Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier
Abstract
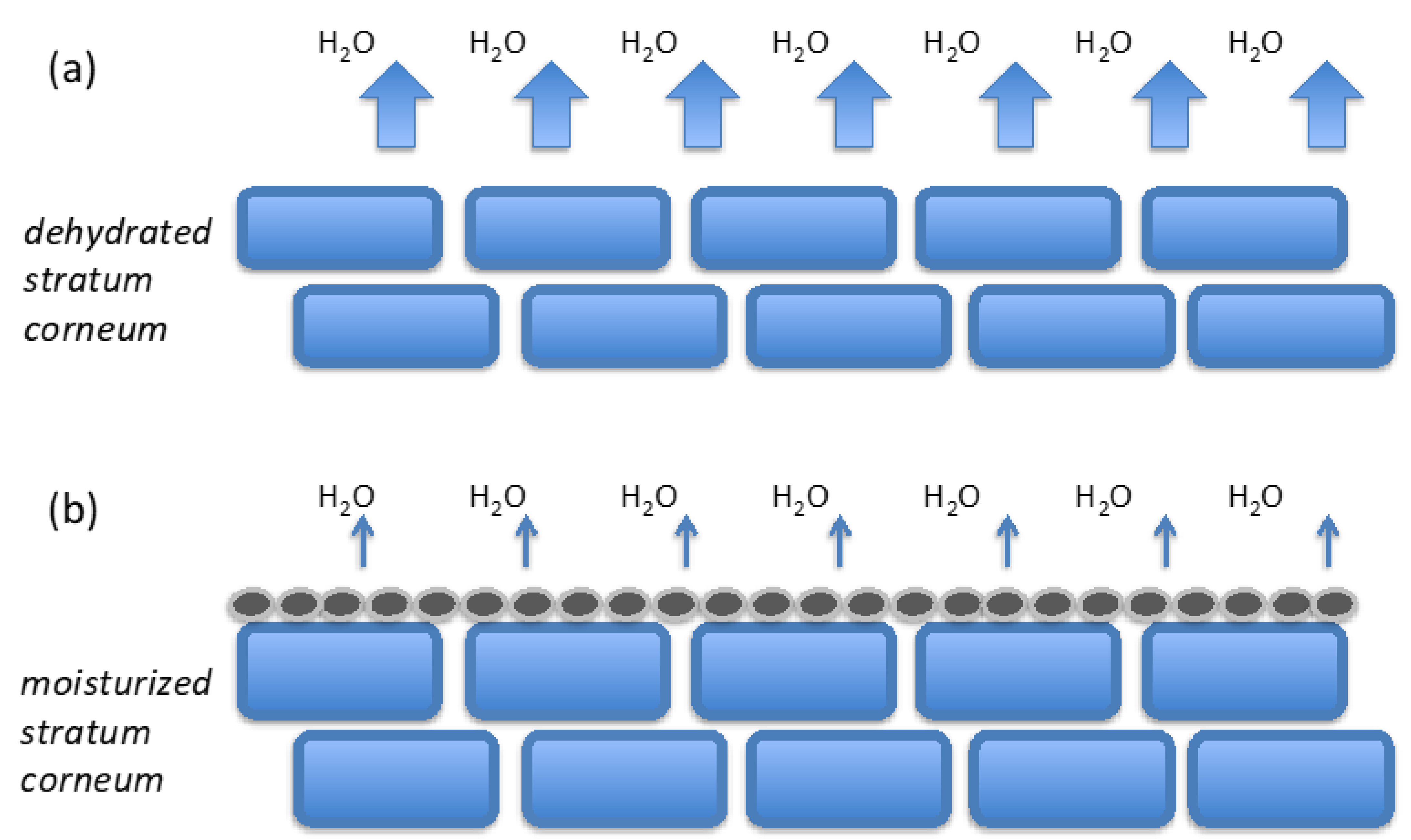
:1. Introduction
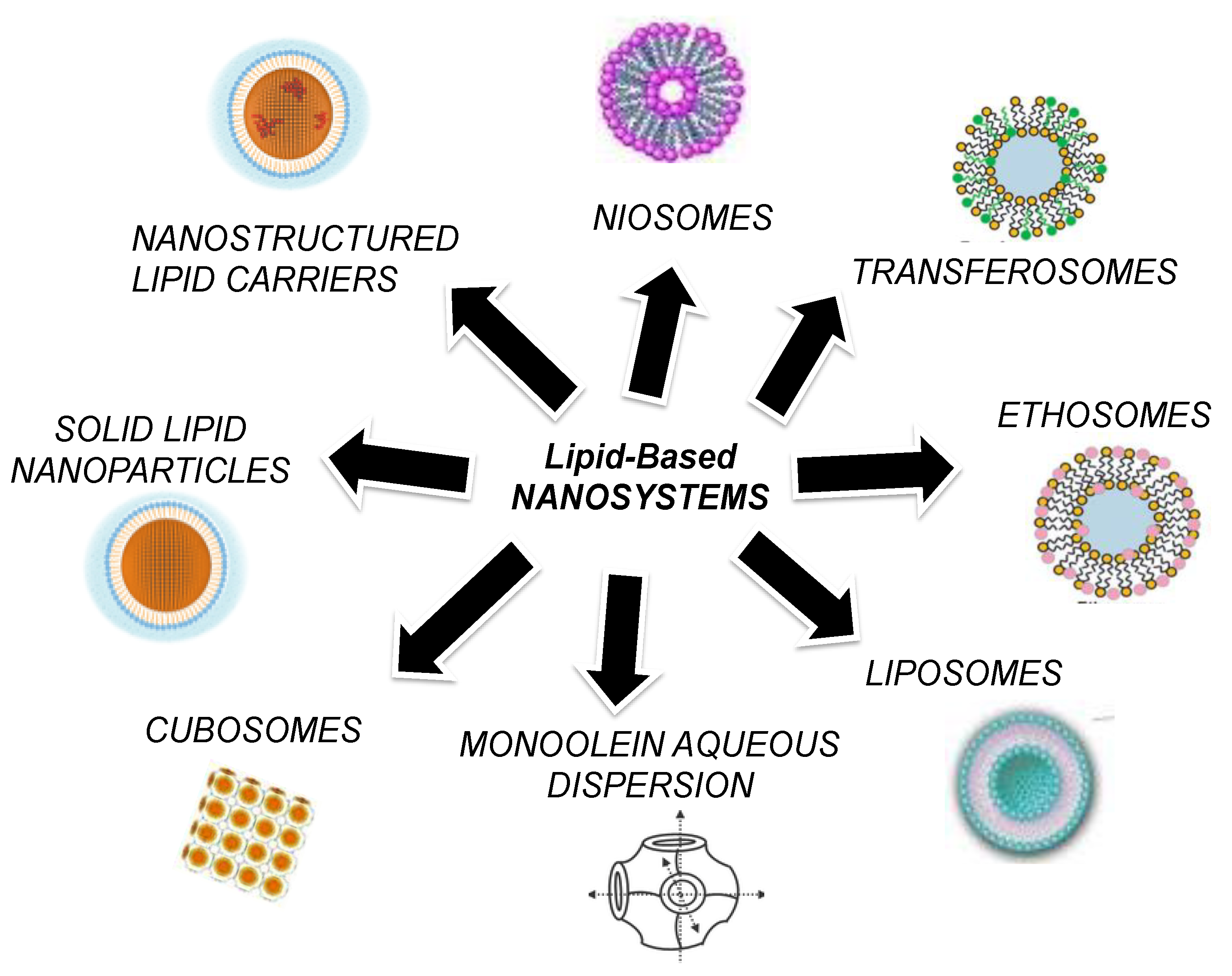
2. Lipid-Based Nanosystems
2.1. Liposomes
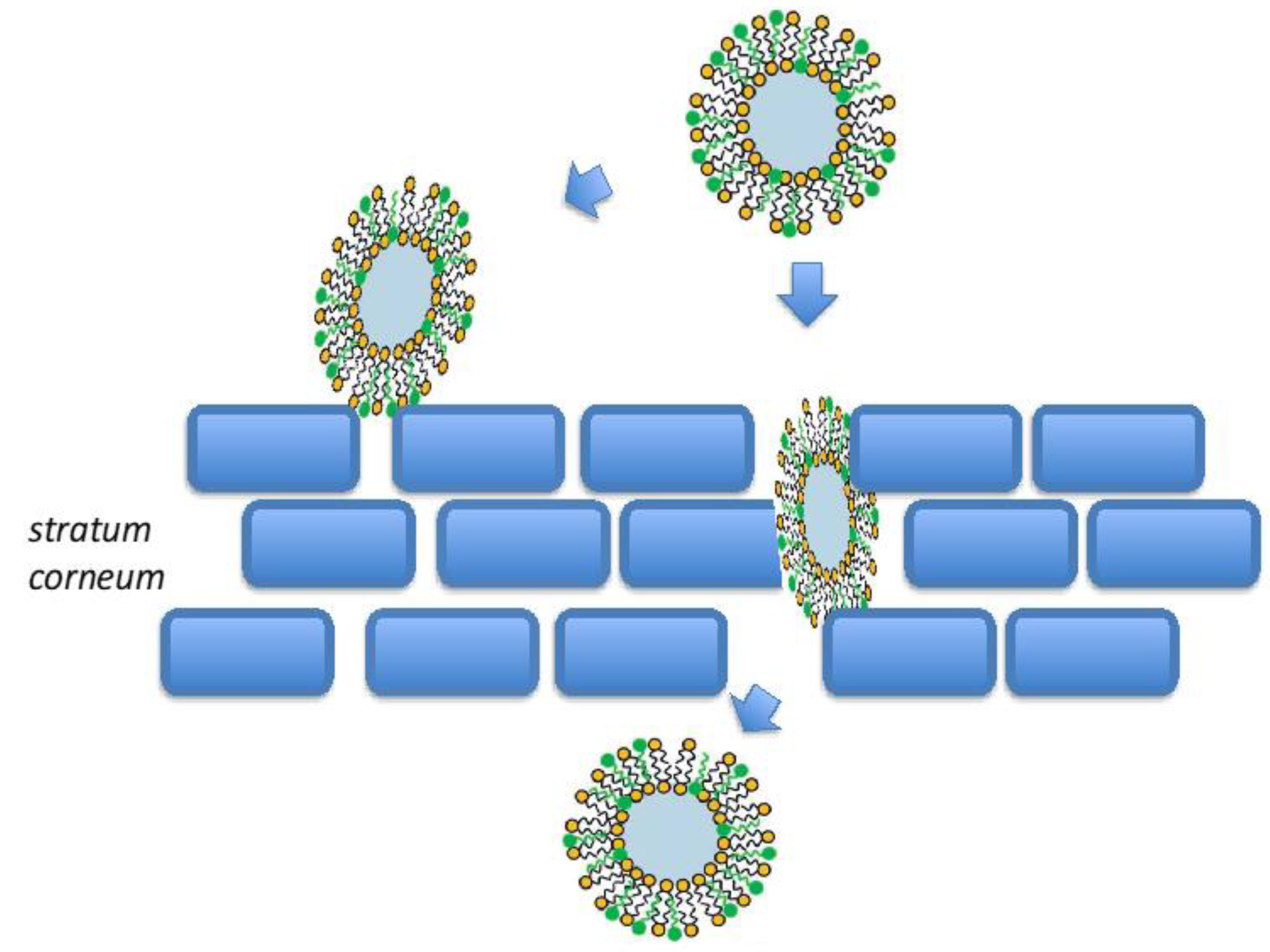
2.2. Transfersomes
2.3. Niosomes
2.4. Ethosomes
2.5. Cubosomes and Monoolein Aqueous Dispersions (MADs)
2.6. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, M. Understanding the structure and function of the skin. Nurs. Times 2003, 99, 46–48. [Google Scholar]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta 2006, 1758, 2080–2095. [Google Scholar] [CrossRef] [Green Version]
- de Souza Guedes, L.; Martinez, R.M.; Bou-Chacra, N.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. An Overview on Topical Administration of Carotenoids and Coenzyme Q10 Loaded in Lipid Nanoparticles. Antioxidants 2021, 10, 1034. [Google Scholar] [CrossRef]
- Fore, J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manag. 2006, 52, 24–35. [Google Scholar]
- Bouwstra, J. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Imam, S.S.; Ahmad, M.Z.; Vuddanda, P.R.; Alshehri, S.; Mahdi, W.A.; Ahmad, J. Recent Progress in Lipid Nanoparticles for Cancer Theranostics: Opportunity and Challenges. Pharmaceutics 2021, 13, 840. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Sharma, G.S.; Goyal, A.K.; Ghosh, G.; Si, S.C.; Rath, G. Recent advances in topical carriers of anti-fungal agents. Heliyon 2020, 6, e04663. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.C.; Schäfer-Korting, M. Carriers in the topical treatment of skin disease. Handb. Exp. Pharmacol. 2010, 435–468. [Google Scholar] [CrossRef]
- Nguyen, T.; Zuniga, R. Skin conditions: New drugs for managing skin disorders. FP Essent. 2013, 407, 11–16. [Google Scholar]
- Kulkarni, V.S.; Shaw, C. Formulating Creams, Gels, Lotions, and Suspensions. In Essential Chemistry for Formulators of Semisolid and Liquid Dosages; Academic Press: New York, NY, USA, 2016; pp. 29–41. ISBN 978-0-12-801024-2. [Google Scholar]
- Araujo, V.H.S.; Delello Di Filippo, L.; Duarte, J.L.; Spósito, L.; Camargo, B.A.F.D.; da Silva, P.B.; Chorilli, M. Exploiting solid lipid nanoparticles and nanostructured lipid carriers for drug delivery against cutaneous fungal infections. Crit. Rev. Microbiol. 2021, 47, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Saraceno, R.; Chiricozzi, A.; Gabellini, M.; Chimenti, S. Emerging applications of nanomedicine in dermatology. Skin Res. Technol. 2013, 19, e13–e19. [Google Scholar] [CrossRef] [Green Version]
- Friedman, A.; Nasir, A. Nanotechnology and dermatology education in the United States: Data from a pilot survey. J. Drugs Dermatol. JDD 2011, 10, 1037–1041. [Google Scholar]
