Phenotypic Effects of Homeodomain-Interacting Protein Kinase 2 Deletion in Mice
Abstract
1. Introduction
2. Results
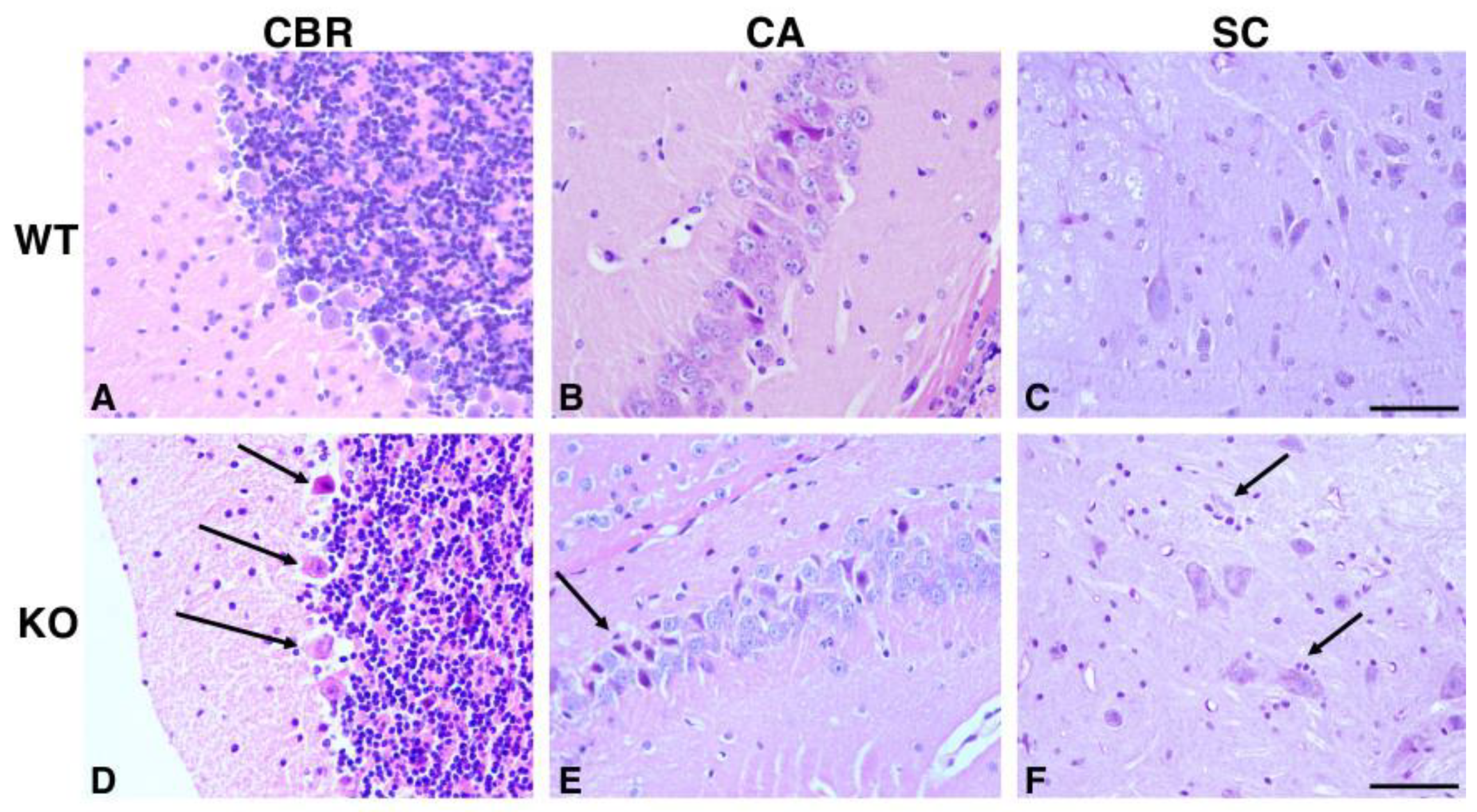
2.1. Hipk2-KO Mice Show Neuronal Alterations
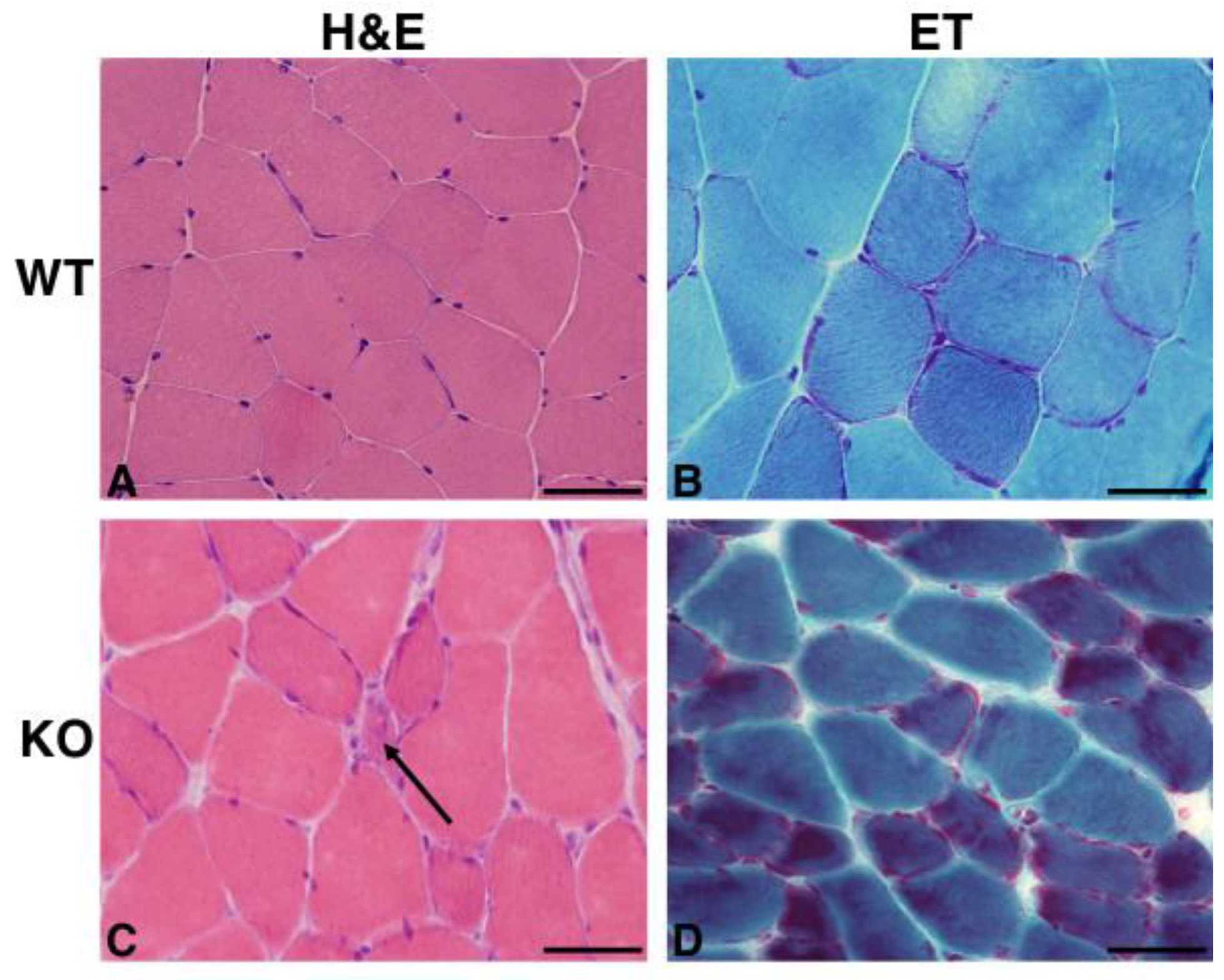
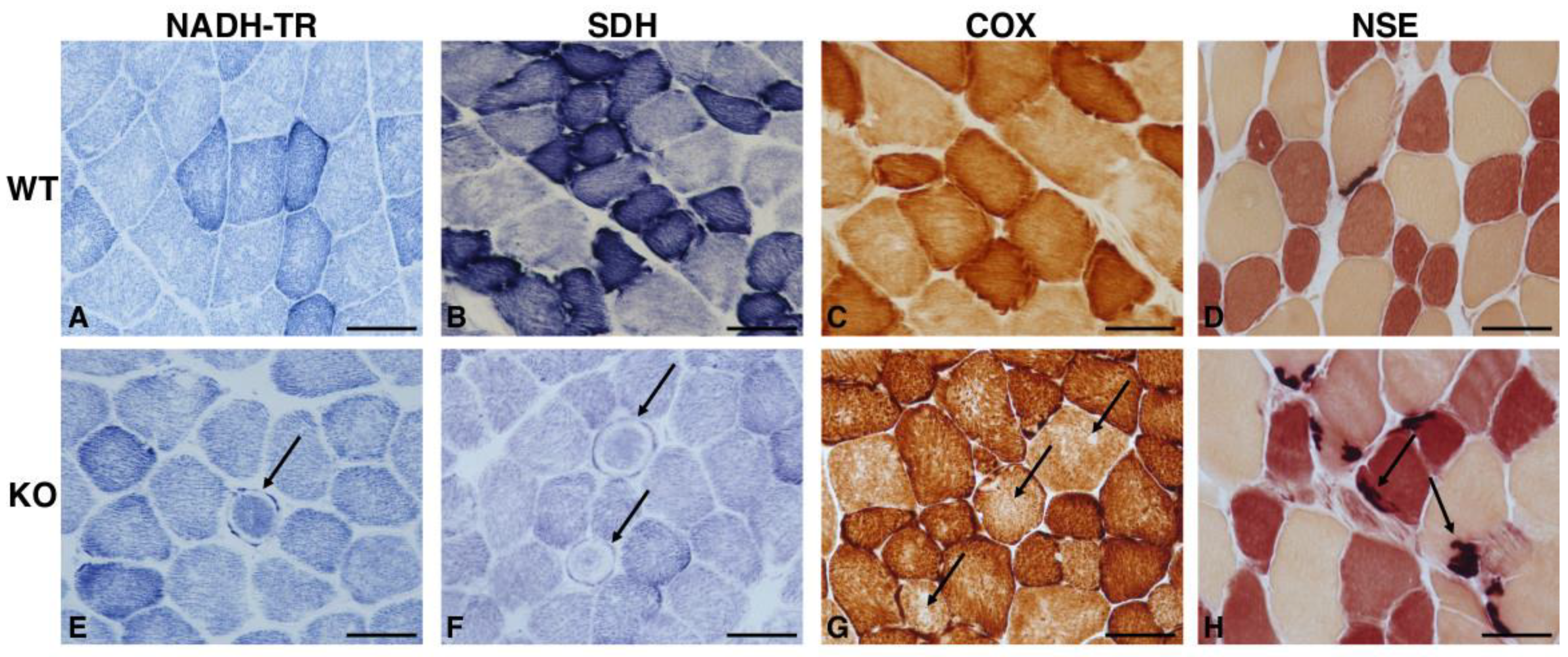
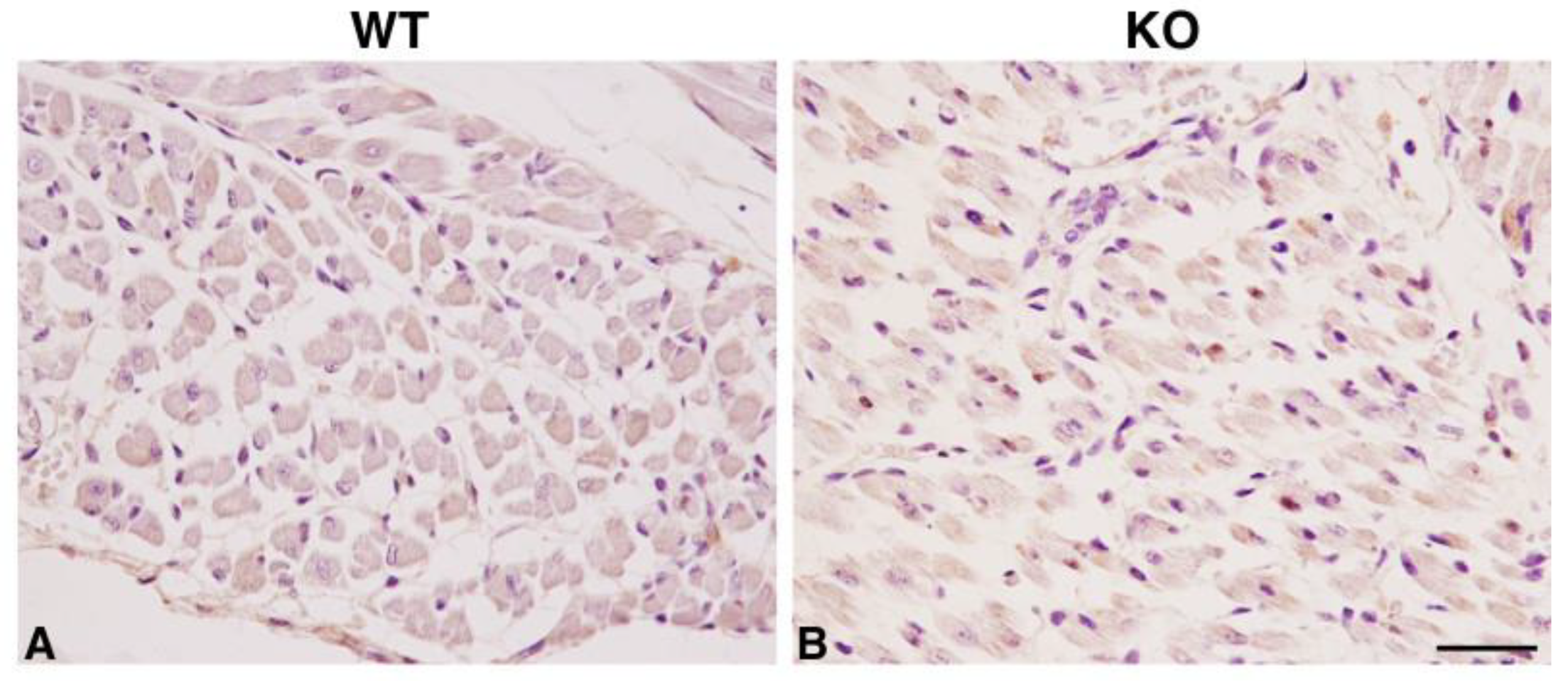
2.2. Hipk2-KO Mice Show a Mild Myopathic Phenotype
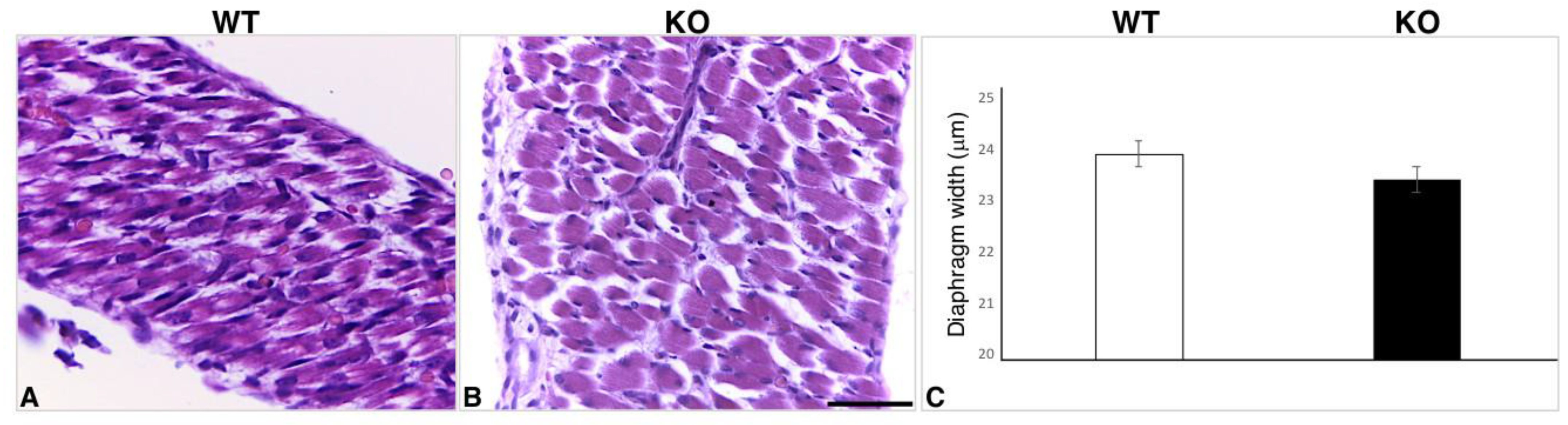
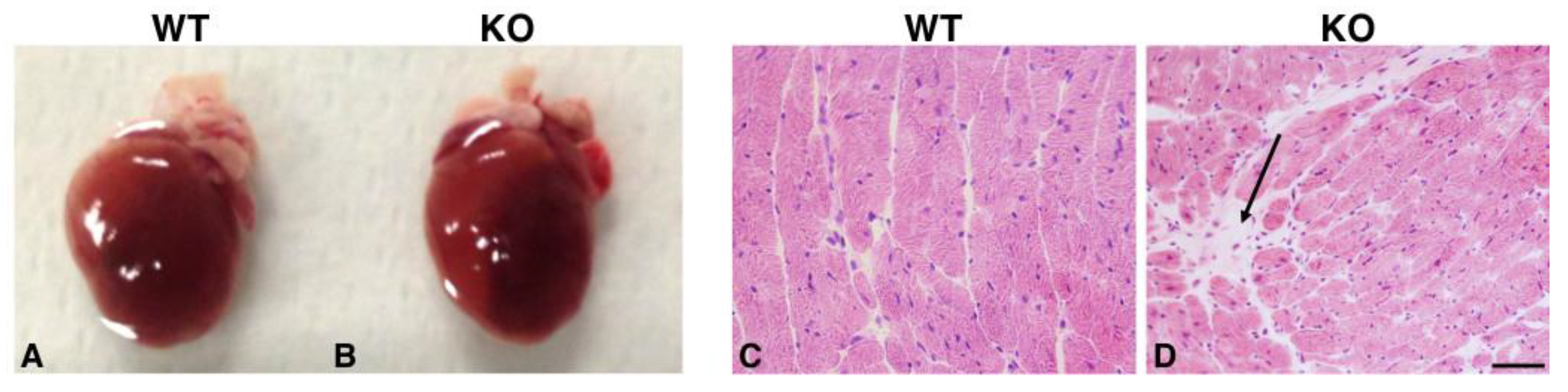
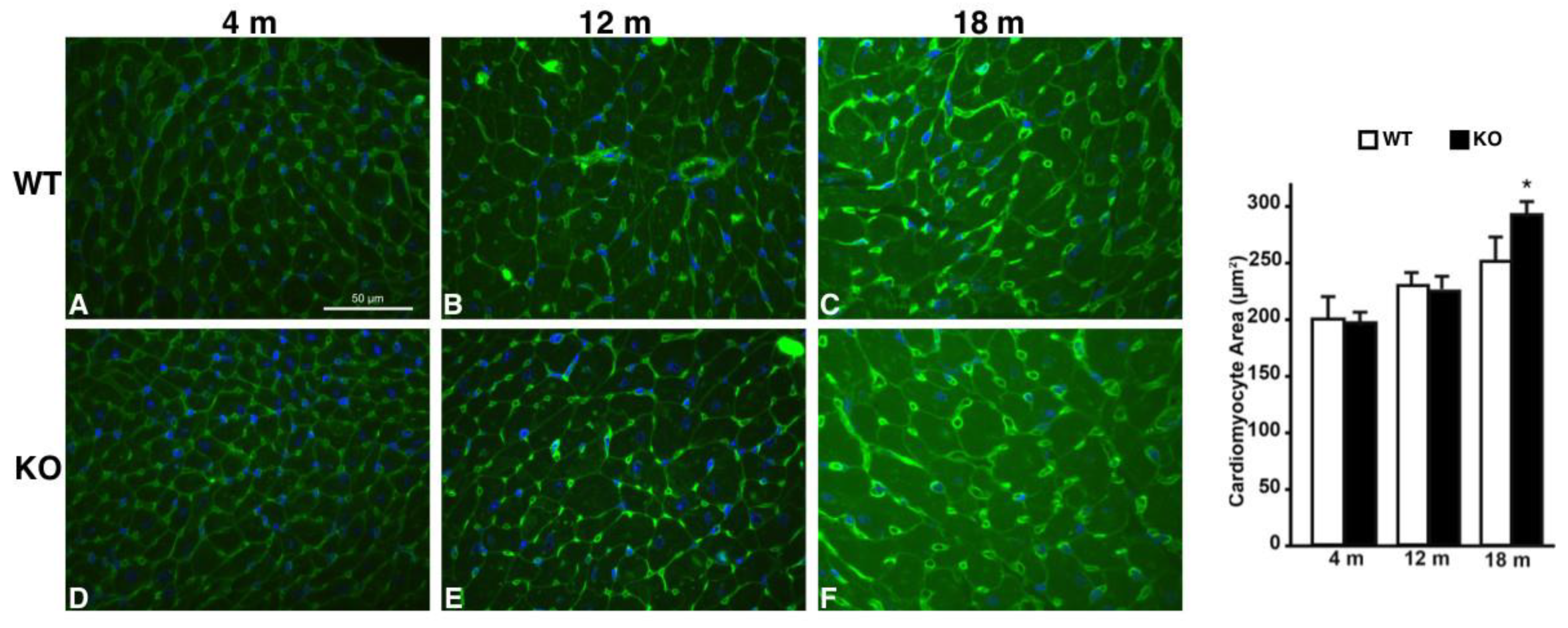
2.3. Hearts of Hipk2-KO Mice Show Increased Cardiomyocyte Cell Size and Fibrosis
3. Discussion
4. Materials and Methods
4.1. Animals and Ethics Statement
- Hipk2-Fw 5′-TAGTACCCAGGTGAACCTTGGAGT-3′
- Hipk2WT-Re 5′-GCTTCTCTCAAACTAAAGACCACGC-3′
- Hipk2KO-Re 5′-CAAAGGGTCTTTG
4.2. Morphology and Histochemistry
- -
- <10% of atrophic fibers/100 fibers at 20× magnification (mild)
- -
- 10–50% of atrophic fibers/100 fibers at 20× magnification (moderate)
- -
- >50% of atrophic fibers/100 fibers at 20× magnification (severe)
4.3. Immunohistochemistry
4.4. RNA Extraction and Real-Time PCR
- mouse-NPPA. Fw: 5′-CACAGATCTGATGGATTTCAAGA-3′
- mouse-NPPA. Re: 5′-CCTCATCTTCTACCGGCATC-3′
- mouse-NPPB. Fw: 5′-GTCAGTCGTTTGGGCTGTAAC-3′
- mouse-NPPB. Re: 5′-AGACCCAGGCAGAGTCAGAA-3′
- mouse-MYH7. Fw: 5′-CGGAAACTGAAAACGGAAAG-3′
- mouse-MYH7. Re: 5′-TCCTCGATCTTGTCGAACTTG-3′
- mouse-Rps18. Fw: 5′-AAACGGCTACCACATCCAAG-3′
- mouse-Rps18. Re: 5′-CCTCCAATGGATCCTCGTTAA-3′
