Photoprotective Role of Photosynthetic and Non-Photosynthetic Pigments in Phillyrea latifolia: Is Their “Antioxidant” Function Prominent in Leaves Exposed to Severe Summer Drought?
Abstract
:1. Introduction
2. Results
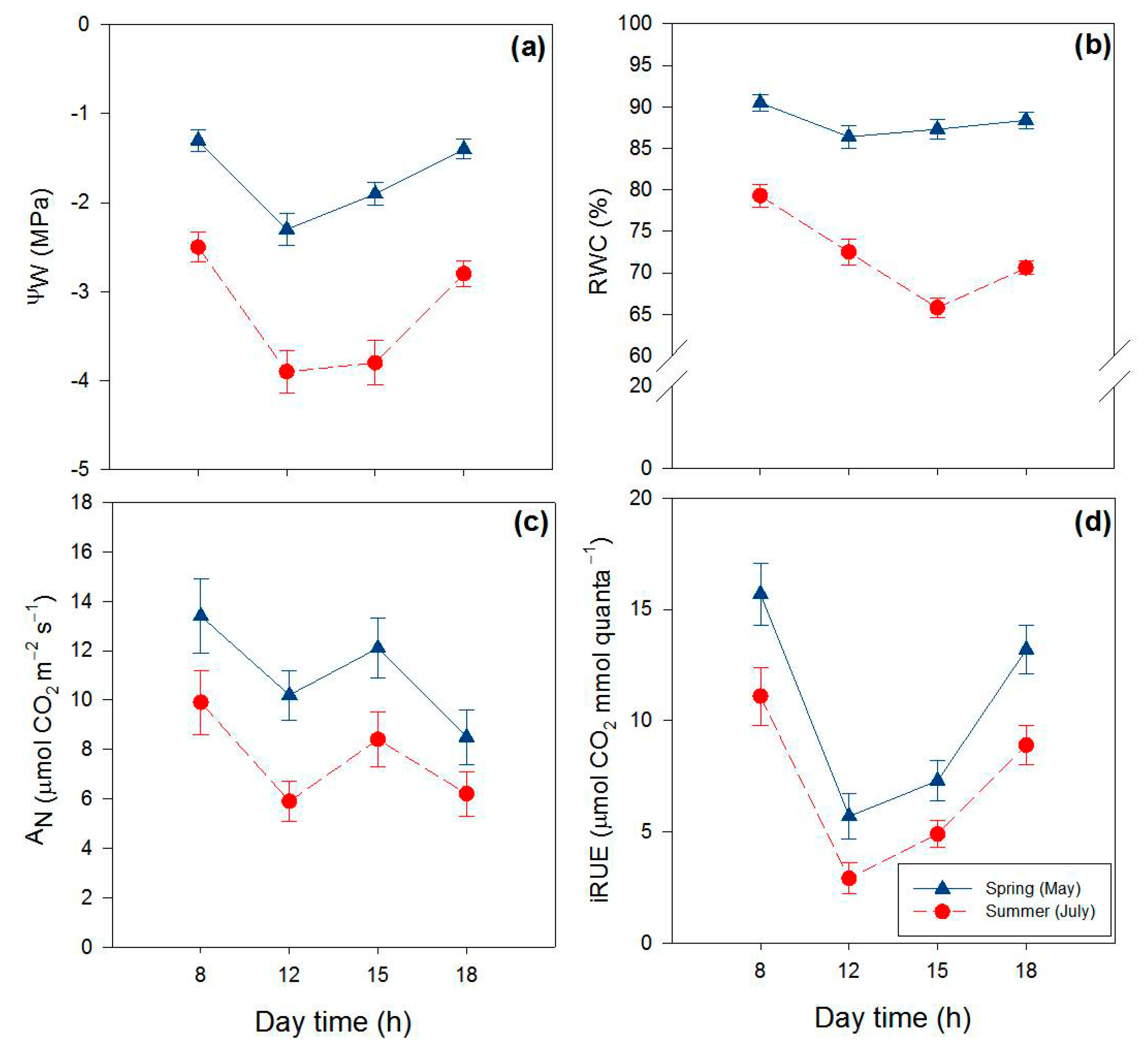
2.1. Effects of Season and Hour of the Day on Water Relations and Gas Exchange
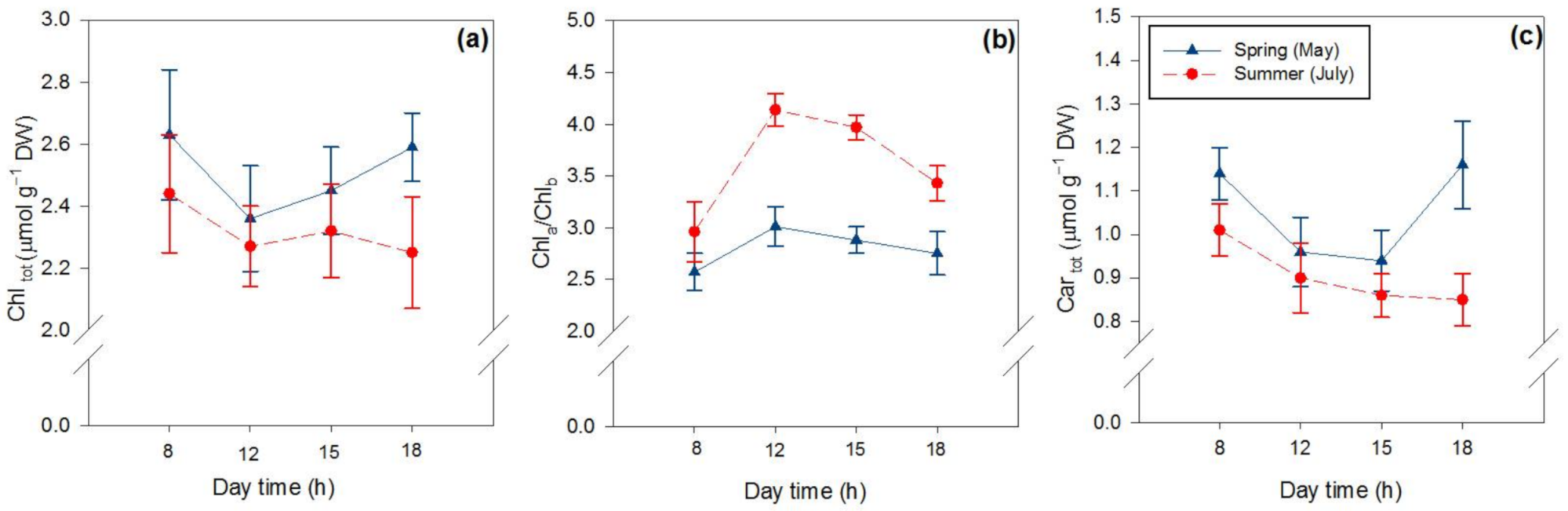
2.2. Effects of Season and Hour of the Day on Photosynthetic and Non-Photosynthetic Pigments
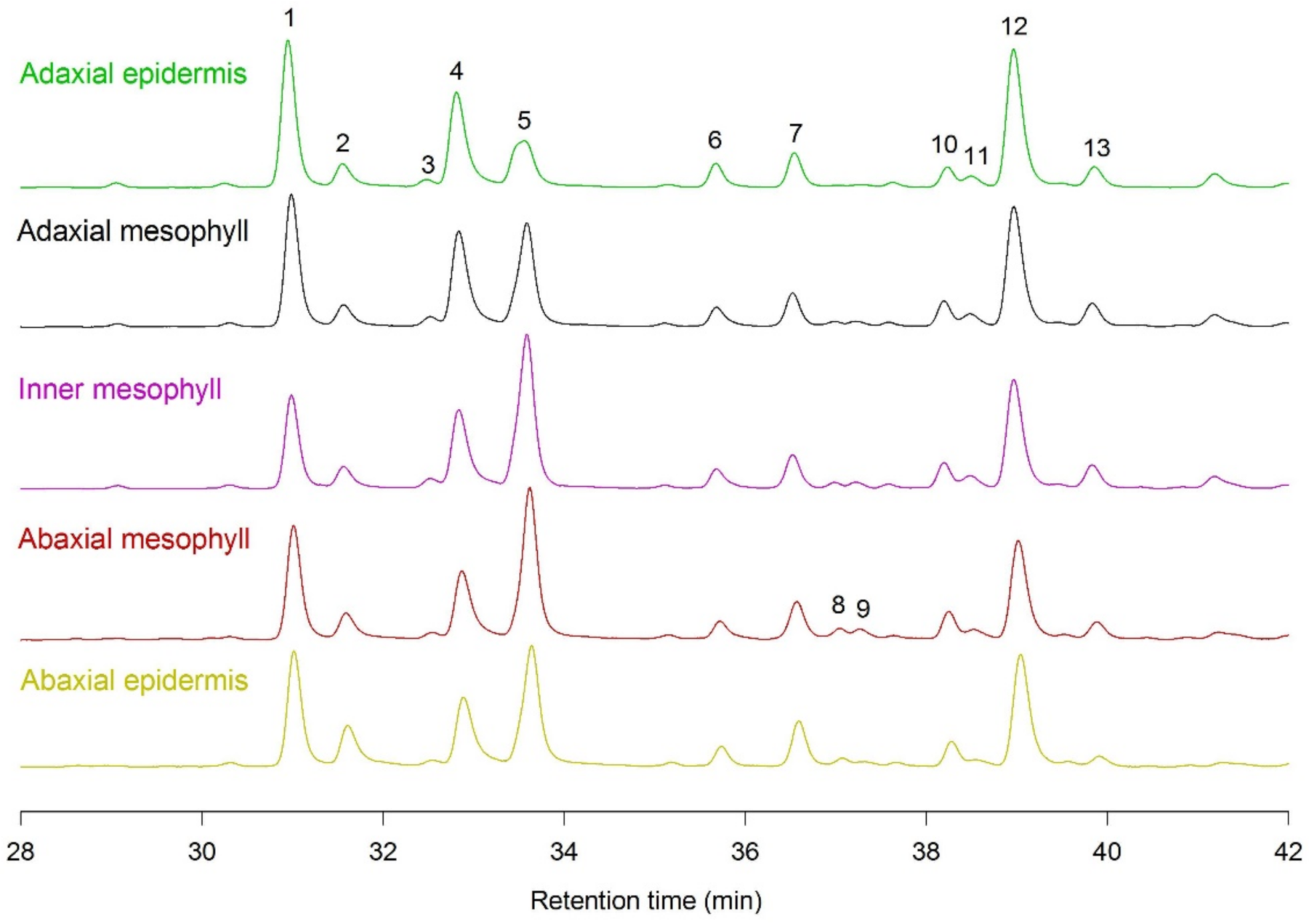
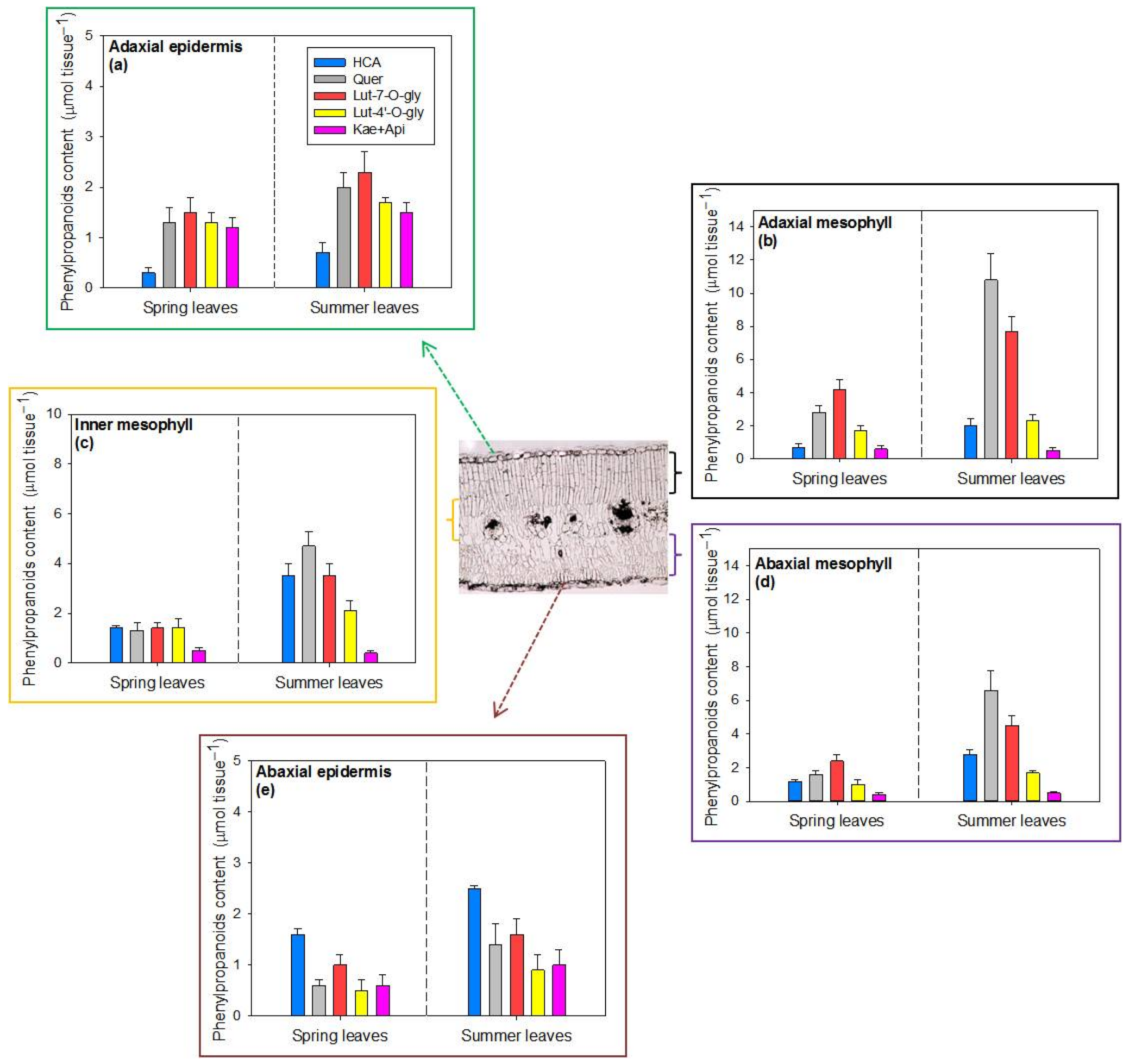
2.3. Tissue Distribution of Hydroxycinnamic Acid Derivatives and Flavonoids in Spring and Summer Leaves
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Water Relations and Gas Exchange Measurements
4.3. Analysis of Photosynthetic Pigments and Phenylpropanoids
4.4. Experimental Design and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tattini, M.; Loreto, F. Plants in Mediterranean areas: “Living in the sun”. Environ. Exp. Bot. 2014, 103, 1–2. [Google Scholar] [CrossRef]
- Fini, A.; Tattini, M.; Esteban, R. Plants’ Responses to Novel Environmental Pressures. Front. Plant Sci. 2017, 8, 2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussotti, F.; Ferrini, F.; Pollastrini, M.; Fini, A. The challenge of Mediterranean sclerophyllous vegetation under climate change: From acclimation to adaptation. Environ. Exp. Bot. 2014, 103, 80–98. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Gallé, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matesanz, S.; Valladares, F. Ecological and evolutionary responses of Mediterranean plants to global change. Environ. Exp. Bot. 2014, 103, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Fahn, A. Structural and functional properties of trichomes of xeromorphic leaves. Ann. Bot. 1986, 57, 631–637. [Google Scholar] [CrossRef]
- Tattini, M.; Matteini, P.; Saracini, E.; Traversi, M.L.; Giordano, C.; Agati, G. Morphology and biochemistry of non-glandular trichomes in Cistus salvifolius L. leaves growing in extreme habitats of the Mediterranean basin. Plant Biol. 2007, 9, 411–419. [Google Scholar] [CrossRef]
- Tattini, M.; Guidi, L.; Morassi-Bonzi, L.; Pinelli, P.; Remorini, D.; Degl’Innocenti, E.; Giordano, C.; Massai, R.; Agati, G. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia to high solar radiation. New Phytol. 2005, 167, 457–470. [Google Scholar] [CrossRef]
- Li, Z.; Wakao, S.; Fischer, B.B.; Nyiogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Ylmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Gravano, E.; Pinelli, P.; Mulinacci, N.; Romani, A. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytol. 2000, 148, 69–77. [Google Scholar] [CrossRef]