- Papakostas, D.; Rancan, F.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Nanoparticles in dermatology. Arch. Dermatol. Res. 2011, 303, 533–550. [Google Scholar] [CrossRef]
- DeLouise, L.A. Applications of nanotechnology in dermatology. J. Investig. Dermatol. 2012, 132, 964–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasoń, E. Topical Administration of Terpenes Encapsulated in Nanostructured Lipid-Based Systems. Molecules 2020, 25, 5758. [Google Scholar] [CrossRef]
- Kirjavainen, M.; Urtti, A.; Valjakka-Koskela, R.; Kiesvaara, J.; Mönkkönen, J. Liposome-skin interactions and their effects on the skin permeation of drugs. Eur. J. Pharm. Sci. 1999, 7, 279–286. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Zhang, Z.; Wo, Y.; Zhang, Y.; Wang, D.; He, R.; Chen, H.; Cui, D. In vitro study of ethosome penetration in human skin and hypertrophic scar tissue. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Alkabes, M.; Eliaz, M. Ethosomes: Novel vesicular carriers for enhanced skin delivery. Pharm. Res. 1997, 14, S305–S306. [Google Scholar]
- Schaferkorting, M.; Mehnert, W.; Korting, H. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Jores, K.; Mehnert, W.; Drechsler, M.; Bunjes, H.; Johann, C.; Mäder, K. Investigations on the structure of solid lipid nanoparticles (SLN) and oil-loaded solid lipid nanoparticles by photon correlation spectroscopy, field-flow fractionation and transmission electron microscopy. J. Control Release 2004, 95, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, B.; Bunjes, H.; Koch, M.H.J.; Westesen, K. Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride–water phases. Int. J. Pharm. 2002, 244, 33–43. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Nyström, A.M.; Fadeel, B. Safety assessment of nanomaterials: Implications for nanomedicine. J. Control. Release 2012, 161, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinology 2009, 1, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neubert, R.H.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Schanzer, S.; Knorr, F.; Meinke, M.; Sterry, W.; Patzelt, A. Penetration and storage of particles in human skin: Perspectives and safety aspects. Eur. J. Pharm. Biopharm. 2011, 77, 465–468. [Google Scholar] [CrossRef]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef]
- Bolzinger, M.-A.; Briançon, S.; Chevalier, Y. Nanoparticles through the skin: Managing conflicting results of inorganic and organic particles in cosmetics and pharmaceutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–IN27. [Google Scholar] [CrossRef]
