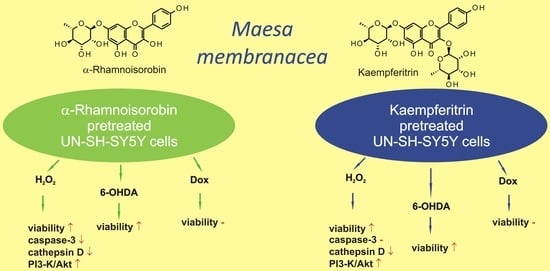
Neuroprotective Properties of Kempferol Derivatives from Maesa membranacea against Oxidative Stress-Induced Cell Damage: An Association with Cathepsin D Inhibition and PI3K/Akt Activation
Abstract
:1. Introduction
2. Results
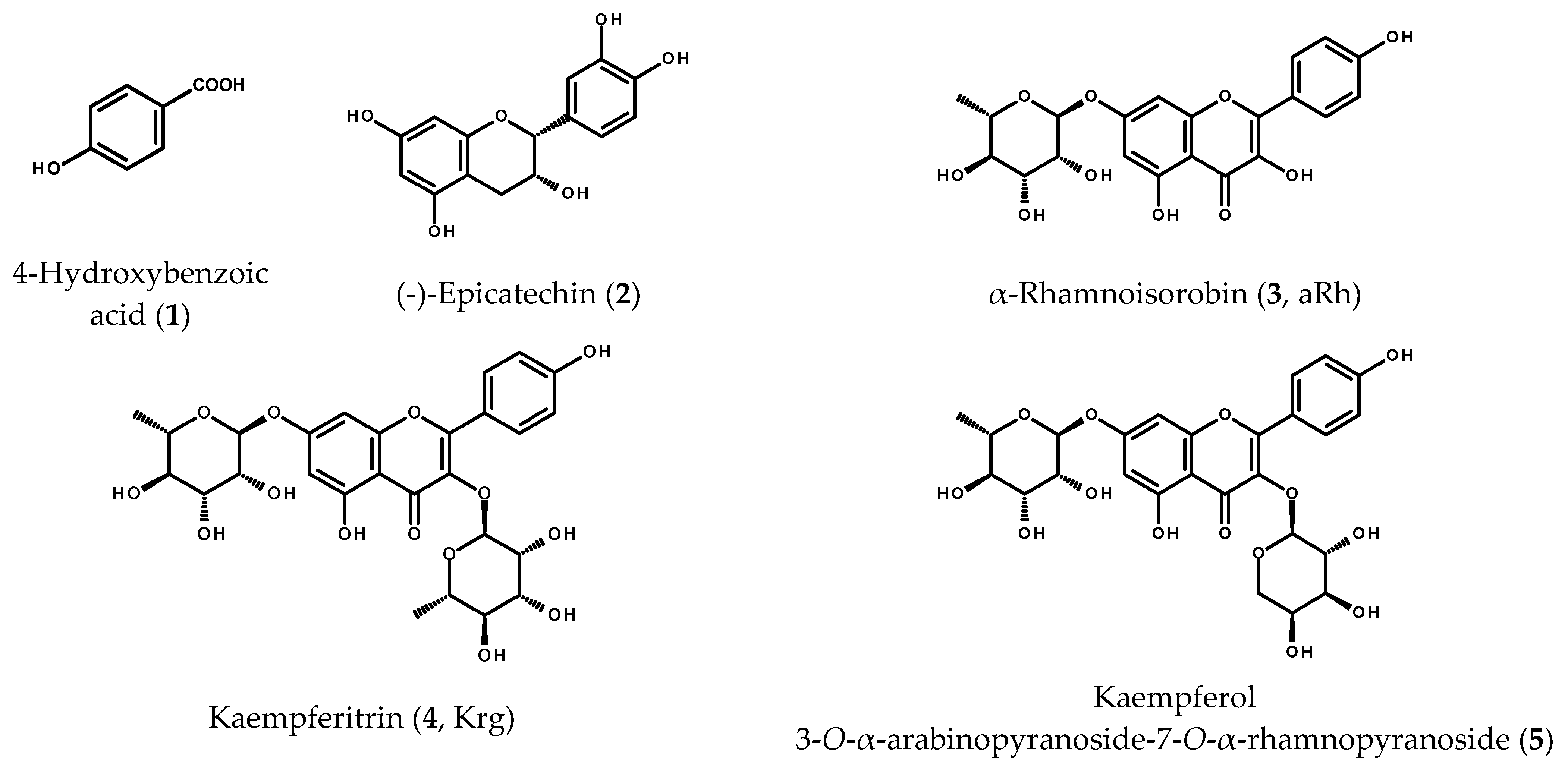
2.1. Phenolic Compounds from the Leaves of M. membranacea
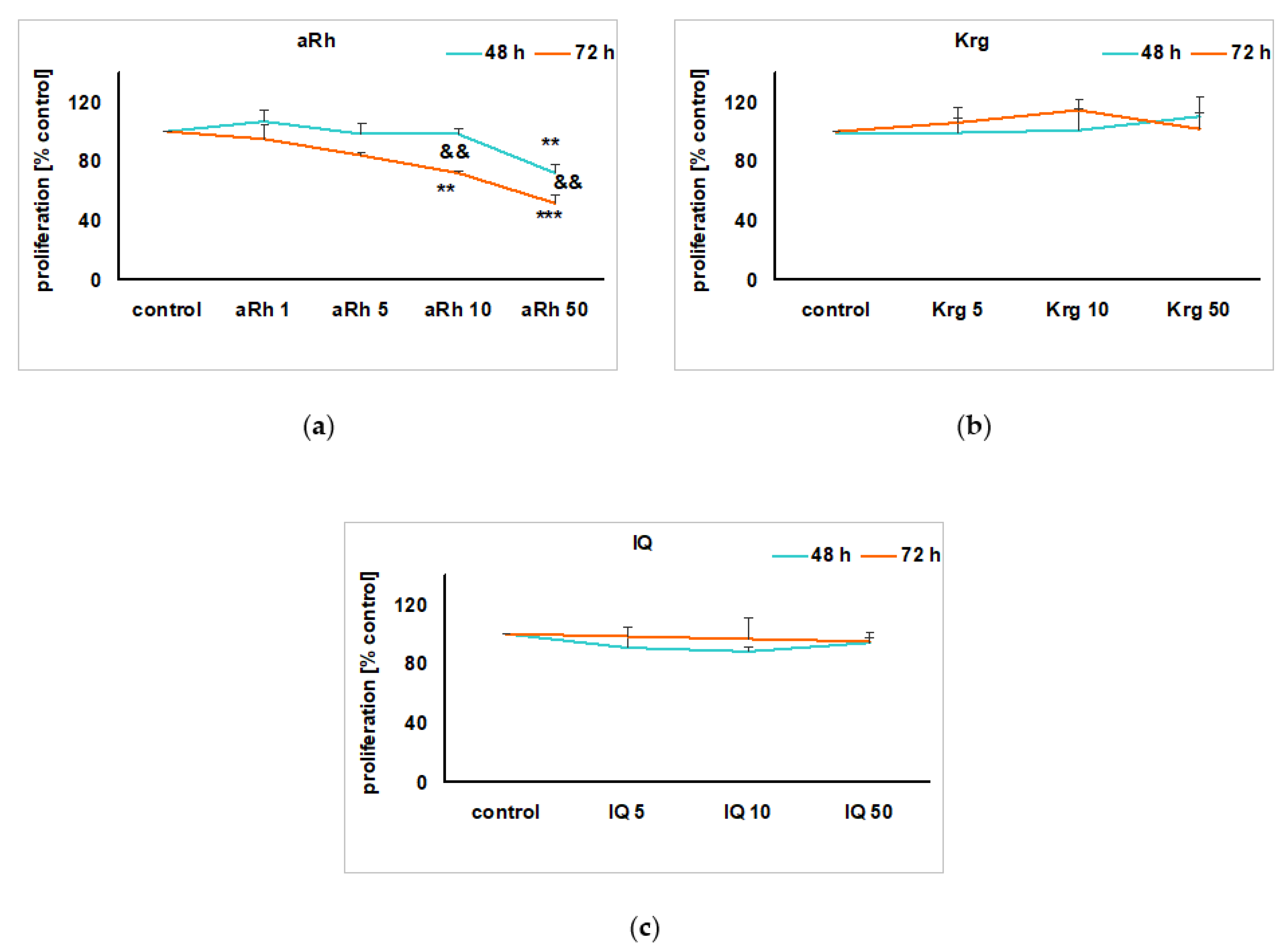
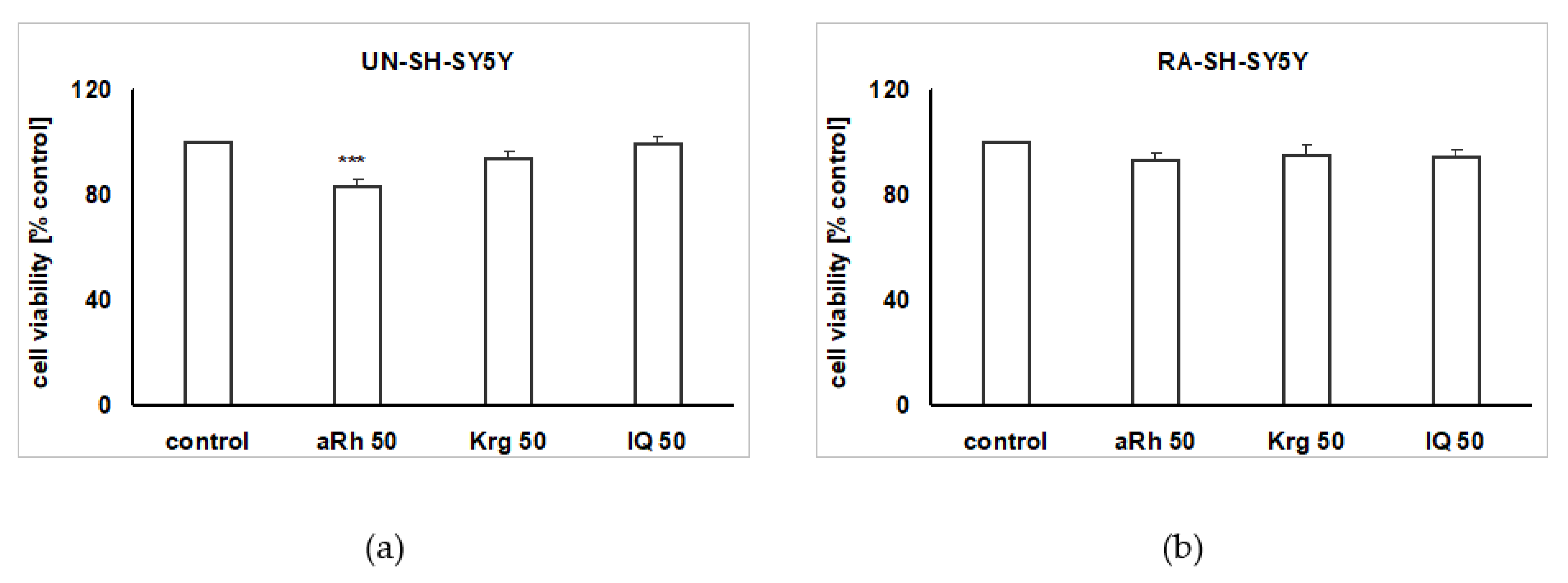
2.2. The Impact of the Tested Flavonols on Cell Proliferation and Biosafety Issues
