
- Bengoa-Vergniory, N.; Roberts, R.; Wade-Martins, R.; Alegre-Abarrategui, J. Alpha-synuclein oligomers: A new hope. Acta Neuropathol. 2017, 134, 819–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayed, R.; Dettmer, U.; Lesné, S.E. Soluble endogenous oligomeric α-synuclein species in neurodegenerative diseases: Expression, spreading, and cross-talk. J. Park. Dis. 2020, 10, 791–818. [Google Scholar] [CrossRef]
- Umeda, T.; Ono, K.; Sakai, A.; Yamashita, M.; Mizuguchi, M.; Klein, W.L.; Yamada, M.; Mori, H.; Tomiyama, T. Rifampicin is a candidate preventive medicine against amyloid-β and tau oligomers. Brain 2016, 139, 1568–1586. [Google Scholar] [CrossRef] [Green Version]
- Ubhi, K.; Rockenstein, E.; Mante, M.; Patrick, C.; Adame, A.; Thukral, M.; Shults, C.; Masliah, E. Rifampicin reduces α-synuclein in a transgenic mouse model of multiple system atrophy. Neuroreport 2008, 19, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Umeda, T.; Tanaka, A.; Sakai, A.; Yamamoto, A.; Sakane, T.; Tomiyama, T. Intranasal rifampicin for Alzheimer’s disease prevention. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Lischke, A.; Grace, S.; Scheele, D.; Ma, Y.; Becker, B. Advances in the field of intranasal oxytocin research: Lessons learned and future directions for clinical research. Mol. Psychiatry 2021, 26, 80–91. [Google Scholar] [CrossRef]
- Lee, M.K.; Stirling, W.; Xu, Y.; Xu, X.; Qui, D.; Mandir, A.S.; Dawson, T.M.; Copeland, N.G.; Jenkins, N.A.; Price, D.L. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 8968–8973. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Covelo, A.; Martell-Martínez, H.; Nanclares, C.; Sherman, M.A.; Okematti, E.; Meints, J.; Teravskis, P.; Gallardo, C.; Savonenko, A.V.; et al. Tau is required for progressive synaptic and memory deficits in a transgenic mouse model of α-synucleinopathy. Acta Neuropathol. 2019, 138, 551–574. [Google Scholar] [CrossRef] [Green Version]
- Tomiyama, T.; Asano, S.; Suwa, Y.; Morita, T.; Kataoka, K.; Mori, H.; Endo, N. Rifampicin Prevents the Aggregation and Neurotoxicity of Amyloid β Protein in Vitro. Biochem. Biophys. Res. Commun. 1994, 204, 76–83. [Google Scholar] [CrossRef]
- Tomiyama, T.; Shoji, A.; Kataoka, K.-I.; Suwa, Y.; Asano, S.; Kaneko, H.; Endo, N. Inhibition of Amyloid β Protein Aggregation and Neurotoxicity by Rifampicin. J. Biol. Chem. 1996, 271, 6839–6844. [Google Scholar] [CrossRef] [Green Version]
- Molloy, D.W.; Standish, T.; Zhou, Q.; Guyatt, G. The DARAD Study Group: A multicenter, blinded, randomized, factorial controlled trial of doxycycline and rifampin for treatment of Alzheimer’s disease: The DARAD trial. Int. J. Geriatr. Psychiatry 2012, 28, 463–470. [Google Scholar] [CrossRef]
- Nicoll, J.; Buckland, G.R.; Harrison, C.; Page, A.; Harris, S.; Love, S.; Neal, J.W.; Holmes, C.; Boche, D. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain 2019, 142, 2113–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.S.; Donohue, M.C.; Sperling, R.; Hansson, O.; Mattsson-Carlgren, N. The A4 study: β-amyloid and cognition in 4432 cognitively unimpaired adults. Ann. Clin. Transl. Neurol. 2020, 7, 776–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimer’s Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Selkoe, D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J. Neurochem. 2020, 154, 583–597. [Google Scholar] [CrossRef]
- Iizuka, T.; Morimoto, K.; Sasaki, Y.; Kameyama, M.; Kurashima, A.; Hayasaka, K.; Ogata, H.; Goto, H. Preventive Effect of Rifampicin on Alzheimer Disease Needs at Least 450 mg Daily for 1 Year: An FDG-PET Follow-Up Study. Dement. Geriatr. Cogn. Disord. Extra 2017, 7, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhu, M.; Rajamani, S.; Uversky, V.N.; Fink, A.L. Rifampicin Inhibits α-Synuclein Fibrillation and Disaggregates Fibrils. Chem. Biol. 2004, 11, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- Low, P.A.; Robertson, D.; Gilman, S.; Kaufmann, H.; Singer, W.; Biaggioni, I.; Freeman, R.; Perlman, S.; Hauser, R.A.; Cheshire, W.; et al. Efficacy and safety of rifampicin for multiple system atrophy: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014, 13, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Wenning, G.K.; Krismer, F.; Poewe, W. Rifampicin for multiple system atrophy. Lancet Neurol. 2014, 13, 237–239. [Google Scholar] [CrossRef]