- Kapoor, B.; Gupta, R.; Gulati, M.; Singh, S.K.; Khursheed, R.; Gupta, M. The Why, Where, Who, How, and What of the vesicular delivery systems. Adv. Colloid Interface Sci. 2019, 271, 101985. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-formulations for transdermal drug delivery: A review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Carita, A.C.; Eloy, J.O.; Chorilli, M.; Lee, R.J.; Leonardi, G.R. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr. Med. Chem. 2018, 25, 606–635. [Google Scholar] [CrossRef]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, E.; Nastruzzi, C.; Sguizzato, M.; Cortesi, R. Nanomedicines to Treat Skin Pathologies with Natural Molecules. Curr. Pharm. Des. 2019, 25, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim. Biophys. Acta BBA Biomembr. 1992, 1104, 226–232. [Google Scholar] [CrossRef]
- Cevc, G. Transfersomes, liposomes and other lipid suspensions on the skin: Permeation enhancement, vesicle penetration, and transdermal drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 1996, 13, 257–388. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, S.; Sharma, D.; Webster, T.; Shafaat, K.; Faruk, A. Elastic liposomes as novel carriers: Recent advances in drug delivery. Int. J. Nanomed. 2017, 12, 5087–5108. [Google Scholar] [CrossRef] [Green Version]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pathak, K.; Bali, V. Ultra-adaptable nanovesicular systems: A carrier for systemic delivery of therapeutic agents. Drug Discov. Today 2012, 17, 1233–1241. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, A.; Oh, Y.-K.; Kim, C.-K. Effect of edge activators on the formation and transfection efficiency of ultradeformable liposomes. Biomaterials 2005, 26, 205–210. [Google Scholar] [CrossRef]
- Ravikumar, P.; Tatke, P. Advances in encapsulated dermal formulations in chemoprevention of melanoma: An overview. J. Cosmet. Dermatol. 2019, 18, 1606–1612. [Google Scholar] [CrossRef]
- Abd El-Alim, S.H.; Kassem, A.A.; Basha, M.; Salama, A. Comparative study of liposomes, ethosomes and transfersomes as carriers for enhancing the transdermal delivery of diflunisal: In vitro and in vivo evaluation. Int. J. Pharm. 2019, 563, 293–303. [Google Scholar] [CrossRef]
- Cevc, G. Transdermal Drug Delivery of Insulin with Ultradeformable Carriers. Clin. Pharmacokinet. 2003, 42, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Rauber, G.; Argenta, D.F.; Caon, T. Emerging Technologies to Target Drug Delivery to the Skin—The Role of Crystals and Carrier-Based Systems in the Case Study of Dapsone. Pharm. Res. 2020, 37, 240. [Google Scholar] [CrossRef] [PubMed]