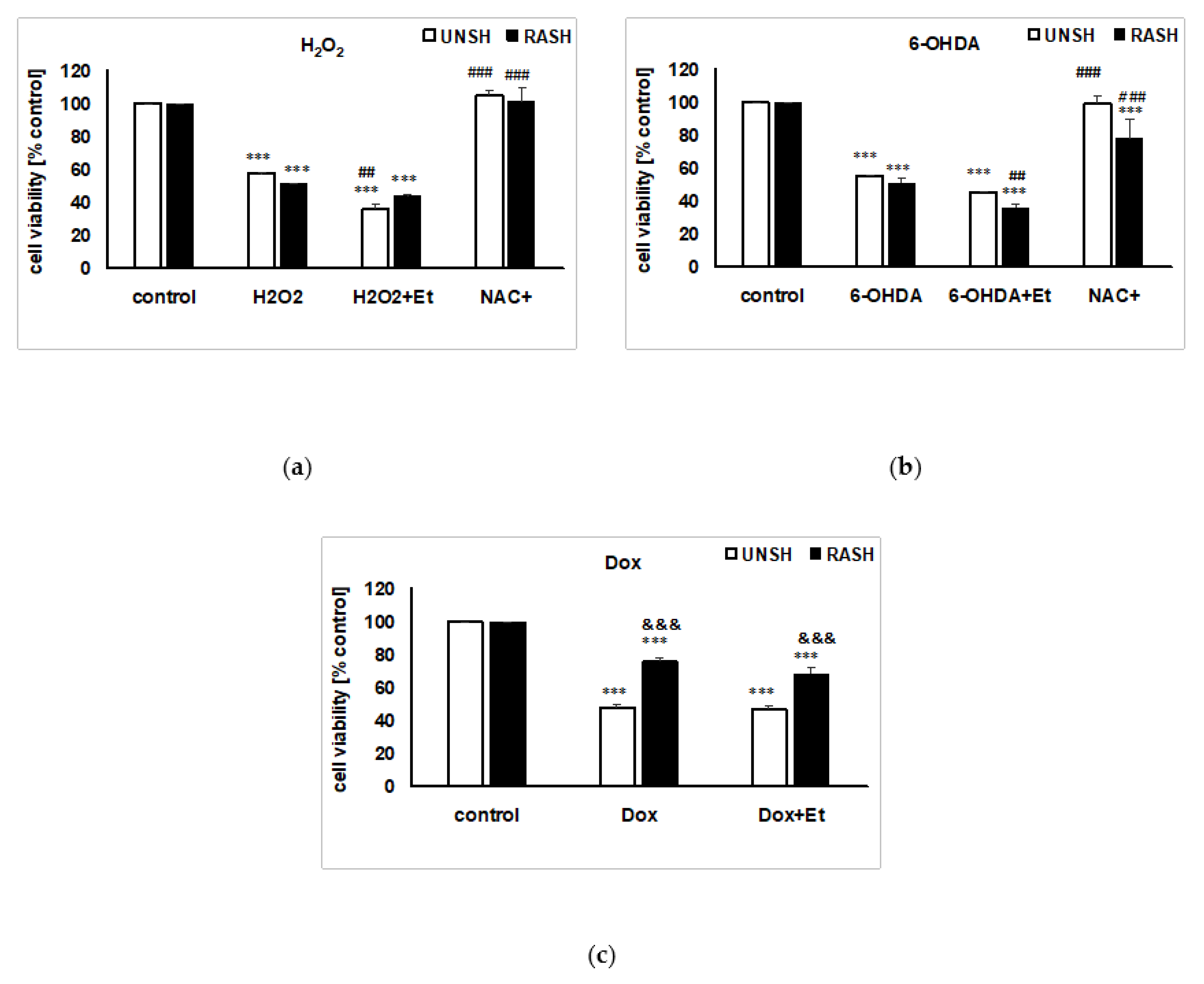
2.3. The Impact of the Vehicle on the Cell Damage Induced by Various Factors in UN- and RA-SH-SY5Y Cells
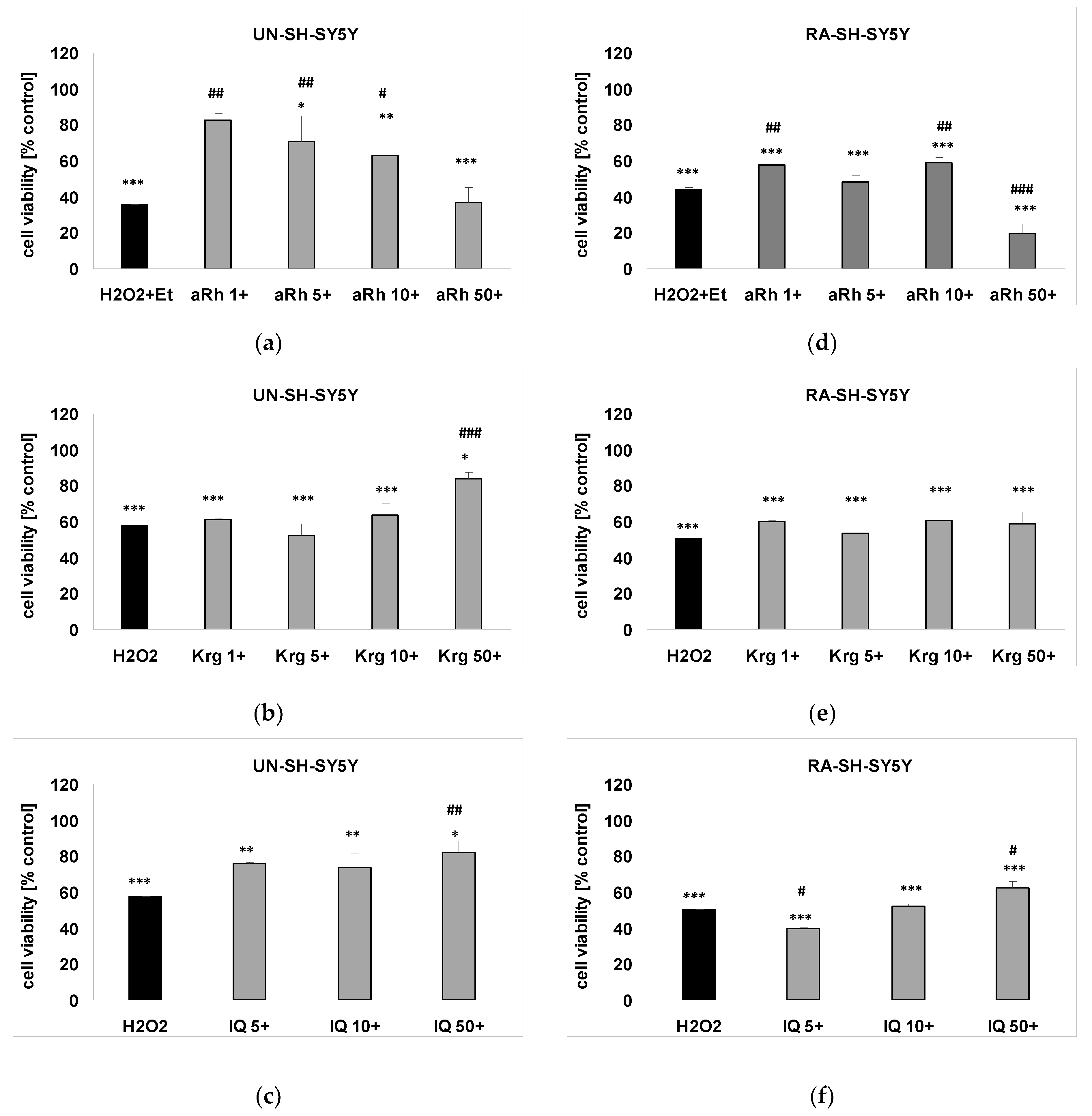
2.4. The Effects of Flavonols on H2O2-induced Cell Damage in UN- and RA-SH-SY5Y Cells
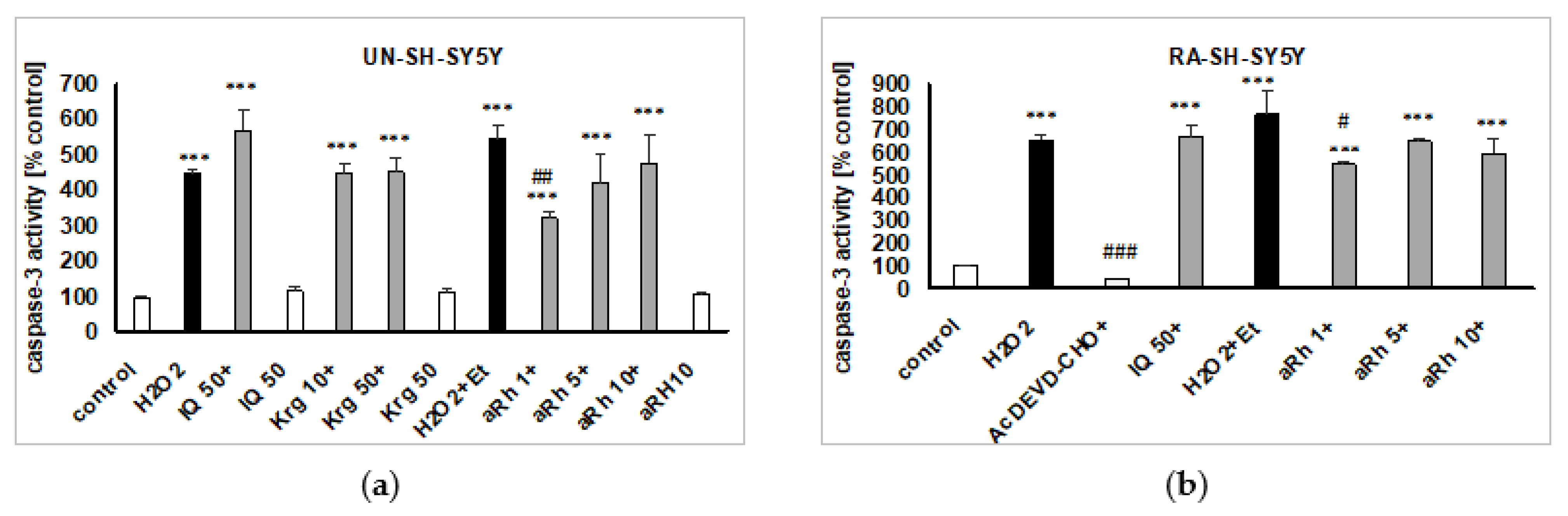
2.5. Mechanisms of aRh-, Krg- and IQ-mediated Protection against H2O2-induced Cell Damage in SH-SY5Y Cells
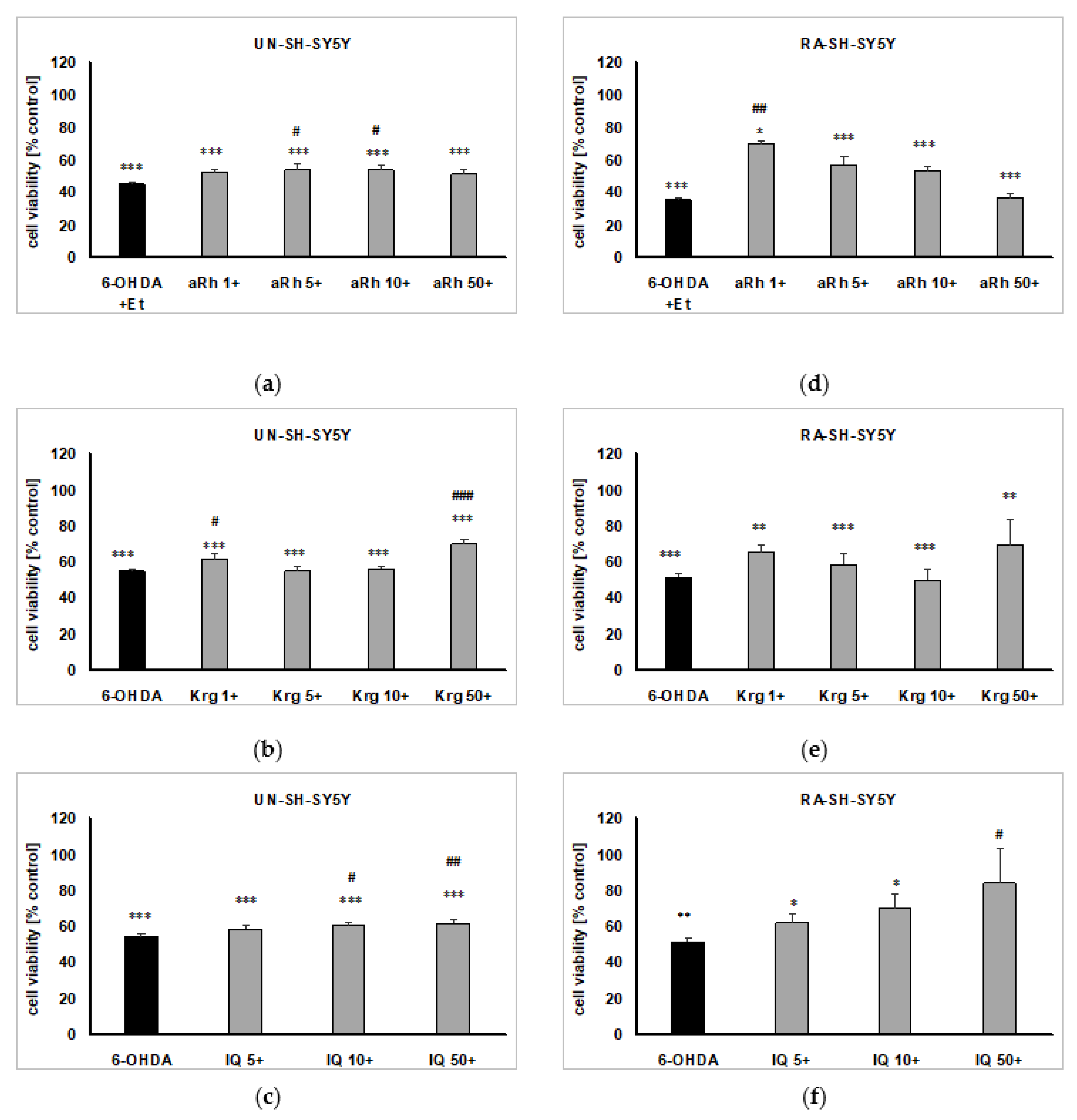