- Adams, W.W.; Zarter, C.R.; Ebbert, V.; Demmig-Adams, B. Photoprotective strategies of overwintering evergreens. Bioscience 2004, 54, 41–49. [Google Scholar] [CrossRef]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Pokhilko, A.; Bou-Torrent, J.; Pulido, P.; Rodríguez-Concepción, M.; Ebenhöh, O. Mathematical modeling of the diurnal regulation of the MEP pathway in Arabidopsis. New Phytol. 2015, 206, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef] [Green Version]
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.L.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M.; Dall’Osto, L.; Cuiné, S.; Giuliano, G.; Bassi, R. The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 13878–13888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and phenylpropanoids are key component of the antioxidant defense system of plants facing severe light stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Tattini, M.; Loreto, F.; Fini, A.; Guidi, L.; Brunetti, C.; Velikova, V.; Gori, A.; Ferrini, F. Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought-stressed Platanus × acerifolia plants during Mediterranean summers. New Phytol. 2015, 207, 613–626. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kollist, H.; García-Plazaola, J.I.; Hernández, A.; Becerril, J.M. Do the capacity and kinetics for modification of xanthophyll cycle pool size depend on growth irradiance in temperate trees? Plant Cell Environ. 2003, 26, 1787–1801. [Google Scholar] [CrossRef]
- Johnson, M.P.; Havaux, M.; Triantaphylidès, C.; Ksas, B.; Pascal, A.A.; Robert, B.; Davison, P.A.; Ruban, A.V.; Horton, P. Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective antioxidant mechanism. J. Biol. Chem. 2007, 282, 22605–22618. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef] [Green Version]
- Beckett, M.; Loreto, F.; Velikova, V.; Brunetti, C.; Di Ferdinando, M.; Tattini, M.; Calfapietra, C.; Farrant, J.M. Photosynthetic limitations and volatile and non-volatile isoprenoids in the poikilochlorophyllous resurrection plant Xerophyta humilis during dehydration and rehydration. Plant Cell Environ. 2012, 35, 2061–2074. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Hernández, A.; Garcia-Plazaola, J.I.; Esteban, R.; Míguez, F.; Artetxe, U.; Gómez-Sagasti, M.T. Photoprotective strategies of Mediterranean plants in relation to morphological traits and natural environmental pressure: A meta-analytical approach. Front. Plant Sci. 2017, 8, 1051. [Google Scholar] [CrossRef] [Green Version]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, N.; Cui, F.; Li, X.; Xiao, J.; Xiong, L. Characterization of the β-carotene hydroxylase gene DSM2 conferring drought and oxidative stress resistance by increasing xanthophylls and abscisic acid synthesis in rice. Plant Physiol. 2010, 154, 1304–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.R.; Wang, Y.H.; Li, T.; Tan, G.F.; Tao, J.P.; Su, X.S.; Xu, Z.S.; Tian, Y.S.; Xiong, A.S. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma 2021, 258, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lu, S. Biosynthesis and regulation of phenylpropanoids in plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Falcone-Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siipola, S.M.; Kotilainen, T.; Sipari, N.; Morales, L.O.; Lindfors, A.V.; Robson, T.M.; Aphalo, P.J. Epidermal UV-A absorbance and whole-leaf flavonoid composition in pea respond more to solar blue light than to solar UV radiation. Plant Cell Environ. 2015, 38, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Stefano, G.; Biricolti, S.; Tattini, M. Mesophyll distribution of antioxidant flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot. 2009, 104, 853–861. [Google Scholar] [CrossRef] [Green Version]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Ferreres, F.; Figueiredo, R.; Bettencourt, S.; Carqueijeiro, I.; Oliveira, J.; Jil-Izqueirdo, A.; Pereira, D.M.; Valentao, P.; Andrade, P.B.; Duarte, P. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: An H2O2 affair? J. Exp. Bot. 2011, 62, 2841–2854. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Agati, G.; Galardi, C.; Gravano, E.; Romani, A.; Tattini, M. Flavonoid distribution in tissues of Phillyrea latifolia L. leaves as estimated by microspectrofluorometry and multispectral fluorescence microimaging. Photochem. Photobiol. 2002, 76, 350–360. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Adams Lii, W.W.; Volk, M.; Hoehn, A.; Demmig-Adams, B. Leaf orientation and the response of the xanthophyll cycle to incident light. Oecologia 1992, 90, 404–410. [Google Scholar] [CrossRef]
- Faria, T.; Garcia-Plazaola, J.I.; Abadia, A.; Cerasoli, S.; Pereira, J.S.; Chaves, M.M. Diurnal changes in photoprotective mechanisms in leaves of cork oak (Quercus suber) during summer. Tree Physiol. 1999, 16, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Adams Lii, W.W.; Demmig-Adams, B.; Logan, B.A.; Barker, D.H.; Osmond, C.B. Rapid changes in xanthophyll cycle-dependent energy dissipation and photosystem II efficiency in two vines, Stephania japonica and Smilax australis, growing in the understory of an open Eucalyptus forest. Plant Cell Environ. 1999, 22, 125–136. [Google Scholar] [CrossRef]
- Kyparissis, A.; Drilias, P.; Manetas, Y. Seasonal fluctuations in photoprotective (xanthophyll cycle) and photoselective (chlorophylls) capacity in eight Mediterranean plant species belonging to two different growth forms. Funct. Plant Biol. 2000, 27, 265–272. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Morales, F.; Flexas, J.; Gil-Pelegrín, E. Differential photosynthetic performance and photoprotection mechanisms of three Mediterranean evergreen oaks under severe drought stress. Funct. Plant Biol. 2009, 36, 453–462. [Google Scholar] [CrossRef] [Green Version]