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kim, Y.H.; Choi, C.Y.; Lee, S.J.; Conti, M.A.; Kim, Y. Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J. Biol. Chem. 1998, 273, 25875–25879. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, C.; Prodosmo, A.; Siepi, F.; Soddu, S. HIPK2: A multitalented partner for transcription factors in DNA damage response and development. Biochem. Cell Biol. 2007, 85, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.L.; Rodriguez-Gil, A.; Hornung, J. Integration of stress signals by homeodomain interacting protein kinases. Biol. Chem. 2014, 395, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.G.; Glas, C.; Bitomsky, N. HIPK2: A tumour suppressor that controls DNA damage-induced cell fate and cytokinesis. Bioessays 2013, 35, 55–64. [Google Scholar] [CrossRef]
- D’Orazi, G.; Rinaldo, C.; Soddu, S. Updates on HIPK2: A resourceful oncosuppressor for clearing cancer. J. Exp. Clin. Cancer Res. 2012, 31, 63. [Google Scholar] [CrossRef]
- Conte, A.; Pierantoni, G.M. Regulation of HIPK proteins by MicroRNAs. MicroRNA 2015, 4, 148–157. [Google Scholar] [CrossRef]
- Conte, A.; Pierantoni, G.M. Update on the regulation of HIPK1, HIPK2 and HIPK3 protein kinases by microRNAs. MicroRNA 2018, 7, 178–186. [Google Scholar] [CrossRef]
- Valente, D.; Bossi, G.; Moncada, A.; Tornincasa, M.; Indelicato, S.; Piscuoglio, S.; Karamitopoulou, E.D.; Bartolazzi, A.; Pierantoni, G.M.; Fusco, A.; et al. HIPK2 deficiency causes chromosomal instability by cytokinesis failure and increases tumorigenicity. Oncotarget 2015, 6, 10320–10334. [Google Scholar] [CrossRef]