2.6. The Effects of Flavonols on 6-OHDA-induced Cell Damage in UN- and RA-SH-SY5Y Cells
2.7. The Lack of Protection against Doxorubicin-induced Cell Damage in UN- and RA-SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solvents
4.2. General Experimental Procedures
4.3. Plant Material
4.4. Isolation and Identification of Phenolic Constituents from Leaves of M. membranacea
4.5. Assessment of Kaempferol 3,7-di-O-glycosides Contents in Extracts from the Leaves of M. membranacea
4.6. SH-SY5Y Cell Culture
4.7. Cell Treatment
4.8. Cell Proliferation Assay
4.9. Cell Viability Assay
4.10. Caspase-3 Activity Assay
4.11. Cathepsin D Activity Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| aRh | α-Rhamnoisorobin (kaempferol 7-O-α-rhamnoside, PubChem CID 25079965) |
| Dox | Doxorubicin |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| IQ | Isoquercitrin (quercetin 3-O-β-glucoside, PubChem CID 5280804) |
| Krg | Kaempferitrin (kaempferol 3,7-di-O-α-rhamnoside, PubChem CID 5486199) |
| MAPK | Mitogen-activated protein kinase |
| 6-OHDA | 6-Hydroxydopamine |
| PI3-K | Phosphoinositide 3-kinase |
| RA | Retinoic acid |
| ROS | Reactive oxygen species |
References
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef] [PubMed]
- Silva dos Santos, J.; Gonçalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- WFO. World Flora Online: Maesa Forssk. 2021. Available online: http://www.worldfloraonline.org/taxon/wfo-4000022843 (accessed on 5 May 2021).