- Ramkar, S.; Sah, A.K.; Bhuwane, N.; Choudhary, I.; Hemnani, N.; Suresh, P.K. Nano-Lipidic Carriers as a Tool for Drug Targeting to the Pilosebaceous Units. Curr. Pharm. Des. 2020, 26, 3251–3268. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G.; Schätzlein, A.; Gebauer, D.; Paul, A. The skin: A pathway for systemic treatment with patches and lipid-based agent carriers. Adv. Drug Deliv. Rev. 1996, 18, 349–378. [Google Scholar] [CrossRef]
- Jadupati, M.; Amites, G.; Kumar, N.A. Transferosomes: An opportunistic carrier for Transdermal drug delivery system. Int. J. Pharm. IRJP 2012, 3, 35–38. [Google Scholar]
- Choi, M.J.; Maibach, H.I. Elastic vesicles as topical/transdermal drug delivery systems. Int. J. Cosmet. Sci. 2005, 27, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef]
- Ijaz, H.; Qureshi, J.; Tulain, U.R.; Iqbal, F.; Danish, Z.; Fayyaz, A.; Sethi, A. Lipid particulate drug delivery systems: A review. Bioinspired Biomim. Nanobiomater. 2018, 7, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Muzzalupo, R.; Tavano, L. Niosomal drug delivery for transdermal targeting: Recent advances. Res. Rep. Transdermal Drug Deliv. 2015, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2. [Google Scholar] [CrossRef]
- Paape, M.J.; Wergin, W.P. The leukocyte as a defense mechanism. J. Am. Vet. Med. Assoc. 1977, 170, 1214–1223. [Google Scholar] [PubMed]
- Liu, M.; Wen, J.; Sharma, M. Solid Lipid Nanoparticles for Topical Drug Delivery: Mechanisms, Dosage Form Perspectives, and Translational Status. Curr. Pharm. Des. 2020, 26, 3203–3217. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, E.A.; Fahmy, H.M. Niosomes and liposomes as promising carriers for dermal delivery of Annona squamosa extract. Braz. J. Pharm. Sci. 2020, 56, e18096. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Hong, C.-T.; Chiu, W.-T.; Wang, Y.-Y. Effect of liposomes and niosomes on skin permeation of enoxacin. Int. J. Pharm. 2001, 219, 61–72. [Google Scholar] [CrossRef]
- Karami, M.A.; Jalili Rad, M.; Zadeh, B.S.M.; Salimi, A. Superoxide dismutase loaded niosomes delivery to hair follicles: Permeation through synthetic membrane and guinea pig skin. Int. J. Appl. Pharm. 2019, 305–312. [Google Scholar] [CrossRef]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal Nanocarriers for Enhanced Skin Delivery of Quercetin with Functions of Anti-Tyrosinase and Antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Rakesh, R.; Anoop, K.R. Ethosomes for transdermal and topical drug delivery. Int. J. Pharm. Pharm. Sci. 2012, 4, 17–24. [Google Scholar]