- Wei, G.; Ku, S.; Ma, G.K.; Saito, S.; Tang, A.A.; Zhang, J.; Mao, J.H.; Appella, E.; Balmain, A.; Huang, E.J. HIPK2 represses beta-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 13040–13045. [Google Scholar] [CrossRef]
- Ritter, O.; Schmitz, M.L. Differential intracellular localization and dynamic nucleocytoplasmic shuttling of homeodomain-interacting protein kinase family members. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1676–1686. [Google Scholar] [CrossRef]
- Pierantoni, G.M.; Fedele, M.; Pentimalli, F.; Benvenuto, G.; Pero, R.; Viglietto, G.; Santoro, M.; Chiariotti, L.; Fusco, A. High mobility group I (Y) proteins bind HIPK2, a serine-threonine kinase protein which inhibits cell growth. Oncogene 2001, 20, 6132–6141. [Google Scholar] [CrossRef]
- Xiao, W.; Jing, E.; Bao, L.; Fan, Y.; Jin, Y.; Wang, A.; Bauman, D.; Li, Z.; Zheng, Y.L.; Liu, R.; et al. Tubular HIPK2 is a key contributor to renal fibrosis. JCI Insight 2020, 5, e136004. [Google Scholar] [CrossRef] [PubMed]
- D’Orazi, G.; Cecchinelli, B.; Bruno, T.; Manni, I.; Higashimoto, Y.; Saito, S.; Gostissa, M.; Coen, S.; Marchetti, A.; Del Sal, G.; et al. Homeodomain- interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol. 2002, 4, 11–19. [Google Scholar] [CrossRef]