- Bhat, R.B.; Jacobs, T.V. Traditional herbal medicine in Transkei. J. Ethnopharmacol. 1995, 48, 2–12. [Google Scholar] [CrossRef]
- Novy, J.W. Medicinal plants of the eastern region of Madagascar. J. Ethnopharmacol. 1997, 55, 119–126. [Google Scholar] [CrossRef]
- De Smet, P.A.G.M. Traditional pharmacology and medicine in Africa. Ethnopharmacological themes in sub-Saharan art objects and utensils. J. Ethnopharmacol. 1998, 63, 1–179. [Google Scholar] [CrossRef]
- Taylor, R.S.L.; Manandhar, N.P.; Hudson, J.B.; Towers, G.H.N. Antiviral activities of Nepalese medicinal plants. J. Ethnopharmacol. 1996, 52, 157–163. [Google Scholar] [CrossRef]
- Faruque, M.O.; Uddin, S.B.; Barlow, J.W.; Hu, S.; Dong, S.; Cai, Q.; Li, X.; Hu, X. Quantitative ethnobotany of medicinal plants used by indigenous communities in the Bandarban District of Bangladesh. Front. Pharmacol. 2018, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WFO. World Flora Online: Maesa membranacea A.DC. 2021. Available online: http://www.worldfloraonline.org/taxon/wfo-0001085875 (accessed on 5 May 2021).
- EFloras. 2008. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200016860 (accessed on 5 May 2021).
- Whitney, C.W.; Min, V.S.; Giang, L.H.; Can, V.V.; Barber, K.; Lanh, T.T. Learning with elders: Human ecology and ethnobotany explorations in northern and central Vietnam. Hum. Organ. 2016, 75, 71–86. [Google Scholar] [CrossRef]
- Manguro, L.O.A.; Lemmen, P.; Ugi, I.; Kraus, W. Flavonol glycosides of Maesa lanceolata leaves. Nat. Prod. Sci. 2002, 8, 77–82. [Google Scholar]
- Shanmugam, S.; Baby, J.P.; Chandran, R.; Thankarajan, S.; Thangaraj, P. Maesa indica: A nutritional wild berry rich in polyphenols with special attention to radical scavenging and inhibition of key enzymes, α-amylase and α-glucosidase. J. Food Sci. Technol. 2016, 53, 2957–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, N.T.; Tran, H.G.; Vu, T.K.O.; Pham, T.D.; Dinh, N.T.; Stojakowska, A.; Truong, B.N. Chemical constituents isolated from stems of Maesa membranacea. Vietnam J. Sci. Technol. Eng. 2020, 62, 15–18. [Google Scholar] [CrossRef]
- Zhang, H.; Conte, M.M.; Huang, X.-C.; Khalil, Z.; Capon, R.J. A search for BACE inhibitors reveals new biosynthetically related pyrrolidones, furanones and pyrroles from a southern Australian marine sponge, Ianthella sp. Org. Biomol. Chem. 2012, 10, 2656–2663. [Google Scholar] [CrossRef]
- Hong, Y.; Qiao, Y.; Lin, S.; Jiang, Y.; Chen, F. Characterization of antioxidant compounds in Eriobotrya fragrans Champ leaf. Sci. Hortic. 2008, 118, 288–292. [Google Scholar] [CrossRef]
- Wu, C.-L.; Chang, H.-T.; Hsui, Y.-R.; Hsu, Y.-W.; Liu, J.-Y.; Wang, S.-Y.; Chang, S.-T. Antioxidant-enriched leaf water extracts of Cinnamomum osmophloeum from eleven provenances and their bioactive flavonoid glycosides. BioResources 2013, 8, 571–580. [Google Scholar] [CrossRef]
- Owczarek, A.; Magiera, A.; Matczak, M.; Piotrowska, D.G.; Olszewska, M.A.; Marchelak, A. Optimisation of preparative HPLC separation of four isomeric kaempferol diglycosides from Prunus spinosa L. by application of the response surface methodology. Phytochem. Lett. 2017, 20, 415–424. [Google Scholar] [CrossRef]
- Jantas, D.; Chwastek, J.; Malarz, J.; Stojakowska, A.; Lasoń, W. Neuroprotective effects of methyl caffeate against hydrogen peroxide-induced cell damage: Involvement of caspase 3 and cathepsin D inhibition. Biomolecules 2020, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Chwastek, J.; Grygier, B.; Lasoń, W. Neuroprotective effects of necrostatin-1 against oxidative stress-induced cell damage: An involvement of cathepsin D inhibition. Neurotox. Res. 2020, 37, 525–542. [Google Scholar] [CrossRef] [Green Version]
- Chwastek, J.; Jantas, D.; Lasoń, W. The ATM kinase inhibitor KU-55933 provides neuroprotection against hydrogen peroxide-induced cell damage via a γH2AX/p-p53/caspase-3-independent mechanism: Inhibition of calpain and cathepsin D. Int. J. Biochem. Cell Biol. 2017, 87, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Ruffels, J.; Griffin, M.; Dickenson, J.M. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: Role of ERK1/2 in H2O2-induced cell death. Eur. J. Pharmacol. 2004, 483, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Yin, J.; Luo, Y.; Huang, S. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int. J. Biochem. Cell Biol. 2009, 41, 1284–1295. [Google Scholar] [CrossRef]
- Jantas, D.; Piotrowski, M.; Lasoń, W. An involvement of PI3-K/Akt activation and inhibition of AIF translocation in neuroprotective effects of undecylenic acid (UDA) against pro-apoptotic factors-induced cell death in human neuroblastoma SH-SY5Y cells. J. Cell Biochem. 2015, 116, 2882–2895. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signaling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- DuPont, M.S.; Day, A.J.; Bennet, R.N.; Mellon, F.A.; Kroon, P.A. Absorption of kaempferol from endive, source of kaempferol-3-glucuronide, in humans. Eur. J. Clin. Nutr. 2004, 58, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods. 2011. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 15 August 2021).