- Maeda, S.; Takashima, A. Tau Oligomers. Adv. Exp. Med. Biol. 2019, 1184, 373–380. [Google Scholar] [CrossRef]
- Hill, E.; Wall, M.J.; Moffat, K.G.; Karikari, T. Understanding the Pathophysiological Actions of Tau Oligomers: A Critical Review of Current Electrophysiological Approaches. Front. Mol. Neurosci. 2020, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Wang, L.; Zhang, T.; Zhang, B.; Chen, L. Sigma-1 receptor knockout increases α-synuclein aggregation and phosphorylation with loss of dopaminergic neurons in substantia nigra. Neurobiol. Aging 2017, 59, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Baek, S.Y.; Park, J.Y.; Kim, Y.W. Rifampicin activates AMPK and alleviates oxidative stress in the liver as mediated with Nrf2 signaling. Chem. Interact. 2020, 315, 108889. [Google Scholar] [CrossRef]
- Bi, W.; Zhu, L.; Wang, C.; Liang, Y.; Liu, J.; Shi, Q.; Tao, E. Rifampicin inhibits microglial inflammation and improves neuron survival against inflammation. Brain Res. 2011, 1395, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Acuña, L.; Hamadat, S.; Corbalán, N.S.; González-Lizárraga, F.; Dos-Santos-Pereira, M.; Rocca, J.; Díaz, J.S.; Del-Bel, E.; Papy-García, D.; Chehín, R.N.; et al. Rifampicin and Its Derivative Rifampicin Quinone Reduce Microglial Inflammatory Responses and Neurodegeneration Induced In Vitro by alphaα-Synuclein Fibrillary Aggregates. Cells 2019, 8, 776. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zheng, D.; Peng, S.; Lin, D.; Jing, X.; Zeng, Z.; Chen, Y.; Huang, K.; Xie, Y.; Zhou, T.; et al. Rifampicin attenuates rotenone-treated microglia inflammation via improving lysosomal function. Toxicol. Vitr. 2020, 63, 104690. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Herman, F.; Westfall, S.; Brathwaite, J.; Pasinetti, G.M. Suppression of Presymptomatic Oxidative Stress and Inflammation in Neurodegeneration by Grape-Derived Polyphenols. Front. Pharmacol. 2018, 9, 867. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, Q.; Hu, Z.; Tang, X. Potential Neuroprotective Treatment of Stroke: Targeting Excitotoxicity, Oxidative Stress, and Inflammation. Front. Neurosci. 2019, 13, 1036. [Google Scholar] [CrossRef] [PubMed]
- Yulug, B.; Kilic, U.; Kilic, E.; Bähr, M. Rifampicin attenuates brain damage in focal ischemia. Brain Res. 2004, 996, 76–80. [Google Scholar] [CrossRef]
- Donald, P. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis 2010, 90, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, T.; Matsuyama, S.; Iso, H.; Umeda, T.; Takuma, H.; Ohnishi, K.; Ishibashi, K.; Teraoka, R.; Sakama, N.; Yamashita, T.; et al. A Mouse Model of Amyloid Oligomers: Their Contribution to Synaptic Alteration, Abnormal Tau Phosphorylation, Glial Activation, and Neuronal Loss In Vivo. J. Neurosci. 2010, 30, 4845–4856. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umeda, T.; Hatanaka, Y.; Sakai, A.; Tomiyama, T. Nasal Rifampicin Improves Cognition in a Mouse Model of Dementia with Lewy Bodies by Reducing α-Synuclein Oligomers. Int. J. Mol. Sci. 2021, 22, 8453. https://doi.org/10.3390/ijms22168453
Umeda T, Hatanaka Y, Sakai A, Tomiyama T. Nasal Rifampicin Improves Cognition in a Mouse Model of Dementia with Lewy Bodies by Reducing α-Synuclein Oligomers. International Journal of Molecular Sciences. 2021; 22(16):8453. https://doi.org/10.3390/ijms22168453
Chicago/Turabian StyleUmeda, Tomohiro, Yukari Hatanaka, Ayumi Sakai, and Takami Tomiyama. 2021. "Nasal Rifampicin Improves Cognition in a Mouse Model of Dementia with Lewy Bodies by Reducing α-Synuclein Oligomers" International Journal of Molecular Sciences 22, no. 16: 8453. https://doi.org/10.3390/ijms22168453
APA StyleUmeda, T., Hatanaka, Y., Sakai, A., & Tomiyama, T. (2021). Nasal Rifampicin Improves Cognition in a Mouse Model of Dementia with Lewy Bodies by Reducing α-Synuclein Oligomers. International Journal of Molecular Sciences, 22(16), 8453. https://doi.org/10.3390/ijms22168453