- Pierantoni, G.M.; Bulfone, A.; Pentimalli, F.; Fedele, M.; Iuliano, R.; Santoro, M.; Chiariotti, L.; Ballabio, A.; Fusco, A. The homeodomain-interacting protein kinase 2 gene is expressed late in embryogenesis and preferentially in retina, muscle, and neural tissues. Biochem. Biophys. Res. Commun. 2002, 290, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Lavra, L.; Rinaldo, C.; Ulivieri, A.; Luciani, E.; Fidanza, P.; Giacomelli, L.; Bellotti, C.; Ricci, A.; Trovato, M.; Soddu, S.; et al. The loss of the p53 activator HIPK2 is responsible for galectin-3 overexpression in well differentiated thyroid carcinomas. PLoS ONE 2011, 6, e20665. [Google Scholar] [CrossRef]
- Hattangadi, S.M.; Burke, K.A.; Lodish, H.F. Homeodomain-interacting protein kinase 2 plays an important role in normal terminal erythroid differentiation. Blood 2010, 115, 4853–4861. [Google Scholar] [CrossRef][Green Version]
- Isono, K.; Nemoto, K.; Li, Y.; Takada, Y.; Suzuki, R.; Katsuki, M.; Nakagawara, A.; Koseki, H. Overlapping roles for homeodomain-interacting protein kinases hipk1 and hipk2 in the mediation of cell growth in response to morphogenetic and genotoxic signals. Mol. Cell. Biol. 2006, 26, 2758–2771. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, C.; Moncada, A.; Gradi, A.; Ciuffini, L.; D’Eliseo, D.; Siepi, F.; Prodosmo, A.; Giorgi, A.; Pierantoni, G.M.; Trapasso, F.; et al. HIPK2 controls cytokinesis and prevents tetraploidization by phosphorylating histone H2B at the midbody. Mol. Cell 2012, 47, 87–98. [Google Scholar] [CrossRef]