- Sauceda, J.U.; Rodriguez, H.G.; Lozano, R.R.; Silva, I.C.; Meza, M.V.; Larga, L. Seasonal trends of chlorophylls a and b and carotenoids in native trees and shrubs of Northeastern Mexico. J. Biol. Sci. 2008, 8, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Plazaola, J.I.; Faria, T.; Abadia, J.; Chaves, M.M.; Pereira, J.S. Seasonal changes in xanthophyll composition and photosynthesis of cork oak (Quercus suber L.) leaves under Mediterranean climate. J. Exp. Bot. 1997, 48, 1667–1674. [Google Scholar] [CrossRef] [Green Version]
- Prinsloo, G.; Nogemane, N. The effects of season and water availability on chemical composition, secondary metabolites and biological activity in plants. Phytochem. Rev. 2018, 17, 889–902. [Google Scholar] [CrossRef]
- Gori, A.; Tattini, M.; Centritto, M.; Ferrini, F.; Marino, G.; Mori, J.; Guidi, L.; Brunetti, C. Seasonal and daily variations in primary and secondary metabolism of three maquis shrubs unveil different adaptive responses to Mediterranean climate. Conserv. Physiol. 2019, 7, coz070. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Nascimento, L.B.S.; Ferrini, F.; Centritto, M.; Brunetti, C. Seasonal and diurnal variation in leaf phenolics of three medicinal Mediterranean wild species: What is the best harvesting moment to obtain the richest and the most antioxidant extracts? Molecules 2020, 25, 956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischbach, R.J.; Kossmann, B.; Panten, H.; Steinbrecher, R.; Heller, W.; Seidlitz, H.K.; Sandermann, H.; Hertkorn, N.; Schnitzler, J.P. Seasonal accumulation of ultraviolet-B screening pigments in needles of Norway spruce (Piceaabies (L.) Karst.). Plant Cell Environ. 1999, 22, 27–37. [Google Scholar] [CrossRef]
- Bouderias, S.; Teszlak, P.; Jakab, G.; Korosi, L. Age- and season-dependent pattern of flavonol glycosides in Cabernet Sauvignon grapevine leaves. Sci. Rep. 2020, 10, 14241. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.J.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlungher, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.W.; Flint, S.D.; Tobler, M.A.; Ryel, R.J. Diurnal adjustment in ultraviolet sunscreen protection is widespread among higher plants. Oecologia 2016, 181, 55–63. [Google Scholar] [CrossRef]
- Barnes, P.W.; Tobler, M.A.; Keefover-Ring, K.; Flint, S.D.; Barkley, A.E.; Ryel, R.J.; Lindroth, R.L. Rapid modulation of ultraviolet shielding in plants is influenced by solar ultraviolet radiation and linked to alterations in flavonoids. Plant Cell Environ. 2016, 39, 222–230. [Google Scholar] [CrossRef]