- Godin, B.; Touitou, E. Ethosomes: New prospects in transdermal delivery. Crit. Rev. Ther. Drug Carrier Syst. 2003, 20, 63–102. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Spinozzi, F.; Benedusi, M.; Cervellati, F.; Cortesi, R.; Drechsler, M.; Prieux, R.; Valacchi, G.; Esposito, E. Ethosomes for Coenzyme Q10 Cutaneous Administration: From Design to 3D Skin Tissue Evaluation. Antioxidants 2020, 9, 485. [Google Scholar] [CrossRef]
- Esposito, E.; Menegatti, E.; Cortesi, R. Ethosomes and liposomes as topical vehicles for azelaic acid: A preformulation study. Int. J. Cosmet. Sci. 2004, 26, 270–271. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and Transethosomes for Mangiferin Transdermal Delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Shen, L.-N.; Wu, Z.-H.; Zhao, J.-H.; Feng, N.-P. Evaluation of Skin Viability Effect on Ethosome and Liposome-Mediated Psoralen Delivery via Cell Uptake. J. Pharm. Sci. 2014, 103, 3120–3126. [Google Scholar] [CrossRef] [PubMed]
- Natsheh, H.; Vettorato, E.; Touitou, E. Ethosomes for Dermal Administration of Natural Active Molecules. Curr. Pharm. Des. 2019, 25, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Godin, B.; Dayan, N.; Weiss, C.; Piliponsky, A.; Levi-Schaffer, F. Intracellular delivery mediated by an ethosomal carrier. Biomaterials 2001, 22, 3053–3059. [Google Scholar] [CrossRef]
- Jain, S.; Tiwary, A.K.; Sapra, B.; Jain, N.K. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS Pharm. Sci. Tech. 2007, 8, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallan, S.S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Balakrishnan, P.; Shim, C.-K.; Chung, S.-J.; Chong, S.; Kim, D.-D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [Green Version]
- Paiva-Santos, A.C.; Silva, A.L.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Castro, R.; Veiga, F. Ethosomes as Nanocarriers for the Development of Skin Delivery Formulations. Pharm. Res. 2021, 38, 947–970. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Abdelbary, A.; Refai, H.; El-Nabarawi, M. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: In vitro, ex vivo, and in vivo evaluation. Int. J. Nanomed. 2019, 14, 1953–1968. [Google Scholar] [CrossRef] [Green Version]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascenso, A.; Raposo, S.; Batista, C.; Cardoso, P.; Mendes, T.; Praça, F.G.; Bentley, M.V.L.B.; Simões, S. Development, characterization, and skin delivery studies of related ultradeformable vesicles: Transfersomes, ethosomes, and transethosomes. Int. J. Nanomed. 2015, 10, 5837–5851. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Cubic Lipid—Water Phase Dispersed into Submicron Particles. Langmuir 1996, 12, 4611–4613. [Google Scholar] [CrossRef]