- Iacovelli, S.; Ciuffini, L.; Lazzari, C.; Bracaglia, G.; Rinaldo, C.; Prodosmo, A.; Bartolazzi, A.; Sacchi, A.; Soddu, S. HIPK2 is involved in cell proliferation and its suppression promotes growth arrest independently of DNA damage. Cell Prolif. 2009, 42, 373–384. [Google Scholar] [CrossRef]
- Wiggins, A.K.; Wei, G.; Doxakis, E.; Wong, C.; Tang, A.A.; Zang, K.; Luo, E.J.; Neve, R.L.; Reichardt, L.F.; Huang, E.J. Interaction of Brn3a and HIPK2 mediates transcriptional repression of sensory neuron survival. J. Cell Biol. 2004, 167, 257–267. [Google Scholar] [CrossRef]
- Zhang, J.; Pho, V.; Bonasera, S.J.; Holtzman, J.; Tang, A.T.; Hellmuth, J.; Tang, S.; Janak, P.H.; Tecott, L.H.; Huang, E.J. Essential function of HIPK2 in TGFbeta-dependent survival of midbrain dopamine neurons. Nat. Neurosci. 2007, 10, 77–86. [Google Scholar] [CrossRef]
- Anzilotti, S.; Tornincasa, M.; Gerlini, R.; Conte, A.; Brancaccio, P.; Cuomo, O.; Bianco, G.; Fusco, A.; Annunziato, L.; Pierantoni, G.M.; et al. Genetic ablation of homeodomain-interacting protein kinase 2 selectively induces apoptosis of cerebellar Purkinje cells during adulthood and generates an ataxic-like phenotype. Cell Death Dis. 2015, 6, e2004. [Google Scholar] [CrossRef]
- Guo, Y.; Sui, J.Y.; Kim, K.; Zhang, Z.; Qu, X.A.; Nam, Y.J.; Willette, R.N.; Barnett, J.V.; Knollmann, B.C.; Force, T.; et al. Cardiomyocyte Homeodomain-Interacting Protein Kinase 2 Maintains Basal Cardiac Function via Extracellular Signal-Regulated Kinase Signaling. Circulation 2019, 140, 1820–1833. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, M.Z.; Wen, S.H.; Sun, Y.F.; Jiang, P.H.; Wang, L.L.; Zhao, R.; Wang, C.L.; Jiang, S.K.; Guan, D.W. The distribution and Time-dependent expression of HIPK2 during the repair of contused skeletal muscle in mice. Histol. Histopathol. 2019, 34, 745–753. [Google Scholar]
- Gerlini, R.; Amendola, E.; Conte, A.; Valente, V.; Tornincasa, M.; Credendino, S.C.; Cammarota, F.; Gentile, C.; Di Guida, L.; Paladino, S.; et al. Double knock-out of Hmga1 and Hipk2 genes causes perinatal death associated to respiratory distress and thyroid abnormalities in mice. Cell Death Dis. 2019, 10, 747. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.J.; Sinclair, D.A.A.; Kim, H.Y.; Kinsey, S.D.; Yoo, B.; Shih, C.R.R.; Wong, K.K.K.; Krieger, C.; Harden, N.; Verheyen, E.M. Homeodomain-interacting protein kinase (Hipk) plays roles in nervous system and muscle structure and function. PLoS ONE 2020, 15, e0221006. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, L.; Hornung, J.; Kremmer, E.; Milanovic, M.; Schmitz, M.L. Homeodomain-interacting protein kinase 2-dependent repression of myogenic differentiation is relieved by its caspase-mediated cleavage. Nucleic Acids Res. 2013, 41, 5731–5745. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; Esposito, F.; De Martino, M.; Pirozzi, C.; Luciano, A.; Palma, G.; Raso, G.M.; Iovane, V.; Marzocco, S.; Fusco, A.; et al. Characterization of inflammatory infiltrate of ulcerative dermatitis in C57BL/6NCrl-Tg(HMGA1P6)1Pg mice. Lab. Anim. 2019, 53, 447–458. [Google Scholar] [CrossRef]