- Csepregi, K.; Teszlak, P.; Korosi, L.; Hideg, E. Changes in grapevine leaf phenolic profiles during the day are temperature rather than irradiance driven. Plant Physiol. Biochem. 2019, 137, 169–178. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Mulinacci, N.; Vincieri, F.F.; Gravano, E.; Tattini, M. HPLC analysis of flavonoids and secoiridoids in leaves of Ligustrum vulgare L. (Oleaceae). J. Agric. Food Chem. 2000, 48, 4091–4096. [Google Scholar] [CrossRef]
- Penuelas, J.; Lluisa, J.; Pinol, J.; Filella, I. Photochemical reflectance index and leaf photosynthetic radiation-use-efficiency assessment in Mediterranean trees. Int. J. Remote Sens. 1997, 18, 2863–2868. [Google Scholar] [CrossRef]
- Havaux, M.; Tardy, F. Loss of chlorophyll with limited reduction of photosynthesis as an adaptive response of Syrian barley landraces to high-light and heat stress. Funct. Plant Biol. 1999, 26, 569–578. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kalaji, H.M. Photosynthetic responses of sun-and shade-grown barley leaves to high light: Is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouřil, R.; Wientjes, E.; Bultema, J.B.; Croce, R.; Boekema, E.J. High-light vs. low-light: Effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim. Biophys. Acta 2013, 1827, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Fini, A.; Ferrini, F.; Di Ferdinando, M.; Brunetti, C.; Giordano, C.; Gerini, F.; Tattini, M. Acclimation to partial shading or full sunlight determines the performance of container-grown Fraxinus ornus to subsequent drought stress. Urban For. Urban Green 2014, 13, 63–70. [Google Scholar] [CrossRef]
- Brunetti, C.; Loreto, F.; Ferrini, F.; Gori, A.; Guidi, L.; Remorini, D.; Centritto, M.; Fini, A.; Tattini, M. Metabolic plasticity in the hygrophyte Moringa oleifera exposed to water stress. Tree Physiol. 2016, 38, 1640–1654. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Havaux, M.; García-Plazaola, J.I. Beyond non-photochemical fluorescence quenching: The overlapping antioxidant functions of zeaxanthin and tocopherols. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria. Advances in Photosynthesis and Respiration (Including Bioenergy and Related Processes); Demmig-Adams, B., Garab, G., Adams Lii, W.W., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 583–603. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Cazzaniga, S.; Havaux, M.; Bassi, R. Enhanced photoprotection by protein-bound vs free xanthophyll pools: A comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Mol. Plant 2010, 3, 576–593. [Google Scholar] [CrossRef] [PubMed]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Mialoundama, A.S.; Havaux, M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot. 2013, 64, 799–805. [Google Scholar] [CrossRef]