- Esposito, E.; Ravani, L.; Mariani, P.; Contado, C.; Drechsler, M.; Puglia, C.; Cortesi, R. Curcumin containing monoolein aqueous dispersions: A preformulative study. Mater Sci. Eng. C 2013, 33, 4923–4934. [Google Scholar] [CrossRef]
- Spicer, P.T.; Hayden, K.L.; Lynch, M.L.; Ofori-Boateng, A.; Burns, J.L. Novel Process for Producing Cubic Liquid Crystalline Nanoparticles (Cubosomes). Langmuir 2001, 17, 5748–5756. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef]
- Esposito, E.; Pecorelli, A.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Cervellati, F.; Nastruzzi, C.; Cortesi, R.; Valacchi, G. Production and Characterization of Nanoparticle Based Hyaluronate Gel Containing Retinyl Palmitate for Wound Healing. Curr. Drug Deliv. 2018, 15, 1172–1182. [Google Scholar] [CrossRef]
- Waghule, T.; Gorantla, S.; Rapalli, V.K.; Shah, P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Emerging Trends in Topical Delivery of Curcumin Through Lipid Nanocarriers: Effectiveness in Skin Disorders. AAPS Pharm. Sci. Tech. 2020, 21, 284. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, Y.; Han, K.; Qin, L.; Dian, L.; Li, G.; Pan, X.; Wu, C. Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. Drug Des. Devel. Ther. 2015, 4209. [Google Scholar] [CrossRef] [Green Version]
- Nasr, M.; Younes, H.; Abdel-Rashid, R.S. Formulation and evaluation of cubosomes containing colchicine for transdermal delivery. Drug Deliv. Transl. Res. 2020, 10, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Nithya, R.; Jerold, P.; Siram, K. Cubosomes of dapsone enhanced permeation across the skin. J. Drug Deliv. Sci. Technol. 2018, 48, 75–81. [Google Scholar] [CrossRef]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Vanic, Z.; Holaeter, A.-M.; Skalko-Basnet, N. (Phospho)lipid-based Nanosystems for Skin Administration. Curr. Pharm. Des. 2015, 21, 4174–4192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13, 549. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Ravani, L.; Contado, C.; Costenaro, A.; Drechsler, M.; Rossi, D.; Menegatti, E.; Grandini, A.; Cortesi, R. Clotrimazole nanoparticle gel for mucosal administration. Mater Sci. Eng. C 2013, 33, 411–418. [Google Scholar] [CrossRef]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Cortesi, R. Progesterone lipid nanoparticles: Scaling up and in vivo human study. Eur. J. Pharm. Biopharm. 2017, 119, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, A.; Savic, S.; Vuleta, G.; Müller, R.H.; Keck, C.M. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): Effects on size, physical stability and particle matrix structure. Int. J. Pharm. 2011, 406, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Uner, M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Die Pharm. Int. J. Pharm. Sci. 2006, 61, 375–386. [Google Scholar]