- Pagano, T.B.; Prisco, F.; De Biase, D.; Piegari, G.; Maurelli, M.P.; Rinaldi, L.; Cringoli, G.; Papparella, S.; Paciello, O. Muscular Sarcocystosis in Sheep Associated with Lymphoplasmacytic Myositis and Expression of Major Histocompatibility Complex Class I and II. Vet. Pathol. 2020, 57, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.D.; Xu, P.Z.; Chen, M.L.; Hahn-Windgassen, A.; Skeen, J.; Jacobs, J.; Sundararajan, D.; Chen, W.S.; Crawford, S.E.; Coleman, K.G.; et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003, 17, 1352–1365. [Google Scholar] [CrossRef]
- Rapa, S.F.; Prisco, F.; Popolo, A.; Iovane, V.; Autore, G.; Di Iorio, B.R.; Dal Piaz, F.; Paciello, O.; Nishijima, F.; Marzocco, S. Pro-Inflammatory Effects of Indoxyl Sulfate in Mice: Impairment of Intestinal Homeostasis and Immune Response. Int. J. Mol. Sci. 2021, 22, 1135. [Google Scholar] [CrossRef] [PubMed]
- Valentino, T.; Palmieri, D.; Vitiello, M.; Pierantoni, G.M.; Fusco, A.; Fedele, M. PATZ1 interacts with p53 and regulates expression of p53-target genes enhancing apoptosis or cell survival based on the cellular context. Cell Death Dis. 2013, 4, e963. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Paladino, S.; Bianco, G.; Fasano, D.; Gerlini, R.; Tornincasa, M.; Renna, M.; Fusco, A.; Tramontano, D.; Pierantoni, G.M. High mobility group A1 protein modulates autophagy in cancer cells. Cell Death Differ. 