- Kotilainen, T.; Tegelberg, R.; Julkunen-Tiitto, R.; Lindfors, A.; O’Hara, R.B.; Aphalo, P.J. Seasonal fluctuations in leaf phenolic composition under UV manipulations reflect contrasting strategies of alder and birch trees. Physiol. Plant 2010, 140, 297–309. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2019, 104, 81–91. [Google Scholar] [CrossRef]
- De Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Xu, Y.; Tang, X.; Huang, Y.; Liu, J.; Deng, X.; Xu, Q. CsCYT75B1, a Citrus CYTOCHROME P450 gene, is involved in accumulation of antioxidant flavonoids and induces drought tolerance in transgenic Arabidopsis. Antioxidants 2020, 9, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mátai, A.; Nagy, D.; Hideg, E. UV-B strengthens antioxidant responses to drought in Nicotiana benthamiana leaves not only as supplementary irradiation but also as pre-treatment. Plant Physiol. Biochem. 2019, 134, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.A.; Lees, H.A.; Lampi, M.A.; Enstone, D.; Brain, R.A.; Greenberg, B.M. Photosynthetic redox imbalance influences flavonoid biosynthesis in Lemma gibba. Plant Cell Environ. 2010, 33, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Akhtar, T.A.; Lampi, M.A.; Tripuranthakam, S.; Dixon, G.R.; Greenberg, B.M. Similar stress responses are elicited by copper and ultraviolet radiation in the aquatic plant Lemma gibba: Implication of reactive oxygen species as common signals. Plant Cell Physiol. 2003, 44, 1320–1329. [Google Scholar] [CrossRef] [Green Version]
- Viola, A.L.; Camoirano, A.; Gonzalez, D.H. Redox-dependent modulation of anthocyanin biosynthesis by the TCP transcription factor TCP15 during exposure to high light intensity conditions in Arabidopsis. Plant Physiol. 2016, 170, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Page, M.; Sultana, N.; Paszkievicz, K.; Florance, H.; Smirno, N. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: Further evidence for redox control of anthocyanin synthesis. Plant Cell Environ. 2012, 35, 388–404. [Google Scholar] [CrossRef]
- Falcone–Ferreyra, M.L.; Rius, S.; Emiliani, J.; Pourcel, L.; Feller, A.; Morohashi, K.; Casati, P.; Grotewold, E. Cloning and characterization of a UV-B-inducible maize flavonol synthase. Plant J. 2010, 62, 77–91. [Google Scholar] [CrossRef]
- Pollastri, S.; Tattini, M. Flavonols: Old compounds for old roles. Ann. Bot. 2011, 108, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fini, A.; Guidi, L.; Ferrini, F.; Brunetti, C.; Di Ferdinando, M.; Biricolti, S.; Pollastri, S.; Calamai, L.; Tattini, M. Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: An excess light stress affair? J. Plant Physiol. 2012, 169, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced biosynthesis of flavonoids and the antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Polle, A. Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling: Computer simulations as a step towards flux analysis. Plant Physiol. 2001, 169, 929–939. [Google Scholar] [CrossRef] [Green Version]