- Maher, P.; Akaishi, T.; Abe, K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl. Acad. Sci. USA 2006, 103, 16568–16573. [Google Scholar] [CrossRef] [Green Version]
- Pu, F.; Mishima, K.; Irie, K.; Motohashi, K.; Tanaka, Y.; Orito, K.; Egawa, T.; Kitamura, Y.; Egashira, N.; Iwasaki, K.; et al. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J. Pharmacol. Sci. 2007, 104, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [Green Version]
- Mecocci, P.; Tinarelli, C.; Schulz, R.J.; Polidori, M.C. Nutraceuticals in cognitive impairment and Alzheimer’s disease. Front. Pharmacol. 2014, 5, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, F.; Lambert, E.; Commeau, L.; Lejeune, F.-X.; Roudier, N.; Fonte, C.; Parker, J.A.; Boddaert, J.; Verny, M.; Baulieu, E.-E.; et al. The stress response factor daf-16/FOXO is required for multiple compound families to prolong the function of neurons with Huntington’s disease. Sci. Rep. 2017, 7, 4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Hwang, L.; Jeong, E.J.; Kim, S.H.; Kim, Y.C.; Sung, S.H. Effect of neuroprotective flavonoids of Agrimonia eupatoria on glutamate-induced oxidative injury to HT22 hippocampal cells. Biosci. Biotechnol. Biochem. 2010, 74, 1704–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-J.; Kim, H.-N.; Kim, C.Y.; Seo, M.-D.; Baek, S.-H. Synergistic protection by isoquercitrin and quercetin against glutamate-induced oxidative cell death in HT22 cells via activating Nrf2 and HO-1 signaling pathway: Neuroprotective principles and mechanisms of Dendropanax morbifera leaves. Antioxidants 2021, 10, 554. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Protective effects of flavonol isoquercitrin, against 6-hydroxydopamine (6-OHDA)-induced toxicity in PC12 cells. BMC Res. Notes 2014, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Resham, K.; Khare, P.; Bishnoi, M.; Sharma, S.S. Neuroprotective effects of isoquercitrin in diabetic neuropathy via Wnt/β-catenin signaling pathway inhibition. BioFactors 2020, 46, 411–420. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Li, H.; Liu, J.; Zhang, P.; Cheng, Y.; Qin, X.; Hu, Y.; Wei, Y. The neuroprotective effects of isoquercitrin purified from apple pomace by high-speed countercurrent chromatography in the MPTP acute mouse model of Parkinson’s disease. Food Funct. 2021, 12, 6091–6101. [Google Scholar] [CrossRef]
- Yu, L.; Chen, C.; Wang, L.-F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.-R. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-kB and STAT3 in transient focal stroke. PLoS ONE 2013, 8, e55839. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Domínguez, F.; Pérez-Ortega, G.; Aguillón, M.; Martínez-Vargas, D.; Almazán-Alvarado, S.; Martínez, A. Justicia spicigera Schltdl. and kaempferitrin as potential anticonvulsant natural products. Biomed. Pharmacother. 2017, 92, 240–248. [Google Scholar] [CrossRef]
- da Paixão, D.; Barboza, R.S.; Valente, L.M.M.; Souza, M.O.; Siani, A.C.; Pereira, R.C.A.; Gallo, B.; Berrueta, L.A. Polyphenol profile and quantitative assessment of the flavonoid kaempferitrin in wild and cultivated Brazilian Amazonian Uncaria guaianensis (Rubiaceae). J. Braz. Chem. Soc. 2021, 32, 1670–1679. [Google Scholar] [CrossRef]
- Tatsimo, S.J.N.; Tamokou, J.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rho, H.S.; Ahn, S.M.; Lee, B.C.; Kim, M.K.; Ghimeray, A.K.; Jin, C.W.; Cho, D.H. Changes on flavonoid content and tyrosinase inhibitory activity in kenaf leaf extract after far-infrared treatment. Bioorg. Med. Chem. Lett. 2010, 20, 7534–7536. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, E.; Zanatta, L.; Seifriz, I.; Creczynski-Pasa, T.B.; Pizzolatti, M.G.; Szpoganicz, B.; Barreto Silva, F.R.M. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(α)-dirhamnoside from Bauhinia forficata leaves. J. Nat. Prod. 2004, 67, 829–832. [Google Scholar] [CrossRef]
- Ku, W.C.; Sridharan, B.; Chen, J.-Y.; Li, J.-Y.; Yang, S.Y.; Lee, M.-J. Kaempferitrin-treated HepG2 differentially expressed exosomal markers and affect extracellular vesicle sizes in the secretome. Biomolecules 2021, 11, 187. [Google Scholar] [CrossRef]
- Wang, M.; Xing, S.; Luu, T.; Fan, M.; Li, X. The gastrointestinal tract metabolism and pharmacological activities of grosvenorine, a major and characteristic flavonoid in the fruits of Siraitia grosvenorii. Chem. Biodiv. 2015, 12, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.-M.; Chen, K.; Rao, Y.K.; Lee, M.-J. Kaempferitrin activates the insulin signaling pathway and stimulates secretion of adiponectin in 3T3-L1 adipocytes. Eur. J. Pharmacol. 2009, 607, 27–34. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Pereira, D.F.; Kappel, V.D.; Folador, P.; Figueiredo, M.B.S.R.; Pizzolatti, M.G.; Silva, F.R.M.B. Insulin signaling: A potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle. Eur. J. Pharmacol. 2013, 712, 1–7. [Google Scholar] [CrossRef]