2017, 24, 1948–1962. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Biase, D.; Valente, V.; Conte, A.; Cammarota, F.; Boccella, N.; D’Esposito, L.; d’Aquino, I.; Paciello, O.; Paladino, S.; Pierantoni, G.M. Phenotypic Effects of Homeodomain-Interacting Protein Kinase 2 Deletion in Mice. Int. J. Mol. Sci. 2021, 22, 8294. https://doi.org/10.3390/ijms22158294
De Biase D, Valente V, Conte A, Cammarota F, Boccella N, D’Esposito L, d’Aquino I, Paciello O, Paladino S, Pierantoni GM. Phenotypic Effects of Homeodomain-Interacting Protein Kinase 2 Deletion in Mice. International Journal of Molecular Sciences. 2021; 22(15):8294. https://doi.org/10.3390/ijms22158294
Chicago/Turabian StyleDe Biase, Davide, Valeria Valente, Andrea Conte, Francesca Cammarota, Nicola Boccella, Lucia D’Esposito, Ilaria d’Aquino, Orlando Paciello, Simona Paladino, and Giovanna Maria Pierantoni. 2021. "Phenotypic Effects of Homeodomain-Interacting Protein Kinase 2 Deletion in Mice" International Journal of Molecular Sciences 22, no. 15: 8294. https://doi.org/10.3390/ijms22158294
APA StyleDe Biase, D., Valente, V., Conte, A., Cammarota, F., Boccella, N., D’Esposito, L., d’Aquino, I., Paciello, O., Paladino, S., & Pierantoni, G. M. (2021). Phenotypic Effects of Homeodomain-Interacting Protein Kinase 2 Deletion in Mice. International Journal of Molecular Sciences, 22(15), 8294. https://doi.org/10.3390/ijms22158294