- Peltzer, D.; Polle, A. Diurnal fluctuations of antioxidative systems in leaves of field-grown beech trees (Fagus sylvatica): Responses to light and temperature. Physiol. Plant 2001, 111, 158–164. [Google Scholar] [CrossRef]
- Peltzer, D.; Dreyer, E.; Polle, A. Differential temperature dependencies of antioxidative enzymes in two contrasting species. Plant Physiol. Biochem. 2002, 40, 141–150. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Gironas-Villaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Samson, G.; Cerovic, Z.G.; El Rouby, W.M.A.; Millet, P. Oxidation of polyphenols and inhibition of photosystem II under acute photooxidative stress. Planta 2020, 251, 16. [Google Scholar] [CrossRef]
- Sakihama, Y.; Mano, J.; Sano, S.; Asada, K.; Yamasaki, H. Reduction of phenoxyl radicals mediated by mono dehydroascorbate reductase. Biochem. Biophys. Res. Commun. 2000, 279, 949–954. [Google Scholar] [CrossRef]
- Zechmann, B. Compartment-specific importance of ascorbate during environmental stress in plants. Antiox. Redox Signal. 2018, 29, 1488–1501. [Google Scholar] [CrossRef]
- Zechmann, B. Subcellular roles of glutathione in mediating plant defense during biotic stress. Plants 2020, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S.; Markham, K.R.; Smith, R.H.; Goris, J.J. Functional roles of anthocyanins in leaves of Quintinia serrata A. Cunn. J. Exp. Bot. 2000, 51, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Schoch, G.; Goepfert, S.; Morant, M.; Hehn, A.; Meyer, D.; Ullmann, P.; Werck-Reichhart, D. CYP98A3 from Arabidopsis thaliana is a 3′-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J. Biol. Chem. 2001, 276, 36566–36574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besseau, S.; Hoffmann, L.; Geoffroy, P.; Lapierre, C.; Pollet, B.; Legrand, M. Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 2007, 19, 148–162. [Google Scholar] [CrossRef] [Green Version]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in Photosynthesis. Plants 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ålenius, C.M.; Vogelmann, T.C.; Bornman, J.F. A three-dimensional representation of the relationship between penetration of UV-B radiation and UV-screening pigments in leaves of Brassica napus. New Phytol. 1995, 131, 297–302. [Google Scholar] [CrossRef]


| Parameter | Fseason | Fday time | Fseason × hour |
|---|---|---|---|
| Ψw (-MPa) | 2958.0 **** | 385.1 **** | 34.9 **** |
| RWC (%) | 1324.5 **** | 325.6 **** | 147.1 **** |
| AN (μmol CO2 m−2 s−1) | 240.8 **** | 23.9 **** | 6.5 * |
| iRUE (μmol CO2 mmol−1 quanta) | 458.6 **** | 425.1 **** | 2.0 n.s. |
| Chltot (μmol g−1 DW) | 41.1 **** | 55.1 **** | 27.8 **** |