- Vivek, K.; Reddy, H.; Murthy, R.S.R. Investigations of the effect of the lipid matrix on drug entrapment, in vitro release, and physical stability of olanzapine-loaded solid lipid nanoparticles. AAPS Pharm. Sci. Tech. 2007, 8, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- Dobreva, M.; Stefanov, S.; Andonova, V. Natural Lipids as Structural Components of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Topical Delivery. Curr. Pharm. Des. 2020, 26, 4524–4535. [Google Scholar] [CrossRef]
- Bunjes, H.; Koch, M.H.J.; Westesen, K. Effect of Particle Size on Colloidal Solid Triglycerides. Langmuir 2000, 16, 5234–5241. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Li, T.S.; Su, H.L.; Lee, P.C.; Wang, H.-M.D. Transdermal Delivery Systems of Natural Products Applied to Skin Therapy and Care. Molecules 2020, 25, 5051. [Google Scholar] [CrossRef]
- Westesen, K.; Bunjes, H.; Koch, M.H.J. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J. Control Release 1997, 48, 223–236. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lin, M.T.-Y.; Ng, A.H.C.; Wong, T.T.; Mehta, J.S. Nanotechnology for the Treatment of Allergic Conjunctival Diseases. Pharmaceuticals 2020, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiß, B.; Schaefer, U.F.; Lehr, C.-M.; Wepf, R. Nanoparticles—An efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Knorr, F.; Lademann, J.; Patzelt, A.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Follicular transport route—Research progress and future perspectives. Eur. J. Pharm. Biopharm. 2009, 71, 173–180. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Mariani, P.; Sivieri, E.; Bozzini, R.; Montesi, L.; Menegatti, E.; Cortesi, R. Nanosystems for skin hydration: A comparative study. Int. J. Cosmet. Sci. 2007, 29, 39–47. [Google Scholar] [CrossRef]
- Esposito, E.; Menegatti, E.; Cortesi, R. Skin care: The innovative nanotechnology to improve the performance of delivery systems. J. Appl. Cosmetol. 2005, 23, 105. [Google Scholar]
- Yong-Tai, Z.; Meng-Qing, H.; Li-Na, S.; Ji-Hui, Z.; Nian-Ping, F. Solid lipid nanoparticles formulated for transdermal aconitine administration and evaluated in vitro and in vivo. J. Biomed. Nanotechnol. 2015, 11, 351–361. [Google Scholar] [CrossRef]