- De Melo, G.O.; Malvar, D.C.; Vanderlinde, F.A.; Rocha, F.F.; Pires, P.A.; Costa, E.A.; de Matos, L.G.; Kaiser, C.R.; Costa, S.S. Antinociceptive and anti-inflammatory kaempferol glycosides from Sedum dendroideum. J. Ethnopharm. 2009, 124, 228–232. [Google Scholar] [CrossRef]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [Green Version]
- Wenker, S.D.; Chamorro, M.E.; Vota, D.M.; Callero, M.A.; Vittori, D.C.; Nesse, A.B. Differential antiapoptotic effect of erythropoietin on undifferentiated and retinoic acid-differentiated SH-SY5Y cells. J. Cell. Biochem. 2010, 110, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Greda, A.; Leśkiewicz, M.; Grygier, B.; Pilc, A.; Lasoń, W. Neuroprotective effects of mGluR II and III activation against staurosporine- and doxorubicin-induced cellular injury in SH-SY5Y cells: New evidence for a mechanism involving inhibition of AIF translocation. Neurochem. Int. 2015, 88, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Miloso, M.; Villa, D.; Crimi, M.; Galbiati, S.; Donzelli, E.; Nicolini, G.; Tredici, G. Retinoic acid-induced neuritogenesis of human neuroblastoma SH-SY5Y cells is ERK independent and PKC dependent. J. Neurosci. Res. 2004, 75, 241–252. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Lau, W.K.; Yu, M.S.; Lai, C.S.; Yeung, S.C.; So, K.F.; Chang, R.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Juszczak, M.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol and its glycoside derivatives as modulators of etoposide activity in HL-60 cells. Int. J. Mol. Sci. 2021, 22, 3520. [Google Scholar] [CrossRef] [PubMed]
- Kłeczek, N.; Malarz, J.; Gierlikowska, B.; Kiss, A.K.; Stojakowska, A. Constituents of Xerolekia speciosissima (L.) Anderb. (Inuleae), and Anti-Inflammatory Activity of 7,10-Diisobutyryloxy-8,9-epoxythymyl Isobutyrate. Molecules 2020, 25, 4913. [Google Scholar] [CrossRef]
- Michalska, K.; Malarz, J.; Paul, W.; Stojakowska, A. Natural products from Tolpis barbata (L.) Gaertn. (Asteraceae, Cichorieae). Biochem. Syst. Ecol. 2019, 86, 103922. [Google Scholar] [CrossRef]
- Statistica, version 13.3; StatSoft, Tibco Software Inc.: Palo Alto, CA, USA, 2017.

| UN-SH-SY5Y | RA-SH-SY5Y | |
|---|---|---|
| Control | 100.00 ± 0.00 | 100.00 ± 0.00 |
| Dox | 48.15 ± 1.15 *** | 76.62 ± 2.44 *** |
| IQ 5+ | 43.40 ± 3.73 *** | 72.24 ± 1.64 *** |
| IQ 10+ | 51.01 ± 4.86 *** | 74.20 ± 4.23 *** |
| IQ 50+ | 53.60 ± 6.32 *** | 72.63 ± 4.26 *** |
| Krg 5+ | 49.97 ± 4.49 *** | 76.99 ± 1.35 *** |
| Krg 10+ | 49.96 ± 2.80 *** | 78.96 ± 1.69 *** |
| Krg 50+ | 51.13 ± 4.32 *** | 73.94 ± 1.85 *** |
| Dox + Et | 46.81 ± 2.28 *** | 68.38 ± 3.34 *** |
| aRh 5+ | 51.25 ± 4.32 *** | 73.38 ± 0.80 *** |
| aRh 10+ | 40.32 ± 2.63 *** | 70.47 ± 0.61 *** |
| aRh 50+ | 41.64 ± 4.10 *** | 66.77 ± 2.79 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantas, D.; Malarz, J.; Le, T.N.; Stojakowska, A. Neuroprotective Properties of Kempferol Derivatives from Maesa membranacea against Oxidative Stress-Induced Cell Damage: An Association with Cathepsin D Inhibition and PI3K/Akt Activation. Int. J. Mol. Sci. 2021, 22, 10363. https://doi.org/10.3390/ijms221910363
Jantas D, Malarz J, Le TN, Stojakowska A. Neuroprotective Properties of Kempferol Derivatives from Maesa membranacea against Oxidative Stress-Induced Cell Damage: An Association with Cathepsin D Inhibition and PI3K/Akt Activation. International Journal of Molecular Sciences. 2021; 22(19):10363. https://doi.org/10.3390/ijms221910363
Chicago/Turabian StyleJantas, Danuta, Janusz Malarz, Thanh Nguyen Le, and Anna Stojakowska. 2021. "Neuroprotective Properties of Kempferol Derivatives from Maesa membranacea against Oxidative Stress-Induced Cell Damage: An Association with Cathepsin D Inhibition and PI3K/Akt Activation" International Journal of Molecular Sciences 22, no. 19: 10363. https://doi.org/10.3390/ijms221910363
APA StyleJantas, D., Malarz, J., Le, T. N., & Stojakowska, A. (2021). Neuroprotective Properties of Kempferol Derivatives from Maesa membranacea against Oxidative Stress-Induced Cell Damage: An Association with Cathepsin D Inhibition and PI3K/Akt Activation. International Journal of Molecular Sciences, 22(19), 10363. https://doi.org/10.3390/ijms221910363