| Chla/Chlb | 212.6 **** | 154.2 **** | 78.9 **** |
| Cartot (μmol g−1 DW) | 54.1 **** | 13.2 *** | 8.6 ** |
| Neoxanthin (mmol mol−1 Chltot) | 1.8 n.s. | 2.4 n.s. | 0.9 n.s. |
| Violaxanthin (V, mmol mol−1 Chltot) | 6.2 * | 256.8 **** | 9.3 ** |
| Antheraxanthin (A, mmol mol−1 Chltot) | 76.9 **** | 285.4 **** | 14.8 *** |
| Zeaxanthin (Z, mmol mol−1 Chltot) | 455.6 **** | 956.0 **** | 64.7 **** |
| Lutein (mmol mol−1 Chltot) | 9.1 ** | 5.4 * | 2.6 n.s. |
| ß-carotene (mmol mol−1 Chltot) | 2.9 n.s. | 16.3 ** | 4.3 * |
| VAZ Chltot−1(mmol mol−1) | 165.4 **** | 38.2 **** | 1.5 n.s. |
| DES | 292.6 **** | 335.1 **** | 25.4 **** |
| HCAtot (μmol g−1 DW) | 435.6 **** | 84.7 **** | 11.5 ** |
| Flavtot (μmol g−1 DW) | 3953.1 **** | 199.7 **** | 64.2 **** |
| Que 3-O-der (μmol g−1 DW) | 3176.3 *** | 175.4 **** | 89.1 **** |
| Lut 7-O-der (μmol g−1 DW) | 1412.6 **** | 58.6 **** | 12.7 ** |
| Lut 4′-O-der (μmol g−1 DW) | 19.3 *** | 12.1 ** | 2.4 n.s. |
| Kae 3-O-der (μmol g−1 DW) | 6.8 * | 1.4 n.s. | 1.1 n.s. |
| Api 7-O-der (μmol g−1 DW) | 5.2 * | 1.1 n.s. | 0.8 n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gori, A.; Brunetti, C.; dos Santos Nascimento, L.B.; Marino, G.; Guidi, L.; Ferrini, F.; Centritto, M.; Fini, A.; Tattini, M. Photoprotective Role of Photosynthetic and Non-Photosynthetic Pigments in Phillyrea latifolia: Is Their “Antioxidant” Function Prominent in Leaves Exposed to Severe Summer Drought? Int. J. Mol. Sci. 2021, 22, 8303. https://doi.org/10.3390/ijms22158303
Gori A, Brunetti C, dos Santos Nascimento LB, Marino G, Guidi L, Ferrini F, Centritto M, Fini A, Tattini M. Photoprotective Role of Photosynthetic and Non-Photosynthetic Pigments in Phillyrea latifolia: Is Their “Antioxidant” Function Prominent in Leaves Exposed to Severe Summer Drought? International Journal of Molecular Sciences. 2021; 22(15):8303. https://doi.org/10.3390/ijms22158303
Chicago/Turabian StyleGori, Antonella, Cecilia Brunetti, Luana Beatriz dos Santos Nascimento, Giovanni Marino, Lucia Guidi, Francesco Ferrini, Mauro Centritto, Alessio Fini, and Massimiliano Tattini. 2021. "Photoprotective Role of Photosynthetic and Non-Photosynthetic Pigments in Phillyrea latifolia: Is Their “Antioxidant” Function Prominent in Leaves Exposed to Severe Summer Drought?" International Journal of Molecular Sciences 22, no. 15: 8303. https://doi.org/10.3390/ijms22158303
APA StyleGori, A., Brunetti, C., dos Santos Nascimento, L. B., Marino, G., Guidi, L., Ferrini, F., Centritto, M., Fini, A., & Tattini, M. (2021). Photoprotective Role of Photosynthetic and Non-Photosynthetic Pigments in Phillyrea latifolia: Is Their “Antioxidant” Function Prominent in Leaves Exposed to Severe Summer Drought? International Journal of Molecular Sciences, 22(15), 8303. https://doi.org/10.3390/ijms22158303