- Gönüllü, Ü.; Üner, M.; Yener, G.; Karaman, E.F.; Aydoğmuş, Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta. Pharm. 2015, 65, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, K.; Anbu, J.; Ravichandiran, V.; Venkateswarlu, V.; Rao, Y. Lipid nanoparticles for transdermal delivery of flurbiprofen: Formulation, in vitro, ex vivo and in vivo studies. Lipids Health Dis. 2009, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, K.; Mohan, C.K.; Lingam, M.; Mohan, S.J.; Venkateswarlu, V.; Rao, Y.M.; Bhaskar, K.; Anbu, J.; Ravichandran, V. Development of SLN and NLC Enriched Hydrogels for Transdermal Delivery of Nitrendipine: In Vitro and In Vivo Characteristics. Drug Dev. Ind. Pharm. 2009, 35, 98–113. [Google Scholar] [CrossRef]
- Schlupp, P.; Blaschke, T.; Kramer, K.D.; Höltje, H.-D.; Mehnert, W.; Schäfer-Korting, M. Drug Release and Skin Penetration from Solid Lipid Nanoparticles and a Base Cream: A Systematic Approach from a Comparison of Three Glucocorticoids. Skin Pharmacol. Physiol. 2011, 24, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Üner, M.; Karaman, E.; Aydoğmuş, Z. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of Loratadine for Topical Application: Physicochemical Stability and Drug Penetration through Rat Skin. Trop. J. Pharm. Res. 2014, 13, 653. [Google Scholar] [CrossRef] [Green Version]
- Ravani, L.; Esposito, E.; Bories, C.; Moal, V.L.-L.; Loiseau, P.M.; Djabourov, M.; Cortesi, R.; Bouchemal, K. Clotrimazole-loaded nanostructured lipid carrier hydrogels: Thermal analysis and in vitro studies. Int. J. Pharm. 2013, 454, 695–702. [Google Scholar] [CrossRef]
- Lombardi Borgia, S.; Regehly, M.; Sivaramakrishnan, R.; Mehnert, W.; Korting, H.C.; Danker, K.; Röder, B.; Kramer, K.D.; Schäfer-Korting, M. Lipid nanoparticles for skin penetration enhancement—Correlation to drug localization within the particle matrix as determined by fluorescence and parelectric spectroscopy. J. Control. Release 2005, 110, 151–163. [Google Scholar] [CrossRef]
- Olechowski, F.; Müller, R.H.; Pyo, S.M. BergaCare SmartLipids: Commercial lipophilic active concentrates for improved performance of dermal products. Beilstein J. Nanotechnol. 2019, 10, 2152–2162. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. Int. J. Mol. Sci. 2021, 22, 8319. https://doi.org/10.3390/ijms22158319
Sguizzato M, Esposito E, Cortesi R. Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. International Journal of Molecular Sciences. 2021; 22(15):8319. https://doi.org/10.3390/ijms22158319
Chicago/Turabian StyleSguizzato, Maddalena, Elisabetta Esposito, and Rita Cortesi. 2021. "Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier" International Journal of Molecular Sciences 22, no. 15: 8319. https://doi.org/10.3390/ijms22158319
APA StyleSguizzato, M., Esposito, E., & Cortesi, R. (2021). Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. International Journal of Molecular Sciences, 22(15), 8319. https://doi.org/10.3390/ijms22158319







